Abstract
The rare entity of primary T-cell lymphoma of thyroid gland may pose great diagnostic and therapeutic challenges to the pathologist and clinician. There are very few case and short series reports of these tumors describing their varied clinicopathologic features in English literature. We report a case of mature T-cell lymphoma of thyroid in a 26 year old male, with unique pseudogranulomatous and lymphohistiocytic Lennert type of morphology, on a background of autoimmune thyroiditis. This man, diagnosed with Hashimoto’s thyroiditis for the previous 2 years, underwent thyroidectomy for sudden onset of pressure symptoms. The diagnosis of T-cell lymphoma was made on the thyroid tissue based on histopathologic and immunophenotypic findings, in concert with the results of T-cell receptor gene rearrangement studies by polymerase chain reaction. Later, after about 3 months, similar findings were confirmed in an excision biopsy from a left cervical lymph node in the patient. The patient has been started on chemotherapy with gemcitabine, dexamethasone, and cisplatin along with involved field radiotherapy; however, he has shown a rapid upstaging of disease from stage IE to IIIE in a short period of 3 months with relatively well preserved clinical parameters until the latest follow up.
Keywords: Lennert type, Primary T-cell lymphoma, Thyroid gland
Background
Primary lymphoma of the thyroid is a rare neoplasm comprising 2–5 % of all thyroid malignancies [1–3]. Almost all primary thyroid lymphomas turn out to be a non-Hodgkin lymphoma (NHL) of B-cell origin of either marginal zone lymphoma of mucosa associated lymphoid tissue (MALT) or diffuse large B-cell lymphoma (DLBCL) type [2–5]. T-cell lymphoma (TCL) in the thyroid gland is distinctly rare with about 20 cases having been reported so far in the literature [6–20]. Age range of these cases was between 34 and 86 years with a male to female ratio of 1.15:1. Most of the reported cases showed a low grade behavior on follow up unlike the aggressive behavior seen in conventional TCL [3, 9]. Microscopic morphology, in these cases, is variable showing most commonly a small lymphocytic infiltrate invading the thyroid follicles, also forming lymphoepithelial bodies in some cases [6–20]. Immunohistochemistry (IHC) of tumors in these cases has often revealed a predominant T-cell population with positivity for CD2, CD3 & CD5, with one case also reporting loss for CD7 [6]. Genetic studies remain important adjuncts for demonstrating TCR gene rearrangement as a marker of T-cell clonality, especially in difficult cases. Correct diagnosis of lymphoma is ever more relevant in this era of targeted therapy and evolving dynamics of lymphoma management.
We report a case of mature TCL of thyroid gland in a 26 year old man, youngest to the best of our knowledge, who was previously diagnosed with Hashimoto’s thyroiditis. Microscopy of the tumor revealed predominant lymphohistiocytic Lennert like morphology, as yet unreported in a case of TCL of thyroid gland.
Case Report
This 26 year old man sought medical consultation for swelling in the front aspect of his neck, of about 2 year duration. On examination, he did not have any features of thyroid dysfunction and had a normal haemogram and complete blood counts with normal erythrocyte sedimentation rate (ESR). On serology, he had elevated Thyroid Stimulating Hormone (TSH) level with normal T3 & T4 levels. Anti-thyroglobulin antibody level was >500 IU/mL (normal range 0–116 IU/mL). The ultrasonographic examination of thyroid gland showed diffuse enlargement of parenchyma with no focal lesions. On fine needle aspiration cytology (FNAC), a diagnosis of Hashimoto’s thyroiditis was made. After about a year, the patient became symptomatic with pressure symptoms in the form of hoarseness of voice, dysphagia and dyspnoea, following which, a total thyroidectomy was performed and the patient received oral levothyroxine supplementation. Histopathologic examination of thyroid gland confirmed the diagnosis of Hashimoto’s thyroiditis along with atypical lymphoid proliferation on its background. The patient presented to our center and a review of slides and blocks from thyroidectomy specimen was carried out. A diagnosis of peripheral T-cell lymphoma, not otherwise specified (PTCL, NOS) of thyroid gland was made based on morphologic and immunophenotypic findings further supported by molecular studies. Fifteen percent of T-cells in peripheral blood of the patient showed a CD4 & CD8 double positive immunophenotype on flow cytometry. Soon, he also developed multiple lymph nodes in bilateral cervical and axillary, mediastinal and abdominal regions, the largest measuring about 3.7 cm, which showed avid metabolic uptake on PET scan. Excision biopsy of an enlarged left cervical node revealed a similar atypical lymphoid proliferation as seen in the thyroid gland with a population of CD20 & CD30 positive immunoblasts in the background. Bone marrow examination revealed no lymphoma infiltration.
Pathologic Findings
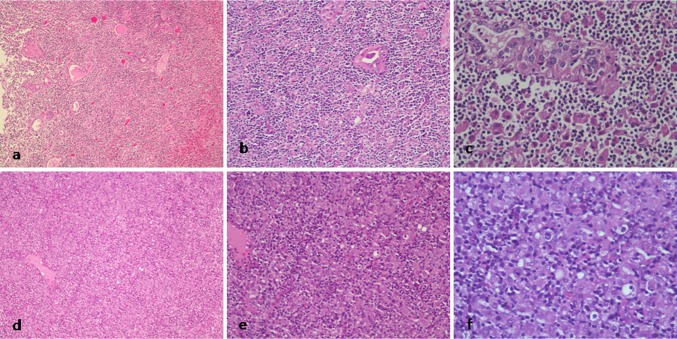
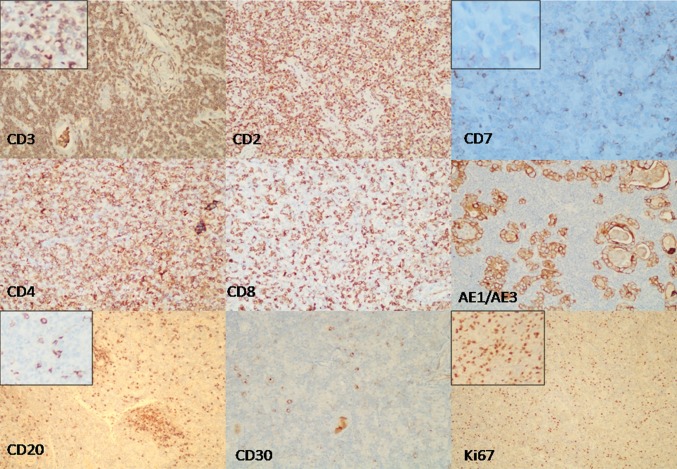
No gross pathologic findings were available for recording with the authors. The microscopic findings revealed features of a thyroid gland overrun by pseudogranulomatous, lymphohistiocytic proliferation of Lennert type, on a background of lymphocytic thyroiditis. Focal Hurthle cell change of thyroid follicular cells was noted. Numerous lymphoepithelial bodies were seen amidst a diffuse population of histiocytes, lymphocytes, plasma cells, and remnant thyroid epithelial cells (Fig. 1). The lymphocytes showed mild to moderate atypia, including irregular, indented and hyperchromatic nuclei, occasional prominence of nucleoli, and some intermediate to large sized cells having a rim of clear cytoplasm. Similar features were also seen in the left cervical lymph node with a relative prominence of larger cells (Fig. 1). No Hodgkin-Reed-Sternberg (HRS) like cells were seen. On IHC, the atypical lymphoid cells in both the thyroid and the lymph node expressed CD45, CD3, CD2, and CD5. No necrosis or extra thyroidal extension was evident. CD4:CD8 ratio was 3:1. The lymphoid cells were negative for CD7, CD20, CD79a, PAX5, CD15, CD30, CD10, bcl6, bcl2, MUM1, TdT, and EBV LMP1 (Fig. 2). Few reactive follicles were highlighted by CD23 & CD20. Scattered immunoblasts were also seen, more so in the lymph node, which were highlighted by CD20, CD30, MUM1, and bcl6 immunostains. Mib1 proliferation index was 40 % (Fig. 2). Thus, a diagnosis of PTCL, NOS, on a background of Hashimoto’s thyroiditis was made.
Fig. 1.
a, b, c Photomicrographs from hematoxylin & eosin (H&E) stained slides of thyroid gland showing ×10, ×20, & ×40 views, respectively. Diffuse lymphohistiocytic proliferation, remnant thyroid follicles and lymphoepithelial bodies are seen. d, e, f Photomicrographs from H&E stained slides of left cervical lymph node showing ×10, ×20, & ×40 views, respectively. Please note similar lymphohistiocytic population of cells with scattered atypical lymphocytes and plasma cells
Fig. 2.
IHC photomicrographs from thyroid tissue; CD3 & CD2 show diffuse positivity in the predominant T-cell population with a loss of CD7 expression.CD4 stains most of lymphoid cells with a CD4:CD8 ratio of 3:1. Pancytokeratin, AE1/AE3, highlights remnant thyroid epithelial follicles; CD20 is seen positively staining B-lymphocytes in occasional small nodular clusters; CD30 is expressed by occasional immunoblasts; Ki67 mitotic index is approximately 40 %. Figures in insets show areas from the left cervical lymph node for respective IHC stains
Genetic Studies
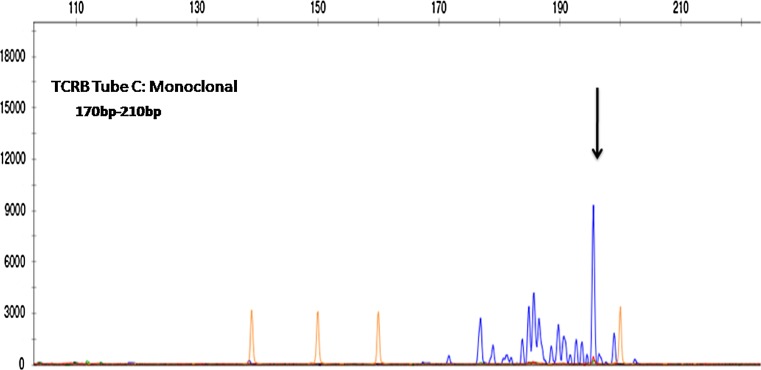
TCR gene rearrangement studies were carried out in succession on paraffin embedded thyroid tissue followed by left cervical lymph node tissue by multiplex PCR based heteroduplex analysis, using in vivo scribe technologies, IdentiClone™ TCR (beta, gamma, and delta) gene clonality assay kit. The products of PCR from thyroid tissue were run for fragment analysis in ABI 3500 gene sequencer and readings recorded with the help of Genemapper software. PCR products from left cervical lymph node were run in polyacrylamide gel, and Veriti thermal cycler was used for PCR with appropriate control. A clonal peak for beta gene in the region of 170–210 base pairs (bp) was detected in tube C for the thyroidal tissue (Fig. 3). Results for the lymph node tissue were uninterpretable on account of a very faint band seen for beta-actin housekeeping gene.
Fig. 3.
Fragment analysis of PCR products from thyroid tissue; TCR gene rearrangement is detected; a prominent peak (within the range of 170–210 bp) is seen against a polyclonal background in tube C of TCR B
Discussion
Primary T-cell lymphoma of thyroid is an extremely rare neoplasm with largely unknown clinicopathological attributes. B–cell lymphomas overwhelmingly outnumber their T-cell counterparts in thyroid gland, with only about twenty cases of TCL having been reported in the literature [2–4, 6–20]. The first case of TCL of thyroid was reported in an English lady in 1977 [12]. Almost four decades later the repertoire of such cases has increased to a modest total of just about 20, underlying the rarity of their occurrence. Watanabe et al. [2] found a single case of T-cell phenotype in a large study from Japan comprising of 171 cases of primary thyroid lymphomas diagnosed in a large cohort of 24,553 patients of Hashimoto’s thyroiditis. Geographical differences in incidence of cases of thyroid TCL, if any, are difficult to establish because of the obvious limitation of the number of cases. The cases reported so far show a mild predilection for women with 12 females and 8 males [6, 8]. The age distribution of cases is wide; 34 years in a male being minimum reported by Cobvic and colleagues in 2007 [6]. We report a case of mature TCL of thyroid in a 26 year old man, the youngest, to the best of our knowledge.
Thyroid function tests and the clinical thyroid status of patients are found to be either euthyroid or hypothyroid goiter in all case reports [6–20]. Our patient was a case of sub-clinical goiter with a sudden onset of pressure symptoms. A history of sudden increase in the size of goiter may be the first clue to an underlying lymphoma in cases with thyroiditis, as was also seen in our patient. This emphasizes the value of thorough history-taking and a high index of suspicion for both the clinician and pathologist.
B-cell NHL of thyroid gland is historically associated with chronic thyroiditis; no such association has been established for TCL [3, 4, 8]. However, the majority of the cases of thyroid TCL also had a history of Hashimoto’s thyroiditis much like B-cell lymphomas where it is an almost universal finding [2, 7, 8]. This patient also had an antecedent history of Hashimoto’s thyroiditis of 2 years duration before the diagnosis of TCL of thyroid.
Most of the cases presented at an early clinical stage (IE or IIE), unlike patients of PTCL of other extra nodal sites, which are usually diagnosed at advanced stage of disease. Though no association has yet been established between stages and prognosis in cases of T-cell thyroid lymphoma, stage remains an important determinant for mode and duration of therapy [3, 4]. Our case presented at early stage (IE) at diagnosis but has shown a rapid upstaging of disease to a clinical stage IIIE during a short period of follow up. This kind of clinical progression has not been reported in the literature and its therapeutic and prognostic significance is being closely pursued in this case.
The peripheral blood FCM in this patient revealed about 15 % of CD4 & CD8 double positive T-cells in circulation. This finding, though non-specific and seemingly associated with the underlying autoimmune disease process, has not been described or studied so far in association with TCL of thyroid [21]. Further studies are needed to enhance our insight into the tumor biology of T-cell thyroid lymphomas and its relation with autoimmune thyroiditis.
TCL of thyroid may show variable histomorphology; however, in most cases a diffuse infiltration of small to intermediate sized lymphocytes predominates [6, 8]. Most of the cases reported are of a mature T-cell phenotype save a case of lymphoblastic lymphoma in a 15 year old boy reported by Chen et al. [8]. The lymphohistiocytic Lennert like morphology is unique to this case, not described so far in literature. This finding possibly underscores the diversity of morphologic variations, which can masquerade as a reactive or inflammatory process in a variety of lymphoid neoplasm, and poses a diagnostic challenge to even experienced pathologists. IHC is a necessary adjunct in such cases to supplement the neoplastic nature of the lymphocytic proliferation.
Thyroid TCL may uncommonly show lymphoepithelial lesions, which are usually a hallmark of MALT lymphoma [3, 4]. This case also showed numerous lymphoepithelial lesions on histomorphology, possibly signifying an association with a background of Hashimoto’s thyroiditis, like in other cases described in literature [7, 9–11, 14, 15, 19]. Definitive reason for association of PTCL of thyroid with autoimmune thyroiditis is not well established, possibly because of very limited data on this subject. However, a plausible explanation by Koida et al. describes the roles of CD4-positive T-helper (Th1) cells and related cytokines CXCR3 and CCR5. This may be driven by reduced suppressor T-cell response and resultant change in the milieu of cytokines, in autoimmune conditions. Th1 cells proliferate under the influence of CXCR3 and CCR5, which in turn helps in initiation and propagation of B-cell response, leading to production of autoantibodies. In the event of chronic stimulation, Th1-cells, like B-cells, may occasionally acquire clonal properties leading to TCL [9]. Rarity of PTCL as compared to MALT lymphoma or DLBCL in these cases is still not explainable. The authors propose that, Th1-cells being the master regulator of autoimmune responses, may be less prone to genetic or epigenetic injuries or better equipped to repair them. At the genetic and molecular levels, Th1-cell proliferation in response to cytokine stimulation seems to be much more meticulously controlled, thereby rendering them less vulnerable to clonal evolution. Further studies in this regard are needed to establish a causative association between autoimmunity and PTCL of thyroid gland.
CD4-positive T helper-cell lymphoma predominates amongst the reported cases with only two cases of CD8-positive cytotoxic T-cell lymphomas identified to date [6, 8]. The neoplastic lymphocytes in this case were also of T helper-cell phenotype, as highlighted by positivity for CD3, CD5, and CD4 antibodies. Loss of CD7 and Ki67 mitotic index (40 %) were of further assistance in identifying the lymphomatous process.
In cases with controversial morphology or inconclusive immunophenotypic features, clonality studies by various methods are routinely being performed to aid in the diagnosis of lymphomas [6, 15]. In our case, clonality study by PCR was done in view of the atypical morphology, and it demonstrated positivity for TCR β gene rearrangement supporting the diagnosis of PTCL, NOS, of thyroid. Yamaguchi et al. [13] have reported the only case of thyroid lymphoma with γ/δ gene rearrangement whereas the rest all tested were positive for α/β genotype.
Treatment options for B-cell NHL of thyroid are relatively well established while regimens for TCL of thyroid have not yet fully evolved and no survival statistics still exist for these cases, clearly owing to their rarity [2, 3, 14, 16]. The patients have been treated by surgery, radiotherapy, chemotherapy, or various combinations thereof primarily based on clinical condition of the patient and the stage of disease [7, 9]. Surgery alone or in combination with loco-regional radiotherapy is the choice of treatment in early stage cases [3, 4, 6]. The most common choice of drugs for chemotherapy used in index cases has included cyclophosphamide, daunorubicin, vincristine, and prednisolone (CHOP) based regimen [6–20]. This case is being treated with 6 cycles of gemcitabine, dexamethasone, and cisplatin followed by involved field radiotherapy. The patient has shown good clinical response with little evidence of toxicity. These are still early days to predict treatment outcome or to comment on prognosis in this case.
Yokoyoma and Chen et al. have described the follow up findings of possibly all reported cases of T-cell thyroid lymphomas and most cases have shown remarkably good prognosis. Only three out of twenty cases died of lymphoma or its complications [6, 8]. Our case has shown a very rapid spread of disease evolving to stage IIIE within 3 months of the diagnosis. However, he remains symptomatically stable with an isolated episode of B symptom, of about 3 kg weight loss over a 3 month period in the recent past. No significant drug-toxicity has been recorded in our case after completion of four cycles of chemotherapy, except for mild to moderate diarrhea and recently a sore throat.
In conclusion, we have reported a case of primary TCL of thyroid in a young male with Lennert like morphology, arising on a background of Hashimoto’s thyroiditis. We emphasize on keeping in mind the rare differential diagnosis of PTCL of thyroid, when faced with unusual histomorphologic patterns of lymphoid proliferation in thyroid gland, in a pertinent clinical context.
Acknowledgments
Authors sincerely thank Ms. Mamta for her excellent technical support in carrying out PCR study in this case.
Compliance with Ethical Standards
Conflicts of interest
The authors have no conflicts of interest to declare.
References
- 1.Jaffe E, Harris NL, Stein H, Vardiman L, editors. World Health Organization classification of tumours, pathology and genetics of tumours of haematopoietic and lymphoid tissues. Lyon: IARC Press; 2004. pp. 109–111. [Google Scholar]
- 2.Watanabe N, Narimatsu Noh J Y, Takeuchi H, Yamaguchi K, Kameyama T, Kobayashi K, et al. Clinicopathological features of 171 cases of primary thyroid lymphoma: a long-term study involving 24 553 patients with Hashimoto’s disease. Br J Haematol. 2011;153:236–243. doi: 10.1111/j.1365-2141.2011.08606.x. [DOI] [PubMed] [Google Scholar]
- 3.Pedersen RK, Pedersen NT. Primary non-Hodgkin’s lymphoma of the thyroid gland: a population based study. Histopathology. 1996;28:25. doi: 10.1046/j.1365-2559.1996.268311.x. [DOI] [PubMed] [Google Scholar]
- 4.Singer JA. Primary lymphoma of the thyroid. Am Surg. 1998;64:334–337. [PubMed] [Google Scholar]
- 5.Ansell SM, Grant CS, Habermann TM. Primary thyroid lymphoma. Semin Oncol. 1999;26:316–323. [PubMed] [Google Scholar]
- 6.Yokoyama J, Ito S, Ohba S, Fujimaki M, Sato E, Komatsu N, et al. Problems of primary T-cell lymphoma of thyroid: a case report. World J Surg Oncol. 2012;10:58–61. doi: 10.1186/1477-7819-10-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim NR, Ko Y-H, Lee Y-D. Primary T-cell Lymphoma of the thyroid associated with Hashimoto’s thyroiditis histologically mimicking MALT-lymphoma. J Korean Med Sci. 2010;25:481–484. doi: 10.3346/jkms.2010.25.3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen C, Yang Y, Jin L, Dong L, Zhang X, Xiang Y, et al. Primary thyroid T-lymphoblastic lymphoma: a case report and review of the literature. Int J Clin Exp Pathol. 2014;7(1):443–450. [PMC free article] [PubMed] [Google Scholar]
- 9.Koida S, Tsukasaki K, Tsuchiya T, Harasawa H, Fukushima T, Yamada Y, et al. Primary T-cell lymphoma of the thyroid gland with chemokine receptors of Th1 phenotype complicating autoimmune thyroiditis. Haematologica. 2007;92:37–40. doi: 10.3324/haematol.10351. [DOI] [PubMed] [Google Scholar]
- 10.Motoi N, Ozawa Y. Malignant T-cell lymphoma of the thyroid gland associated with Hashimoto’s thyroiditis. Pathol Int. 2005;55:425–430. doi: 10.1111/j.1440-1827.2005.01848.x. [DOI] [PubMed] [Google Scholar]
- 11.Dunbar JA, Lyall MH, MacGillivray JB, Potts RC. T-cell lymphoma of the thyroid. BMJ. 1977;2:679–681. doi: 10.1136/bmj.2.6088.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abdul-Rahman ZH, Gogas HJ, Tooze JA, Anderson B, Mansi J, Sacks NP, Finlayson CJ. T-cell lymphoma in Hashimoto’s thyroiditis. Histopathology. 1996;29:455–459. doi: 10.1046/j.1365-2559.1996.d01-515.x. [DOI] [PubMed] [Google Scholar]
- 13.Yamaguchi M, Ohno T, Kita K. γ/δ Tcell lymphoma of the thyroid gland. N Engl J Med. 1997;336:1391–1392. doi: 10.1056/NEJM199705083361915. [DOI] [PubMed] [Google Scholar]
- 14.Mizukami Y, Michigishi T, Nonomura A, Nakamura S, Hashimoto T, Katsuda S, et al. Primary lymphoma of the thyroid: a clinical, histological and immunohistochemical study of 20 cases. Histopathology. 1990;17:201–209. doi: 10.1111/j.1365-2559.1990.tb00708.x. [DOI] [PubMed] [Google Scholar]
- 15.Haciyanli M, Erkan N, Yorukoglu K, Sagol O, Harmancioglu O. Primary non-Hodgkin’s T-cell lymphoma of the thyroid gland complicating Hashimoto’s thyroiditis: a case report. Thyroid. 2000;10:717–720. doi: 10.1089/10507250050137824. [DOI] [PubMed] [Google Scholar]
- 16.Sun TQ, Zhu XL, Wang ZY, et al. Characteristics and prognosis of primary thyroid non-Hodgkin’s lymphoma in Chinese patients. J Surg Oncol. 2010;101:545. doi: 10.1002/jso.21543. [DOI] [PubMed] [Google Scholar]
- 17.Forconi F, Bocchia M, Marconcini S, Bigazzi C, Milani M, Fraternali-Orcioni G, Lauria F. CD30 positive (nonanaplastic) peripheral T-cell lymphoma of the thyroid gland. Haematologica. 1999;84:946–948. [PubMed] [Google Scholar]
- 18.Freeman HJ. T-cell lymphoma of the thyroid gland in celiac disease. Can J Gastroenterol. 2000;14:635–636. doi: 10.1155/2000/582364. [DOI] [PubMed] [Google Scholar]
- 19.Raftpoulos I, Vanuno D, Kouraklis G. Two unusual sites of colon cancer metastases and rare thyroid arising in a background of Hashimoto’s thyroiditis. J Clin Oncol. 2001;19:3576–3580. doi: 10.1200/JCO.2001.19.15.3576. [DOI] [PubMed] [Google Scholar]
- 20.Coltrera MD. Primary T-cell lymphoma of the thyroid. Head Neck. 1999;21:160–163. doi: 10.1002/(SICI)1097-0347(199903)21:2<160::AID-HED10>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 21.Parell Yann, Chizollini Calo. CD4+ CD8+ double positive T-cells in health and disease. Autoimmun Rev. 2004;3:215–220. doi: 10.1016/j.autrev.2003.09.001. [DOI] [PubMed] [Google Scholar]