Abstract
Conserved prolines in the transmembrane helices of G protein-coupled receptors (GPCRs) are often considered to function as hinges that divide the helix into two segments capable of independent motion. Depending on their potential to hydrogen-bond, the free C=O groups associated with these prolines can facilitate conformational flexibility, conformational switching or stabilize receptor structure. To address the role of conserved prolines in family A GPCRs, we focus on bovine rhodopsin, a GPCR in the visual receptor subfamily, using solid-state NMR spectroscopy. The free backbone C=O groups on helices H5 and H7 are found to stabilize the inactive rhodopsin structure through hydrogen-bonds to residues on adjacent helices. In response to light-induced isomerization of the retinal chromophore, hydrogen-bonding interactions involving these C=O groups are released facilitating H5 and H7 repacking onto the transmembrane core of the receptor. These results provide insights into the multiple structural and functional roles prolines play in membrane proteins.
Prolines within the transmembrane (TM) helices of membrane proteins can function in several ways. First, they may facilitate protein dynamics as flexible hinges and play a functional role in guiding large scale conformational changes1–3. Second, prolines may be key elements in stabilizing protein structure. Proline-induced kinks can facilitate tighter packing of membrane proteins by allowing the helices to adopt optimal side chain interactions4,5 and the backbone carbonyls at the i-4 position relative to TM prolines are free to form strong stabilizing interhelical hydrogen bonds. Third, prolines and the associated free C=O groups can facilitate (reversible) switching between distinct protein conformations6.
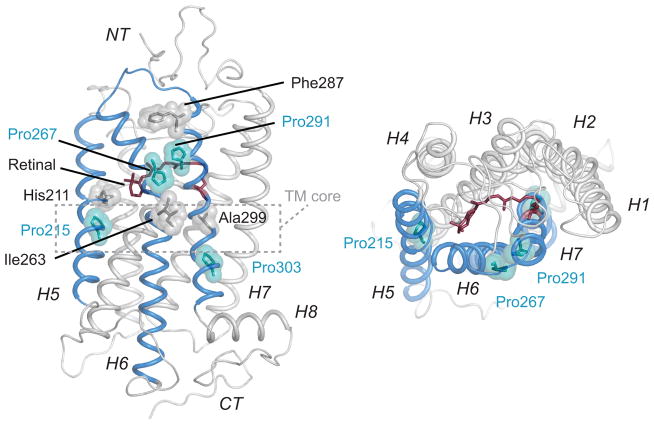
To address the role of prolines in the structure and function of GPCRs, we focus on the visual receptor rhodopsin. Rhodopsin serves as an on-off switch for light detection in the vertebrate retina7. Light energy absorbed by the retinal chromophore in rhodopsin drives the receptor from an inactive to an active conformation. The hallmark of the active state of rhodopsin (Metarhodopsin II or Meta II) is the outward rotation of the intracellular end of TM helix H6. Coupled with H6 motion are changes in the orientations of the adjacent helices H5 and H7. Helices H5, H6, and H7 each contains a proline residue in the middle of the TM sequence: Pro2155.50, Pro2676.50 and Pro3037.50, respectively (residues are designated throughout the text according to the Ballesteros-Weinstein universal numbering system8)(Fig. 1). These residues are the most conserved in each of these helices in the family A GPCRs. An additional proline (Pro2917.38) with high sequence conservation within the visual receptor subfamily occurs at the extracellular end of H7.
Figure 1.
Crystal structure of the visual receptor rhodopsin (PDB ID 1U19 9) showing the positions of Pro2155.50, Pro2676.50, Pro2917.38 and Pro3037.50. These prolines are located on helices H5, H6 and H7. The lack of an NH group results in a carbonyl group at the i-4 position from these prolines that is free to form interhelical hydrogen-bonds. The residues with the free carbonyl (His2115.46, Ile2636.46, Phe2877.34, and Ala2997.46) are shown in grey. The three free C=O groups that are conserved across the family A GPCRs (His2115.46, Ile2636.46, and Ala2997.46) lie within or near the TM core of the receptor.
The defining feature of a proline in a TM helix is that it is unable to form a backbone hydrogen bond to the carbonyl group one helical turn away. In the case of Pro2155.50 and Pro3037.50 on H5 and H7, respectively, the free i-4 backbone carbonyls form hydrogen-bonds with strongly polar residues on adjacent helices. However, the free backbone carbonyls associated with Pro2676.50 and Pro2917.38 are oriented toward the membrane lipids and do not hydrogen bond in the crystal structures of inactive rhodopsin9,10, active opsin11 or Meta II12,13, suggesting instead that they allow the helical segments to easily swivel. Coordinated motion of the extracellular ends of H6 and H7 has been proposed as part of a general mechanism for GPCR activation14,15 raising the possibility that the sequence stretching from Pro2676.50 to Pro2917.38, which includes extracellular loop (EL3), pivots upon activation.
Early studies on rhodopsin using FTIR spectroscopy indicated that conformational changes at one or more prolines occurred upon activation16. FTIR difference spectra revealed a large shift of the amide I vibration associated with a peptide bond adjacent to the amino-terminal side of a proline. The authors left open the possibility that the observed shift was due to cis-trans proline isomerization. More recent studies using non-native amino acid substitutions at conserved prolines in the D2 dopamine receptor ruled out the idea that cis-trans proline isomerization occurs, at least in this specific GPCR17. They found that the main function of proline was to introduce a break in the helix by removing a backbone NH. Introducing either cyclic, R-hydroxy, or N-methyl residues unable to form NH hydrogen bonds at the position of proline resulted in receptors with wild-type function. Consistent with this role, in several GPCRs mutation of proline to residues that are able to form backbone hydrogen bonds disrupts expression18 and/or function18,19.
Solid-state NMR spectroscopy in combination with isotope labeling of rhodopsin can be used to clarify the role of specific prolines by targeting the carbonyl groups that are four amino acids upstream of conserved prolines. These C=O groups are free to form alternative hydrogen bonds due to the lack of an NH hydrogen bond partner along their own helix backbone. Our focus is on the highly conserved i-4 free C=O groups on helices H5, H6 and H7 whose 13C chemical shifts are sensitive to both secondary structure and hydrogen bonding. Each of these free carbonyls is in a functionally important region of the protein (Supplementary Note 1). The His2115.46 carbonyl associated with conserved Pro2155.50 on H5 is located within the retinal-binding site, and is a key determinant of the high sensitivity of the dim light photoreceptors in rod cells, as compared to the color photoreceptors in cone cells20. The Ile2636.46 carbonyl associated with conserved Pro2676.50 is bracketed by conserved residues (Phe2616.44 and Trp2656.48) on TM helix H6 that are part of a transmission switch that couples retinal isomerization to conformational changes on the intracellular side of the receptor21,22. Finally, the Ala2997.46 carbonyl associated with conserved Pro3037.50 serves to orient the side chain of Asn551.50, the most conserved residue within the family A GPCRs7. The 13C chemical shift changes of these free carbonyls upon receptor activation reveal that they play both structural roles in guiding how the TM helices pack in the inactive receptor, as well as functional roles in allowing the helices to reassemble in response to light-induced isomerization of the retinal chromophore. These results highlight the importance of hydrogen-bonding interactions involving the free C=O groups associated with TM prolines in membrane proteins.
RESULTS
The TM core of rhodopsin is highly conserved and serves to couple structural changes on the extracellular side of the receptor containing the retinal chromophore to the intracellular G protein binding region (Fig. 1). Crystal structures of the inactive9,10 and active11–13 states of rhodopsin reveal the positions of the conserved prolines and their associated free C=O groups relative to surrounding amino acids and structural water. These structures provide an essential framework for understanding how changes in hydrogen bonding guide the rearrangement of TM helices H5-H7. NMR spectroscopy was used here to target the free carbonyl groups associated with the conserved prolines within the TM core of rhodopsin. NMR measurements of specific 13C=O chemical shifts report on the strength of the hydrogen bonding interactions in the inactive state and how they change upon activation with and without the Gα peptide.
Pro2155.50
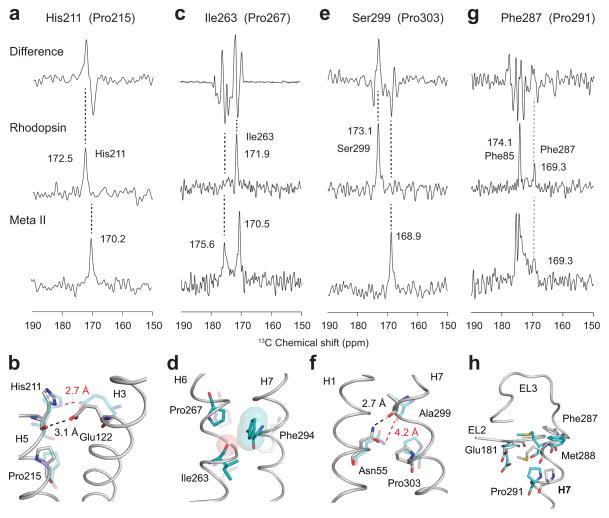
The conserved proline on helix H5 at position 215 in rhodopsin frees the carbonyl of His2115.46. There are six histidine residues in rhodopsin that must be considered. The magic angle spinning (MAS) NMR difference spectrum between the inactive (dark) state of rhodopsin and the active Meta II intermediate showed that at least one His 13C=O resonance shifts to lower frequency upon activation (Fig. 2a). The 13C=O resonance that changes chemical shift can be assigned to His2115.46 by using rotational echo double resonance (REDOR)23 NMR filtering of rhodopsin specifically labeled with 1-13C histidine and 15N-phenylalanine. This labeling strategy generates a single 13C-15N labeled peptide bond as there is only a single His-Phe pair in the rhodopsin sequence, namely His2115.46-Phe2125.47. The REDOR experiment allows one to measure 13C…15N dipolar couplings in the solid-state NMR experiments, and the strong dipolar coupling resulting from the directly bonded 13C-15N pair allows one to selectively observe only the single 13C=O resonance in the REDOR filtered spectrum23. Using the REDOR filtering experiment, we observed the 13C=O frequency of H2115.46 at 172.5 ppm in rhodopsin and at 170.2 ppm upon conversion to Meta II (Fig. 2a).
Figure 2.
REDOR NMR as a probe of hydrogen bonding changes of carbonyl residues at the i-4 positions of Pro2155.50, Pro2676.50, Pro2917.38 and Pro3037.50. (a, c, e, g) One-dimensional MAS NMR difference spectra between rhodopsin and Meta II are shown in the top panel. REDOR filtered spectra of rhodopsin and Meta II are shown in the middle and lower panels, respectively. The spectra highlight the i-4 for carbonyls associated with His2115.46 using rhodopsin labeled with 1-13C His, 15N Phe (a), Ile2636.46 using rhodopsin labeled with 1-13C Ile, 15N Cys (c), Ser2997.46 using rhodopsin labeled with 1-13C Ser, 15N Val (e), and Phe2877.34 using rhodopsin labeled with 1-13C Phe, 15N Met (g). (b, d, f, h) Crystal structures of rhodopsin (gray, PDB ID 1U199) and Meta II (cyan, PDB ID 3PQR13) are shown in the region of interest.
The decrease in the His2115.46 13C=O chemical shift in the transition to Meta II can result from changes in hydrogen bonding, backbone torsion angles or both. Table 1 lists the 13C chemical shifts of the free C=O groups on H5, H6 and H7 along with the backbone torsion angles associated with these residues in inactive and active structures. The chemical shift changes associated with changes in backbone conformation versus hydrogen bonding can be comparable in magnitude (4–5 ppm)24–26. The X-ray crystal structure of rhodopsin shows that the C=O of His2115.46 is directly hydrogen-bonded to the Glu1223.37 COOH side chain9,10. Nevertheless, the observed chemical shift of 172.5 ppm is lower than that normally observed for hydrogen-bonded C=O groups in α-helices suggesting that the lower chemical shift is caused by non-helical backbone torsion angles (bold, italics in Table 1). Indeed in the crystal structure of rhodopsin, the ϕ and ψ torsion angles show a strong distortion compared to standard α-helices (ϕ= −60° and ψ = −45°). Upon activation, the His2115.46 C=O hydrogen bond with Glu1223.37 is broken and a new hydrogen bond is formed between Glu1223.37 and the His2115.46 imidazole nitrogen11,27 (see Fig. 2b). The direct His2115.46 - Glu1223.37 interaction can already be sensed using FTIR spectroscopy in the Meta I intermediate28. In the Meta II crystal structure12,13, the helix is less distorted than in rhodopsin (the backbone torsion angles of His2115.46 are closer to standard α-helix values), which would favor a downfield shift in the His2115.46 13C=O resonance. The observed upfield chemical shift of the His2115.46 C=O in Meta II is in the opposite direction and consequently is attributed to the loss of Glu1223.37 hydrogen bonding. This change suggests that the His2115.46 C=O functions as a hydrogen-bonding switch in receptor activation.
Table 1.
NMR 13C=O chemical shifts and crystal structure ϕ, ψ torsion angles in rhodopsin and Meta II
| Residue | State | Chemical shift (ppm)1 | ϕ2 | ψ |
|---|---|---|---|---|
| His211 | Rho Meta II |
172.5 170.23 |
−115/−1064 −1074 |
53/224 −34 |
| Ile263 | Rho Meta II |
171.93 170.5/175.6 |
−55/−66 −48 |
−44/−41 −41 |
| Ser299 | Rho Meta II |
173.1 168.93 |
−86/−824 −65 |
0/−84 −20 |
| Phe287 | Rho Meta II |
169.33 169.33 |
−35/−59 −56 |
−60/−41 −34 |
| Standard α-helix | 175–177 | −60 | −45 | |
Rhodopsin and Meta II 13C chemical shifts are taken from REDOR filtering experiments.
Torsion angles are taken from the rhodopsin crystal structures (PDB ID 1U199 and PDB ID 1GZM10) and demarcated by slashes (1U19/1GZM). Torsion angles for Meta II are taken from the Meta II crystal structure (PDB ID 3PQR13).
Low chemical shift values in bold are attributed to loss of or weaker C=O hydrogen bonding since the torsion angles are close to those for standard helices or, in the case of His211, the torsion angles become less distorted in Meta II.
Torsion angles in bold and italics are distorted from those of standard α-helices.
Pro2676.50
Pro2676.50, the conserved proline in TM helix H6, is associated with the free carbonyl of Ile2636.46. There are 22 isoleucine residues in rhodopsin. In contrast to the histidine C=O difference spectrum (Fig. 2a), the MAS NMR difference between rhodopsin and Meta II containing 1-13C isoleucine showed that several isoleucines change chemical shift upon activation (Fig. 2c). We took advantage of the unique Ile2636.46-Cys264 pair to identify the Ile2636.46 resonance in this spectrum. In the REDOR NMR filtered spectrum of rhodopsin (middle panel) labeled with 1-13C-isoleucine and 15N-cysteine, we observed a single resonance at 171.9 ppm assigned to Ile2636.46. The Ile2636.46 C=O group is oriented away from the helical bundle (Fig. 2d), toward the lipids, and the 171.9 ppm chemical shift likely reflects the absence of a hydrogen-bonding partner since the backbone ϕ and ψ torsion angles of Ile2636.46 are close to values observed in standard α-helices.
Upon activation, we found that the Ile2636.46 resonance split into two components at 170.5 and 175.6 ppm. In the Meta II crystal structure12,13, the backbone ϕ and ψ torsion angles are still close to values in standard α-helices (Table 1). The upfield (170.5 ppm) component of the Ile2636.46 13C=O resonance is consistent with a non-hydrogen bonded C=O and the small (1.4 ppm) upfield chemical shift would be consistent with a small distortion of the backbone from standard ϕ and ψ torsion angles. In contrast, the most likely explanation for the resonance with the large downfield chemical shift is that the Ile2636.46 C=O establishes a strong hydrogen bonding interaction upon activation. The crystal structures of Meta II12,13 reveal that the only side chain near the Ile2636.46 C=O is the aromatic ring of Phe2947.41.
Pro3037.50
Conserved Pro3037.50 on helix H7 frees the carbonyl of Ala2997.46 in rhodopsin. With the HEK293S expression system used for 13C-labeling rhodopsin, alanine is scrambled and cannot be specifically labeled. To target this carbonyl, we mutated Ala2997.46 to serine. The A299S rhodopsin mutant exhibits a 500 nm absorption band, and the photobleaching behavior and stability of Meta II are similar to the wild-type pigment (data not shown). The new Ser2997.46 - Val3007.47 dipeptide sequence is unique. Using the REDOR filtering experiment on 1-13C Ser, 15N-Val-labeled rhodopsin, we observed the Ser299 13C=O resonance at 173.1 ppm in rhodopsin and at 168.9 ppm in Meta II (Fig. 2e).
The Ala2997.46 C=O group is hydrogen bonded to the side chain NH2 of Asn551.50 (Fig. 2f). Like His2115.46, the backbone ϕ and ψ torsion angles of Ala2997.46 in rhodopsin are different from those in standard α-helices (Table 1), but in Meta II the values are consistent with α-helix. The large decrease in chemical shift of the Ser299 13C=O resonance upon activation is therefore attributed to a loss of hydrogen bonding to Asn551.50 (indicated by bold highlight in Table 1). Given the high conservation of both Asn551.50 and the free C=O group at position 299 across the family A GPCRs, this structural change upon activation is likely related to receptor function.
Pro2917.38
Pro2917.38 at the extracellular end of H7 is highly conserved (82%) in the visual receptor subfamily, and frees the carbonyl at Phe2877.34. There are two Phe-Met pairs, and correspondingly two peaks in the REDOR spectrum of rhodopsin at 174.1 ppm and 169.3 ppm (Fig. 2g). We assigned the 174.1 ppm to Phe85 in helix H2. This chemical shift is consistent with the helical secondary structure and backbone hydrogen-bonding of Phe85 in the rhodopsin crystal structure. The 169.3 ppm resonance is assigned to the Phe2877.34 C=O on the basis of the ϕ and ψ torsion angles and the lack of a clear hydrogen bonding interaction. The Phe2877.34 C=O is oriented toward the surrounding lipid and does not form inter-residue hydrogen bonds in the rhodopsin crystal structure9,10 (Fig. 2h). Upon activation, we observed that the 174.1 ppm resonance splits into two components at 174 and 175.3 ppm and the resonance at 169 ppm broadens. We attribute the broadening (and lower intensity) to increased disorder in the backbone structure at Phe2877.34, and unlike the other conserved prolines discussed above, this result is consistent with Pro2917.38 functioning as a flexible hinge. The splitting of the 174.1 ppm resonance is tentatively assigned to conformational changes in the region of Gly892.56-Gly902.57, one helical turn from Phe852.52 in the rhodopsin sequence (see Discussion).
Induced fit of the C-terminal Gα peptide influences H6
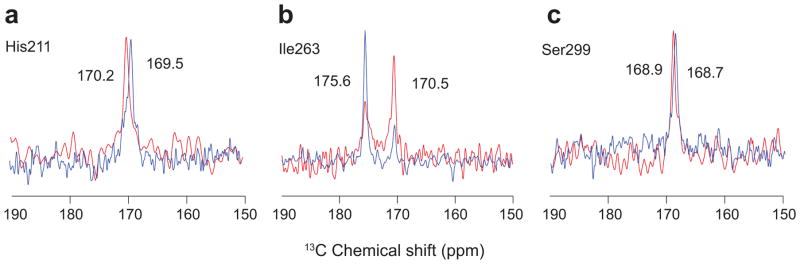
The change in the 13C=O resonance of Ile2636.46 upon light activation is the most unusual of the three sites associated with the conserved prolines described above. A component of this 13C=O resonance shifts downfield in frequency, but is not associated with a clear hydrogen-bonding partner in the crystal structures of active opsin11 or Meta II12,13. Comparison of the crystal structures of rhodopsin and Meta II shows that the backbone torsion angles of Ile2636.46 do not change appreciably upon activation. To better understand the structural changes occurring in this region of H6, we have obtained REDOR NMR spectra of the free carbonyls at His2115.46, Ile2636.46 and Ser2997.46 in the presence of the transducin Gα peptide.
It is known that binding of the transducin Gα peptide to Meta II stabilizes the active structure29. The C=O chemical shifts of His2115.46, Ile2636.46 and Ser2997.46 do not move appreciably upon Gα peptide binding (Figs. 3a–c). However, there is a marked increase in the intensity of the Ile2636.46 C=O with the unusual downfield chemical shift. The change in populations suggests there is an induced fit of the Gα peptide into its intracellular binding site, which is allosterically coupled to the region containing the Ile2636.46 C=O.
Figure 3.
13C…15N REDOR NMR experiments of Meta II in the presence and absence of the Gα peptide of transducin. REDOR filtered spectra are shown for His2115.46 (a), Ile2636.46 (b) and Ser2997.46 (c). The experiments on rhodopsin without (red) and with (blue) added Gα peptide were carried out in DDM micelles and mixed DDM/DOPS micelles, respectively.
DISCUSSION
Prolines are unique in lacking a backbone NH, which effectively eliminates the ability of the carbonyl group at the i-4 position to form an α-helical hydrogen bond. The three prolines in rhodopsin on helices H5, H6 and H7 are among the most conserved residues in the family A GPCRs, and consequently the free C=O groups at the i-4 positions associated with these prolines are highly conserved as well. Since breaking a backbone hydrogen bond in a hydrophobic environment is estimated to cost ~4–5 kcal/mol of energy30, the free i-4 carbonyl group represents an energetically favorable site for hydrogen bond formation. Depending on their ability to hydrogen bond, the free C=O groups can stabilize protein structure (strong hydrogen bonds) or facilitate conformational dynamics (weak or no hydrogen bonds). Conformational switching of hydrogen-bonding interactions may substantially lower the energetic barrier for breaking interhelical contacts and as such mediate the conformational changes that underlie protein function6. Our studies focus on the hydrogen bonding interactions of the conserved free C=O groups on H5, H6 and H7 in rhodopsin to address their role in receptor structure and function. These prolines and their associated free C=O groups exhibit several different hydrogen-bonding interactions and consequently provide insights into the possible roles of prolines in membrane proteins.
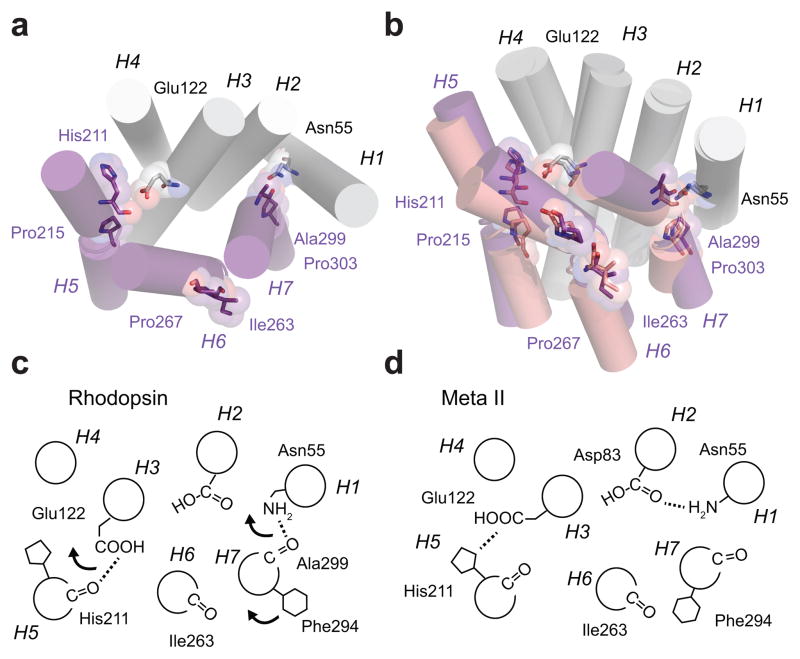
The structure of rhodopsin reflects the evolutionary requirements of night vision, namely a photoreceptor finely tuned to balance low dark noise (via a stable structure) and high sensitivity (through rapid receptor dynamics upon single photon absorption). The first four TM helices provide the stable framework onto which helices H5, H6 and H7 pack in the dark-state structure7. Helices H5, H6, and H7 each contain highly conserved prolines (Pro2155.50, Pro2676.50 and Pro3037.50) and undergo the largest structural changes upon activation (Fig. 4a,b). The free C=O groups associated with these prolines are all roughly in the same plane within or near the receptor’s TM core (Fig. 1). The TM core is highly conserved and involved in both receptor structure and function7.
Figure 4.
Receptor activation leads to repacking of helices H5–H7 on the TM core of rhodopsin. (a) Inactive structure of rhodopsin. Helices H1–H4 form a scaffold onto which helices H5–H7 pack. The key packing contacts on H5 and H7 are associated with Pro2155.50 and Pro3037.50 and their corresponding i-4 carbonyls. Retinal isomerization disrupts both interactions. For H5, the β-ionone ring has a steric clash at the position of the His2115.46-Glu1223.37 hydrogen bond upon conversion the all-trans configuration. For H7, the retinal is covalently attached to Lys2967.43. Upon isomerization, Trp2656.48 on H6 rotates away from H7 and disrupts a water mediated hydrogen bond with Asn3027.49, which is part of a hydrogen bonding network stretching from Asn551.50 and Asp832.50 to the Ala2997.46 C=O. (b) Overlap of the crystal structures of rhodopsin (1GZM10, purple) and Meta II (3PQR13, light purple) showing the positions of the TM helices. The disruption of the interactions of the His2115.46 C=O and Ala2997.46 C=O with the H1–H4 scaffold allows helices H5–H7 to reorient. (c,d) Schematic of the hydrogen bonding changes occurring between inactive rhodopsin and active Meta II rhodopsin.
Structural roles likely exist for all three highly conserved prolines. As the receptor folds during protein synthesis, the free carbonyls at His2115.46 and Ala2997.46 in the middle of the TM helices are in a position to hydrogen bond to the strongly polar Glu1223.37 and Asn551.50 side chains on helices H3 and H1, respectively. Computational studies on receptor stability and unfolding highlight His2115.46 and Ala2997.46 as the major TM residues that contribute to the overall structural integrity of rhodopsin31. These studies, which assessed receptor stability after removing single TM hydrogen bonds, provide strong support for the role of the free C=O groups on H5 and H7 in stabilizing receptor structure. Although His2115.46 and Ala2997.46 were identified using two slightly different computational approaches, both results are consistent with His2115.46 and Ala2997.46 being sites of autosomal dominant retinitis pigmentosa (ADRP) mutations, which are typically associated with protein misfolding 32. Mutation of Pro2676.50 also leads to protein misfolding and ADRP. Since the free C=O associated with Pro2676.50 does not hydrogen bond in inactive rhodopsin, its structural role may be to allow the helical segments on each side of Pro2676.50 to optimize contacts with the other TM helices and possibly prevent non-native (misfolded) interactions33.
Functional roles of the three highly conserved prolines likely involve conformational switching rather than conformational dynamics. Upon activation, both the His2115.46 and Ser2997.46 C=O groups lose their stabilizing hydrogen bonding interactions, which appear to act as molecular linchpins in rhodopsin. That is, they are stabilizing when in place, but result in a rearrangement of interhelical interactions and helix orientations when broken (Fig. 4c,d). The balance between the active and inactive conformations is dependent on whether the retinal-binding site is occupied by an 11-cis retinal PSB (inverse agonist) or all-trans retinal SB (agonist) chromophore. For H5, the new helix orientation is stabilized not by an alternative hydrogen bonding partner for the His2115.46 C=O, but instead by the formation of two new interhelical hydrogen bonds, one involving the His2115.46 side chain with Glu1223.37, and the other involving the interaction of Tyr2235.58 with Arg1353.50. Conservative mutations of either Glu1223.37 or Tyr2235.58 decrease the stability of Meta II20,34, i.e. the active H5 conformation reverts back to an inactive conformation more readily. For H7, the loss of the hydrogen bonding interaction with Asn551.50 allows H7 to become more helical. Mutations that favor a helical (non-distorted) conformation, as in the P303A mutant, lead to hyperactivity of the receptor35. The presence of molecular switches involving free C=O groups may also exist at other positions that have high subfamily conservation in GPCRs. For example, a recent NMR study suggested that the free C=O associated with conserved Pro4.60 in the β1-adrenergic receptor is part of a complex hydrogen-bonding network linking H4 and H5 that is modulated by ligand binding36.
The observation of two distinct chemical shifts for Ile2636.46 13C=O resonance suggests that Pro2676.50 provides a hinge in H6, but that it is not conformationally dynamic. The 13C=O resonances are sharp, rather than broadened as observed for Phe2877.34. Consistent with this idea, position 6.46 is 80% conserved as Ile, Val or Leu and only 2% conserved as glycine, which is thought to facilitate dynamics when located at the i-4 position relative to TM prolines2. The mixture of chemical shift changes of the Ile2636.46 13C=O resonance indicates that H6 does not toggle to a fully active conformation until binding of the Gα peptide. In contrast to the C=O groups on H5 and H7, one component of the Ile2636.46 13C=O resonance shifts downfield consistent with an increase in hydrogen bonding. Furthermore, the chemical shifts or intensities of the free C=O groups associated with H5 and H7 do not markedly change with the addition of the Gα peptide, suggesting that H5 and H7 have adopted their fully active conformations prior to G protein binding.
Of the four prolines studied, only Pro2917.38 appears to be conformationally dynamic in the active state. The broadened NMR resonances indicate that there is not a distinct conformation in this region of the active receptor. For Pro2155.50 and Pro3037.50 on H5 and H7, the identity of the side chain at the i-4 position may contribute to the hinging motion of these helices. Glycine and proline are two residue types that may confer flexibility when present at the i-4 position relative to proline2. Glycine occurs most often at position 5.46 (17%) in the family A GPCRs, with serine being the next highest conserved at this position (11%). Olfactory receptors are excluded from this analysis since position 5.50 is only moderately conserved as a proline (38%) in the olfactory receptor subfamily. For position 7.46, serine has the highest conservation (56%) in the family A GPCRs excluding the olfactory receptors. Interestingly, both positions 7.46 (94%) and 7.50 (98%) are highly conserved as proline in the olfactory receptors. The presence of glycine or other small residues at position 5.46 in some class A GPCRs and proline at position 7.46 in the olfactory receptors may allow increased flexibility in H5 and H7 of these receptors, respectively.
Together, the NMR data presented here provide deeper insight into the diverse roles that proline can perform in rhodopsin. Key to understanding the functions of these prolines are the possible hydrogen bonding interactions involving the free i-4 carbonyls. The free i-4 carbonyls can form stabilizing interactions in rhodopsin and function as conformational switches in the conversion to the active Meta II intermediate. As such, these findings are of broad significance in the structure and function of membrane proteins in general. In a survey of high-resolution membrane protein structures from the Protein Data Bank, we have found that ~75% of the free carbonyls have a polar residue nearby in a position to directly hydrogen bond or hydrogen bond via a water molecule (Supplementary Note 2). Many of these prolines occur in regions that are important in protein function, and they appear in membrane proteins as diverse as transporters, enzymes, and ion channels (Supplementary Note 2).
ONLINE METHODS
Expression and purification of 13C labeled rhodopsin
Isotope enriched bovine opsin was expressed using inducible HEK293S cell lines. The original cell lines were obtained from Jeremy Nathans (Johns Hopkins University), but not authenticated or tested for mycoplasma contamination. HEK293S cells are widely used for production of recombinant proteins and viruses. The expressed opsin was generated into rhodopsin through incubation with ~30 micromolar 11-cis retinal, extracted from membranes using 1% (w/v) n-β-D dodecyl maltopyranoside (DDM) in PBS pH 7.4, and purified using Rho-1D4-Sepharose resin37. 13C labeled retinal was prepared synthetically. Resin-bound rhodopsin was washed with 50 column volumes of 0.02% DDM in PBS pH 7.2, equilibrated with 0.02% DDM, 2 mM sodium phosphate pH 6.0. Rhodopsin was eluted in 0.02% DDM, sodium phosphate pH 6.0, 100 μM 9-mer elution peptide (TETSQVAPA) and concentrated to a volume of 1 ml using centricon (Millipore) centrifugation devices with a molecular weight cutoff of 30 kDa. The volume was further reduced to < 60 μl under a gentle stream of argon gas and packed into a 4 mm MAS rotor.
Solid-state NMR spectroscopy
Solid-state NMR experiments were conducted at static field strengths of either 500 or 600 MHz using a three-channel 4 mm MAS probe with a spinning rate of 10 kHz. Spectra were collected using a 2 ms contact pulse during cross polarization. SPINAL64 decoupling was used during acquisition with a 1H RF field strength between 70–90 kHz. REDOR spectra were obtained with a dephasing period of 20 rotor cycles at 10 KHz MAS rate (2 ms)38. The REDOR filtered spectra (ΔS) were obtained by subtracting spectra with (S) and without (S0) rotor-synchronized 15N π pulses (10–11 μs). To reduce artifacts, S and S0 spectral acquisition was interleaved and difference spectra were acquired scan-by-scan. ΔS spectra were summed over 60–100K scans using ~5–6 mgs of rhodopsin in a typical sample.
For rhodopsin and Meta II spectra, states were cryo-trapped at 190 K as described previously39. The spectra shown were generally repeated at least twice on samples purified from different cell growths without deviations of more than ±0.2 ppm in the reported chemical shifts. The 13C high-resolution NMR and solid-state MAS NMR spectra were externally referenced to the 13C resonance of neat TMS at 0 ppm at room temperature. Using TMS as the external reference, we calibrated the carbonyl resonance of solid glycine at 176.46 ppm. The chemical shift difference between 13C of DSS in D2O relative to neat TMS is 2.01 ppm.
Gα peptide synthesis and reconstitution with rhodopsin
The 15-mer C-terminal peptide of the Gα subunit of transducin (TDIIIKENLKDCGLF) was synthesized using solid-phase methods (Keck Small Scale Peptide Synthesis Facility, Yale University) and purified by reverse phase HPLC. The experiments on rhodopsin with added Gα peptide were carried in mixed micelles using DDM and dioleoylphosphoserine (DOPS). Solubilization of rhodopsin in DDM/DOPS mixed micelles has been shown to facilitate the interaction between rhodopsin and the full heterotrimeric form of transducin40. DOPS was added to rhodopsin in DDM micelles in a 1:100 rhodopsin to lipid ratio, and the Gα peptide to rhodopsin ratio was 8:1. The stability of the Meta II state was monitored by fluorescence quenching interaction between the indole of Trp265 and the β-ionone ring of the retinal chromophore41.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health (NIH) grant GM41412 (to S.O.S.) We thank H. Sasaki and X. Zhou (Institute of Protein Research, Osaka University) for expression and purification of several of the 15N, 13C labeled rhodopsin samples, and J. Goncalves for preliminary experiments with the Gα peptide.
Footnotes
Author Contributions
M.E., P.J.R and S.O.S conceived the study; N.K, A.P, and M.E. made samples; M.E. and M.Z. collected and analyzed NMR data; A.P. and O.B.S.R. analyzed the protein database for proline interactions; C.A.O constructed rhodopsin mutants; N.K., A.P., P.J.R. and S.O.S wrote the manuscript.
Competing Financial Interests
The authors declare no competing financial interests.
References
- 1.Sansom MSP, Weinstein H. Hinges, swivels and switches: The role of prolines in signalling via transmembrane α-helices. Trends Pharmacol Sci. 2000;21:445–451. doi: 10.1016/s0165-6147(00)01553-4. [DOI] [PubMed] [Google Scholar]
- 2.Cordes FS, Bright JN, Sansom MSP. Proline-induced distortions of transmembrane helices. J Mol Biol. 2002;323:951–960. doi: 10.1016/s0022-2836(02)01006-9. [DOI] [PubMed] [Google Scholar]
- 3.Williams KA, Deber CM. Proline residues in transmembrane helices: structural or dynamic role? Biochemistry. 1991;30:8919–8923. doi: 10.1021/bi00101a001. [DOI] [PubMed] [Google Scholar]
- 4.von Heijne G. Proline kinks in transmembrane α-helices. J Mol Biol. 1991;218:499–503. doi: 10.1016/0022-2836(91)90695-3. [DOI] [PubMed] [Google Scholar]
- 5.Fu Q, et al. Structural basis and functional role of intramembrane trimerization of the Fas/CD95 death receptor. Mol Cell. 2016;61:602–613. doi: 10.1016/j.molcel.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cao Z, Bowie JU. Shifting hydrogen bonds may produce flexible transmembrane helices. Proc Natl Acad Sci USA. 2012;109:8121–8126. doi: 10.1073/pnas.1201298109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith SO. Structure and activation of the visual pigment rhodopsin. Annual Review of Biophysics. 2010;39:309–328. doi: 10.1146/annurev-biophys-101209-104901. [DOI] [PubMed] [Google Scholar]
- 8.Ballesteros JA, Weinstein H. Integrated methods for the construction of three dimensional models and computational probing of structure-function relations in G-protein coupled receptors. Meth Neurosci. 1995;25:366–428. [Google Scholar]
- 9.Palczewski K, et al. Crystal structure of rhodopsin: A G protein-coupled receptor. Science. 2000;289:739–745. doi: 10.1126/science.289.5480.739. [DOI] [PubMed] [Google Scholar]
- 10.Li J, Edwards PC, Burghammer M, Villa C, Schertler GFX. Structure of bovine rhodopsin in a trigonal crystal form. J Mol Biol. 2004;343:1409–38. doi: 10.1016/j.jmb.2004.08.090. [DOI] [PubMed] [Google Scholar]
- 11.Park JH, Scheerer P, Hofmann KP, Choe HW, Ernst OP. Crystal structure of the ligand-free G-protein-coupled receptor opsin. Nature. 2008;454:183–187. doi: 10.1038/nature07063. [DOI] [PubMed] [Google Scholar]
- 12.Deupi X, et al. Stabilized G protein binding site in the structure of constitutively active metarhodopsin-II. Proc Natl Acad Sci USA. 2012;109:119–124. doi: 10.1073/pnas.1114089108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choe HW, et al. Crystal structure of metarhodopsin II. Nature. 2011;471:651–655. doi: 10.1038/nature09789. [DOI] [PubMed] [Google Scholar]
- 14.Elling CE, et al. Metal ion site engineering indicates a global toggle switch model for seven-transmembrane receptor activation. J Biol Chem. 2006;281:17337–17346. doi: 10.1074/jbc.M512510200. [DOI] [PubMed] [Google Scholar]
- 15.Schwartz TW, Frimurer TM, Holst B, Rosenkilde MM, Elling CE. Molecular mechanism of 7TM receptor activation - A global toggle switch model. Annu Rev Pharmacol Toxicol. 2006;46:481–519. doi: 10.1146/annurev.pharmtox.46.120604.141218. [DOI] [PubMed] [Google Scholar]
- 16.Ganter UM, Gärtner W, Siebert F. Rhodopsin-lumirhodopsin phototransition of bovine rhodopsin investigated by Fourier transform infrared difference spectroscopy. Biochemistry. 1988;27:7480–7488. doi: 10.1021/bi00419a046. [DOI] [PubMed] [Google Scholar]
- 17.Van Arnam EB, Lester HA, Dougherty DA. Dissecting the functions of conserved prolines within transmembrane helices of the D2 dopamine receptor. ACS Chem Biol. 2011;6:1063–1068. doi: 10.1021/cb200153g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wess J, Nanavati S, Vogel Z, Maggio R. Functional role of proline and tryptophan residues highly conserved among G protein-coupled receptors studied by mutational analysis of the m3 muscarinic receptor. EMBO J. 1993;12:331–338. doi: 10.1002/j.1460-2075.1993.tb05661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stitham J, Martin KA, Hwa J. The critical role of transmembrane prolines in human prostacyclin receptor activation. Mol Pharmacol. 2002;61:1202–1210. doi: 10.1124/mol.61.5.1202. [DOI] [PubMed] [Google Scholar]
- 20.Imai H, et al. Single amino acid residue as a functional determinant of rod and cone visual pigments. Proc Natl Acad Sci USA. 1997;94:2322–2326. doi: 10.1073/pnas.94.6.2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deupi X, Standfuss J. Structural insights into agonist-induced activation of G-protein-coupled receptors. Curr Opin Struct Biol. 2011;21:541–551. doi: 10.1016/j.sbi.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 22.Deupi X. Relevance of rhodopsin studies for GPCR activation. Biochim Biophys Acta. 2014;1837:674–82. doi: 10.1016/j.bbabio.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 23.Gullion T, Schaefer J. Rotational-echo double-resonance NMR. J Magn Reson. 1989;81:196–200. doi: 10.1016/j.jmr.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 24.Saito H. Conformation-dependent C-13 chemical-shifts - a new means of conformational characterization as obtained by high-resolution solid-state C-13 NMR. Magn Reson Chem. 1986;24:835–852. [Google Scholar]
- 25.Gu ZT, Zambrano R, McDermott A. Hydrogen-bonding of carboxyl groups in solid state amino acids and peptides: comparison of carbon chemical shielding, infrared frequencies, and structures. J Am Chem Soc. 1994;116:6368–6372. [Google Scholar]
- 26.Szilagyi L. CHEMICAL-SHIFTS IN PROTEINS COME OF AGE. Progress in Nuclear Magnetic Resonance Spectroscopy. 1995;27:325–443. [Google Scholar]
- 27.Scheerer P, et al. Crystal structure of opsin in its G-protein-interacting conformation. Nature. 2008;455:497–502. doi: 10.1038/nature07330. [DOI] [PubMed] [Google Scholar]
- 28.Beck M, Sakmar TP, Siebert F. Spectroscopic evidence for interaction between transmembrane helices 3 and 5 in rhodopsin. Biochemistry. 1998;37:7630–7639. doi: 10.1021/bi9801560. [DOI] [PubMed] [Google Scholar]
- 29.Hamm HE, et al. Site of G protein binding to rhodopsin mapped with synthetic peptides from the alpha subunit. Science. 1988;241:832–835. doi: 10.1126/science.3136547. [DOI] [PubMed] [Google Scholar]
- 30.White SH, Wimley WC. Membrane protein folding and stability: Physical principles. Annu Rev Biophys Biomol Struct. 1999;28:319–365. doi: 10.1146/annurev.biophys.28.1.319. [DOI] [PubMed] [Google Scholar]
- 31.Rader AJ, et al. Identification of core amino acids stabilizing rhodopsin. Proc Natl Acad Sci USA. 2004;101:7246–7251. doi: 10.1073/pnas.0401429101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sung CH, Davenport CM, Nathans J. Rhodopsin mutations responsible for autosomal-dominant retinitis-pigmentosa - clustering of functional classes along the polypeptide-chain. J Biol Chem. 1993;268:26645–26649. [PubMed] [Google Scholar]
- 33.Wigley WC, et al. A protein sequence that can encode native structure by disfavoring alternate conformations. Nat Struct Biol. 2002;9:381–388. doi: 10.1038/nsb784. [DOI] [PubMed] [Google Scholar]
- 34.Goncalves JA, et al. Highly conserved tyrosine stabilizes the active state of rhodopsin. Proc Natl Acad Sci USA. 2010;107:19861–19866. doi: 10.1073/pnas.1009405107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fritze O, et al. Role of the conserved NPxxY(x)(5,6)F motif in the rhodopsin ground state and during activation. Proc Natl Acad Sci USA. 2003;100:2290–2295. doi: 10.1073/pnas.0435715100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Isogai S, et al. Backbone NMR reveals allosteric signal transduction networks in the beta(1)-adrenergic receptor. Nature. 2016;530:237-+. doi: 10.1038/nature16577. [DOI] [PubMed] [Google Scholar]
- 37.Goncalves J, et al. Magic angle spinning nuclear magnetic resonance spectroscopy of G protein-coupled receptors. Meth Enzymol. 2013;522:365–89. doi: 10.1016/B978-0-12-407865-9.00017-0. [DOI] [PubMed] [Google Scholar]
- 38.Gullion T, Schaefer J. Detection of weak heteronuclear dipolar coupling by rotational-echo double-resonance NMR. In: Warren WS, editor. Advances in Magnetic Resonance; Conference on “High Resolution NMR in Solids”; January 19–21, 1989; San Diego, California, USA; London, England, UK: Academic Press, Inc; 1989. pp. 57–84. [Google Scholar]
- 39.Eilers M, Ying WW, Reeves PJ, Khorana HG, Smith SO. Magic angle spinning nuclear magnetic resonance of isotopically labeled rhodopsin. Meth Enzymol. 2002;343:212–222. doi: 10.1016/s0076-6879(02)43137-0. [DOI] [PubMed] [Google Scholar]
- 40.Jastrzebska B, Goc A, Golczak M, Palczewski K. Phospholipids are needed for the proper formation, stability, and function of the photoactivated rhodopsin-transducin complex. Biochemistry. 2009;48:5159–5170. doi: 10.1021/bi900284x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Farrens DL, Khorana HG. Structure and function in rhodopsin. Measurement of the rate of metarhodopsin II decay by fluorescence spectroscopy. J Biol Chem. 1995;270:5073–5076. doi: 10.1074/jbc.270.10.5073. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.