Abstract
Objective
Endothelial dysfunction, including upregulation of inflammatory adhesion molecules and impaired vasodilatation, is a key element in cardiovascular disease. Aging and estrogen withdrawal in women are associated with endothelial inflammation, vascular stiffness and increased cardiovascular disease. Epoxyecosatrienoic acids (EETs), the products of arachidonic acid metabolism mediated by cytochrome P450 (CYP) 2J, 2C and other isoforms, are regulated by soluble epoxide hydrolase (sEH)-catalyzed conversion into less active diols. We hypothesized that 11,12-EETs would reduce the endothelial dysfunction associated with aging and estrogen loss.
Approach/results
When stabilized by an sEH inhibitor (seHi), 11,12-EET at a physiologically low dose (0.1 nM) reduced cytokine-stimulated upregulation of adhesion molecules on human aorta endothelial cells (HAEC) and monocyte adhesion under shear flow through marked depolarization of the HAEC when combined with TNFα. Mechanistically, neither 11,12-EETs nor 17β-estradiol (E2) at physiologic concentrations prevented activation of NFκB by TNFα. E2 at physiological concentrations reduced sEH expression in HAEC, but did not alter CYP expression, and when combined with TNFα depolarized the cell. We also examined vascular dysfunction in adult and aged ovariectomized Norway brown rats (with and without E2 replacement) using an ex-vivo model to analyze endothelial function in an intact segment of artery. sEHi and 11,12-EET with or without E2 attenuated phenylephrine induced constriction and increased endothelial-dependent dilation of aortic rings from ovariectomized rats.
Conclusions
Increasing 11,12-EETs through sEH inhibition effectively attenuates inflammation and may provide an effective strategy to preserve endothelial function and prevent atherosclerotic heart disease in postmenopausal women.
Keywords: EETs, Estrogen, Vascular, Endothelial cell, Inflammation, Adhesion, Aging
1. Introduction
Estrogen has many anti-inflammatory properties, which contribute to the delayed onset of cardiovascular disease in women compared to men [1,2]. Loss of estrogen leads to increased expression of inflammatory genes and proteins [3]. Similarly, epoxyecosatrienoic acids (EETs), which are the products of arachidonic acid metabolism via the cytochrome P450(CYP) pathway are anti-inflammatory. Previously we identified no change in EETs levels with aging and loss of estrogen, although gender differences have been reported in other models [4]. We hypothesized that increasing EETs through inhibition of soluble epoxide hydrolase (sEH) might be an alternative approach to the use of estrogen to improve vascular function and reduce the risk of coronary disease in post-menopausal women. Increased EETs could decrease inflammation and reduce vascular disease associated with aging. 11,12-EETs have been shown to improve vascular dysfunction, but other studies have shown no effect [5–7]. To investigate this, we examined the effect of increased 11,12-EETs, which based on the literature have the most effect on the vasculature of the four EETs [5,8], and sEH inhibition on inflammatory monocyte adhesion to a monolayer of endothelial cells under shear stress, an early step in atherosclerosis, and on vascular function, in aged and adult rat aortas from ovariectomized (ovx) rats with and without estrogen replacement.
The importance of arachidonic acid metabolism through the COX (cycloxygenase) and LOX (lipoxygenase) pathways, which produce prostaglandins and leukotrienes, among other mediators, is well-established in the cardiovascular system; however, the role of the CYP pathway has yet to be fully characterized [9,10]. CYP2J and CYP2C are the Cyp isoforms expressed in vascular endothelial cells, contributing to the biosynthesis of the 4 isomers, 5,6-, 8,9-, 11,12-, and 14,15-EET, which to varying degrees inhibit inflammation and promote vascular relaxation and angiogenesis [11,12]. In the heart EETs reduce ischemic injury [13–15]. EETs are primarily regulated by rapid conversion into their less active diols, DHETs (dihydroxyeicosatrienoic acids), by sEH. It has been unclear whether DHETs are just less potent/inactive compared to their respective EETs, or whether DHETs actually have deleterious properties. Inhibition of sEH raises EET levels, decreases levels of the corresponding DHETs, and amplifies their biologic effects to attenuate inflammation and preserve blood pressure [16,17]. In humans, polymorphisms of genes encoding both sEH and CYPs are associated with elevated risk for cardiovascular disease [18,19]. In animal models of cardiovascular diseases pharmaceutical inhibition or genetic depletion of sEH antagonized the formation of abdominal aortic aneurysm, athero-sclerotic plaque and pathological neointima [20,21].
We hypothesized that 11,12-EETs would have an anti-inflammatory effect and mitigate vascular dysfunction and the endothelial inflammation associated with aging and estrogen loss. We tested this with comprehensive studies of vascular function by using isolated aortic rings from aging and ovx rats and an ex vivo model of TNFα-induced endothelial inflammation.
2. Methods
2.1. Animal protocol
Norway Brown rats were purchased from the National Institute of Aging and housed under standard conditions. Adult (5 months) and aged (22 months) female rats were ovariectomized (rats develop a state of sustained estrus with aging) and half immediately replaced with 0.36 mg 17β-estradiol (E2) 90 day slow-release pellets (Innovative Research of America, Sarasota, FL) subcutaneously, as previously described [3]. The animal protocol was approved by the University of California, Davis, Animal Research Committee (17411) in accordance with the NIH Guide for the Care and Use of Laboratory Animals. 9 weeks after ovariectomy, at 7 and 24 months, the rats were euthanized with ketamine and xylazine. Plasma and tissues were collected. The descending thoracic aorta was excised, adhering tissue removed, and cut into 3–4 mm aortic rings. The rings were treated with vehicle control, the sEH inhibitor (sEHi), TUPS [1-(1-methylsulfonyl piperidin-4-yl)-3-4-(4-trifluoromethoxy-phenyl)-urea (also known as 1709)] [22] alone or in combination with 11,12-EET (Cayman Chemical, Ann Arbor, Michigan) for 4 h at 37° in a CO2 incubator before studies of vascular function.
2.2. Isometric tension studies
Isometric tension was measured in descending thoracic aortic rings using a standard Radnoti apparatus (Radnoti Glass Technology, Inc., Monrovia, CA) as previously described [23,24]. A passive load of 2.0 g was applied, and the aortic segments were equilibrated for 1 h with frequent readjustment of tension until reaching a stable baseline. KCl (70 mM)-induced contractions were performed to evaluate endothelial cell-independent contraction. Rings were washed and equilibrated for 40 min. The contractile response to l-phenylephrine (PE, Sigma, 1 nM to 1 μM) was done as previously detailed, and 100 nM PE was used to assess vasodilation to acetylcholine (ACh, Sigma). For each rat, treatments were performed in duplicate, the results were averaged and considered as one independent experiment (n = 1). All aortas were studied with intact endothelium and incubated with vehicle, sEHi alone or simultaneously with 11,12-EET for the duration of the procedure. Data were collected and analyzed using PowerLab software (AD Instruments, Colorado Springs, CO).
2.3. Flow cytometry
Low passage (passage 4–6) human aortic endothelial cells (HAEC) derived from an adult female donor (Invitrogen, Grand Island, NY) were pretreated with vehicle control, sEHi alone or simultaneously with 11,12-EET or 11,12-DHET for 30 min followed by a 4 h treatment with 0.3 ng/mL TNFα (R & D Systems, Minneapolis). HAEC were detached with 0.5 mM EDTA (pH 7.4), Fc blocked with normal IgG, labeled with fluorescein-conjugated antibodies against human E-selectin, ICAM-1, VCAM-1, or isotype-matched IgG control, and analyzed with FACScan flow cytometer (BD, Franklin Lakes, NJ) equipped with CellQuest software.
2.4. Monocyte adhesion assay
HAEC monolayers were pretreated with sEHi and 11,12-EET or DHET, and stimulated with TNFα as above. For consistency, monocytes in all the experiments were obtained from a single, healthy male human donor, who was not on any medication, according to Institutional Review Board-approved protocols under informed consent. Briefly, mononuclear cells (MNC) were isolated from fasting blood by sedimentation over Lymphosep density separation media (MP) and depleted of platelets by repetitive centrifugation. 106 MNC were resuspended in 1 mL HBSS buffer with Ca2+/Mg2+ plus 0.1% human serum albumin and perfused over preconditioned HAEC monolayers at a shear stress of 2 dyne/cm2 for 2 min in a custom-made parallel-plate flow channel, as previously described [25]. The arrested monocytes were identified with an Alexa fluor 488-labeled antibody to the monocytic marker CD14. For each experiment, the results from 5 to 6 fields/channel of 1–2 channels/condition was averaged and counted as n = 1.
2.5. NFκB activation
NFκB activation was assessed in nuclear extracts using a DNA binding assay (Pierce), as previously described [26].
2.6. Membrane potential measurement
The membrane potentials of HAEC were measured using bis-(1,3-dibutylbarbituric acid) Trimethine Oxonol (DiBAC4 (3) or bis-oxonol (Life Technologies) and a Zeiss LSM 700 confocal microscope at room temperature. The cells were cultured on glass coverslips and mounted on a recording chamber which was continuously perfused with cell culture medium buffered with 10 mM Hepes. Cells were pre-incubated in the culture medium containing 1 μM bis-oxonol in cell culture incubator for 30 min. Treatments were added to the cell culture medium. The time lapse image acquisitions were performed with fast solution exchanges. The bis-oxonol loaded cells were excited by a 488 nm laser and the emission spectra at >530 nm wavelength were acquired. To quantify the image intensity, the cytoplasmic fluorescence signals (excluding the nucleus region) were measured and the averaged intensity was calculated after subtracting background fluorescence intensity. The signals were normalized to that measured in control medium. According to the manufacturer's information, the bis-oxonol sensitivity to membrane potential is typically ~1% per mV. Cell membrane depolarization results in more influx of the dye and an increase in fluorescence signals. Conversely, hyperpolarization induces a decrease in fluorescence. Thus membrane potential changes could be estimated by the percentage changes of the fluorescence signals. Data analysis was performed with Image J (NIH) and Origin 6.1 (OriginLab) software. A paired t-test and a one-way ANOVA were used to determine the significant differences between two groups (TNFα effects) and three groups (EETs and subsequent TNFα effects), respectively.
2.7. Western blot analysis
HAEC were treated with 0.3 or 3 ng/mL TNFα for 4 h or E2 (1 nM or 100 nM, Steraloids Inc., Newport, RI) for 72 h and then lysed in radioimmunoprecipitation assay (RIPA) buffer containing protease inhibitor cocktail (Sigma). Westerns were performed as previously described [23]. The blots were incubated overnight at 4 °C with 1:500 anti-sEH (Cayman 10010146), 1:1000 anti-CYP2J2 and 1:1000 anti-CYP2C9 (Santa Cruz Biotech, sc-67276 and sc-53245, respectively) followed by 1.5 h incubation with an HRP-secondary antibody (Jackson). After development with chemiluminescent substrate (Pierce), signals were analyzed with a ChemiDoc MP gel imaging system (Bio-Rad). The membrane was reprobed with 1:10,000 anti-GAPDH mouse antibody (Novus Biologicals) as a loading control.
2.8. Statistics
Data are expressed as mean ± standard error of the mean (SEM), and were analyzed with GraphPad Prism version 5.0 (GraphPad Software Inc., San Diego, CA). Multiple groups were analyzed by ANOVA or and differences were assessed post-hoc with a Student–Newman–Keuls test. Where appropriate, such as the aortic ring data, a repeated measure two-way ANOVA was used. A one-way repeated ANOVA was used to compare the effects on the three groups (control, sEHi and sEhi+11,12-EET) at the same dose of PE (or Ach) followed by Bonferroni's post hoc test. Two experimental groups were compared using Student's t-test, with pairing where appropriate. Two-tailed p values of ≤0.05 were considered statistically significant unless otherwise indicated.
3. Results
3.1. sEH inhibitor and 11,12-EET decrease TNFα-induced CAM expression
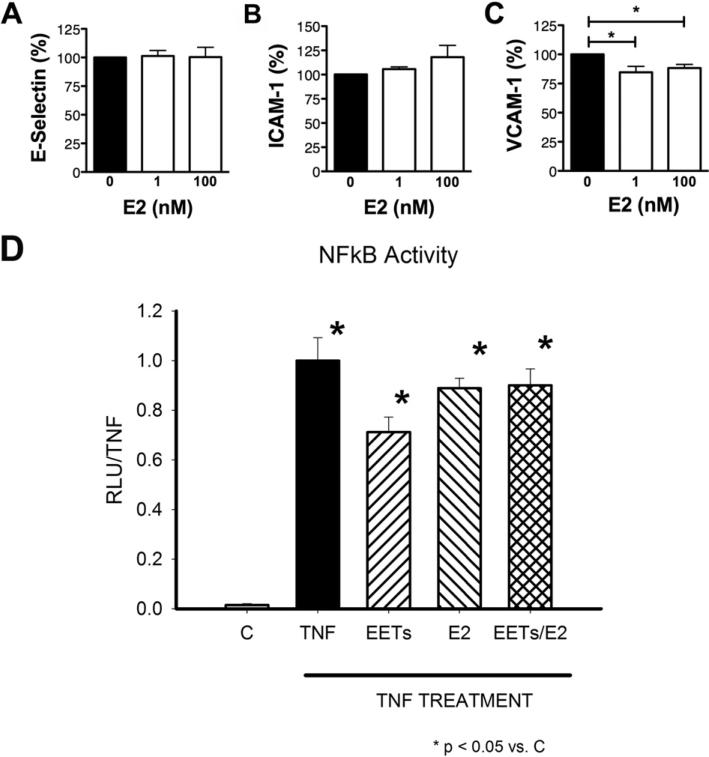
EETs are synthesized from arachidonic acid (AA) in a reaction catalyzed by the cytochrome P450 (CYP) oxidases, and the predominant metabolism pathway for EETs is rapid hydration to DHETs by sEH. Inhibition of sEH increases the ratio of EETs to DHETs by stabilizing EETs. We examined the effect of 11,12-EET on the inflammatory response of HAEC to TNFα. We focused on 11,12-EET because previous papers have reported conflicting results in the context of inflammatory vascular disease [5–7]. Given that the physiological level of 11,12-EET remains controversial [27–32], a dose response study was performed. HAEC monolayers were treated with 0.3 ng/mL TNFα, the EC50 for up-regulation of membrane adhesion receptors (VCAM-1, ICAM-1, or E-selectin) on HAEC [33]. Treatment with TNFα alone resulted in a 59-, 6- and 8-fold increase in E-selectin, ICAM-1 and VCAM-1 (not shown), respectively. Inhibition of 11,12-EET conversion to DHET by TUPS, a highly selective sEHi, decreased TNFα-induced E-selectin expression (12.5%) but not ICAM-1 or VCAM-1 expression (Fig. 1A–C). In the presence of sEHi, 11,12-EET at a dose as low as 0.1 nM further inhibited E-selectin by 11.4% (Fig. 1D, left). The same combination significantly reduced VCAM-1 expression (22.6%, Fig. 1F, left). Experiments without the inhibitor confirmed the inhibitory effect on VCAM-1 expression was mainly from 11,12-EET because this EET at 0.1 or 1 nM decreased VCAM-1 expression at a similar level as observed in the presence of sEHi. Without sEHi, 11,12-EET had no effect on E-selectin expression, indicating this adhesion molecule is more sensitive to the inhibition of EET conversion. With or without sEHi, 11,12-EET had no effect on ICAM-1 expression (Fig. 1E). Surprisingly, with higher 11,12-EET concentrations the inhibitory effect disappeared (Fig. 1D–F). These data suggest the most effective dose of 11,12-EET in inhibiting VCAM-1 expression ranges from 0.1–1 nM. In contrast, 11,12-DHET at these doses had no effect on the TNFα-induced inflammatory response in HAEC regardless of the presence of sEHi (Fig. S1).
Fig. 1.
11,12-EET, sEHi, and inhibition of TNFα-induced CAM expression. HAEC monolayers were pretreated with DMSO or 125 nM sEHi in the absence or presence of different concentrations of 11,12-EET or DHET, followed by a 4-hour treatment with TNFα (0.3 ng/mL). E-selectin, ICAM-1 and VCAM-1 expression were assessed by flow cytometry. A–C, HAEC were treated with sEHi or vehicle (DMSO); D–F) 11,12-EET plus sEHi (left) vs. 11,12-EET alone (right). n ≥ 3. *p ≤ 0.05; **p ≤ 0.05.
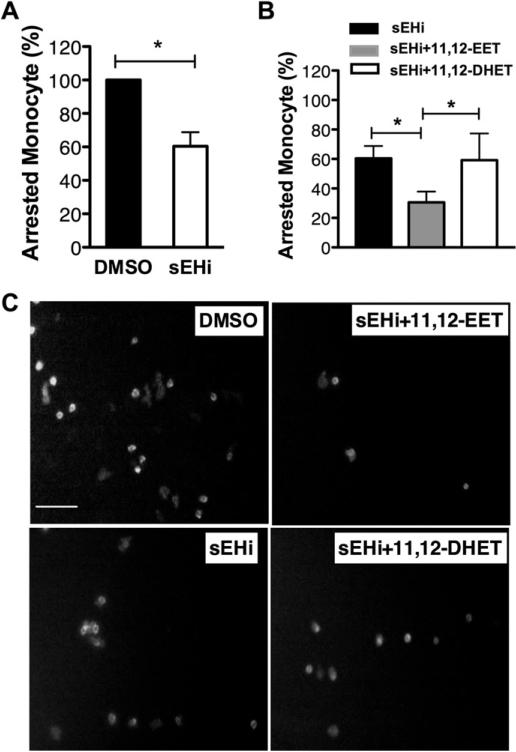
3.2. 11,12-EET inhibits monocyte recruitment to HAEC monolayer under shear stress
We used our well-established model where we pretreat HAEC monolayers and quantify cell arrest under physiologically relevant shear stress to evaluate the effects of EETs [33,34]. Pretreatment with sEHi significantly attenuated monocyte adhesion onto TNFα-stimulated HAEC (by 39.9%, Fig. 2A, C). Addition of 0.1 nM 11,12-EET led to a further 29.9% reduction in monocyte recruitment (Fig. 2B, C). This is consistent with the fact that both E-selectin ligands and VLA-4 (α4β1 integrin) are expressed on the monocyte and their binding to endothelial E-selectin and VCAM-1 contribute to monocyte-endothelial cell interaction [35]. 11,12-DHET treatment of HAEC did not alter monocyte recruitment compared to vehicle control (Fig. 2B, C).
Fig. 2.
sEH inhibitor and 11,12-EET, but not 11,12-DHET, decrease TNFα-induced monocyte adhesion under shear stress. HAEC monolayers were pretreated with sEHi in the absence or presence of 11,12-EET or 11,12-DHET for 30 min, followed by 4 h treatment with 0.3 ng/mL TNFα. Then the monolayers were exposed to human monocytes under flow of 2 dyne/cm2 for 2 min in a parallel-plate flow channel. The arrested monocytes were identified with an Alexa fluor 488-labeled antibody to the monocytic marker CD14. A and B, Monocyte arrest was quantified and presented as percentage of DMSO control. 3 separate experiments assessed multiple monocyte arrests. *p ≤ 0.05. C) Representative images of CD14-positive monocytes adhered to a HAEC monolayer. Scale bar = 50 μm. p ≤ 0.05.
3.2.1. E2 and adhesion molecule expression
Consistently, 1 nM and 100 nM E2 treatment of HAEC resulted in a significant decrease of 15.7% and 11.9%, respectively in TNFα - induced VCAM-1 expression while ICAM-1 and E-selectin were not obviously affected (Fig. 3A–C).
Fig. 3.
A–C) Surface expression of E-selectin, ICAM-1 and VCAM-1, respectively, by flow cytometry. HAEC monolayers were pretreated with DMSO (0) or E2 for 72 h, followed by 4 h treatment with TNFα. *p ≤ 0.05 vs. control (0, panel C). D) Effect of E2 and 11,12 EETs on NFκB activation by TNFα. Neither 11,12-EETs nor E2 at physiologic concentrations inhibited NFκB activation. Values are means ± SEM; n = 3–12/group.*p ≤ 0.05 vs. control (C) cells.
3.3. EETs, E2 and NFκB activation by TNFα
A number of studies have reported inhibition of NFκB by E2, but used high levels of E2. 11,12-EETs, as well as 8,9- and 14,15-EETs have been reported to inhibit NFκB by preventing nuclear translocation of p65. [5,36] [37,38] However, these studies used 100 nM4 to 1 μM EETs concentrations, which greatly exceeds in vivo concentrations. In another approach, the sEH inhibitor, 1-adamantan-3-(5-(2-(2-ethylethoxy)ethoxy)pentyl)urea (AEPU), blocked activation of NFκB in a TAC model of hypertrophy [17]. However, it was not established that activation of NFκB is inhibited with physiologic levels of EETs.
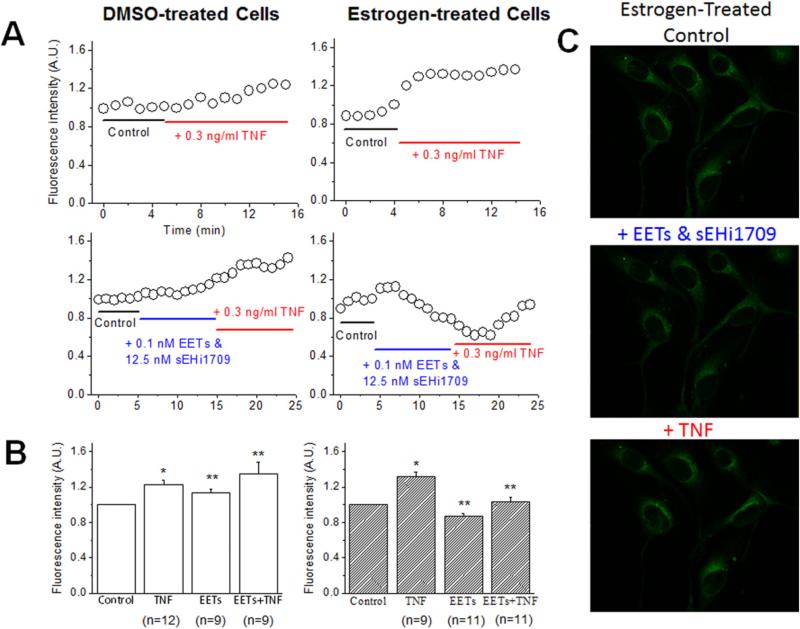
We investigated whether treatment with 0.5 nM E2 (136 pg/mL) would be effective, as it is more reflective of mean estrogen levels in mature females. Neither E2 nor E2 plus 11,12-EETs at a concentration that inhibited monocyte adhesion (0.1 nM), significantly reduced NFκB activation by TNFα, as assessed by a DNA binding assay using nuclear fractions (Fig. 3D). Thus, NFκB inhibition was not the mechanism reducing VCAM-1 and E-selectin expression on endothelial cells in response to TNFα stimulation. We hypothesized that changes in membrane potentials could be a mechanism by which TNFα induced upregulation of VCAM-1 and E-selectin was inhibited. HAEC were cultured on glass coverslips and loaded with bis-oxanol, a cell permeable fluorescent dye, which increases fluorescence intensity as membrane potentials become depolarized. We pretreated cells with E2 or vehicle (DMSO) and then added 0.1 nM 11,12-EETS with TUPS, as in previous experiments. After treatment with inhibitors, monolayers were stimulated with TNFα, as depicted. A special setup on the microscope stage allowed rapid solution changes. We monitored fluorescence using confocal microscopy throughout the experiments. As shown in Fig. 4A–C, in control DMSO treated cells, the addition of TNFα caused mild membrane depolarization. In contrast, pretreatment with EETs led to significant depolarization when TNFα was added. Cells were grown in E2 for 4 days to model chronic E2 effects rather than the well-described rapid, protective response to E2, and then studied. Addition of TNFα caused marked depolarization (Fig. 4A, upper right panel), similar to that seen with acute treatment with 11,12-EETs in DMSO control group (Fig. 4A, lower left panel). If 11,12-EETs alone were added to E2 treated cells, this caused membrane hyperpolarization (Fig.4A, lower right panel). These results suggest that TNFα added to 11,12-EETs or E2 treated cells results in marked depolarization of the cell membrane, which could interfere with the trafficking of intracellular proteins to the cell membrane. Interestingly, the combination of 11,12-EETs and E2 together with TNFα caused less depolarization (Fig. 4A, lower right panel).
Fig. 4.
EETs hyperpolarizes the membrane potential of the E2-treated HAECs. A) Representative traces of the bis-oxonol fluorescence signals from HAECs treated with either DMSO or E2. TNFα only, or EETs & sEHi TUPS (1709) and subsequent TNFα were applied to test their effects on the fluorescence signal, an indicator of the membrane potential. B) Summary of the effects of TNF and EETs & sEHi TUPS on membrane potentials (*p < 0.05, compared to control group by paired t-test; ** p < 0.05, compared to control by one-way ANOVA). C) Representative images of the bis-oxonol loaded cell with estrogen treatment at different conditions. Data is sum of 3 experiments studying 9–12 cells/group.
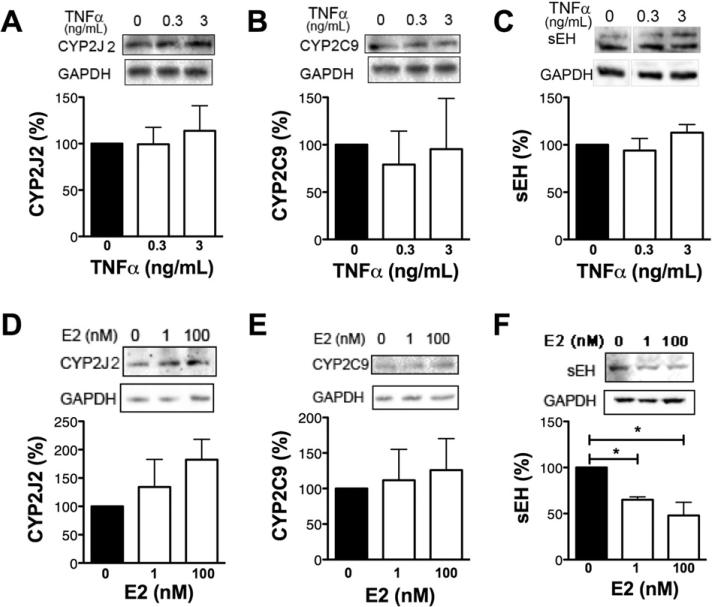
3.4. TNFα, 17β-estradiol and the expression of CYPs and sEH, enzymes responsible for 11,12-EET production and hydration to 11,12-DHET
CYPs are widely expressed, and metabolize arachidonic acids to EETs. Different CYPs have distinct catalytic efficiency. The CYP2C and CYP2J oxidase enzymes have been implicated in olefin oxidation of arachidonic acid to its four EET isomers (5,6-, 8,9-, 11,12-, 14,15-EET) in the cardiovascular system [9,10]. Previous work focused on human umbilical vein EC (HUVECs), cell lines and nonhuman EC. We examined CYP2C9 and CYP2J2 expression in HAEC lysates following 4 h of stimulation with TNFα. To investigate whether E2 increases CYP expression cells were treated with E2 for 72 h., but there was no significant difference compared with control cells (Fig. 5A, B, D, E). TNFα stimulation did not alter sEH expression (Fig. 5C). However, sEH expression was significantly decreased by 72-hour-treatment with E2 either at a physiological (1 nM) or a pharmacologic (100 nM) concentration (Fig. 5F). Furthermore, this increase in sEH due to E2 treatment correlated with the significant decrease in TNFα-induced VCAM-1 expression (Fig. 3A–C).
Fig. 5.
17β-Estradiol, TNFα and CYP/sEH expression in HAEC - HAEC monolayers were pretreated with TNFα (A–C) for 4 h or E2 (D–F) for 72 h. Panel A–C and D–F images from the same blot. n ≥ 3.*p ≤ 0.05.
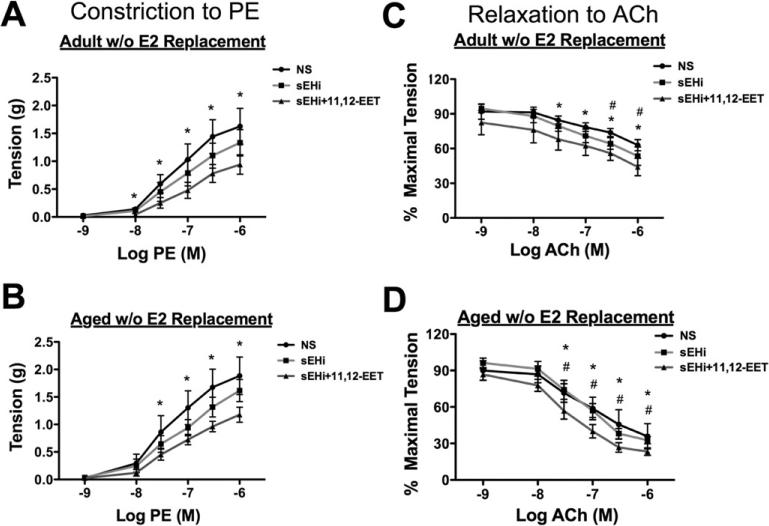
3.5. sEHi and 11,12-EET attenuated vascular stiffness caused by E2-withdrawal or aging
EETs have vasodilatory properties. Previously we reported that aging is associated with increased constriction in response to PE, and that aging combined with estrogen loss leads to impaired relaxation secondary to decreased soluble guanylyl cyclase (sGC) α and β, resulting in impaired relaxation in response to nitric oxide(NO) [23]. We hypothesized that TUPS or TUPS plus 11,12-EETs would improve vascular function. Aortic rings were prepared from ovx aged and adult Norway Brown rat with and without E2 replacement, as previously described [23]. We confirmed our previously reported increase in constriction with KCl (Fig. S2) in aged rats with and without E2 replacement, which was improved by TUPS and by TUPS + 11,12-EETs. We also confirmed our previously reported increase in constriction with PE (data not shown) in aged rats without E2 replacement, and then examined whether TUPS or TUPs plus 11,12-EET would mitigate increased constriction [23]. Developed tension was significantly decreased by the combination of TUPS and 11,12-EETs in both groups without E2 (Fig. 6A,B); however TUPS and 11,12-EETs had no effect in E2 groups (Fig. S3). Similarly, TUPS plus 11,12-EETs improved vascular relaxation to acetyl choline only in groups without E2 (Fig. 6C,D, Fig. S3). TUPS plus 11,12-EETs was superior to TUPS alone (Fig. 6C,D). These data suggest deregulation of EETs through sEH is, at least partly, responsible for vascular dysfunction that is associated with estrogen withdrawal during aging.
Fig. 6.
Effect of 11,12-EETs on the aortic function in adult and aged rats with estrogen withdrawal. Aged (22 months) and Adult (5 months) Norwegian brown rats were ovariectomized with or without E2 slow-release pellets implanted sc and studied 9 weeks later. 11,12-EETs had no added effect in E2 replaced group (Supplemental Fig. 2). A) Isometric tension developed in a dose response to PE. B) Dose-response curves to ACh were obtained in 100 nM PE pre-contracted aortic rings. Each group contains 5–6 rats (n = 5–6). *p ≤ 0.05, NS vs. sEHi+EET. #p ≤ 0.05, sEHi vs. sEHi+EET.
4. Discussion
Endothelial inflammation and impaired vasodilation play critical roles in atherosclerotic heart disease. Endothelial inflammation is manifest as upregulated expression of CAMs leading to monocyte recruitment and infiltration into the arterial wall. Although the vasodilatory effect of 11,12-EETs has been recognized, aspects of the anti-inflammatory properties remain controversial. 11,12-EETs at a concentration as high as 100 nM blocked monocyte recruitment to inflamed endothelium through down regulation of adhesion molecule expression, including E-selectin, ICAM-1 and VCAM-1 by inhibiting NFκB activity [5]. However, the physiologic levels of 11,12 EETs used here did not inhibit NFκB. Others found that 14,15-EET increased U937 (monocyte cell line) cell adhesion to HUVECs [39]. Minimally oxidized LDL induced EETs and promoted adhesion [6,7]. 11,12-EET or 14,15-EET given at the time of reperfusion mitigated the increase in endothelial permeability with reperfusion in ischemia induced lung injury [40]. In a model of arteriovenous graft stenosis pre-treatment of monocytes with an sEHi reduced the release of MCP-1 and TNFα, but not MCP-1 or IL-6 in response to LPS [41]. Most interestingly, Kundu et al. have reported that DHETs are essential for monocyte recruitment by MCP-1 [42]. In other studies pretreatment with 11,12-EET or 14,15 had no effect [41]. In the current study, we found that physiologic concentrations of 11,12-EETs and E2 led to mild hyperpolarization of the endothelial cell membrane. Both EETs and E2 are known to activate the large conductance Ca-activated K+ channel (BK channels) in both vascular smooth muscle cells and EC, which may contribute to membrane polarization changes [43,44]. When TNFα was added to EETs or E2, the membrane was markedly depolarized. This change in membrane polarization could explain the anti-inflammatory mechanisms of EET and E2 through interference with the trafficking of adhesion molecules to the membrane.
Endothelium-dependent vasodilatation in premenopausal women correlates with increased circulating estrogen levels, and blood pressure is lower in premenopausal women than in age-matched men [45]. Accordingly, pre-menopausal women have lower cardiovascular risk than men. The difference disappears after menopause when cardiovascular disease becomes the predominant cause of death in women [46]. Induction of vasodilation is also one of the most important cardioprotective effects of EETs. Studies have primarily focused on models in young animals, while increased vasoconstriction is a problem of aging. EETs relax preconstricted mesenteric arteries, renal arteries, cerebral arteries, and coronary arteries [47,48]. Endothelial overexpression of CYPs lowers blood pressure and attenuates hypertension-induced renal injury in mice [49]. 11,12-EET has been reported to increase cAMP levels and activate protein phosphatase 2A (PP2A), and these signaling pathways contribute to the activation of the BKCa channel and vasodilation in mesenteric resistance arteries and renal microvessels [43]. In porcine coronary vasculature, 11,12-EET activates both smooth muscle cell BKCa channel and endothelial cell small (SKCa) and intermediate (IKCa) conductance calcium-activated K+ channels [43]. Thus a number of studies support that 11,12-EETs can activate the BKCa channel. Furthermore, previous work [50], as well as the current study, supports that E2 increases EETs through inhibition of sEH expression and also potentiates the effects of EETs. Combined with previous results, our data imply that both estrogen itself and its effects on 11,12-EET metabolism contribute to the beneficial effect of estrogen replacement on vascular function via discrete mechanisms in postmenopausal females.
5. 11,12-EET and monocyte adhesion
Applying a state of the art atherosclerosis-relevant ex vivo model using HAEC stimulated with low dose of TNFα and pretreated over a large dose range of 11,12-EET, we observed differential changes in the expression of the vascular adhesion molecules, as well as a biphasic response. sEH inhibition alone or with 11,12-EET had no effect on ICAM-1 expression. While sEH inhibition alone is sufficient to inhibit E-selectin expression, 11,12-EET plus sEHi is required to inhibit VCAM-1 expression. This is consistent with distinct transcriptional regulation of VCAM-1 compared to E-selectin or ICAM-1 expression on the endothelial plasma membrane. Moreover, in the presence of sEH inhibition, 11,12-EET at a dose of 0.1 nM was most effective in inhibiting expression of E-selectin and VCAM-1. The disparity between our results and the previous studies may be due to the different conditions applied for the experiments [5]. For example, 30-fold less TNFα (i.e., 0.3 ng/mL vs. 10 ng/mL) was used in the current study. Flow cytometry, rather than ELISA, was employed to assess the changes in membrane expressed adhesion molecules. To prevent the conversion of 11,12-EET to 11,12-DHET, we used an sEH inhibitor. Our data further indicate that 11,12-DHET has little anti-inflammatory effect, but is not detrimental to adhesion molecule expression and monocyte function.
The precise concentration of EETs in the blood remains controversial because of the use of different models and different measurement techniques. Furthermore, plasma/tissue concentrations may not reflect localized tissue concentrations. In published reports, 14,15-EET ranges from 3.9 ng/mL (~13.0 nM) to 0.101 ng/mL (~0.3 nM) in human plasma; and 11,12-EET from 2 ng/mL (6.7 nM) to <0.1 ng/mL (<0.3 nM) [27–30,32]. Based on recent studies with HLPC–MS/MS, the total concentration of EETs in the plasma of healthy humans is ~ 0.19 ng/mL(~ 0.6 nM) and 14,15-EET is 0.1 ng/mL (~ 0.3 nM) while 11,12-EET is below the lower limit of detection in both healthy individuals and patients with coronary artery disease [31]. Although the effective dose of 11,12-EET found in our study is far lower than that reported by other groups, it is closer to physiological concentrations in human as well as in experimental animals models. The availability of sEH inhibitors makes it possible to increase EETs levels, and their efficacy is currently being investigated in clinical trials for the treatment of hypertension [51].
6. Conclusions
In the current study we found that both 11,12-EETs and E2 at physiologic concentrations led to marked endothelial cell depolarization upon stimulation with TNFα. This depolarization was associated with a decrease in the translocation of specific CAMs to the cell membrane in response to TNFα. E2 pretreatment decreased VCAM-1 upregulation on the endothelial cell membrane in response to TNFα. Inhibition of the metabolism of EETs to DHETs with TUPS, a selective sEH inhibitor, reduced E-selectin upregulation on the endothelial cell in response to TNFα. The combination of TUPS and 11,12-EETs treatment not only decreased E-selectin, but also VCAM-1 presentation on the cell membrane. Collectively, these findings suggest that CYP-mediated arachidonic acid metabolism is altered in endothelial cells during aging and estrogen loss. Modulation of 11,12-EET metabolism may represent an effective strategy to preserve endothelial function and prevent atherosclerotic heart disease in postmenopausal women.
Supplementary Material
Acknowledgements
We thank Dr. Keri Hayakawa and Sean Ott from the Eiserich lab for invaluable help in vessel studies. This work was supported by a Merit Award (5101BX000839)from the U.S. Department of Veterans’ Affairs, Office of Research and Development, Biomedical Laboratory Research Program (AAK) and by an American Heart Association Western States Affiliate Beginning Grant-in-Aid (14BGIA18870087, XZ). Partial support was provided by RO1ES002710 (BDH) and NIH support HL082689 (SIS). Disclosure - Nothing to disclose.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.yjmcc.2016.03.019.
Disclaimer
The contents reported do not represent the views of the Department of Veterans Affairs or the United States Government.
References
- 1.Knowlton AA, Lee AR. Estrogen and the cardiovascular system. Pharmacol. Ther. 2012;135(1):54–70. doi: 10.1016/j.pharmthera.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xing D, Nozell S, Chen YF, Hage F, Oparil S. Estrogen and mechanisms of vascular protection. Arterioscler. Thromb. Vasc. Biol. 2009 Mar 1;29(3):289–295. doi: 10.1161/ATVBAHA.108.182279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pechenino AS, Lin L, Mbai FN, Lee AR, He XM, Stallone JN, et al. Impact of aging vs. estrogen loss on cardiac gene expression: late estrogen replacement and inflammation. Physiol. Genomics. 2011;43:1065–1073. doi: 10.1152/physiolgenomics.00228.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pinot F, Grant DF, Spearow JL, Parker AG, Hammock BD. Differential regulation of soluble epoxide hydrolase by clofibrate and sexual hormones in the liver and kidneys of mice. Biochem. Pharmacol. 1995 Aug 8;50(4):501–508. doi: 10.1016/0006-2952(95)00167-x. [DOI] [PubMed] [Google Scholar]
- 5.Node K, Huo Y, Ruan X, Yang B, Spiecker M, Ley K, et al. Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science. 1999;285:1276–1279. doi: 10.1126/science.285.5431.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang T, Peng R, Guo Y, Shen L, Zhao S, Xu D. The role of 14,15-dihydroxyeicosatrienoic acid levels in inflammation and its relationship to lipo-proteins. Lipids iHealth Dis. 2013;12(1):151. doi: 10.1186/1476-511X-12-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Honda HM, Leitinger N, Frankel M, Goldhaber JI, Natarajan R, Nadler JL, et al. Induction of monocyte binding to endothelial cells by MM-LDL: role of lipoxygenase metabolites. Arterioscler. Thromb. Vasc. Biol. 1999 Mar 1;19(3):680–686. doi: 10.1161/01.atv.19.3.680. [DOI] [PubMed] [Google Scholar]
- 8.Gill SS, Hammock BD. Distribution and properties of a mammalian soluble epoxide hydrase. Biochem. Pharmacol. 1980;29(3):389–395. doi: 10.1016/0006-2952(80)90518-3. [DOI] [PubMed] [Google Scholar]
- 9.Fleming I. DiscrEET regulators of homeostasis: epoxyeicosatrienoic acids, cytochrome P450 epoxygenases and vascular inflammation. Trends Pharmacol. Sci. 2007 Sep;28(9):448–452. doi: 10.1016/j.tips.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 10.Spector AA. Arachidonic acid cytochrome P450 epoxygenase pathway. J. Lipid Res. 2009 Oct 23;50:s52–s56. doi: 10.1194/jlr.R800038-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Imig JD. Epoxides and soluble epoxide hydrolase in cardiovascular physiology. Physiol. Rev. 2012 Jan 1;92(1):101–130. doi: 10.1152/physrev.00021.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yan G, Chen S, You B, Sun J. Activation of sphingosine kinase-1 mediates induction of endothelial cell proliferation and angiogenesis by epoxyeicosatrienoic acids. Cardiovasc. Res. 2008 May 1;78(2):308–314. doi: 10.1093/cvr/cvn006. [DOI] [PubMed] [Google Scholar]
- 13.Gross GJ, Gauthier KM, Moore J, Falck JR, Hammock BD, Campbell WB, et al. Effects of the selective EET antagonist, 14,15-EEZE, on cardioprotection produced by exogenous or endogenous EETs in the canine heart. Am. J. Physiol. Heart Circ. 2008 Jun 1;294(6):H2838–H2844. doi: 10.1152/ajpheart.00186.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Motoki A, Merkel MJ, Packwood WH, Cao Z, Liu L, Iliff J, et al. Soluble epoxide hydrolase inhibition and gene deletion are protective against myocardial ischemia-reperfusion injury in vivo. Am. J. Physiol. Heart Circ. 2008 Nov 1;295(5):H2128–H2134. doi: 10.1152/ajpheart.00428.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seubert JM, Zeldin DC, Nithipatikom K, Gross GJ. Role of epoxyeicosatrienoic acids in protecting the myocardium following ischemia/reperfusion injury. Prostaglandins Other Lipid Mediat. 2007;82:50–59. doi: 10.1016/j.prostaglandins.2006.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmelzer KR, Kubala L, Newman JW, Kim IH, Eiserich JP, Hammock BD. Soluble epoxide hydrolase is a therapeutic target for acute inflammation. PNAS. 2005 Jul 12;102(28):9772–9777. doi: 10.1073/pnas.0503279102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu D, Li N, He Y, Timofeyev V, Lu L, Tsai HJ, et al. Prevention and reversal of cardiac hypertrophy by soluble epoxide hydrolase inhibitors. PNAS. 2006 Dec 5;103(49):18733–18738. doi: 10.1073/pnas.0609158103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fornage M, Lee CR, Doris PA, Bray MS, Heiss G, Zeldin DC, et al. The soluble epoxide hydrolase gene harbors sequence variation associated with susceptibility to and protection from incident ischemic stroke. Hum. Mol. Genet. 2005;14:2829–2837. doi: 10.1093/hmg/ddi315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wei Q, Doris PA, Pollizotto MV, Boerwinkle E, Jacobs DR, Siscovick DS, et al. Sequence variation in the soluble epoxide hydrolase gene and subclinical coronary atherosclerosis: interaction with cigarette smoking. Atherosclerosis. 2007;190:26–34. doi: 10.1016/j.atherosclerosis.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 20.Zhang LN, Vincelette J, Cheng Y, Mehra U, Chen D, Anandan SK, et al. Inhibition of soluble epoxide hydrolase attenuated atherosclerosis, abdominal aortic aneurysm formation, and dyslipidemia. Arterioscler. Thromb. Vasc. Biol. 2009 Sep 1;29(9):1265–1270. doi: 10.1161/ATVBAHA.109.186064. [DOI] [PubMed] [Google Scholar]
- 21.Revermann M, Schloss M, Barbosa-Sicard E, Mieth A, Liebner S, Morisseau C, et al. Soluble epoxide hydrolase deficiency attenuates neointima formation in the femoral cuff model of hyperlipidemic mice. Arterioscler. Thromb. Vasc. Biol. 2010 May 1;30(5):909–914. doi: 10.1161/ATVBAHA.110.204099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsai HJ, Hwang SH, Morisseau C, Yang J, Jones PD, Kasagami T, et al. Pharmaco-kinetic screening of soluble epoxide hydrolase inhibitors in dogs. Eur. J. Pharm. Sci. 2010 Jun 14;40(3):222–238. doi: 10.1016/j.ejps.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stice JP, Eiserich JP, Knowlton AA. Role of aging vs. the loss of estrogens in the reduction in vascular function in female rats. Endocrinology. 2009 Jan 1;150:212–219. doi: 10.1210/en.2008-0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eiserich J, Baldus S, Brennan M, Ma W, Zhang C, Tousson A, et al. Myeloperoxidase, a leuykocyte-derived vascular NO oxidase. Science. 2002;296:2391–2394. doi: 10.1126/science.1106830. [DOI] [PubMed] [Google Scholar]
- 25.Foster GA, Gower RM, Stanhope KL, Havel PJ, Simon SI, Armstrong EJ. On-chip phenotypic analysis of inflammatory monocytes in atherogenesis and myocardial infarction. Proc. Natl. Acad. Sci. U. S. A. 2013 Aug 20;110(34):13944–13949. doi: 10.1073/pnas.1300651110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stice JP, Chen L, Kim SC, Jung JS, Tran AL, Liu TT, et al. 17β-Estradiol, aging, inflammation and the stress response in the female heart. Endocrinology. 2011;152:1589–1598. doi: 10.1210/en.2010-0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deng Y, Edin ML, Theken KN, Schuck RN, Flake GP, Kannon MA, et al. Endothelial CYP epoxygenase overexpression and soluble epoxide hydrolase disruption attenuate acute vascular inflammatory responses in mice. FASEB J. 2011 Feb 1;25(2):703–713. doi: 10.1096/fj.10-171488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang H, Quilley J, Doumad AB, Zhu AG, Falck JR, Hammock BD, et al. Increases in plasma trans-EETs and blood pressure reduction in spontaneously hypertensive rats. Am. J. Physiol. Heart Circ. 2011 Jun 1;300(6):H1990–H1996. doi: 10.1152/ajpheart.01267.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee AR, Pechenino AS, Dong H, Hammock BD, Knowlton AA. Aging, estrogen loss and epoxyeicosatrienoic acids (EETs) PLoS One. 2013 Aug 13;8(8):e70719. doi: 10.1371/journal.pone.0070719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Minuz P, Jiang H, Fava C, Turolo L, Tacconelli S, Ricci M, et al. Altered release of cytochrome P450 metabolites of arachidonic acid in renovascular disease. Hypertension. 2008 May 1;51(5):1379–1385. doi: 10.1161/HYPERTENSIONAHA.107.105395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Theken KN, Schuck RN, Edin ML, Tran B, Ellis K, Bass A, et al. Evaluation of cytochrome P450-derived eicosanoids in humans with stable atherosclerotic cardiovascular disease. Atherosclerosis. 2012 Jun 27;222(2):530–536. doi: 10.1016/j.atherosclerosis.2012.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu P, Peck B, Licea-Perez H, Callahan JF, Booth-Genthe C. Development of a semi-automated LC/MS/MS method for the simultaneous quantitation of 14,15-epoxyeicosatrienoic acid, 14,15-dihydroxyeicosatrienoic acid, leukotoxin and leukotoxin diol in human plasma as biomarkers of soluble epoxide hydrolase activity in vivo. J. Chromatogr. B. 2011 Sep 1;879(25):2487–2493. doi: 10.1016/j.jchromb.2011.06.042. [DOI] [PubMed] [Google Scholar]
- 33.Ting HJ, Stice JP, Schaff UY, Hui DY, Rutledge JC, Knowlton AA, et al. Triglyceride-rich lipoproteins prime aortic endothelium for an enhanced inflamma-tory response to tumor necrosis factor-{alpha} Circ. Res. 2007 Feb 16;100(3):381–390. doi: 10.1161/01.RES.0000258023.76515.a3. [DOI] [PubMed] [Google Scholar]
- 34.Gower RM, Wu H, Foster GA, Devaraj S, Jialal I, Ballantyne CM, et al. CD11c/CD18 expression is upregulated on blood monocytes during hypertriglyceridemia and enhances adhesion to vascular cell adhesion molecule-1. Arterioscler. Thromb. Vasc. Biol. 2011;31(1):160–166. doi: 10.1161/ATVBAHA.110.215434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Foster GA, Xu L, Chidambaram AA, Soderberg SR, Armstrong EJ, Wu H, et al. CD11c/CD18 signals very late antigen-4 activation to initiate foamy monocyte recruitment during the onset of hypercholesterolemia. J. Immunol. 2015 Oct 30; doi: 10.4049/jimmunol.1501077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen G, Xu R, Zhang S, Wang Y, Wang P, Edin ML, et al. CYP2J2 overexpression attenuates nonalcoholic fatty liver disease induced by high-fat diet in mice. Am. J. Physiol. Endocrinol. Metab. 2015 Jan 15;308(2):E97–E110. doi: 10.1152/ajpendo.00366.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moshal KS, Zeldin DC, Sithu SD, Sen U, Tyagi N, Kumar M, et al. Cytochrome P450 (CYP) 2 J2 gene transfection attenuates MMP-9 via inhibition of NF-κB in hyperhomocysteinemia. J. Cell. Physiol. 2008 Jun 1;215(3):771–781. doi: 10.1002/jcp.21356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dai M, Wu L, He Z, Zhang S, Chen C, Xu X, et al. Epoxyeicosatrienoic acids regulate macrophage polarization and prevent LPS-induced cardiac dysfunction. J. Cell. Physiol. 2015 Sep 1;230(9):2108–2119. doi: 10.1002/jcp.24939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pritchard J, Tota RR, Stemerman MB, Wong PYK. 14,15-Epoxyeicosatrienoic acid promotes endothelial cell dependent adhesion of human monocytic tumor U937 cells. Biochem. Biophys. Res. Commun. 1990 Feb 28;167(1):137–142. doi: 10.1016/0006-291x(90)91741-a. [DOI] [PubMed] [Google Scholar]
- 40.Townsley MI, Morisseau C, Hammock B, King JA. Impact of epoxyeicosatrienoic acids in lung ischemia/reperfusion injury. Microcirculation. 2010 Feb 1;17(2):137–146. doi: 10.1111/j.1549-8719.2009.00013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sanders WG, Morisseau C, Hammock BD, Cheung AK, Terry CM. Soluble epoxide hydrolase expression in a porcine model of arteriovenous graft stenosis and anti-inflammatory effects of a soluble epoxide hydrolase inhibitor. Am. J. Phys. Cell Phys. 2012 Aug 1;303(3):C278–C290. doi: 10.1152/ajpcell.00386.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kundu S, Roome T, Bhattacharjee A, Carnevale KA, Yakubenko VP, Zhang R, et al. Metabolic products of soluble epoxide hydrolase are essential for monocyte chemo-taxis to MCP-1 in vitro and in vivo. J. Lipid Res. 2013 Feb 1;54(2):436–447. doi: 10.1194/jlr.M031914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carroll MA, Doumad AB, Li J, Cheng MK, Falck JR, McGiff JC. Adenosine2A receptor vasodilation of rat preglomerular microvessels is mediated by EETs that activate the cAMP/PKA pathway. Am. J. Physiol. Ren. 2006 Jun 7;291(1):F155–F161. doi: 10.1152/ajprenal.00231.2005. [DOI] [PubMed] [Google Scholar]
- 44.Weston AH, Félétou M, Vanhoutte PM, Falck JR, Campbell WB, Edwards G. Bradykinin-induced, endothelium-dependent responses in porcine coronary arteries: involvement of potassium channel activation and epoxyeicosatrienoic acids. Br. J. Pharmacol. 2005 Jul 16;145(6):775–784. doi: 10.1038/sj.bjp.0706256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kovacic JC, Moreno P, Hachinski V, Nabel EG, Fuster V. Cellular senescence, vascular disease, and aging: part 1 of a 2-part review. Circulation. 2011 Apr 19;123(15):1650–1660. doi: 10.1161/CIRCULATIONAHA.110.007021. [DOI] [PubMed] [Google Scholar]
- 46.Barrett-Connor E. Menopause, atherosclerosis, and coronary artery disease. Curr. Opin. Pharmacol. 2013 Apr;13(2):186–191. doi: 10.1016/j.coph.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zeldin DC. Epoxygenase pathways of arachidonic acid metabolism. J. Biol. Chem. 2001 Sep 28;276(39):36059–36062. doi: 10.1074/jbc.R100030200. [DOI] [PubMed] [Google Scholar]
- 48.Roman RJ. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol. Rev. 2002 Jan 1;82(1):131–185. doi: 10.1152/physrev.00021.2001. [DOI] [PubMed] [Google Scholar]
- 49.Lee CR, Imig JD, Edin ML, Foley J, DeGraff LM, Bradbury JA, et al. Endothelial expression of human cytochrome P450 epoxygenases lowers blood pressure and attenuates hypertension-induced renal injury in mice. FASEB J. 2010 Oct 5;24(10):3770–3781. doi: 10.1096/fj.10-160119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koerner IP, Zhang W, Cheng J, Parker S, Hurn PD, Alkayed NJ. Soluble epoxide hydrolase: regulation by estrogen and role in the inflammatory response to cerebral ischemia. Front. Biosci. 2008;13:2833–2841. doi: 10.2741/2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chiamvimonvat N, Ho C, Tsai HJ, Hammock BD. The soluble epoxide hydrolase as a pharmaceutical target for hypertension. J. Cardiovasc. Pharmacol. 2007;50:225–237. doi: 10.1097/FJC.0b013e3181506445. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.