Abstract
We present the second reported mammary analog secretory carcinoma (MASC) apparently arising in the thyroid and propose a potential close relationship to ETV6-NTRK3 fusion papillary thyroid carcinoma. The patient, a 36 year old woman, presented with a neck mass of 1 year’s duration. Imaging studies showed a tumor involving most of the thyroid with enlarged regional lymph nodes. FNA biopsy yielded a diagnosis of “papillary thyroid carcinoma”. Resection revealed a 4.5 cm infiltrative tumor. Final diagnosis was “papillary thyroid carcinoma (PTC) consistent with diffuse sclerosing variant” with positive lymph nodes (2+/4) and margins. Histologic features included mixed microcystic, solid, follicular and papillary architecture, prominent nucleoli, abundant nuclear grooves and rare nuclear pseudo-inclusions. Despite radioactive iodine, radiotherapy and multiagent chemotherapy, the patient progressed over 6 years with local recurrence and additional lymph node involvement finally developing widespread distant metastases. Prompted by the breast carcinoma-like histopathology of a metastasis, immunohistochemical staining was performed and revealed strong expression of GATA3 and mammaglobin with no reactivity for thyroglobulin or TTF-1. The original tumor was then tested and showed the same immunoprofile. RT-PCR confirmed the presence of an ETV6-NTRK3 fusion consistent with a diagnosis of MASC. Our patient’s clinical, imaging and morphologic features remarkably mimicked papillary thyroid carcinoma. At the molecular level, the ETV6-NTRK3 fusion in this patient involved exons reported in the rare “papillary thyroid carcinoma” with this translocation. Given the immunophenotype of this case, it is possible that at least some ETV6-NTRK3 fusion positive PTC are actually MASC masquerading as papillary thyroid carcinoma.
Keywords: MASC, Thyroid carcinoma, Papillary thyroid carcinoma, ETV6-NTRK3 fusion, Genetics
Background
Mammary analog secretory carcinoma (MASC) is a recently described carcinoma occurring in the major and minor salivary glands of adults [1]. Other than one possible prior case, it has not been reported as arising in the thyroid. MASC of the salivary glands is rare and represents approximately 4 % of primary salivary gland carcinomas [2]. The tumor may be mistaken for numerous other salivary gland tumors including benign tumors such as pleomorphic and monomorphic adenomas and carcinomas such as acinic cell carcinoma, mucoepidermoid carcinoma, adenocarcinoma NOS, salivary duct carcinoma, clear cell cribriform carcinoma and cystadenocarcinoma, among others. This long list of mistaken identities highlights the morphologic variety of MASC which usually includes microcystic but also tubular, solid, pseudopapillary, true papillary, trabecular and follicular patterns [3]. Tumor cells are typically minimally pleomorphic with a low to medium N/C ratio and eosinophilic, slightly granular to bubbly cytoplasm. Mammary analog secretory carcinoma was most often previously misdiagnosed as a zymogen granule-poor acinic cell carcinoma.
Given its morphologic variety that considerably overlaps with other tumors, immunohistochemical staining is essential in correctly diagnosing MASC. Mammary analog secretory carcinoma expresses mammaglobin, GATA3 and variably expresses gross cystic disease fluid protein-15 (GCDFP-15) similarly to many primary breast carcinomas. Other antigens expressed include S-100 protein (diffuse in most cases), vimentin and high molecular weight keratins with variable expression reported for p63 [1, 3–7]. In their original description of MASC, Skalova and colleagues found an ETV6-NTRK3 fusion in all cases [1]. Subsequent studies by Skalova and others verified that an ETV6-NTRK3 rearrangement is almost always present in MASC. However, Skalova and collaborators also have recently reported rare cases that show ETV6 fused to an unknown gene partner (“Gene X”) [8]. Consequently, ETV6-NTRK3 or ETV6-Gene X gene rearrangement confirms the diagnosis in the correct histologic and immunohistochemicalsetting, as the ETV6-NTRK3 fusion is not unique to MASC but also characterizes secretory carcinoma of the breast, congenital fibrosarcoma, cellular or mixed mesoblastic nephroma, a few myeloid leukemias, 1–4 % of sporadic papillary thyroid carcinoma and 10–14 % of PTC arising after radiation exposure [9–13]. Intriguingly, in 2016, Skalova and colleagues also reported a case of salivary gland MASC with an ETV6-NTRK3 fusion involving the same exons as found in “papillary thyroid carcinoma” with ETV6-NTRK3 fusion [8].
Recently, one case of mammary analog secretory carcinoma has been reported as arising in (or near) the thyroid gland [7]. Our report details a second patient with mammary analog secretory carcinoma apparently arising in the thyroid with diagnosis confirmed on PCR for an ETV6-NTRK3 fusion and with characteristic immunohistochemical features. Our patient’s ETV6-NTRK3 fusion involves the exons also involved in “papillary thyroid carcinoma” with ETV6-NTRK3 fusion [13].
Case Report
Clinical Presentation
A 36 year old woman presented with a 1 year history of a neck mass. She had no history of radiation exposure. Imaging studies revealed a mass involving the left lobe and right lower lobe of the thyroid with enlarged cervical lymph nodes and mediastinal extension (Fig. 1). Fine-needle aspiration biopsy was performed showing “papillary thyroid carcinoma” prompting a total thyroidectomy 2 months after the initial FNA. The resected thyroid contained a 4.5 cm tumor that on histologic review was described as having features of both the diffuse sclerosing variant and tall cell variant of PTC. Extracapsular extension was present, surgical margins were positive, two of four lymph nodes contained metastatic tumor, and the patient was adjuvantly treated with 159 mCi of radioactive iodine. Nevertheless, fine-needle aspiration of the thyroid bed, performed 14 months later, was called “suspicious” for papillary thyroid carcinoma, and surgical biopsies of para-tracheal and para-esophageal soft tissue were positive for recurrent/residual PTC 20 months after initial surgery. At this point, the patient began treatment with the kinase-inhibitor sorafenib, followed by 3500 cGy of palliative external beam radiation to the thyroid bed. A second kinase inhibitor, sunitinib, was also added to the chemotherapeutic regimen. Despite these treatments, 44 months after initial surgery the patient had a second recurrence of tumor in the thyroid bed. Evidence of distant disease followed, with metastasis to the right deltoid muscle 53 months after initial surgery and subsequent involvement of the soft tissue surrounding the right tibia 65 months after initial surgery. Simultaneously, multiple lung, liver and other soft tissue nodules were identified by imaging and presumed to be metastatic disease and not biopsied. The patient subsequently succumbed to complications of widespread progressive disease 107 months (8.9 years) after her initial diagnosis.
Fig. 1.
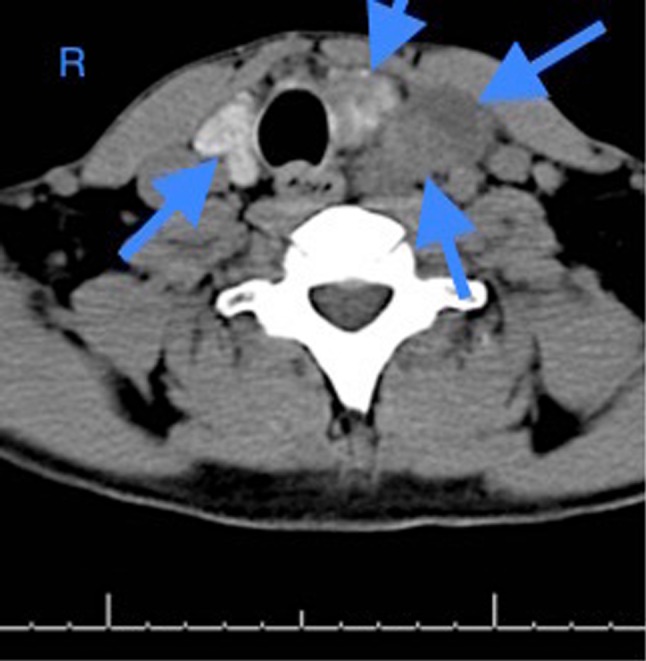
Axial CT scan image of the patient’s large thyroid mass prior to resection. The tumor is heterogeneous, involves the majority of the thyroid and extends into the mediastinum causing tracheal and esophageal deviation (arrows show tumor outlines). Radiologic interpretation was “possible primary thyroid carcinoma or large, multinodular goiter”
Materials and Methods
Immunohistochemistry
Table 1 lists the manufacturer, dilutions, incubation times, and type of antigen retrieval for the antibodies used in this study: thyroid transcription factor 1 (TTF-1), thyroglobulin, mammaglobin, GATA-3, S-100 protein and estrogen receptor.
Table 1.
Antibodies used
| Antibody | Clone | Dilution | Pre-treatment | Manufacturer |
|---|---|---|---|---|
| TTF-1 | SPT24 | 1:100 | CC1 (36′) | Novocastra |
| Thyroglobulin | DAK-TgG | 1:200 | CC1 (36′) | Dako |
| Mammaglobin | 304-1A5 | 1:100 | CC1 (36′) | Dako |
| GATA-3 | L50-823 | Pre-diluted from vendor | CC1 (64′) | Cell Marque |
| ER | SP1 | Pre-diluted from vendor | CC1 (64′) | Ventana |
| S-100 protein | DR96 + BC96 | 1:400 | CC1 (36′) | Biocare |
TTF-1 thyroid transcription factor 1, GCDFP-15 gross cystic disease fluid protein-15, ER estrogen receptor
All immunohistochemical (IHC) staining was performed on five micron-thick mounted sections cut from formalin-fixed, paraffin-embedded blocks of tumor using appropriate positive and negative controls. IHC staining was performed using a Ventana Benchmark™ automated immunostainer (Ventana Medical Systems, Roche, Tuscon, AZ).
Molecular Analysis
Formalin-fixed, paraffin-embedded (FFPE) tissue, one target tumor case, one positive control, and two negative controls, were collected for this study. All the tissues were reviewed and histologically confirmed by surgical pathologists and obtained from the University of New Mexico Hospital. Total RNA was extracted using PureLink™ FFPE Total RNA Isolation Kit (Thermo Fisher Scientific, Cat. No. K1560-02) from two sections of each FFPE at 10 µm thickness. The RNA was treated with DNase I. RNA concentration was evaluated on the NanoDrop and Qubit 2.0. Complementary DNA (cDNA) was synthesized using High-Capacity RNA-to-cDNA™ Kit (Thermo Fisher Scientific, Cat. No. 4387406). All procedures were performed according to the manufacturer’s standard protocols.
ETV6 Exon 5-NTRK3 Exon 13 Fusion
Forward primer TEL971 (5′-ACCACATCATGGTCTCTGTCTCCC-3′) and reverse primer TRKC1059 (5′-CAGTTCTCGCTTCAGCACGATG-3′) were chosen to amplify ETV6 exon 5-NTRK3 exon 13 fusion region [1]. The PCR was performed using AmpliTaq Gold® Fast PCR Master Mix (Thermo Fisher Scientific, Cat. No. 4390939). The target tumor, the positive control, two negative control cDNAs, and a non-template control (nucleotide-free water) were used as template. Briefly, 3ul cDNA or water and a 0.2 μM final concentration of each primer were used for a 20 µl PCR reaction. The PCR was achieved in a 9700 thermal cycle under the following conditions: 95 °C for 10 min followed by 45 cycles at 96 °C for 3 s, 60 °C for 3 s, 68 °C for 5 s, and a final extension at 72 °C for 10 min. PCR product was evaluated on a 2 % agarose gel. The positive control showed a 110 base pair amplicon as expected; the other PCR reactions were negative. The target tumor and two negative control cDNAs were evaluated using Beta-actin amplification with forward primer (5′-GCTCGTCGTCGACAACGGCTC-3′) and reverse primer (5′-CAAACATGATCTGGGTCATCTTCTC-3′) [14]. A 353 base pair amplicon showed up well for all three cases, which represented good cDNA quality. We tested two different fusion regions.
ETV6 Exon 4-NTRK3 Exon 14 Fusion
Forward primer (5′-CATTCTTCCACCCTGGAAAC-3′) and reverse primer (5′-TCCTCACCACTGATGACAGC-3′) were chosen to amplify ETV6 exon 4-NTRK3 exon 14 fusion region [13]. The tumor and the positive control cDNA were used as templates. The PCR conditions were similar as above; however, the thermal cycler program was 35 cycles with annealing temperature at 55 °C for 3 s. The tumor cDNA yielded a 97 base pair amplified product.
Sanger Sequencing
The successfully amplified PCR product of the ETV6 and NTRK3 fusion genes were purified with ExoSAP-IT (Affymetrix USB, Cat. No. 78200). The purified PCR product was directly sequenced using BigDye® Direct Cycle Sequencing Kit (Thermo Fisher Scientific, Cat. No. 4458687) from both directions. The sequencing reactions were run on an Applied Biosystems’ 3130XL sequencer.
Fluorescence In Situ Hybridization
The patient’s 2007 tumor was further studied by FISH using an with an ETV6 (labeled green)-NTRK3 (labeled red) dual color fusion probe (Empire Genomics, Buffalo, NY).
An ETV6-NTRK3 fusion negative control and an ETV6-NTRK3 fusion positive control were tested in parallel to the patient’s tumor. Tissue sections were cut at 0.4 µM thickness. FISH was performed using standard methodology according to the manufacturer’s instructions. A fluorescence microscope was used to count signals per nucleus.
Results
Pathologic Findings
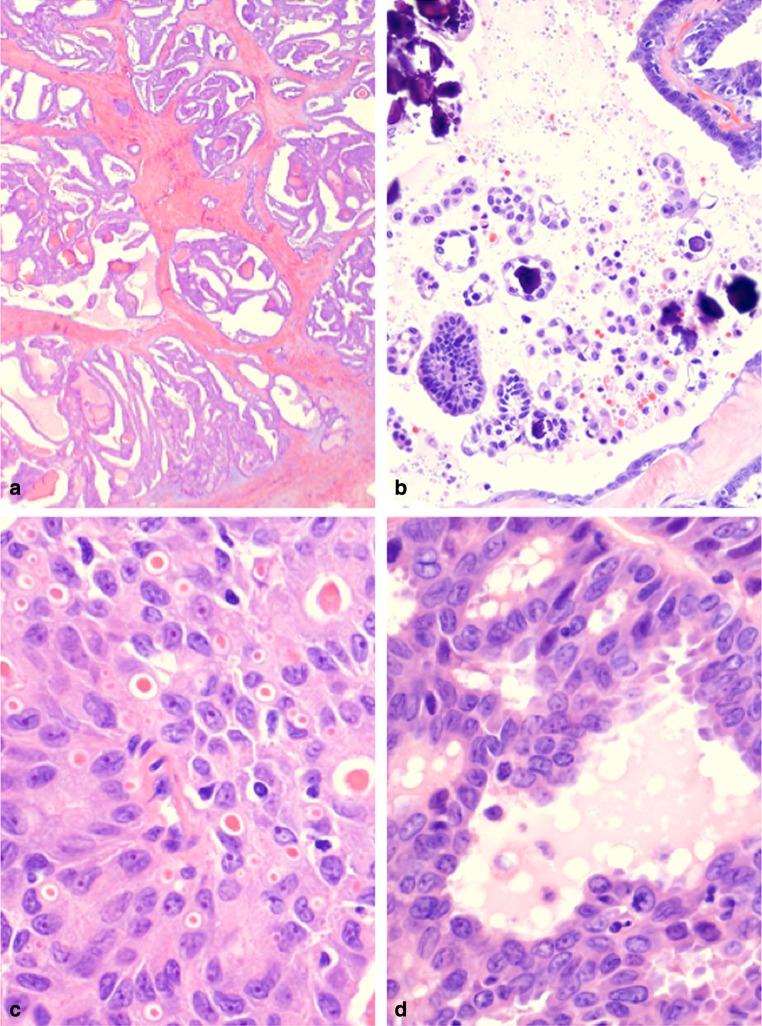
The patient’s resection grossly comprised a 4.5 cm, unencapsulated solid tumor that diffusely involved the right and left thyroid lobes. Microscopic examination revealed an architecturally complex tumor arranged in multiple nodules embedded in sclerotic fibrous stroma (Fig. 2a). Growth patterns included follicular, microcystic secretory, trabecular, solid, and papillary, the last even with a few psammoma bodies (Fig. 2b). Eosinophilic secretions mimicking thyroid colloid ranged in size from dot-like intracytoplasmic to intercellular, thyroid follicle-sized (Fig. 2c). Some of these pseudofollicles contained peripheral clear bubbles further mimicking true thyroid follicles (Fig. 2d). Other follicles displayed apocrine-like capitation activity in the lining cells (Fig. 3a). High magnification revealed round to columnar epithelial cells with pale eosinophilic to markedly eosinophilic slightly granular cytoplasm simulating oncocytic change. Tumor nuclei were oval and vesicular with single macronucleoli and numerous nuclear grooves (Fig. 3b). Nuclear pseudoinclusions were rare. The prominent nucleoli, larger than typical in PTC, were generally centrally rather than eccentrically placed. Mitotic figures were rare (<1/30 hpf). Necrosis was absent. Chronic lymphocytic thyroiditis was also present. At histologic review, the tumor retained the same appearance from original resection to metastases with no evidence of transformation to a high grade carcinoma (Fig. 3c, d).
Fig. 2.
a MASC of thyroid. Low magnification of the primary tumor shows multiple nodules of tumor embedded in a fibrous matrix. The tumor nodules exhibit follicular, papillary, microcystic, trabecular and fused papillary patterns (H&E stain). b MASC of thyroid. The primay tumor focally forms true papillae with psammomatous calcifications (H&E stain). c MASC of thyroid (2007). At high magnification, a microcystic secretory pattern is seen in this field with intra-cellular and extra-cellular globules of eosinophilic material. Tumor cells have slightly granular eosinophilic cytoplasm, vesicular nuclei and numerous nuclear grooves (H&E stain). d MASC of thyroid (2007). In many areas, the primary tumor forms follicle-like spaces with peripheral clear bubbles mimicking colloid (H&E stain)
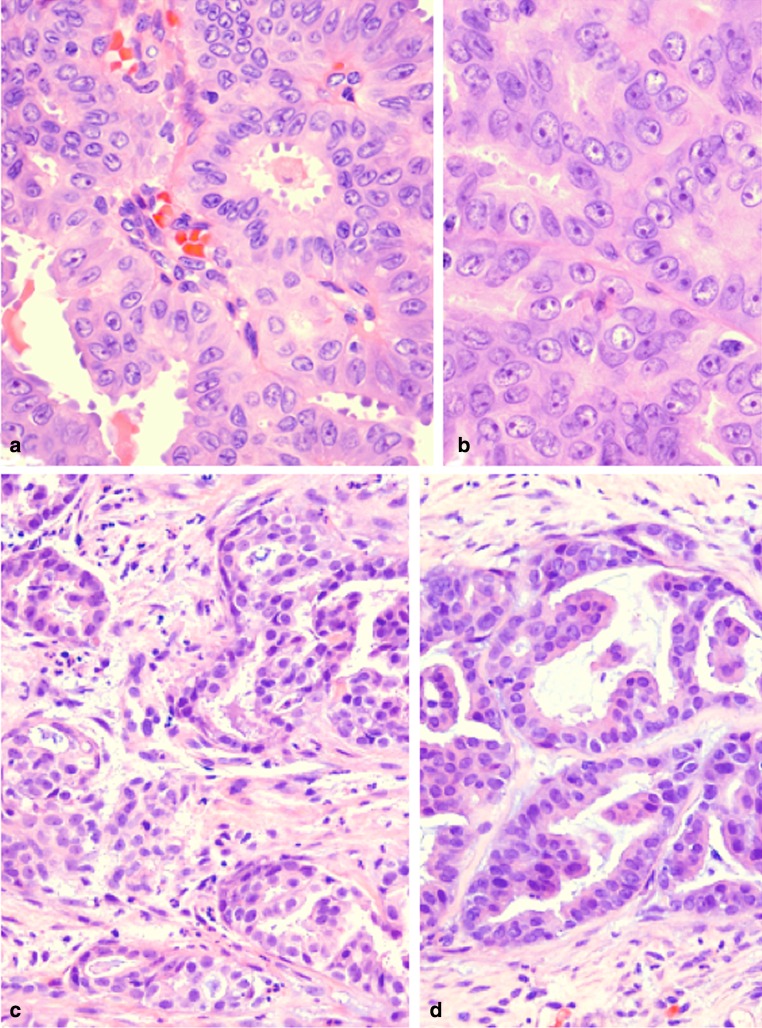
Fig. 3.
a MASC of thyroid (2007). Apocrine-like capitation by lining cells is present in this field (H&E stain). (H&E stain). b MASC of thyroid (2007). Prominent single, centrally placed nucleoli and numerous nuclear grooves are a common feature in all architectural patterns of the tumor (H&E stain). c Metastatic MASC to deltoid muscle (2014). The tumor comprises nests of eosinophilic cells embedded in fibrotic stroma. There is no evidence of transformation to a higher grade (H&E stain). d Metastatic MASC to soft tissue of lower leg (2015). The tumor continues to show similar histology to the original tumor without dedifferentiation (H&E stain)
Table 2 details the immunohistochemical staining results of the original and biopsied metastases. Notably, tumor cells variably expressed mammaglobin and GATA3 and completely lacked expression of thyroglobulin and TTF-1 (Fig. 4a, b). This immunophenotype was consistent from original resection to biopsies of metastases obtained up to 7 years after original resection. S-100 protein expression was patchy in the initial tumor but strong and diffuse in the patient’s two soft tissue metastases.
Table 2.
Immunohistochemical Results in Primary and Metastatic Tumors
| Immunohistochemical stain | 2007 Original thyroid carcinoma | 2014 Metastatic carcinoma | 2015 Metastatic carcinoma |
|---|---|---|---|
| TTF-1 | NEG | NEG | NEG |
| Thyroglobulin | NEG | NEG | NEG |
| Mammaglobin | POS diffuse | POS diffuse | POS diffuse |
| GATA-3 | POS patchy | POS diffuse | POS diffuse |
| S-100 protein | POS patchy | POS diffuse | POS diffuse |
| Estrogen receptor | NEG | NEG | NEG |
TTF-1 thyroid transcription factor-1, NEG negative, POS positive
Fig. 4.
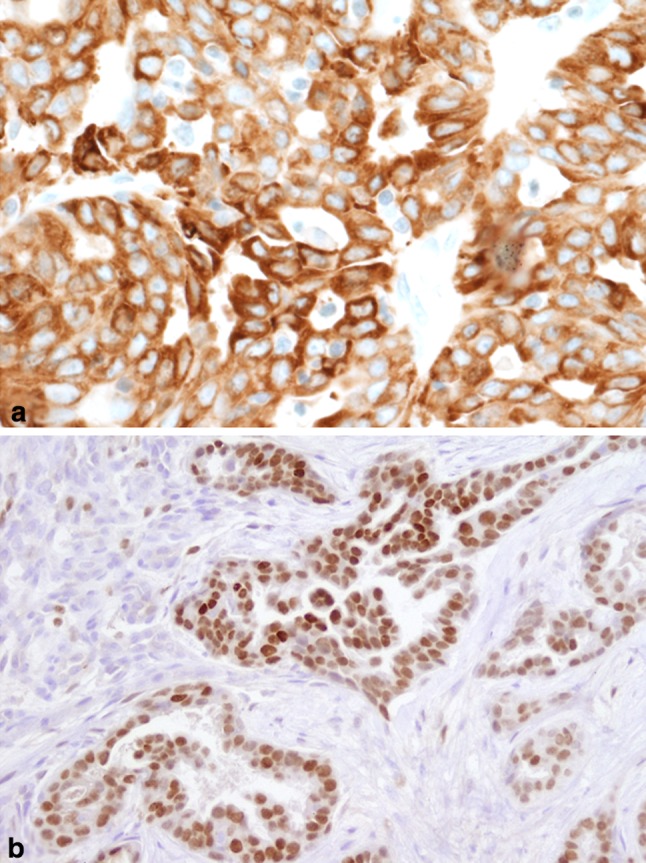
a MASC of thyroid (2007). Mammaglobin expression is diffuse and strong in the primary tumor (Mammaglobin IHC stain). b Metastatic MASC to deltoid muscle (2014). GATA3 is strongly expressed in ~ 50 % of tumor cells (GATA3 IHC stain)
RT-PCR Results
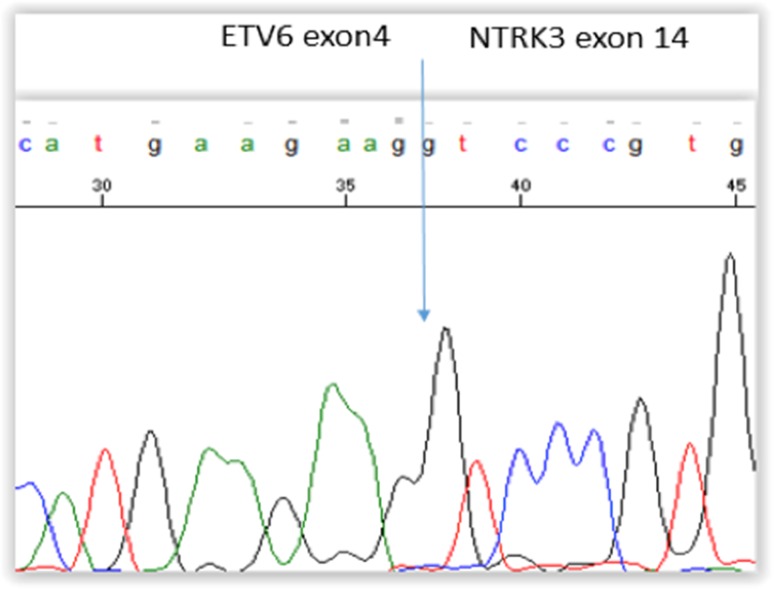
Sanger sequencing of specific PCR product derived from the patient’s original tumor confirmed the presence of an ETV6-NTRK3 fusion at exon 4 of ETV6 and exon 14 of NTRK3 (Fig. 5). The tumor was negative for fusion of exon 5 of ETV6 to exon 13 of NTRK3.
Fig. 5.
Sanger sequencing of specific PCR product from 2007 tumor confirms the fusion of exon 4 of ETV6 to exon 14 of NTRK3 as is reported in many cases of ETV6-NTRK3 fusion positive papillary thyroid carcinomas
Fish Confirmation of Sanger Sequencing
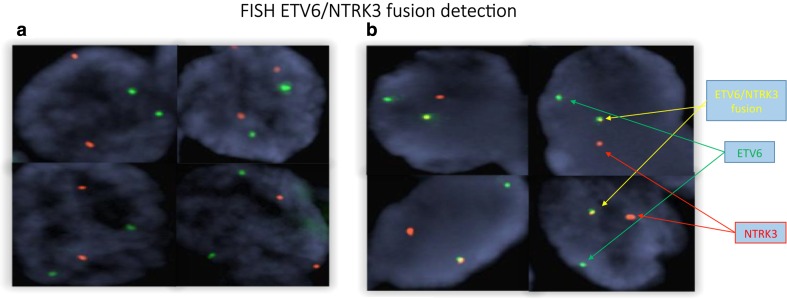
Two red and two green signals were observed from the normal control (Fig. 6a). A fused green and red signal, one red signal, and one green signal were observed from the patient and the positive control (Fig. 6b). The FISH results confirmed the finding of an ETV6-NTRK3 fusion as detected in Sanger sequencing of the patient’s tumor.
Fig. 6.
a Normal control tissue two red and two green signals are observed. b The patient’s 2007 tumor shows a fused green and red signal appearing yellow, one red signal, and one green signal
Discussion
This patient developed a mammary analogy secretory carcinoma apparently arising in the thyroid confirmed by IHC and molecular testing. The histologic features, while remarkably reminiscent of papillary thyroid carcinoma, differed significantly at both the architectural and cytologic levels. The tumor showed architectural variety as is common in papillary thyroid carcinoma. However, novel findings included: (1) numerous small, intracytoplasmic eosinophilic secretions and (2) cells lining some of the pseudofollicles with capitation secretions. Apocrine type capitation is found in the newly described hobnail variant of PTC but is paired with micropapillary architecture that was absent in our patient’s tumor [15]. Nuclear features included single, central macronucleoli with numerous nuclear grooves but very rare nuclear pseudo-inclusions. Immunophenotypically, the tumor expressed antigens characteristic of MASC and completely lacked expression of thyroglobulin and TTF-1.
Correct diagnosis may be essential because of two treatment implications: (1) possibly more aggressive behavior, and (2) probable non-response to the typical post-surgical therapy for papillary thyroid carcinoma: radioactive iodine. Our patient’s tumor behaved aggressively, a highly unusual course for salivary MASC which exhibits overall low local recurrence and distant metastsis rates [2]. Thus compared to our patient’s tumor, the majority of MASC arising in the salivary glands behave as low grade carcinomas.
Although most MASC behave indolently, Skalova and colleagues recently described three cases of mammary analog secretory carcinoma with high grade transformation in which all patients succumbed to disease [6]. The morphologic features of these transformed MASC comprised typical low grade areas juxtaposed with high grade tumor with comedo necrosis and nuclear pleomorphism. Another case of MASC with high grade transformation occurring in a 41 year old woman also exhibited cellular pleomorphism with diagnosis confirmed by ETV6 gene rearrangement [16]. In contrast to these cases of high grade transformed MASC with aggressive behavior, our patient’s tumor behaved aggressively in the absence of high grade transformation: over 7 years of multiple biopsies, the patient’s tumor remained histologically identical without cellular pleomorphism, increased mitotic activity or necrosis.
At consecutive diagnostic assessment points, our patient’s tumor mimicked papillary thyroid carcinoma. At the clinical and imaging points, the tumor occurred in the typical age group of PTC and presented with probable positive lymph nodes on imaging, another common finding in PTC.
At the microscopic point of diagnostic assessment, our patient’s tumor resembled PTC to a remarkable degree. Histologically, MASC displays a plethora of morphologic patterns which includes papillary, pseudopapillary, microcystic, macrocystic and cribriform patterns all of which can be seen in papillary thyroid carcinoma. Notably, multiple authors have described some patterns found in MASC as appearing similar to thyroid colloid-like deposition or a follicular pattern of thyroid carcinoma [1, 3, 7, 17]. Nuclear features of our patient’s tumor also resembled closely those of PTC in that the nuclei were vesicular with numerous nuclear grooves. Thus, the microscopic differential diagnosis is difficult and may not be considered when the tumor arises in the thyroid, albeit thyroid origin may be an exceptional occurrence. It is possible that this tumor arose in perithyroidal heterotopic salivary gland tissue. However, even if this were the source of this MASC, the tumor presented as a thyroid neoplasm, was surgically resected assuming it was a thyroid carcinoma, and histologically showed features highly overlapping with and in fact nearly identical to papillary thyroid carcinoma. Thus, it closely resembled PTC at multiple clinical and pathologic levels.
At the molecular level of diagnostic assessment, our patient’s tumor conformed to the findings seen in ETV6-NTRK3 fusion “papillary thyroid carcinoma”. However, our patient had no history of exposure to radiation. Exposure history is relevant, because up to 14.5 % of patients with ETV6-NTRK3 fusion papillary thyroid carcinoma have been exposed to a high level of environmental radiation such as subsequent to the 1986 Chernobyl nuclear reactor accident [18]. In sporadic papillary thyroid carcinoma, the incidence of EVT6-NTRK3 gene fusion ranges from approximately 1.2–4 % [19–21]. However, the prevalence may be higher in children and adolescents based on a recent small series of 28 patients aged 6–18 years with no history of radiation exposure and with “papillary thyroid carcinoma” whose tumors were assessed by a targeted next-generation sequencing panel (ThyroSeq version 2) revealing a 26 % rate of NTRK fusions (7/27 cases; 5 comprising the ETV6-NTRK3 fusion) [22]. Notably, the ETV6-NTRK3 fusion in the radiation and sporadic papillary thyroid carcinomas differs from the ETV6-NTRK3 fusion reported in most MASC in the exon breakpoint in the NTRK3 gene (exon 14 in thyroid tumors and exon 13 in MASC of salivary glands) [1, 20, 21]. However, Skalova and colleagues recently reported a case of MASC of the salivary glands with the same fusion breakpoints as found in “papillary thyroid carcinoma” with the ETV6-NTRK3 fusion [8]. Thus, there is now precedent for these exon breakpoints to occur in a bona fide example of a case of MASC occurring in the salivary glands. Intriguingly, the MASC-like immunohistochemical findings in our patient’s tumor but with breakpoints in ETV6 and NTRK3 as reported in “papillary thyroid carcinoma” suggest that at least some ETV6-NTRK3 “papillary thyroid carcinomas” may represent mammary analog secretory carcinoma of the thyroid.
Although the fusion partner in NTRK fusion positive “papillary thyroid carcinoma” is predominantly ETV6, other fusions have been described including TPR-NTRK1 and NTRK3-unknown fusion partner [22]. Due to the low number of NTRK fusion positive “papillary thyroid carcinomas”, it is not possible to determine the definitive relative incidence of the various NTRK fusions in these papillary appearing thyroid carcinomas.
Morphologically, “papillary thyroid carcinoma” associated with the EVT6-NTRK3 fusion is described most commonly as either the follicular variant or mixed classic and follicular variants of papillary thyroid carcinoma [19–21]. Further descriptions of the histomorphology of these tumors are generally not provided other than to note a solid component in some cases, especially those associated with radiation exposure. Descriptions of five examples of pediatric ETV6-NTRK3 fusion positive “papillary thyroid carcinoma” noted a mixture of architectures typically including solid growth (present in 6/7 cases with NTRK fusion “PTC”), sclerotic background and follicle formation (including 2/7 cases with “diffuse follicular variant PTC”), nodular growth (4/5 cases with ETV6-NTRK3 fusions) with uncommon psammoma bodies and papillae [22]. Importantly, the studies of ETV6-NTRK3 fusion positive PTC do not provide immunohistochemical staining results of the tumors with the antibodies we used and which would be expected to distinguish true PTC from MASC (e.g. antibodies to thyroglobulin, TTF-1, mammoglobin, GATA3, or S-100 protein) [18–22].
Studies reviewing the genetic findings of the papillary thyroid carcinomas with the ETV6-NTRK3 fusion have not generally reported outcomes in these patients. Possibly the outcome may differ from the typical papillary thyroid carcinoma which has a 5-year survival of greater than 95 % [15]. Importantly, MASC originating in the thyroid, as it is not differentiating to follicular thyroid cells, would not be expected to respond to radioactive iodine therapy. Intriguingly, among five pediatric patients with ETV6-NTRK3 fusion positive “papillary thyroid carcinomas”, Prasad and co-workers reported one patient who developed recurrent tumor in the bed of the thyroid 2 years after initial resection despite radioactive iodine therapy [22]. Possibly, NTRK fusion positve “papillary thyroid carcinomas”, whether immunophenotypically PTC or immunophenotypically MASC, may be better treated by a specific targeted therapy, especially in aggressively behaving and/or high stage cases. For example, entrectinib (RXDS-101) and LOXO-101 are two new orally delivered tyrosine kinase inhibitors undergoing early clinical trials in patients with NTRK fusion positive tumors which may potentially show efficacy in MASC, whether arising in the salivary glands or apparently in the thyroid (see clinicaltrials.gov).
Stevens and colleagues reported in 2015 one patient with MASC in which the tumor appeared to infiltrate the thyroid and perilaryngeal soft tissue and which was hypothesized to have possibly arisen in ectopic minor salivary gland tissue [7]. The patient’s tumor was confirmed as MASC by ETV6 translocation identified on fluorescence in situ hybridization. The specific fusion breakpoints were not reported. It co-expressed mammaglobin and S-100 protein and was negative for TTF-1 and thyroglobulin [7].
In summary, we report the second known case of mammary analog secretory carcinoma apparently arising in the thyroid proven by PCR and FISH to bear the ETV6-NTRK3 fusion and with expected immunohistochemical reactivity as for MASC. Correct diagnosis impacts choice of adjuvant therapy, as radioactive iodine would be predicted to be ineffective. Further work entails a review of more cases to determine the incidence of MASC in the thyroid, define its relationship to ETV6-NTRK3 fusion positive thyroid carcinomas, analyze its behavior when arising in the thyroid, and optimize treatment.
References
- 1.Skálová A, Vanecek T, Sima R, et al. Mammary analogue secretory carcinoma of salivary glands, containing the ETV6-NTRK3 fusion gene: a hitherto undescribed salivary gland tumor entity. Am J Surg Pathol. 2010;34:599–608. doi: 10.1097/PAS.0b013e3181d9efcc. [DOI] [PubMed] [Google Scholar]
- 2.Majewska H, Skálová A, Stodulski D, et al. Mammary analogue secretory carcinoma of salivary glands: a new entity associated with ETV6 gene rearrangement. Virchows Arch. 2015;466(3):245–254. doi: 10.1007/s00428-014-1701-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Connor A, Perez-Ordoñez B, Shago M, et al. Mammary analog secretory carcinoma of salivary gland origin with the ETV6 gene rearrangement by FISH: expanded morphologic and immunohistochemical spectrum of a recently described entity. Am J Surg Pathol. 2012;36:27–34. doi: 10.1097/PAS.0b013e318231542a. [DOI] [PubMed] [Google Scholar]
- 4.Projetti F, Lacroix-Triki M, Serrano E, et al. A comparative immunohistochemistry study of diagnostictools in salivary gland tumors: usefulness of mammaglobin, gross cystic disease fluid protein 15, and p63 cytoplasmic staining for the diagnosis of mammary analog secretory carcinoma? J Oral Pathol Med. 2015;44:244–251. doi: 10.1111/jop.12226. [DOI] [PubMed] [Google Scholar]
- 5.Bishop JA. Unmasking MASC: bringing to light the unique morphologic, immunohistochemical and genetic features of the newly recognized mammary analogue secretory carcinoma of salivary glands. Head Neck Pathol. 2013;7:35–39. doi: 10.1007/s12105-013-0429-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Skálová A, Vanecek T, Majewska H, et al. Mammary analogue secretory carcinoma of salivary glands with high-grade transformation: report of 3 cases with the ETV6-NTRK3 gene fusion and analysis of TP53, β-catenin, EGFR, and CCND1 genes. Am J Surg Pathol. 2014;38:23–33. doi: 10.1097/PAS.0000000000000088. [DOI] [PubMed] [Google Scholar]
- 7.Stevens TM, Kovalovsky AO, Velosa C, et al. Mammary analog secretory carcinoma, low-grade salivary duct carcinoma, and mimickers: a comparative study. Mod Pathol. 2015;28:1084–1100. doi: 10.1038/modpathol.2015.64. [DOI] [PubMed] [Google Scholar]
- 8.Skálová A, Vanacek T, Simpson RH, et al. Mammary analogue secretory carcinoma of salivary glands: molecular analysis of 25 ETV6 gene rearranged tumors with lack of detection of classical ETV6-NTRK3 fusion transcript by standart RT-PCR: report of four cases harboring ETV6-X gene fusion. Am J Surg Pathol. 2016;40:3–13. doi: 10.1097/PAS.0000000000000537. [DOI] [PubMed] [Google Scholar]
- 9.Tognon C, Knezevich SR, Huntsman D, et al. Expression of the ETV6-NTRK3 gene fusion as a primary event in human secretory breast carcinoma. Cancer Cell. 2002;2:367–376. doi: 10.1016/S1535-6108(02)00180-0. [DOI] [PubMed] [Google Scholar]
- 10.Rubin BP, Chen CJ, Morgan TW, et al. Congenital mesoblastic nephroma t(12;15) is associated with ETV6-NTRK3 gene fusion: cytogenetic and molecular relationship to congenital (infantile) fibrosarcoma. Am J Pathol. 1998;153:1451–1458. doi: 10.1016/S0002-9440(10)65732-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knezevich SR, McFadden DE, Tao W, et al. A novel ETV6-NTRK3 gene fusion in congenital fibrosarcoma. Nat Genet. 1998;18:184–187. doi: 10.1038/ng0298-184. [DOI] [PubMed] [Google Scholar]
- 12.Eguchi M, Eguchi-Ishimae M, Tojo A, et al. Fusion of ETV6 to neurotrophin-3 receptor TRKC in acute myeloid leukemia with t(12;15)(p13;q25) Blood. 1999;93:1355–1363. [PubMed] [Google Scholar]
- 13.Leeman-Neill RJ, Kelly LM, Liu P, et al. ETV6-NTRK3 is a common chromosomal rearrangement in radiation-associated thyroid cancer. Cancer. 2014;120:799–807. doi: 10.1002/cncr.28484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsieh M-S, Chou Y-H, Yeh S-J, et al. Papillary-cystic pattern is characteristic in mammary analogue secretory carcinomas but is rarely observed in acinic cell carcinomas of the salivary gland. Virchows Arch. 2015;467:145–153. doi: 10.1007/s00428-015-1786-8. [DOI] [PubMed] [Google Scholar]
- 15.Lubitz CC, Economopoulos KP, Pawlak AC, et al. Hobnail variant of papillary thyroid carcinoma: an institutional case series and molecular profile. Thyroid. 2014;24(6):958–965. doi: 10.1089/thy.2013.0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luo W, Lindley SW, Lindley PH, et al. Mammary analog secretory carcinoma of salivary gland with high-grade histology arising in hard palate, report of a case and review of literature. Int J Clin Exp Pathol. 2014;7:9008–9022. [PMC free article] [PubMed] [Google Scholar]
- 17.Skálová A. Mammary analog secretory carcinoma of salivary gland origin: an update and expanded morphological and immunohistochemical spectrum of recently described entity. Head Neck Pathol. 2013;7:S30–S36. doi: 10.1007/s12105-013-0455-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Costa V, Esposito R, Ziviello C, et al. New somatic mutations and WNK1-B4GALNT3 gene fusion in papillary thyroid carcinoma. Oncotarget. 2015;6:11242–11251. doi: 10.18632/oncotarget.3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cancer Genome Atlas Research Network Integrated genomic characterization of papillary thyroid carcinoma. Cell. 2014;159:676–690. doi: 10.1016/j.cell.2014.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Santoro M, Carlomagno F. Oncogenic rearrangements driving ionizing radiation-associated human cancer. J Clin Invest. 2013;123:4566–4568. doi: 10.1172/JCI72725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ricarte-Filho JC, Li S, Garcia-Rendueles ME, et al. Identification of kinase fusion oncogenes in post-chernobyl radiation-induced thyroid cancers. J Clin Invest. 2013;123:4935–4944. doi: 10.1172/JCI69766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prasad ML, Vyas M, Home MJ, Virk RK, et al. NTRK fusion oncogenes in pediatric papillary thyroid carcinoma in northeast United States. Cancer. 2016;122:1097–1107. doi: 10.1002/cncr.29887. [DOI] [PubMed] [Google Scholar]