Abstract
The APOE ε4 allele is associated with cognitive deficits and brain atrophy in older adults, but studies in younger adults are mixed. We examined APOE genotype effects on cognition and brain structure in younger adults and whether genotype effects differed by age and with presence of depression. 157 adults (32% ε4 carriers, 46% depressed) between 20–50 years of age completed neuropsychological testing, 131 of which also completed 3T cranial MRI. We did not observe a direct effect of APOE genotype on cognitive performance or structural MRI measures. A significant genotype by age interaction was observed for executive function, where age had less of an effect on executive function in ε4 carriers. Similar interactions were observed for the entorhinal cortex, rostral and caudal anterior cingulate cortex and parahippocampal gyrus, where the effect of age on regional volumes was reduced in ε4 carriers. There were no significant interactions between APOE genotype and depression diagnosis. The ε4 allele benefits younger adults by allowing them to maintain executive function performance and volumes of cingulate and temporal cortex regions with aging, at least through age fifty years.
Keywords: Aging, depression, cognition, MRI, APOE
Background
There is a need to identify early biomarkers indicative of Alzheimer’s disease risk as the presentation of clinically evident symptoms lag behind the development of neuropathology (Jack, et al. 2013, Risacher and Saykin 2013). Supporting this goal, it is important to understand how genetic differences that increase Alzheimer’s disease risk may influence cognitive performance and brain structure in pre-clinical populations. This is particularly relevant to younger adult populations who could benefit from preventive strategies. When considering genotypic effects, it is important to test not only for direct relationships but also whether genetic influences alter the effect of age on clinical or imaging measures. Similarly, it is important to examine interactive effects of common conditions that are themselves associated with an increased risk of dementia, such as depression (Byers, et al. 2012).
Apolipoprotein E (apoE) is a very-low-density lipoprotein involved in transporting cholesterol from the blood. In the central nervous system, the apoE lipoprotein is involved in mobilization of cholesterol and fatty acids, aids in recovery from injury and promotes synaptic plasticity (Mahley and Huang 2012, Nathan, et al. 1994). The human APOE gene coding for this protein is located on chromosome 19 and has 3 allelic variants: ε2, ε3, and ε4. A large body of work demonstrates that the APOE ε4 allele is the primary genetic risk factor for the development of Alzheimer’s disease in later life (Corder, et al. 1993, Strittmatter, et al. 1993) and, compared with non-carriers, even healthy older ε4 carriers exhibit differences on cognitive testing (Caselli, et al. 2004). Similarly, healthy older ε4 carriers exhibit smaller volumes and greater rates of atrophy in multiple cortical and subcortical regions (Donix, et al. 2010, Honea, et al. 2009, Risacher, et al. 2010, Taylor, et al. 2014, Wishart, et al. 2006). In contrast, the APOE ε2 allele may have protective effects, as it is associated with reduced age-related cognitive decline and lower risk for developing Alzheimer’s disease (Suri, et al. 2013).
Studies investigating the APOE ε4 allele’s effects on cognition in younger adult carriers are less consistent. Compared with noncarriers, several reports found that younger adult ε4 carriers exhibited better cognitive performance (Schultz, et al. 2008, Yu, et al. 2000) on tasks of attention (Rusted, et al. 2013), verbal memory (delayed recall) (Mondadori, et al. 2007), verbal fluency (Alexander, et al. 2007, Marchant, et al. 2010), and prospective memory (Evans, et al. 2014, Marchant, et al. 2010). In context with observations in geriatric populations, these findings support a theory that APOE ε4 exhibits antagonistic pleiotropy, or different effects on cognition at different ages (Han and Bondi 2008). However, other studies failed to observe differences on cognitive performance based on APOE genotype (Bunce, et al. 2011, Ihle, et al. 2012, Jorm, et al. 2007, Matura, et al. 2014, Richter-Schmidinger, et al. 2011), raising questions about this theory and whether APOE genotype has any effect on cognition in younger adults.
There is less work in younger adults examining the effects of APOE genotype on brain structure. Studies examining small samples report that young adult and midlife ε4 carriers exhibit smaller hippocampal volumes (Lind, et al. 2006, O’Dwyer, et al. 2012) while others report that younger adult carriers exhibit increased entorhinal cortex volumes (DiBattista, et al. 2014). Analyses utilizing voxel-based approaches are mixed: some find no difference in cortical gray matter volume (Dowell, et al. 2013, Matura, et al. 2014) while others report thinner cortex in frontal regions (Fennema-Notestine, et al. 2011). Such differences may be reconciled by observations that the ε4 allele may be associated with regionally different effects, as a study examining gray matter networks in younger carriers found the ε4 allele was associated with gray matter reductions in frontal and cingulate regions, but relative increases in other regions including the hippocampus (Alexander, et al. 2012).
Just as aging is a risk factor for developing Alzheimer’s disease, so is depression (Byers, et al. 2012, Diniz, et al. 2013). Depression increases the risk of dementia even occurring over a decade before dementia onset, although the effect of earlier life depression is primarily in APOE ε4 carriers (Karlsson, et al. 2015). Early work concluded that APOE genotype is not a risk factor for depression (Steffens, et al. 2003), but recent longitudinal population-based studies found that the ε4 allele was associated with prospectively identified depressive symptoms (Skoog, et al. 2015). Studies in depressed older populations report that ε4 carriers exhibit greater depression severity, poorer cognitive performance, and smaller frontotemporal volumes (Corsentino, et al. 2009, Kim, et al. 2002, Lavretsky, et al. 2003, Niti, et al. 2009, Qiu, et al. 2009, Rajan, et al. 2014, Yuan, et al. 2010). This work suggests there is an interactive or additive effect of depression and APOE genotype on cognitive decline, brain atrophy and risk of dementia (Rajan, et al. 2014). It is unclear if such relationships exist in younger adult populations where depression is associated with structural alterations in frontal, temporal, and cingulate regions (Kempton, et al. 2011, Pizzagalli 2011) and neurocognitive differences (McClintock, et al. 2010). Given the overlap in depressive and cognitive symptoms in older adults, it is important to examine if depression and APOE genotype may have interactive effects in younger adults.
The purpose of the study was to examine influences of APOE genotype on cognition and gray matter structure in a young to midlife adult sample. Given the nature of the relationships between APOE ε4 genotype and pathological aging, we hypothesized we would observe an interactive effect between APOE genotype and age on cognition and brain structure. We also sought to determine whether APOE genotype’s effects on cognition and brain structure differs in individuals with current major depressive disorder.
Methods
Participants
Participants between the ages of 20 and 50 years were enrolled at Duke University and Vanderbilt University. Depressed participants had a DSM-IV diagnosis of recurrent MDD, as assessed by the Mini-International Neuropsychiatric Interview (MINI, version 5.0) (Sheehan, et al. 1998) and interview with a study psychiatrist. Additional entry criteria included onset of first depressive episode before age 35 years and a Montgomery-Asberg Depression Rating Scale (MADRS) (Montgomery and Asberg 1979) of 15 or greater. Entry criteria specified no antidepressant use in the last month; however, most subjects reported no antidepressant use for at least three months or longer. Eligible control subjects had neither any lifetime history of psychiatric disorders nor any history of psychotropic medication use.
Exclusion criteria included other lifetime DSM-IV Axis I disorders including substance abuse or dependence. Subjects were excluded for Axis II disorders determined by the Structured Clinical Interview for DSM-IV Axis II Personality Disorders (SCID-II) (First, et al. 1997). Additional exclusion criteria included: history of psychosis, acute suicidality, use of illicit substances in the last month, ECT in the last 6 months, a family history of bipolar disorder, any unstable medical condition, any history of neurological illness or head injury, or MRI contraindications.
Both the Duke University Medical Center Institutional Review Board and the Vanderbilt University Institutional Review Board approved this study. All study participants provided informed consent.
Neuropsychological Testing
Participants completed a battery of neuropsychological tests that covered cognitive domains relevant to depression. A trained psychometric technician supervised by a licensed clinical psychologist administered neuropsychological testing.
Similar to our past approach in late-life depression (Sheline, et al. 2010), we created rationally constructed composite domain variables from a broad test battery. To combine tasks, we created Z-scores for each measure based on the performance of all participants and averaged the Z-scores for all tests within each domain for each individual. Internal consistency for each domain was assessed using Cronbach’s coefficient alpha (CoA). This resulted in four composite neuropsychological measures: a) episodic memory (Logical Memory 1 and 2; Benton Visual Retention Test, number correct; Rey’s Verbal Learning Test, total I-V and total VII, CoA = 0.87); b) executive function (Controlled Oral Word Association [COWA] test (total score); Trails B time (reverse scored time to completion); verbal fluency (total phonological and semantic); Stroop Color-Word interference condition (number completed); CoA = 0.75); c) processing speed (Symbol-Digit Modality (number completed); Trails A (reverse scored time to completion); Stroop color naming condition (number completed); CoA = 0.70); and d) working memory (Digit span forward (number of trials correctly completed); digit span backward (number of trials correctly completed); CoA = 0.75).
MRI Acquisition
To simplify data analyses across two sites using different MRI scanners by different manufacturers, only subjects with MRI data acquired at Duke University was included in these analyses. Cranial MRI was performed using the eight-channel parallel imaging head coil on a whole-body MRI system (Trio, Siemens Medical Systems, Malvern, PA). Parallel imaging was employed with an acceleration factor of 2. Duplicate T1-weighted image sets were acquired during the scan session using a sagittal MPRAGE sequence with TR/TE = 2300/3.46 msec, a 240 Hz/pixel bandwidth, a 256 x 256 matrix, a 240 mm diameter field-of-view, 160 slices with a 1.2 mm slice thickness, yielding an image with voxel sizes of 0.9 x 0.9 x 1.2mm.
Analyses of Volumetric and Cortical Thickness MRI Data
All volumetric measures were calculated using FreeSurfer (version 5.1) software running in a high-performance Linux cluster environment. The FreeSurfer methods used to derive cortical and subcortical brain volumes have been previously described.(Dale, et al. 1999, Fischl, et al. 2002, Fischl, et al. 2004a, Fischl, et al. 2004b) Cortical parcellation used the Desikan-Killiany Atlas;(Desikan, et al. 2006) in each hemisphere, this method identified 33 cortical and 7 subcortical gray matter regions (nucleus accumbens, amygdala, caudate, hippocampus, pallidum, putamen, and thalamus). Intracranial volume was assessed using the method implemented in FreeSurfer. We visually inspected the data by overlaying the surfaces and subcortical segmentations over the T1 data. Individual slices in each orientation were assessed for errors. No manual corrections were needed.
We also tested for differences in cortical thickness using Freesurfer’s QDEC module. This was an atheoretical approach to test for differences not captured in atlas-based comparisons. In this method, cortical thickness is computed as the shortest distance between any point on the pial surface and the gray/white boundary and vice-versa; these two values are averaged (Fischl and Dale 2000). Maps were smoothed using the standard Gaussian kernel of 10mm. We used a general linear model (GLM) to test for differences in cortical thickness while correcting for multiple comparisons using the Monte Carlo simulation method. Data were tested against an empirical null distribution of maximum cluster size by running 10,000 synthesized Gaussian noise simulations with an initial threshold of p < 0.01. Right and left hemispheres were tested separately.
Selection of a priori regions of interest
Selection of a priori volumetric regions for primary analyses was based on work that identified APOE genotype effects on regional volumes. These included: the amygdala, hippocampus, entorhinal cortex, parahippocampal gyrus, precuneus, and cingulate cortex (Donix, et al. 2010, Honea, et al. 2009, Hostage, et al. 2014, O’Dwyer, et al. 2012, Reinvang, et al. 2013, Risacher, et al. 2010, Taylor, et al. 2014). For the cingulate cortex, we examined anterior divisions due to its implication in depression (Pizzagalli 2011) and posterior divisions due to APOE genotype’s influence on metabolism in midlife populations (Protas, et al. 2013).
Genotyping
Genotyping was conducted on blood samples. APOE alleles (corresponding to allele combinations at single nucleotide polymorphism (SNP) +3937/rs429358 and SNP+4075/rs7412) were genotyped using the ABI 7900 Taqman (Applied Biosystems, Foster City, California, USA) system. The two APOE single nucleotide polymorphisms exist at amino acid 112 and 158, which are targeted by the Taqman probes. The individual genotypes at the two sites are then combined to create a single standard APOE genotype.
Analytic Plan
Our a priori hypotheses involved interactive effects between APOE ε4 genotype, age, and depression diagnosis, so we took a stepwise approach. All analyses were conducted with SAS 9.4 (Cary, NC) using PROC MIXED. Our primary analyses included all participants regardless of APOE genotype, however as the APOE ε2 allele may have protective effects (Suri, et al. 2013) we also conducted follow-up analyses where ε2 carriers were excluded.
First, we created initial models that did not include interaction terms but instead tested for a direct effect of APOE ε4 genotype. In these models, either cognitive domain z-scores or MRI measures were the dependent variable, with APOE ε4 genotype (presence or absence of an ε4 allele), diagnosis (depressed / never depressed), age, sex, and race (white or minority). Analyses of cognitive data included the additional covariate of years of education, while analyses of imaging data included total intracranial volume and hemisphere. After testing for initial effects, we subsequently created additional models that included either an APOE by age interaction term or an APOE by diagnosis interaction term. Final models tested for a three-way age by APOE by depression diagnosis interaction.
For analyses of cognition, we focused on our a priori plan of examining the z-transformed summary measures of processing speed, working memory, episodic memory, and executive function. For domains with a statistically significant genotype effect or interaction term, we planned for subsequent analyses that would examine the individual z-transformed tests within those domains.
For analyses of volumetric MRI data, we initially focused on our a priori regions as described above. Subsequent analyses also considered other brain regions not specified in our a priori hypotheses but quantified by our image analysis method. Given the large number of regions identified, for analyses of regions not included in our a priori hypotheses we corrected for multiple comparisons using the false discovery rate (FDR) method. When testing for differences in cortical thickness using FreeSurfer’s QDEC module, we used a general linear model (GLM) to test for differences between genotype groups, including age as an additional independent variable. This allowed us to examine the direct effects of APOE genotype on cortical thickness as well as test for statistical interactions between genotype and age.
To elucidate the relationship between cognition and structure, we examined cognitive domains and brain regions identified as exhibiting statistically significant relationship in the above planned analyses. In these analyses, we examined the z-transformed cognitive domain score as the dependent variable. Independent variables included age, sex, diagnosis, race, and education. We also included brain regions as independent variables, utilizing the mean volume across the hemispheres, normalized for total intracranial volume.
Results
APOE Genotype Effects on Cognitive Performance
The sample for cognitive analyses included participants recruited at both the Duke and Vanderbilt sites. This consisted of 107 APOE ε4 negative individual (48% depressed, N=51) and 50 APOE ε4 positive individuals (42% depressed, N = 21; Table 1). There was no significant difference in APOE ε4 frequency between diagnostic groups (χ2 = 0.44, p = 0.5070). The complete genotypic breakdown consisted of: 2/2, N=2; 2/3, N=14; 2/4, N=4; 3/3, N=87; 3/4, N=45; 4/4, N=5). The depressed cohort was significantly older and exhibited greater severity of depressive symptoms by MADRS than the nondepressed cohort, but there was no significant difference in age or MADRS score within each diagnostic cohort between those who were and were not ε4 carriers. After controlling for demographic covariates, we did not observe any statistically significant differences in the z-transformed cognitive domain scores between individuals identified by both genotype and diagnosis (Table 1).
Table 1.
Demographic and cognitive domain score differences by APOE genotype and diagnostic group
| APOE ε4 − Depressed (N = 51) | APOE ε4 − Nondepressed (N = 56) | APOE ε4 + Depressed (N = 21) | APOE ε4 + Nondepressed (N = 29) | Test Statistic | p value | |
|---|---|---|---|---|---|---|
| Age (y) | 36.5 (8.8) | 29.2 (8.2) | 36.8 (8.8) | 31.4 (10.1) | F = 7.69 | p < 0.0001 |
| Sex (% female) | 66.7% (34) | 58.9% (33) | 76.2% (16) | 68.9% (20) | χ2 = 2.32 | p = 0.5088 |
| Race (% Caucasian) | 64.7% (33) | 51.8% (29) | 61.9% (13) | 51.7% (15) | χ2 = 2.39 | p = 0.4955 |
| Education (y) | 15.5 (2.5) | 15.8 (1.9) | 14.7 (2.1) | 16.1 (2.4) | F = 1.83 | p = 0.1434 |
| MADRS | 23.9 (4.2) | 0.6 (0.9) | 24.4 (5.2) | 1.31 (1.4) | F = 692.83 | p < 0.0001 |
| Episodic Memory | −0.76 (4.16) | 0.92 (4.02) | −1.35 (4.22) | 0.62 (3.40) | F = 0.38 | p = 0.7675 |
| Executive Function | −0.95 (3.07) | 0.49 (3.12) | 0.13 (2.97) | 0.40 (3.06) | F = 1.67 | p = 0.1762 |
| Processing Speed | −0.76 (2.35) | 0.57 (2.39) | −0.28 (1.89) | 0.30 (2.46) | F = 1.21 | p = 0.3094 |
| Working Memory | −0.28 (1.68) | 0.48 (1.75) | −0.10 (1.68) | −0.15 (1.72) | F = 1.13 | p = 0.3383 |
Continuous measures presented as mean (standard deviation). Group differences in demographic variables (age, education, MADRS score) tested using analysis of variance (ANOVA) where the model had a total 156 degrees of freedom. Categorical variables presented as percentage (number), differences tested using chi-square with 3 degrees of freedom. Cognitive domain data presented as z-transformed scores, mean (SD). These cognitive domain models tested for 4-way group differences and included 147 degrees of freedom, controlling for age, sex, education, and race.
In primary models without interaction terms, we did not find direct effects of either APOE genotype or diagnosis on any cognitive domain score. We also did not observe any statistically significant interactions between depression diagnosis and genotype on cognitive domain scores. Similarly, we did not find statistically significant three-way interactions between APOE genotype, age, and depression.
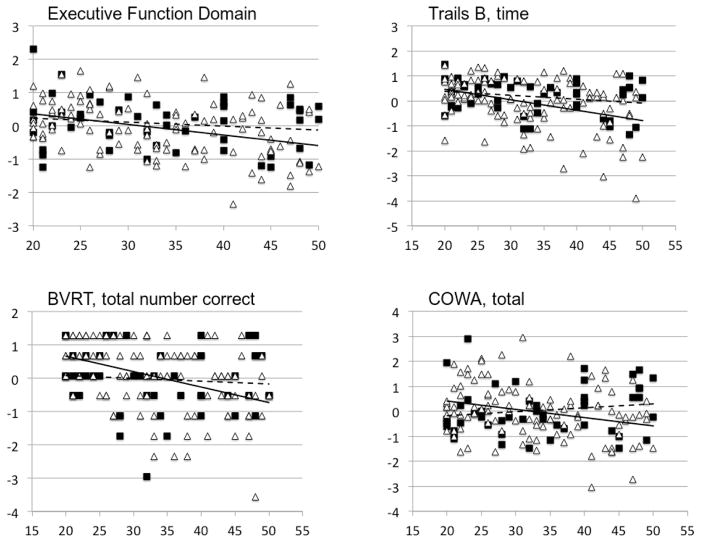
On testing for an interaction between age and APOE genotype, we found a significant interaction for executive function (F1,149 = 4.23, p = 0.0415) and a trend for episodic memory (F1,149 = 3.16, p = 0.0779) but no difference for other domains (working memory: F1,149 = 1.90, p = 0.1707; processing speed: F1,149 = 0.01, p = 0.9038). In analysis of executive function, APOE ε4 noncarriers exhibited worse executive function with increasing age than did the ε4 carriers (Figure 1). In other words, presence of the APOE ε4 allele reduced the effect of age on executive function.
Figure 1.
Differential effect of age between APOE ε4 carriers and noncarriers on z-transformed cognitive performance
Data presented are the domain and tests exhibiting statistically significant age by genotype interactive effects on performance, after controlling for depression diagnosis, sex, race, and education. The executive function domain is the averaged z-score over multiple neuropsychological tests, including Trails Part B, COWA, verbal fluency and the Stroop color-word condition. The individual tests presented are those with statistically significant interactions between genotype and age, and included BVRT from the episodic memory domain. APOE ε4 noncarriers are triangles / solid line, APOE ε4 carriers are squares / dashed line. Z-transformed test performance score on the y-axis, age in years on x-axis.
We conducted additional analyses for the executive function and episodic memory domains, examining the z-transformed individual test items included in the composite domain scores. Controlling for the same covariates as in primary models, for the executive function domain we found significant interactions between age and genotype for Trails B (time to completion; F1,149 = 4.39, p = 0.0378) and COWA total score (F1,149 = 8.97, p = 0.0032), but not verbal fluency (F1,149 = 0.48, p = 0.4880) or Stroop color-word interference (F1,149 = 0.08, p = 0.7722). For the episodic memory domain, there was a significant interaction only for the Benton Visual Retention Test (number correct; F1,149 = 7.00, p = 0.0090). The interactions were similar to those observed with the executive function composite score, where ε4 carriers exhibited less reduction in performance with age (Figure 1). In the absence of the age by genotype interaction term, APOE genotype did not have a direct effect on test measures.
Secondary analyses where we excluded the twenty ε2 carriers were largely concordant with the results described above. We continued to observe a significant interaction between age and genotype for executive function (F1,129 = 5.36, p = 0.0222), but there was no support for a trend with episodic memory (F1,129 = 2.63, p = 0.1074). Analyses of specific cognitive tests resulted in findings similar to those reported above, aside from Trails B time to completion that no longer achieved statistical significance (F1,129 = 3.84, p = 0.0523). Again, ε4 carriers exhibited less reduction in performance with age and in the absence of the age by genotype interaction term, APOE genotype did not have a direct effect on any test measure.
APOE Genotype Effects on Regional Brain Volumes
To simplify analyses of MRI data across sites with different MRI manufacturers, we only included MRI data from the Duke site. Notably, 9 participants recruited at Duke either could not complete MRI or had MRI data of poor quality and so could not be included in analyses. The sample with MRI and APOE genotype data thus included 58 individuals with MDD and 73 never-depressed comparison subjects. There was no difference in APOE ε4 frequency between depressed and nondepressed groups in the MRI sample (ε4 −, MDD N=41; ε4 −, no MDD N=47; ε4 +, MDD N=17; ε4 +, no MDD N=26; χ2 = 0.58, p = 0.4452). Other demographic comparisons were comparable to the larger sample (Supplemental Table 1).
In primary models examining a priori defined regional brain volumes, we did not find direct effects of either APOE genotype or depression diagnosis on any region. We also did not observe any statistically significant interactions between depression diagnosis and genotype on regional volumes. Similarly, we did not find statistically significant three-way interactions between APOE genotype, age, and depression.
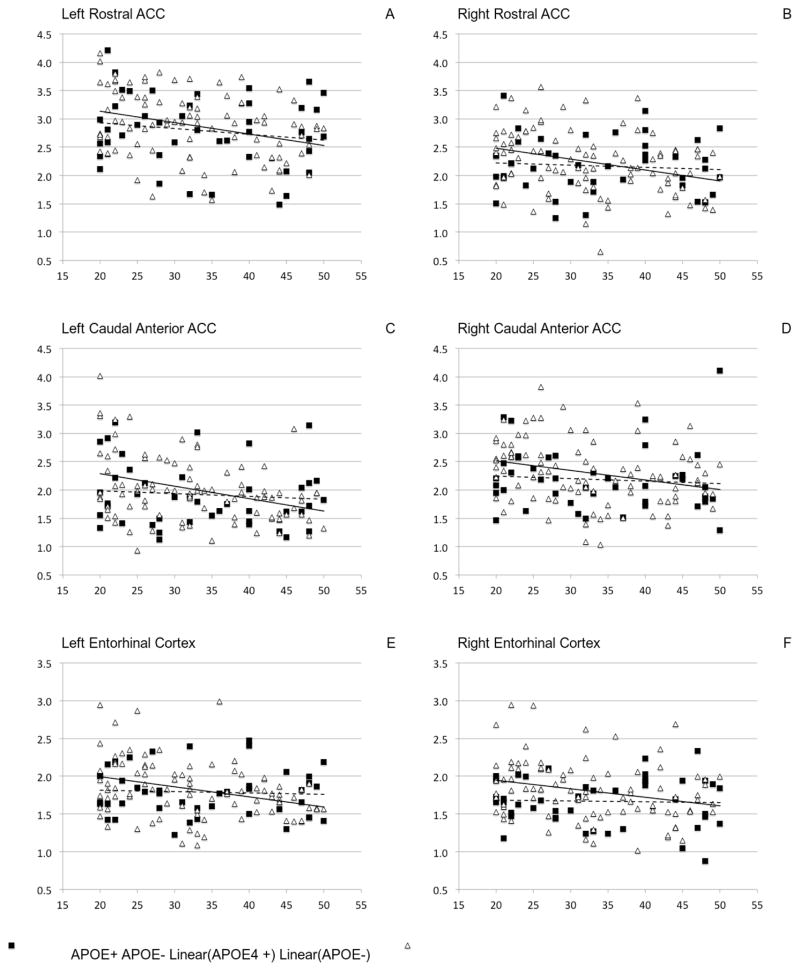
In analyses of our a priori defined regions, we observed statistically significant interactions between APOE genotype and age for the entorhinal cortex, rostral and caudal cingulate cortex (Table 2). Similar to what is observed in the cognitive analyses, the APOE ε4 allele reduced the effect of age on regional volumes (Figure 2). In other words, the relationship between age and volume exhibits a more negative slope in APOE ε4 noncarriers compared with ε4 carriers. These findings persisted when removing the seventeen APOE ε2 allele carriers, and we additionally observed an interaction between age and genotype for the parahippocampal gyrus.
Table 2.
Interactive effects of APOE genotype and age on a priori brain region volumes
| All MRI Subjects (N= 131) | APOE ε3 and ε4 carriers only (N= 114) | |||
|---|---|---|---|---|
| Region | F value | p value | F value | p value |
| Amygdala | F = 3.37 | p = 0.0685 | F = 2.09 | p = 0.1514 |
| Cingulate, Caudal Anterior | F = 5.94 | p = 0.0161 | F = 4.18 | p = 0.0434 |
| Cingulate, Rostral Anterior | F = 5.08 | p = 0.0260 | F = 3.94 | p = 0.0496 |
| Cingulate, Isthmus | F = 0.14 | p = 0.7057 | F = 0.62 | p = 0.4338 |
| Cingulate, Posterior | F = 0.86 | p = 0.3565 | F = 1.16 | p = 0.2838 |
| Entorhinal cortex | F = 6.64 | p = 0.0111 | F = 5.35 | p = 0.0226 |
| Hippocampus | F = 3.10 | p = 0.0808 | F = 2.71 | p = 0.1025 |
| Parahippocampal gyrus | F = 3.06 | p = 0.0828 | F = 3.92 | p = 0.0503 |
| Precuneus | F = 0.45 | p = 0.5028 | F = 0.15 | p = 0.7009 |
Variables for the entire sample analyses had 1,129 df. Variables in analyses where ε2 carriers were excluded had 1,112 df. Mixed models included the independent variables of APOE ε4 genotype, diagnosis (depressed / never depressed), age, sex, race, intracranial volume, and hemisphere.
Figure 2.
Differences in age effects on regional brain volumes between APOE ε4 positive and negative individuals
Data presented are those regions exhibiting statistically significant age by genotype interactive effects on regional volumes, after controlling for depression diagnosis, sex, race, intracranial volume and hemisphere. Similar findings were observed unilaterally for the hippocampus, parahippocampus, and amygdala (figures not shown). APOE ε4 noncarriers are triangles / solid line, APOE ε4 carriers are squares / dashed line. Z-transformed test performance score on the y-axis, age in years on x-axis. Regional volume in milliliters on the y-axis, age in years on x-axis.
Additional analyses examined other regions measured by FreeSurfer that were not part of our a priori hypotheses, where we again created similar models testing for direct and interactive effects of APOE genotype. After FDR correction for multiple comparisons, we observed neither statistically significant primary effects nor interactive effects of APOE genotype.
APOE Genotype Effects on Cortical Thickness
We found no statistically significant effects where APOE genotype was directly associated with differences in cortical thickness. When examining age by genotype interactions, we did not observe any regions where the effect of age on cortical thickness differed significantly between the APOE genotype groups. Similarly, we observed no differences when testing for different effects of genotype between depressed and nondepressed participants.
Relationship between Cognitive Domains and Regional Brain Volumes
Finally we sought to examine the relationship between cognitive domains and regional volumes identified in the above analyses. These analyses focused on the z-transformed executive function domain score, examining the entorhinal cortex, parahippocampal gyrus, and caudal and rostral anterior cingulate cortex volumes. After controlling for demographic differences, normalized parahippocamapal gyrus (F1,124 = 7.66, p = 0.0065) and rostral anterior cingulate cortex volumes (F1,124 = 6.18, p = 0.0143) were significantly associated with executive function. There was also a trend for the caudal anterior cingulate cortex volume (F1,124 = 3.86, p = 0.0518) but no relationship with entorhinal cortex volume (F1,124 = 2.06, p = 0.1536).
Discussion
Our major finding is that in a younger adult population, the APOE ε4 allele reduces the effect of aging on executive function and regional brain volumes in temporal and anterior cingulate regions. Overall APOE ε4 carriers exhibited less reduction in executive function and regional volumes with age than did APOE ε4 noncarriers. We did not observe a direct effect of APOE genotype on cognition or brain morphology. We also did not find evidence to support that depression diagnosis interacted with APOE genotype to result in greater deficits in cognition or altered brain morphology. These findings were persistent across the entire sample, then also when we limited the sample to individuals who did not carry the ε2 allele.
Integration with aging literature
Our analyses of APOE genotype’s effect in younger adults are concordant with and extend past work. These findings support the theory of antagonistic pleiotropy (Han and Bondi 2008), or that the ε4 allele has different effects on cognition at different ages. This may represent superior compensatory function during midlife. Had our sample’s age extended past age 50 years, we anticipate that we would observe a downward curve in the age-cognition relationship in older ε4 carriers. Recent work suggests this more rapid rate of cognitive decline in ε4 carriers occurs around age 70 years (Jack, et al. 2015), well beyond our current sample.
There is still uncertainty about what cognitive domains may be differentially affected by APOE genotype. We found that genotype influenced the effect of age in our executive function domain (specifically performance on the Trails B and COWA) and visual memory measured with the BVRT. These findings are supported by past studies (Marchant, et al. 2010), although APOE effects on attention (Rusted, et al. 2013) may also be influencing performance on our executive function tests.
Our neuroimaging findings follow a pattern similar to our cognitive findings. These results are discrepant with a previous study that did not observe interactive effects of APOE genotype and age on structural measures (Filippini, et al. 2011). However, that study examined a smaller sample with two distinct age populations rather than a young to midlife adult sample with a continuous age range.
This work supports that aging is associated with poorer cognitive performance and smaller brain volumes, even in young to midlife adults. Our observations occur before ages where significant increases in amyloidosis occur (Jack, et al. 2015) and such aging effects are reduced in APOE ε4 carriers. Brain aging in younger adults may be due to multiple causes, including vascular risk factors, early tauopathy (Crary, et al. 2014, Jack, et al. 2013), or occur in the absence of any specific pathology (Jagust 2013). Further work is needed to better understand what processes are modified in APOE ε4 allele carriers and how that benefit to cognition and brain structure is obtained prior to later amyloid deposition and cognitive decline.
Integration with depression literature
It is clear that neuropathology related to cognitive decline can occur decades before the onset of clinical symptoms (Jack, et al. 2013) and that earlier-life onset is associated with increased dementia risk (Byers, et al. 2012). However, there are few studies examining the relationship between depression and risk factors for Alzheimer’s disease in younger adult populations. We observed neither differential effects of APOE genotype nor different genotype by age interactions between depressed and nondepressed individuals. This does not support a theory that cognitive or morphological differences related to APOE genotype may adversely affect neural circuitry in such a way that it would increase a predisposition to depression. Overall this suggests that the ultimate pathways by which depression increases dementia risk does not occur through apolipoprotein E mediated mechanisms in younger adults. However, the underlying nature of the relationship between depression and dementia remains an important question and alternative pathways should be considered, such as the relationship between depression and proinflammatory processes.
Study strengths and limitations
The study has several strengths, including a large sample size and comprehensive neuropsychological testing battery. Moreover image analyses utilized a combination of hypothesis-testing and hypothesis-generating approaches. These methods are complementary, as we found no relationship between study measures and cortical thickness, but did find volumetric differences in cingulate cortex regions and subcortical structures. Finally, in post-hoc analyses, we demonstrated a relationship between the identified cognitive domain (executive function) and identified brain regions (parahippocampal gyrus and rostral anterior cingulate cortex).
The study also has limitations. Although we have a broad cognitive battery, specific domains such as attention (Rusted, et al. 2013) were not assessed. Additionally, this was a well-educated sample and it is unclear if these results would generalize to populations with lower education. Moreover, although we examine age effects, this remains a cross-sectional study. Ultimately longitudinal studies are necessary to examine change in such measures and aging trajectories. Finally, there are notable limitations related to the sample, including a lower number of APOE ε4 allele carriers than ε4 noncarriers, although this limitation applies to much of the existent literature. The age difference between the diagnostic cohorts is also important. The strong effect of age on both cognitive and brain aging may have negatively affected our ability to detect a diagnosis by genotype interaction.
It should be emphasized that despite examining a relatively large sample, we made numerous statistical comparisons. For our a priori hypotheses, we did not control for multiple comparisons, thus increasing the risk for a type 1 error. Given the significance levels observed, correcting for multiple comparisons would have resulted in null findings. Thus while our findings fit within past literature, they should be viewed cautiously.
Despite these limitations, this study advances our understanding of the aging process by demonstrating that the APOE ε4 allele mitigates the effect of age on cognitive processes and brain structure in early- to midlife-adulthood. This occurs prior to significant increases in amyloid deposition as detected by PET scanning (Jack, et al. 2015), suggesting that the ε4 allele may modify other processes influencing brain aging. More work is needed to determine what those processes are, but also to determine how depression may increase risk of dementia.
Supplementary Material
Acknowledgments
This project was supported by National Institute of Mental Health (NIMH) grant R01 MH077745. It was further supported by CTSA award UL1TR000445 from the National Center for Advancing Translational Sciences (NCATS) and conducted in part using the resources of the Advanced Computing Center for Research and Education at Vanderbilt University, Nashville, TN.
Footnotes
Disclosures
All authors (including Dr. Taylor, Mr. Boyd, Ms. Turner, Mr. McQuoid, Dr. Ashley-Koch, Dr. MacFall, Dr. Saleh, and Dr. Potter) declare they have no conflict of interest.
Informed Consent Statement
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, and the applicable revisions at the time of the investigation. Informed consent was obtained from all patients for being included in the study.
Preliminary data were previously presented at the 53rd Annual Meeting of the American College of Neuropsychopharmacology.
References
- Alexander DM, Williams LM, Gatt JM, Dobson-Stone C, Kuan SA, Todd EG, et al. The contribution of apolipoprotein E alleles on cognitive performance and dynamic neural activity over six decades. Biol Psychol. 2007;75(3):229–238. doi: 10.1016/j.biopsycho.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Alexander GE, Bergfield KL, Chen K, Reiman EM, Hanson KD, Lin L, et al. Gray matter network associated with risk for Alzheimer’s disease in young to middle-aged adults. Neurobiol Aging. 2012;33(12):2723–2732. doi: 10.1016/j.neurobiolaging.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunce D, Anstey KJ, Burns R, Christensen H, Easteal S. Does possession of apolipoprotein E varepsilon4 benefit cognitive function in healthy young adults? Neuropsychologia. 2011;49(7):1693–1697. doi: 10.1016/j.neuropsychologia.2011.02.042. [DOI] [PubMed] [Google Scholar]
- Byers AL, Covinsky KE, Barnes DE, Yaffe K. Dysthymia and depression increase risk of dementia and mortality among older veterans. Am J Geriatr Psychiatry. 2012;20(8):664–672. doi: 10.1097/JGP.0b013e31822001c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caselli RJ, Reiman EM, Osborne D, Hentz JG, Baxter LC, Hernandez JL, Alexander GG. Longitudinal changes in cognition and behavior in asymptomatic carriers of the APOE e4 allele. Neurology. 2004;62(11):1990–1995. doi: 10.1212/01.wnl.0000129533.26544.bf. [DOI] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261(5123):921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- Corsentino EA, Sawyer K, Sachs-Ericsson N, Blazer DG. Depressive symptoms moderate the influence of the apolipoproteine epsilon4 allele on cognitive decline in a sample of community dwelling older adults. Am J Geriatr Psychiatry. 2009;17(2):155–165. doi: 10.1097/JGP.0b013e31818f3a6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crary JF, Trojanowski JQ, Schneider JA, Abisambra JF, Abner EL, Alafuzoff I, et al. Primary age-related tauopathy (PART): a common pathology associated with human aging. Acta neuropathologica. 2014;128(6):755–766. doi: 10.1007/s00401-014-1349-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9(2):179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- DiBattista AM, Stevens BW, Rebeck GW, Green AE. Two Alzheimer’s disease risk genes increase entorhinal cortex volume in young adults. Front Hum Neurosci. 2014;8:779. doi: 10.3389/fnhum.2014.00779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diniz BS, Butters MA, Albert SM, Dew MA, Reynolds CF., 3rd Late-life depression and risk of vascular dementia and Alzheimer’s disease: systematic review and meta-analysis of community-based cohort studies. Br J Psychiatry. 2013;202(5):329–335. doi: 10.1192/bjp.bp.112.118307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donix M, Burggren AC, Suthana NA, Siddarth P, Ekstrom AD, Krupa AK, et al. Longitudinal changes in medial temporal cortical thickness in normal subjects with the APOE-4 polymorphism. Neuroimage. 2010;53(1):37–43. doi: 10.1016/j.neuroimage.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowell NG, Ruest T, Evans SL, King SL, Tabet N, Tofts PS, Rusted JM. MRI of carriers of the apolipoprotein E e4 allele-evidence for structural differences in normal-appearing brain tissue in e4+ relative to e4- young adults. NMR Biomed. 2013;26(6):674–682. doi: 10.1002/nbm.2912. [DOI] [PubMed] [Google Scholar]
- Evans S, Dowell NG, Tabet N, Tofts PS, King SL, Rusted JM. Cognitive and neural signatures of the APOE E4 allele in mid-aged adults. Neurobiol Aging. 2014;35(7):1615–1623. doi: 10.1016/j.neurobiolaging.2014.01.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fennema-Notestine C, Panizzon MS, Thompson WR, Chen CH, Eyler LT, Fischl B, et al. Presence of ApoE epsilon4 allele associated with thinner frontal cortex in middle age. J Alzheimers Dis. 2011;26(Suppl 3):49–60. doi: 10.3233/JAD-2011-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippini N, Ebmeier KP, MacIntosh BJ, Trachtenberg AJ, Frisoni GB, Wilcock GK, et al. Differential effects of the APOE genotype on brain function across the lifespan. Neuroimage. 2011;54(1):602–610. doi: 10.1016/j.neuroimage.2010.08.009. [DOI] [PubMed] [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Williams JB, Benjamin LS. Structured Clinical Interview for DSM-IV Axis II Personality Disorders (SCID-II) American Psychiatric Press, Inc; Washington, D.C: 1997. [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, van der Kouwe AJ, Makris N, Segonne F, Quinn BT, Dale AM. Sequence-independent segmentation of magnetic resonance images. Neuroimage. 2004a;23(Suppl 1):S69–84. doi: 10.1016/j.neuroimage.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, et al. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004b;14(1):11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Han SD, Bondi MW. Revision of the apolipoprotein E compensatory mechanism recruitment hypothesis. Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2008;4(4):251–254. doi: 10.1016/j.jalz.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Honea RA, Vidoni E, Harsha A, Burns JM. Impact of APOE on the healthy aging brain: a voxel-based MRI and DTI study. J Alzheimers Dis. 2009;18(3):553–564. doi: 10.3233/JAD-2009-1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostage CA, Choudhury KR, Murali Doraiswamy P, Petrella JR Alzheimer’s Disease Neuroimaging I. Mapping the effect of the apolipoprotein E genotype on 4-year atrophy rates in an Alzheimer disease-related brain network. Radiology. 2014;271(1):211–219. doi: 10.1148/radiol.13131041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihle A, Bunce D, Kliegel M. APOE epsilon4 and cognitive function in early life: a meta-analysis. Neuropsychology. 2012;26(3):267–277. doi: 10.1037/a0026769. [DOI] [PubMed] [Google Scholar]
- Jack CR, Jr, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, et al. Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12(2):207–216. doi: 10.1016/S1474-4422(12)70291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Wiste HJ, Weigand SD, Knopman DS, Vemuri P, Mielke MM, et al. Age, Sex, and APOE epsilon4 Effects on Memory, Brain Structure, and beta-Amyloid Across the Adult Life Span. JAMA neurology. 2015;72(5):511–519. doi: 10.1001/jamaneurol.2014.4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagust W. Vulnerable neural systems and the borderland of brain aging and neurodegeneration. Neuron. 2013;77(2):219–234. doi: 10.1016/j.neuron.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorm AF, Mather KA, Butterworth P, Anstey KJ, Christensen H, Easteal S. APOE genotype and cognitive functioning in a large age-stratified population sample. Neuropsychology. 2007;21(1):1–8. doi: 10.1037/0894-4105.21.1.1. [DOI] [PubMed] [Google Scholar]
- Karlsson IK, Bennet AM, Ploner A, Andersson TM, Reynolds CA, Gatz M, Pedersen NL. Apolipoprotein E epsilon4 genotype and the temporal relationship between depression and dementia. Neurobiol Aging. 2015;36(4):1751–1756. doi: 10.1016/j.neurobiolaging.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempton MJ, Salvador Z, Munafo MR, Geddes JR, Simmons A, Frangou S, Williams SC. Structural Neuroimaging Studies in Major Depressive Disorder: Meta-analysis and Comparison With Bipolar Disorder. Arch Gen Psychiatry. 2011;68(7):675–690. doi: 10.1001/archgenpsychiatry.2011.60. [DOI] [PubMed] [Google Scholar]
- Kim DH, Payne ME, Levy RM, MacFall JR, Steffens DC. APOE genotype and hippocampal volume change in geriatric depression. Biol Psychiatry. 2002;51:426–429. doi: 10.1016/s0006-3223(01)01272-0. [DOI] [PubMed] [Google Scholar]
- Lavretsky H, Ercoli L, Siddarth P, Bookheimer S, Miller K, Small G. Apolipoprotein epsilon4 allele status, depressive symptoms, and cognitive decline in middle-aged and elderly persons without dementia. Am J Geriatr Psychiatry. 2003;11(6):667–673. doi: 10.1176/appi.ajgp.11.6.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind J, Larsson A, Persson J, Ingvar M, Nilsson LG, Backman L, et al. Reduced hippocampal volume in non-demented carriers of the apolipoprotein E epsilon4: relation to chronological age and recognition memory. Neurosci Lett. 2006;396(1):23–27. doi: 10.1016/j.neulet.2005.11.070. [DOI] [PubMed] [Google Scholar]
- Mahley RW, Huang Y. Apolipoprotein e sets the stage: response to injury triggers neuropathology. Neuron. 2012;76(5):871–885. doi: 10.1016/j.neuron.2012.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant NL, King SL, Tabet N, Rusted JM. Positive effects of cholinergic stimulation favor young APOE epsilon4 carriers. Neuropsychopharmacology. 2010;35(5):1090–1096. doi: 10.1038/npp.2009.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matura S, Prvulovic D, Jurcoane A, Hartmann D, Miller J, Scheibe M, et al. Differential effects of the ApoE4 genotype on brain structure and function. Neuroimage. 2014;89:81–91. doi: 10.1016/j.neuroimage.2013.11.042. [DOI] [PubMed] [Google Scholar]
- McClintock SM, Husain MM, Greer TL, Cullum CM. Association between depression severity and neurocognitive function in major depressive disorder: a review and synthesis. Neuropsychology. 2010;24(1):9–34. doi: 10.1037/a0017336. [DOI] [PubMed] [Google Scholar]
- Mondadori CR, de Quervain DJ, Buchmann A, Mustovic H, Wollmer MA, Schmidt CF, et al. Better memory and neural efficiency in young apolipoprotein E epsilon4 carriers. Cereb Cortex. 2007;17(8):1934–1947. doi: 10.1093/cercor/bhl103. [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Nathan BP, Bellosta S, Sanan DA, Weisgraber KH, Mahley RW, Pitas RE. Differential effects of apolipoproteins E3 and E4 on neuronal growth in vitro. Science. 1994;264(5160):850–852. doi: 10.1126/science.8171342. [DOI] [PubMed] [Google Scholar]
- Niti M, Yap KB, Kua EH, Ng TP. APOE-epsilon4, depressive symptoms, and cognitive decline in Chinese older adults: Singapore Longitudinal Aging Studies. J Gerontol A Biol Sci Med Sci. 2009;64(2):306–311. doi: 10.1093/gerona/gln013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dwyer L, Lamberton F, Matura S, Tanner C, Scheibe M, Miller J, et al. Reduced hippocampal volume in healthy young ApoE4 carriers: an MRI study. PLoS ONE. 2012;7(11):e48895. doi: 10.1371/journal.pone.0048895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA. Frontocingulate Dysfunction in Depression: Toward Biomarkers of Treatment Response. Neuropsychopharmacology. 2011;36:183–206. doi: 10.1038/npp.2010.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protas HD, Chen K, Langbaum JB, Fleisher AS, Alexander GE, Lee W, et al. Posterior cingulate glucose metabolism, hippocampal glucose metabolism, and hippocampal volume in cognitively normal, late-middle-aged persons at 3 levels of genetic risk for Alzheimer disease. JAMA neurology. 2013;70(3):320–325. doi: 10.1001/2013.jamaneurol.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu A, Taylor WD, Zhao Z, MacFall JR, Miller MI, Key CR, et al. APOE related hippocampal shape alteration in geriatric depression. Neuroimage. 2009;44:620–626. doi: 10.1016/j.neuroimage.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan KB, Wilson RS, Skarupski KA, Mendes de Leon CF, Evans DA. Gene-behavior interaction of depressive symptoms and the apolipoprotein E {varepsilon}4 allele on cognitive decline. Psychosom Med. 2014;76(2):101–108. doi: 10.1097/PSY.0000000000000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinvang I, Espeseth T, Westlye LT. APOE-related biomarker profiles in non-pathological aging and early phases of Alzheimer’s disease. Neurosci Biobehav Rev. 2013;37(8):1322–1335. doi: 10.1016/j.neubiorev.2013.05.006. [DOI] [PubMed] [Google Scholar]
- Richter-Schmidinger T, Alexopoulos P, Horn M, Maus S, Reichel M, Rhein C, et al. Influence of brain-derived neurotrophic-factor and apolipoprotein E genetic variants on hippocampal volume and memory performance in healthy young adults. J Neural Transm. 2011;118(2):249–257. doi: 10.1007/s00702-010-0539-8. [DOI] [PubMed] [Google Scholar]
- Risacher SL, Saykin AJ. Neuroimaging and other biomarkers for Alzheimer’s disease: the changing landscape of early detection. Annu Rev Clin Psychol. 2013;9:621–648. doi: 10.1146/annurev-clinpsy-050212-185535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risacher SL, Shen L, West JD, Kim S, McDonald BC, Beckett LA, et al. Longitudinal MRI atrophy biomarkers: relationship to conversion in the ADNI cohort. Neurobiol Aging. 2010;31(8):1401–1418. doi: 10.1016/j.neurobiolaging.2010.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusted JM, Evans SL, King SL, Dowell N, Tabet N, Tofts PS. APOE e4 polymorphism in young adults is associated with improved attention and indexed by distinct neural signatures. Neuroimage. 2013;65:364–373. doi: 10.1016/j.neuroimage.2012.10.010. [DOI] [PubMed] [Google Scholar]
- Schultz MR, Lyons MJ, Franz CE, Grant MD, Boake C, Jacobson KC, et al. Apolipoprotein E genotype and memory in the sixth decade of life. Neurology. 2008;70(19 Pt 2):1771–1777. doi: 10.1212/01.wnl.0000286941.74372.cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Inventory (M.I.N.I.): the development and validation of a structured diagnostic interview for DSM-IV and ICD-10. J Clin Psychiatry, Suppl. 1998;20:22–33. [PubMed] [Google Scholar]
- Sheline YI, Pieper CF, Barch DM, Welsh-Boehmer K, McKinstry RC, MacFall JR, et al. Support for the vascular depression hypothesis in late-life depression: results of a 2-site, prospective, antidepressant treatment trial. Arch Gen Psychiatry. 2010;67(3):277–285. doi: 10.1001/archgenpsychiatry.2009.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoog I, Waern M, Duberstein P, Blennow K, Zetterberg H, Borjesson-Hanson A, et al. A 9-Year Prospective Population-Based Study on the Association Between the APOE*E4 Allele and Late-Life Depression in Sweden. Biol Psychiatry. 2015;78(10):730–736. doi: 10.1016/j.biopsych.2015.01.006. [DOI] [PubMed] [Google Scholar]
- Steffens DC, Norton MC, Hart AD, Skoog I, Corcoran C, Breitner JC. Apolipoprotein E genotype and major depression in a community of older adults. The Cache County Study. Psychol Med. 2003;33:541–547. doi: 10.1017/s0033291702007201. [DOI] [PubMed] [Google Scholar]
- Strittmatter WJ, Saunders AM, Schmechel D, Pericak-Vance M, Enghild J, Salvesen GS, Roses AD. Apolipoprotein E: high-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc Natl Acad Sci USA. 1993;90:1977–1981. doi: 10.1073/pnas.90.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suri S, Heise V, Trachtenberg AJ, Mackay CE. The forgotten APOE allele: a review of the evidence and suggested mechanisms for the protective effect of APOE varepsilon2. Neurosci Biobehav Rev. 2013;37(10 Pt 2):2878–2886. doi: 10.1016/j.neubiorev.2013.10.010. [DOI] [PubMed] [Google Scholar]
- Taylor JL, Scanlon BK, Farrell M, Hernandez B, Adamson MM, Ashford JW, et al. APOE-epsilon4 and aging of medial temporal lobe gray matter in healthy adults older than 50 years. Neurobiol Aging. 2014;35(11):2479–2485. doi: 10.1016/j.neurobiolaging.2014.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wishart HA, Saykin AJ, McAllister TW, Rabin LA, McDonald BC, Flashman LA, et al. Regional brain atrophy in cognitively intact adults with a single APOE epsilon4 allele. Neurology. 2006;67(7):1221–1224. doi: 10.1212/01.wnl.0000238079.00472.3a. [DOI] [PubMed] [Google Scholar]
- Yu YW, Lin CH, Chen SP, Hong CJ, Tsai SJ. Intelligence and event-related potentials for young female human volunteer apolipoprotein E epsilon4 and non-epsilon4 carriers. Neurosci Lett. 2000;294(3):179–181. doi: 10.1016/s0304-3940(00)01569-x. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Zhang Z, Bai F, You J, Yu H, Shi Y, Liu W. Genetic variation in apolipoprotein E alters regional gray matter volumes in remitted late-onset depression. J Affect Disord. 2010;121(3):273–277. doi: 10.1016/j.jad.2009.07.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.