Abstract
Background
An increasing number of injectable biologics are now available for the treatment of psoriasis. However, for individuals who have never received this therapy, the process of performing a self-injection can be daunting. There is lack of patient educational material on how to perform and optimize this treatment.
Objective
The objective of this study is to present a freely available online guide and video on biologic injections that is informative to patients and increases the success and compliance of patients starting this therapy.
Methods
The self-injection technique taught at the University of California—San Francisco Psoriasis and Skin Treatment Center as well as available information from the literature were reviewed to design a practical guide for patients receiving biologic injections.
Results
We created a printable guide and video resource that describes how to improve the injection process, pain management, travel planning, and common concerns with biologic injectables.
Conclusion
This guide is beneficial for patients who wish to improve their experience with biologic self-injections, for healthcare providers who prescribe these treatments, and for trainees learning about this modality.
Keywords: Adalimumab, Biologic agents, Biologics, Etanercept, Ixekizumab, Patient education, Psoriasis, Secukinumab, Ustekinumab, Video guide
Introduction
Psoriasis is a common chronic inflammatory skin condition that affects 3–4% of the adult United States population [1, 2]. The pathogenesis of psoriasis is multifactorial and thought to be a combination of genetic susceptibility, immune dysregulation, and environmental factors [3]. Key cytokines involved in the pathogenesis of psoriasis include tumor necrosis factor alpha (TNF-α) and interleukins (IL-12, IL-17, IL-22, IL-23) [4]. In recent years, there has been a focus on the development of treatment agents targeting these inflammatory cytokines. Many of these therapies are biologic injectable agents and are administered via subcutaneous injection. Biologic injectable agents currently available for the treatment of moderate-to-severe plaque psoriasis include the following: Etanercept (Enbrel®, Amgen Inc.), adalimumab (Humira®, AbbVie Inc.), ustekinumab (Stelara®, Janssen Biotech, Inc.), secukinumab (Cosentyx®, Novartis Pharmaceuticals Corporation) and ixekizumab (Taltz®, Eli Lilly and Company.). These treatments have proven to be highly efficacious in the treatment of psoriasis with significant improvement seen in 50–75% of patients and have become more commonly used in clinical practice [5, 6]. Etanercept, adalimumab, secukinumab, and ixekizumab are each available in the form of either an auto-injector pen or a prefilled syringe [7–10]. Ustekinumab is available only as a prefilled syringe [11]. Below we will describe flow of treatment, injection techniques, and practical tips for biologic injectable agents.
Methods
We reviewed the biologic injectable agent protocol used at the University of California—San Francisco Psoriasis and Skin Treatment Center. In addition, the PubMed database was searched using the term “psoriasis” combined with the terms “biologic” “etanercept”, “adalimumab, “ustekinumab”, “secukinumab”, and “ixekizumab” to identify relevant articles to design a comprehensive guide for patients receiving biologic injectable treatment for psoriasis.
This article does not involve any new studies of human or animal subjects performed by any of the authors. All photos are printed with the consent of the subject(s).
Results and Discussion
Overview
The guide below will cover the supplies needed for biologic injectable agents, injection procedure techniques, how to plan injections, and how to travel with medications. Biologic injectable agents have been demonstrated to be effective treatments for psoriasis [12]. It is important for patients and physicians to discuss in detail the treatment options, patient history, and patient preferences when considering biologic injectable agents for the treatment of psoriasis (Tables 1, 2).
Table 1.
Currently available injectable biologic agents for the treatment of psoriasis [7]
| Treatment type | Secukinumab (Cosentyx®) | Ixekizumab (Taltz®) | Etanercept (Enbrel®) | Adalimumab (Humira®) | Ustekinumab (Stelara®) |
|---|---|---|---|---|---|
| Mechanism of action: Biologics work by blocking specific proteins in the immune system | IL-17A | IL-17A | TNF-α | TNF-α | IL-12 and IL-23 |
| Method of delivery: How the drug is given or taken | Subcutaneous self-injection (pen, syringe) | Subcutaneous self-injection (pen, syringe) | Subcutaneous self-injection (pen, syringe) | Subcutaneous self-injection (pen, syringe) | Subcutaneous injection by a health care professional, or self-injection (syringe) |
| Frequency: How often the drug must be taken | Weeks 0, 1, 2, 3, and 4 and every 4 weeks thereafter | Weeks 0, 2, 4, 6, 8, 10, 12 and every 4 weeks thereafter | Two times weekly for the first 3 months, then weekly thereafter | Two injections on weeks 0 then 1 injection every 2 weeks thereafter starting at week 1 | Week 0, week 4, then every 12 weeks thereafter |
| Common side effects: Side effects may vary for each individual | Cold symptoms (>1%), diarrhea (>1%), upper respiratory infection (>1%) | Injection site reactions (≥1%), upper respiratory tract infections (≥1%), nausea (≥1%), tinea infections (≥1%) | Respiratory infection (>5%), injection site reaction (>5%) | Upper respiratory infection (>10%), injection site reaction (>10%), headache (>10%) | Upper respiratory infection (>3%), headache (>3%), fatigue (>3%) |
| Possible risks: the possible risks listed include serious adverse events that have been reported in association with these medications during and after clinical trials |
Serious infection, mucocutaneous candidiasis, Rarely observed: inflammatory bowel disease |
Serious infection Rarely observed: rhinitis, oral candidiasis, urticaria, influenza, conjunctivitis, inflammatory bowel disease, angioedema |
Serious infection Rarely observed: Exacerbation of multiple sclerosis, congestive heart failure, lupus |
Serious infection Rarely observed: Exacerbation of multiple sclerosis, congestive heart failure, lupus |
Serious infections Rarely observed: Serious allergic reactions, reversible posterior leukoencephalopathy syndrome |
| Monitoring | Yearly tuberculosis screening, initial hepatitis screening, symptoms of inflammatory bowel disease | Yearly tuberculosis screening, initial hepatitis screening, symptoms of inflammatory bowel disease | Yearly tuberculosis screening, initial hepatitis screening, blood count and liver function tests | Yearly tuberculosis screening, initial hepatitis screening, blood count and liver function tests | Yearly tuberculosis screening, initial hepatitis screening, blood count and liver function tests |
IL interleukin, TNF tumor necrosis factor
Table 2.
Workflow for injectable biologic agents
Medication Management
The medication should be stored in a refrigerator and kept at approximately 4 °C (39 °F) to maintain its integrity. It should never be frozen as this can inactivate the drug. Unfortunately, patients commonly experience discomfort when injecting the medication that has been stored at cold temperature. Therefore, we recommend patients prepare their injection by removing it from refrigeration and then waiting 10–20 min for the medication to reach room temperature. Additionally, it can be helpful for patients to warm up the medication in their hands to help reach a more comfortable temperature for injection.
Target Injection Site Selection
Selection of the injection site is a personal preference depending on what is the easiest for each individual patient. We have found that some sites are easier to inject than others (Fig. 1). When choosing between the arm, abdomen, and thighs many patients report that injecting in the thighs is the easiest. This location tends to be easier for several reasons. First, it is easily accessible and is within arm’s reach. The thigh can also be injected on either the right or left side without difficulty. In addition, this location tends to be less painful than other injection sites. Hence, thigh injection can be performed while seated, which provides a flat surface and allows for an easier set up.
Fig. 1.
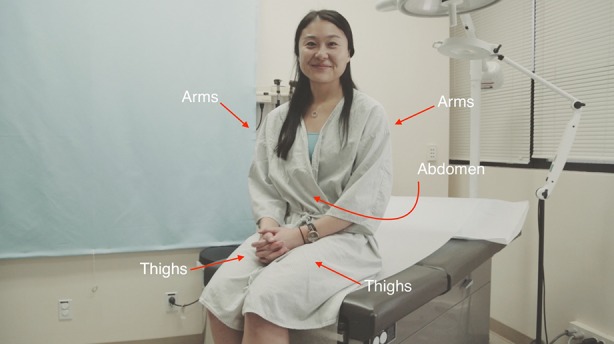
Locations for target injection sites
Pain Management and Preparing for Injection
Pain is a common concern for patients. An ice pack (like the one that may come with the medication upon delivery) can be used to help numb the injection site prior to injection. Patients can apply the ice pack to the area for several seconds or until sufficiently numbed. When the patient is ready to inject, the injection site should be cleaned off with an alcohol wipe prior to injection (Fig. 2).
Fig. 2.
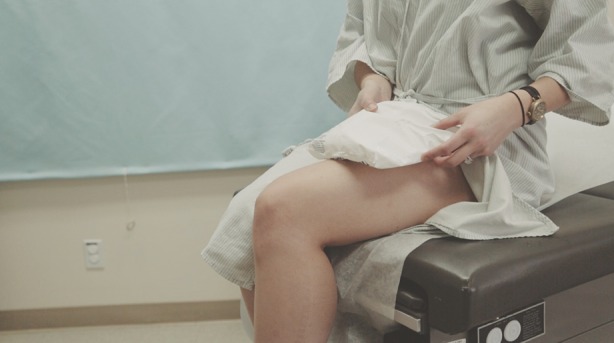
Applying ice pack
Skin Pinch Technique
This technique involves pinching up excess skin at the target injection site and injecting directly into the pinched skin (Fig. 3). The thigh is a viable option in almost all patients with proper technique.
Fig. 3.
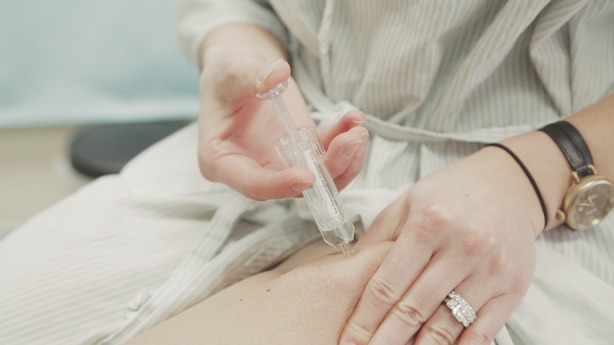
Skin pinch technique
Administering the Medication
To administer the medication please follow the instructions for the individual medication (adalimumab, etanercept, ixekizumab, secukinumab, ustekinumab). Medication is best held in the dominant hand to maximize control and dexterity during injection. Prefilled syringes are best administered at a 45° angle to the skin while using the skin pinch technique. Auto-injector pens are best administered at a 90° angle to the skin. Make sure to review the injection instructions unique to each medication.
Injection Site Reactions
Injection site reactions are relatively common side effects for injectable biologic agents. An injection site reaction is redness, rash, swelling, itching, or bruising at the site of injection. Injection site reactions typically present within a day after injection, and commonly resolve within several days. It can be useful to ice the area to reduce swelling and alleviate symptoms, as well as use an antihistamine to reduce swelling and itch.
Disposing of the Medication
After injection of the medication it is important to dispose of syringes in an appropriate sharps container. Sharps containers can be obtained at a local pharmacy or at some doctors’ offices. When the sharps container is full it can be disposed of in safe container disposal sites located at the doctors’ office and other healthcare facilities. If a sharps container is not available, a coffee tin can be used as an alternative until a sharps container is acquired.
Traveling with Biologic Injectable Medications
While we advise patients not to miss any medication doses, sometimes it can be difficult to maintain the injection schedule during times of travel. There are several options to modulate the injection regimen if the scheduled injection happens to fall during travel time:
For trips which will delay the injection by less than 1 week of the scheduled injection day, it is can be easier to defer the injection until returning from the trip. This will guarantee that the medication remains adequately refrigerated and in optimal condition without risking the integrity of the medications during travel.
For trips which will delay the injection by more than 1 week of the scheduled injection day, the patient will have to travel with the medication. It is crucial to find adequate storage and refrigeration for the medication during travel. The medication should be kept cold in an insulated bag with an ice pack at all times. If the patient is traveling on a long flight, a flight attendant may be able to store the medication in the refrigerator of the airplane. A doctor’s note or copy of the prescription information can be helpful when traveling through airport security. Patients should make sure that their accommodations at their travel destination provide a secure place to refrigerate the medication. The goal is to ensure that that medication is safe, secure, and at an adequate temperature.
Illness
While we advise patients not to miss any medication doses, patients may encounter a situation where their scheduled injection falls during times of illness (flu, infection, fever, etc.) Biologic agents are medications that can modulate the immune system. Depending on the severity of the illness, it is reasonable to delay the injection until after recovering from the illness; however, this should always be done in consultation with a medical doctor.
Conclusions
Biologic injectable agents are safe and effective treatment options for patients with moderate-to-severe psoriasis, and while they are becoming more common throughout medical practice there has been a paucity of guidance for patients and clinicians on how to optimize the treatment experience. We hope that this guide will be a valuable resource for patients and clinicians preparing for treatment with biologic injectable agents.
Acknowledgments
We would like to thank Tim Sarmiento for producing, directing, and editing the educational video that accompanies this manuscript. We would also like to thank the amazing staff and nurses from the UCSF Psoriasis and Skin Treatment Center for inspiring and helping make the video possible. We thank Olivia Chen for her help reviewing the Spanish translation of the accompanying video. No funding or sponsorship was received for publication of this article. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
Disclosures
John Koo is a speaker for AbbVie, Leo, and Celgene, and conducts research for Amgen, Janssen, Novartis, Photomedex, Galderma, Pfizer and Merck. Tina Bhutani is an advisor for Cutanea, and conducts research for AbbVie, Janssen, and Merck. Wilson Liao conducts research for AbbVie, Janssen, Novartis, and Pfizer, and receives funding from the NIH (R01AR065174, U01AI119125). John Koo, Tina Bhutani, and Wilson Liao have no stocks, employment or board memberships with any pharmaceutical company. Michael Abrouk, Benjamin Farahnik, Mio Nakamura, Tian Hao Zhu, Rasnik K. Singh, Kristina M. Lee, and Margareth V. Jose have nothing to disclose.
Compliance with Ethics Guidelines
This article does not involve any new studies of human or animal subjects performed by any of the authors. All photos are printed with the consent of the subject(s).
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Enhanced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/C9D4F0600C816B7E.
References
- 1.Parisi R, Symmons DP, Griffiths CE, Ashcroft DM. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377–385. doi: 10.1038/jid.2012.339. [DOI] [PubMed] [Google Scholar]
- 2.Gupta, R. et al. The Goeckerman regimen for the treatment of moderate to severe psoriasis. J Vis Exp e50509 (2013). doi:10.3791/50509. [DOI] [PMC free article] [PubMed]
- 3.Mitra A, Fallen RS, Lima HC. Cytokine-based therapy in psoriasis. Clin Rev Allergy Immunol. 2013;44:173–182. doi: 10.1007/s12016-012-8306-2. [DOI] [PubMed] [Google Scholar]
- 4.Raychaudhuri SP. Role of IL-17 in psoriasis and psoriatic arthritis. Clin Rev Allergy Immunol. 2013;44:183–193. doi: 10.1007/s12016-012-8307-1. [DOI] [PubMed] [Google Scholar]
- 5.Takeshita J, et al. Psoriasis in the US medicare population: prevalence, treatment, and factors associated with biologic use. J Invest Dermatol. 2015;135:2955–2963. doi: 10.1038/jid.2015.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Puig L, Vilarrasa, Puig López. Efficacy of biologics in the treatment of moderate-to-severe plaque psoriasis: a systematic review and meta-analysis of randomized controlled trials with different time points. J Eur Acad Dermatol Venereol. 2014;28:1633–1653. doi: 10.1111/jdv.12238. [DOI] [PubMed] [Google Scholar]
- 7.National Psoriasis Foundation. Moderate to severe psoriasis and psoriatic arthritis: biologic drugs. https://www.psoriasis.org/about-psoriasis/treatments/biologics.
- 8.Leonardi C, et al. Long-term safety and efficacy of etanercept in patients with psoriasis: an open-label study. J Drugs Dermatol. 2010;9:928–937. [PubMed] [Google Scholar]
- 9.Langley R, et al. Secukinumab in plaque psoriasis—results of two Phase 3 Trials. N Engl J Med. 2014;371:326–338. doi: 10.1056/NEJMoa1314258. [DOI] [PubMed] [Google Scholar]
- 10.Farahnik B, et al. Ixekizumab for the Treatment of Psoriasis: a Review of Phase III Trials. Dermatol Ther (Heidelb) 2016;6:25–37. doi: 10.1007/s13555-016-0102-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Langley RG, et al. Long-term efficacy and safety of ustekinumab, with and without dosing adjustment, in patients with moderate-to-severe psoriasis: results from the PHOENIX 2 study through 5 years of follow-up. Br J Dermatol. 2015;172(5):1371–83. doi: 10.1111/bjd.13469. [DOI] [PubMed] [Google Scholar]
- 12.Leon A, Nguyen A, Letsinger J, Koo J. An attempt to formulate an evidence-based strategy in the management of moderate-to-severe psoriasis: a review of the efficacy and safety of biologics and prebiologic options. Expert Opin Pharmacother. 2007;8:617–632. doi: 10.1517/14656566.8.5.617. [DOI] [PubMed] [Google Scholar]



