Abstract
Perioperative blood transfusions are common in total hip arthroplasty because of preoperative anemia and perioperative blood loss. Perioperative anemia and the need for allogeneic blood transfusion are related with increased morbidity. To reduce perioperative allogeneic blood transfusion, keeping the preoperative hemoglobin level above 12.0 g/dL is important in orthopedic patients. By using the anti-fibrinolytic agent or perioperative cell salvage, reduce intraoperative blood loss is very important for the reduction of perioperative blood loss. As a transfusion trigger, low hemoglobin is another important target to reduce the transfusion rate. Because blood management is closely connected with prognosis, it has become a new challenge in orthopedic surgery.
Keywords: Blood management, Total hip arthroplasty, Allogenic transfusion
INTRODUCTION
The frequency of orthopedic surgery, especially hip and knee arthroplasty, is increasing in Korea. This phenomenon may be related with the trend toward an aging society in Korea. Total hip arthroplasty (THA) is associated with a large amount of blood loss. According to Sehat et al.1), THA patients had a mean total true blood loss of 1,510 mL, with a calculated hidden loss of 471 mL.
Perioperative blood transfusions are common in total hip and knee arthroplasty because of preoperative anemia and perioperative blood loss. It is well known that hip and knee replacement arthroplasty is a representative surgery needing substantial transfusion. According to Browne et al.2) in the United States, transfusion rates following THA have consistently increased from 18.12% in 2005 to 21.21% in 2008. In general, there is no difference in blood management between the two surgeries. However, intra-operative blood management may be more important in case of THA than the total knee arthroplasty (TKA), because a tourniquet can be used in TKA surgery. In contrast to the advances in knowledge of blood management, efforts for reducing blood transfusion have reached a standstill in practice. As orthopedic surgeons have recognized the presence of adverse surgical outcomes, patient blood management has become a new challenge in orthopedic surgery.
NEED FOR PATIENT BLOOD MANAGEMENT
Preoperative and postoperative anemia is common in surgical patients3). Also anemic patients are more likely to receive blood transfusion than non-anemic patients4,5). What is the effect of allogenic blood transfusion on human immune function? Allogeneic blood transfusions generally cause up regulation of humoral immunity and down regulation of macrophage and T-cell immunity. Increased alloantibody formation to human leukocyte antigen-A and B (major histocompatibility complex class I) antigens and decreased cutaneous delayed-type hypersensitivity, T-cell proliferation, and natural killer cell function are observed in transfused patients and experimental animals6). Blood transfusions have been found to increase the rate of glucose and glutamine metabolism, nucleotide triphosphate concentrations, and the level of adenosine deaminase activity. This increased level of lymphocyte metabolism in the face of immunosuppression appears to indicate the occurrence of transfusion-induced immunosuppression7). Moreover, allogeneic transfusion decreases the phagocytic activity of macrophages and elevates glucocorticoid level; this can suppress the human immune system. A hemolytic transfusion reaction is a serious complication that can occur after a transfusion of blood. Febrile reaction, anaphylaxis, transfusion related acute lung injury, and graft-versus-host disease can also occur after a transfusion8).
There is a high incidence of preoperative and postoperative anemia in surgical patients, with a coincident increase in blood utilization. These factors are associated with an increased risk for perioperative infection and adverse outcome (mortality) in surgical patients. According to Dunne et al.9), increased blood transfusion rates were associated with increased mortality, increased postoperative pneumonia, and increased hospital length of stay. Perioperative anemia and the need for allogeneic blood transfusion are related with increased morbidity. According to Husted et al.10), blood transfusion was the most important predictor of discharge around the third day of admission, as patients receiving blood had a three-fold increased risk of staying more than 3 days.
Peri-prosthetic joint infection (PJI) is one of the most challenging complications of joint arthroplasty. Because of the economic and psychological burden of this complication, strategies to minimize or prevent PJI may be needed. It has been well documented that preoperative anemia and increased transfusion rates were independently associated with an increased risk of perioperative adverse outcomes, such as increased postoperative infections. In a study about PJI, Pulido et al.11) reported that of 9,245 patients who underwent TKA or THA, the quantity of allogeneic blood units that was transfused was an independent predictor of PJI after primary joint arthroplasty.
THE OLD CONCEPT OF TRANSFUSION
Many physicians do yet have a old concept of Adam and Lundi's rule (hemoglobin [Hb] level <10 g/dL, hematocrit <30%)12). Although Adams and Lundi's 10/30 rule has been reported as an inadequate transfusion trigger, physicians and other healthcare providers have unconsciously thought that the oxygen transport might be impaired if Hb falls below 10 g/dL or the hematocrit is below 30%. They hold to the attitude that blood transfusion saves life and continue to have a firm belief regarding the need for transfusion. Too few have changed from the transfusion-based culture. Postoperative Hb level was consistently much lower (mean, 4 g/dL) than before surgery after total arthroplasty. Therefore, efforts to maintain preoperative Hb levels at 13 g/dL or greater seems to be recommended for reducing postoperative transfusion. Strategies for early detection, evaluation, and management of preoperative anemia should be identified. However, the significance regarding preoperative correction of anemia and optimization of red cell mass is easily overlooked.
RISK FACTORS OF ALLOTRANSFUSION
There are many studies about risks of transfusion. Generally, blood transfusion has been associated with increasing age, female sex, co-morbidities, low pre- and post-operative Hb levels, increased ASA (American Society of Anesthesiologists) score, and the use of anti-coagulation medication13). We consider these risk factors prior to proceeding with THA (Table 1).
Table 1. Risk Factors of Allotransfusion.
BLOOD CONSERVATION STRATEGIES
1. Optimization of Preoperative Hemoglobin Levels
Postoperative morbidity and mortality are significantly associated with the presence of preoperative anemia14). One of those factors, a pre-operative Hb level of less than 12 g/dL, increases the likelihood of transfusion three-fold in THA patients. In addition, according to our previously mentioned study, to reduce perioperative allogeneic blood transfusion, keeping the preoperative Hb level above 12.0 g/dL is important in orthopedic patients15). In orthopedic surgery, hematologic evaluation and treatment for anemia is recommended 3-4 weeks before the operation, thus allowing time for the correction of anemia before surgery. If patients have anemia, laboratory testing will be performed for further evaluation of nutritional deficiencies, chronic renal insufficiency, and/or chronic inflammatory disease. If a screening blood count detects anemia, evaluation should begin with an assessment of iron status. Moreover, serum iron, transferrin, and ferritin levels can also be evaluated. When absolute iron deficiency is detected, referral to a gastroenterologist is indicated to rule out gastrointestinal malignancy as a source of chronic blood loss. If laboratory evaluation or a diagnostic trial of iron therapy rules out absolute iron deficiency, measurement of serum creatinine and glomerular filtration rate may indicate chronic kidney disease and the need for referral to a nephrologist.
Iron supplementation is indicated in the presence of confirmed iron deficiency anemia. The effectiveness of oral iron in the management of preoperative anemia has been demonstrated in patients undergoing orthopedic and colorectal cancer surgery; patients with preoperative anemia due to iron deficiency or chronic disease should receive preoperative treatment with oral or intravenous iron, depending on the time scale before surgery, tolerance of oral iron, and iron status. We also consider the amount of iron, absorption rate, body weight, and Hb levels.
The use of erythropoietin-stimulating agent (ESA) therapy in patients undergoing major, elective surgery is well established on the basis of randomized controlled trials and is approved for use in this setting. A meta-analysis of 41 published studies (eight studies of ESA therapy alone, 22 studies of ESA therapy augmented with preoperative autologous blood donation [PABD], seven studies of ESA therapy compared with PABD, and four studies of ESA therapy and other comparators) was performed. Pooled estimates of transfusion exposure demonstrated a clinically important benefit for both recombinant human erythropoietin (rHuEPO) alone (relative risk [RR], 0.44; 95% confidence interval [CI], 0.31-0.64) and rHuEPO augmented with PABD (RR, 0.61; 95% CI, 0.49-0.75)16). With erythropoietin (EPO; n=339), mean erythrocyte use was 0.50 U/patient with a transfusion rate of 16%; without EPO (n=344), values were 0.71 U/patient and 26%, respectively17). It is more effective if EPO is administered with oral or intravenous iron18,19).
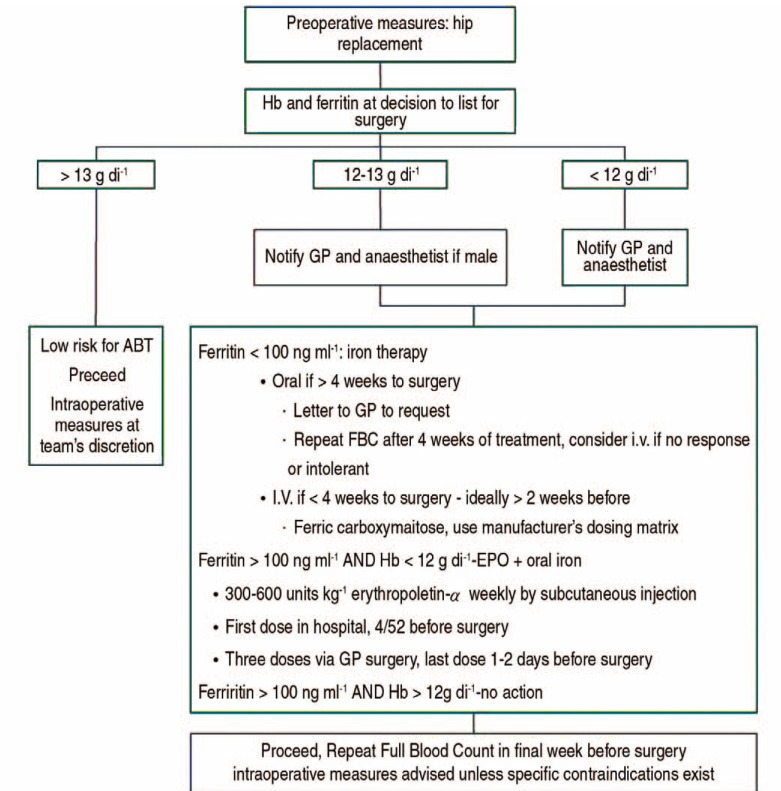
However, this implementation and management is difficult for Korean physicians, because Korean patients have a tendency to not allow enough weeks for the correction of preoperative anemia. Recent studies demonstrate that a very short-term treatment with ESA and intravenous iron initiated only two days before surgery helps reduce perioperative red blood cell transfusions20,21). An example of preoperative patient blood management is as follows22) (Fig. 1).
Fig. 1. Example of preoperative patient blood management. GP: general practitioner, ABT: allogenic blood transfusion, FBC: full blood count, Hb: hemoglobin, EPO: erythropoietin, I.V.: intravenous.
2. Reduce Intraoperative Blood Loss
There is a need to investigate the bleeding tendency of a patient, whether there is a medical history such as von Willebrand disease, or if the patient is taking medication such as aspirin or warfarin. A meticulous surgical technique, use of a pneumatic tourniquet, hypotensive anesthesia, adequate positioning of the patient, and maintenance of the patient's body temperature during surgery significantly contributes to minimizing blood loss. Simply by keeping the patient's body temperature at normal levels, the patient not only loses 20-25% less blood, but also has improved wound healing. When a patient becomes hypothermic, a profound hemostatic defect develops. This is caused by platelet dysfunction. According to Schmied et al.23), warming may be beneficial in patients undergoing THA. There is some debate regarding the number, thickness, shape, and duration in closed suction wound drainage to reduce blood loss. According to Parker et al.24), closed suction surgical wound drainage after orthopedic surgery does not help to reduce blood loss and allogeneic blood transfusion. It is better to make decisions based on the patient's condition and each surgeon's preferences.
As a lysine analog, tranexamic acid suppresses fibrinolytic activity by competitively inhibiting the binding of plasminogen and plasmin to fibrin. By blocking access to the fibrin template, this substantially decreases the kinetic rate of plasmin formation as well as the plasmin-mediated degradation of fibrin and fibrinogen25).
In some meta-analyses, patients treated with intravenous tranexamic acid were less likely to require allogeneic blood transfusion than those treated with placebo (11 randomized controlled trials; pooled odds ratio [OR] 0.16, 95% CI 0.09-0.26). Furthermore, tranexamic acid reduces the total amount of blood loss (weighted mean difference [WMD], 460 mL; 95% CI, 274-626 mL) and the total number of units of allogeneic blood transfused (WMD, 0.85 U; 95% CI, 0.36-1.33 U)26,27). Meanwhile, in a study evaluating the efficacy of topical tranexamic acid in THA, the final reduction in Hb and hematocrit in the tranexamic acid group was less than that in the control group (P=0.01). In addition, the blood transfusion rate was lower in the tranexamic acid group (17%) than that in the control group (35%) (P<0.001)28).
There are still concerns with regards to the increased risk of thromboembolic events with the use of tranexamic acid. Tranexamic acid does not increase the risk of thromboembolic complications such as deep vein thrombosis (DVT), pulmonary embolism, thrombotic cerebral vascular accident, or myocardial infarction26,27). Another study evaluating 701 patients showed that preoperative use of tranexamic acid in primary THA and TKA does not increase the incidence of DVT and pulmonary embolism. In a large case study, a total of 13,262 elective TKA or THA procedures in 11,175 unique patients were analyzed. A total of 196 venous thromboembolic (VTE) events were identified for an overall frequency of 1.48%. Thirty-seven VTE events (1.3%) were identified among 2,785 procedures in which patients received tranexamic acid, while 159 VTE events (1.5%) were identified among 10,477 procedures in which patients did not receive tranexamic acid. There was no statistical difference in the adjusted odds of a clinically significant VTE event in patients that did or did not receive tranexamic acid (OR, 0.98; 95% CI, 0.67-1.45; P=0.939)29,30).
There is some disagreement, but in most intravenous injection cases, 10-20 mg/kg is given 30 minutes before surgery or before tourniquet deflation, and an additional 10-15 mg/kg is given every 8 hours for 24 hours or every 24 hours for 3 days. In the case of oral administration, 1 g is given before surgery and 1 g is given every 6 hours for 18 hours after surgery. With topical administration, an intraoperative or postoperative intra-articular injection will be given using 1 to 3 g tranexamic acid in 100 mL normal saline31). An exact comparison among intravenous, oral, and topical treatment is not possible. It is thought that a good decision is based on the patient's condition and each surgeon's preferences. Moreover, it is important to consider the patient's underlying disease, such as liver cirrhosis, chronic kidney disease, or cerebrovascular accident. Many studies show that tranexamic acid does not increase the risk of DVT, but that there is a need for extra care when the patient has a medical history such as DVT or myocardial infarction.
However, unlike most studies, in some studies, only intraoperative and total blood loss were shown to be significantly reduced in comparison to a placebo. The other outcome measures, including units of blood transfused and the proportion of patients requiring allogeneic transfusions, were not statistically significant compared with placebo32). Future studies should evaluate the effectiveness of tranexamic acid in reducing allogeneic blood transfusions as well as the cost-effectiveness of tranexamic acid.
Intraoperative blood salvage is a good indication for selective cases, especially if any massive hemorrhaging is expected during surgery. Accordingly, intraoperative cell salvage is useful for a revision arthroplasty or simultaneous bilateral primary TKA with an anticipated blood loss of more than 1,000 mL. According to Greenky et al.33), advanced age (P=0.03), higher body mass index (P=0.01), revision requiring exchange of both the femoral and acetabular components (P<0.01), and revision surgeries with a trochanteric osteotomy (P<0.02) were all associated with successful postoperative reinfusion with intraoperative blood salvage.
Postoperative cell salvage is not always effective, because an accurate estimate of blood loss is not possible. Nonetheless, it is effective if the patient has a small total blood volume. Based on a prospective randomized controlled trial by Amin et al.34), there was no difference in the need for allogeneic blood between a control group (15.1%) and a retransfusion group (13%; P=0.439). The incidence of postoperative complications, such as wound infection, DVT, and chest infection, was also comparable between the groups. There were no adverse reactions associated with the retransfusion of autologous blood34). However, according to Smith35), significantly fewer patients with postoperative Hb values less than 9.0 g/dL and significantly fewer patients who required transfusion of homologous blood in the retransfusion group. Maximize effectiveness can be achieved through the selective use.
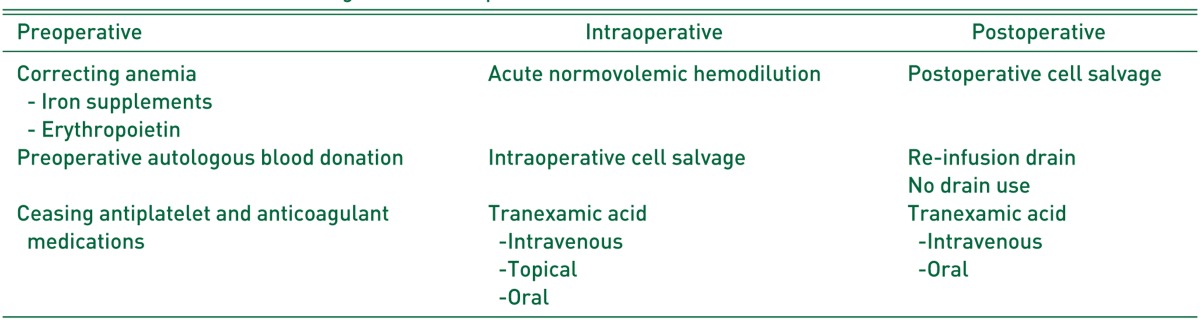
PABD was found to significantly reduce the requirement for allogeneic blood transfusions36,37). However, there is a disadvantage: because the procedure is complex, it may have low compliance. Several studies regarding autologous retransfusion in THA showed allogeneic transfusion rates of 6-15%35,38,39). Notwithstanding, this is still a very low level compared to other studies in which the allogeneic transfusion rate was 57% in THA without the retransfusion of autologous blood40) (Table 2).
Table 2. Application of Each Drug.
IV; intravenous, EPO: erythropoietin.
3. Postoperative Patient Blood Management
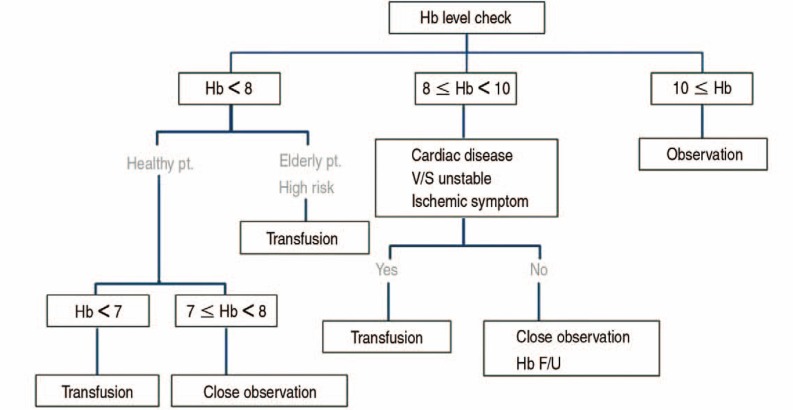
As a transfusion trigger, low Hb is another important target to reduce the transfusion rate. Allogeneic blood transfusion is inappropriate if the Hb is >8.0 g/dL in the absence of symptoms attributable to tissue oxygen deficit or continuing bleeding. Low Hb transfusion triggers, such as <7.0 g/dL in general surgical patients and <8.0 g/dL in elderly high-risk patients, are well tolerated with adequate fluid management. Judicious fluid management enables the low Hb trigger. Careful monitoring of a patient's physical condition is essential, including pulse rate, blood pressure, and the presence of dyspnea symptoms41) (Table 3, Fig. 2).
Table 3. Blood Conservation Strategies-Three Steps.
Fig. 2. Algorithm about the postoperative patient blood management. Hb: hemoglobin, pt.: patient, F/U: follow up.
CONCLUSION
To reduce transfusion rates, patient blood management needs a comprehensive preoperative, intraoperative, and postoperative approach, considering the state of each patient. Because blood management is closely connected with prognosis, it has become a new challenge in management is useful for artificial joint replacement surgery.
References
- 1.Sehat KR, Evans RL, Newman JH. Hidden blood loss following hip and knee arthroplasty. Correct management of blood loss should take hidden loss into account. J Bone Joint Surg Br. 2004;86:561–565. [PubMed] [Google Scholar]
- 2.Browne JA, Adib F, Brown TE, Novicoff WM. Transfusion rates are increasing following total hip arthroplasty: risk factors and outcomes. J Arthroplasty. 2013;28(8 Suppl):34–37. doi: 10.1016/j.arth.2013.03.035. [DOI] [PubMed] [Google Scholar]
- 3.Shander A, Knight K, Thurer R, Adamson J, Spence R. Prevalence and outcomes of anemia in surgery: a systematic review of the literature. Am J Med. 2004;116(Suppl 7A):58S–69S. doi: 10.1016/j.amjmed.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 4.Karkouti K, Wijeysundera DN, Beattie WS Reducing Bleeding in Cardiac Surgery (RBC) Investigators. Risk associated with preoperative anemia in cardiac surgery: a multicenter cohort study. Circulation. 2008;117:478–484. doi: 10.1161/CIRCULATIONAHA.107.718353. [DOI] [PubMed] [Google Scholar]
- 5.Beattie WS, Karkouti K, Wijeysundera DN, Tait G. Risk associated with preoperative anemia in noncardiac surgery: a single-center cohort study. Anesthesiology. 2009;110:574–581. doi: 10.1097/ALN.0b013e31819878d3. [DOI] [PubMed] [Google Scholar]
- 6.Blumberg N, Heal JM. Immunomodulation by blood transfusion: an evolving scientific and clinical challenge. Am J Med. 1996;101:299–308. doi: 10.1016/S0002-9343(96)00124-6. [DOI] [PubMed] [Google Scholar]
- 7.Waymack JP, Gugliuzza K, Dong YL, Herndon DN. Effect of blood transfusion on immune function. IX. Effect on lymphocyte metabolism. J Surg Res. 1993;55:269–272. doi: 10.1006/jsre.1993.1139. [DOI] [PubMed] [Google Scholar]
- 8.Choat JD, Maitta RW, Tormey CA, et al. Transfusion reactions to blood and cell therapy products. In: Hoffman R, Benz EJ Jr, Silberstein LE, Heslop HE, Weitz JI, et al., editors. Hematology: basic principles and practice. Philadelphia, PA: Saunders/Elsevier; 2013. [Google Scholar]
- 9.Dunne JR, Malone D, Tracy JK, Gannon C, Napolitano LM. Perioperative anemia: an independent risk factor for infection, mortality, and resource utilization in surgery. J Surg Res. 2002;102:237–244. doi: 10.1006/jsre.2001.6330. [DOI] [PubMed] [Google Scholar]
- 10.Husted H, Holm G, Jacobsen S. Predictors of length of stay and patient satisfaction after hip and knee replacement surgery: fast-track experience in 712 patients. Acta Orthop. 2008;79:168–173. doi: 10.1080/17453670710014941. [DOI] [PubMed] [Google Scholar]
- 11.Pulido L, Ghanem E, Joshi A, Purtill JJ, Parvizi J. Periprosthetic joint infection: the incidence, timing, and predisposing factors. Clin Orthop Relat Res. 2008;466:1710–1715. doi: 10.1007/s11999-008-0209-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adams RC, Lundy JS. Anesthesia in cases of poor surgical risk: some suggestions for decreasing the risk. Anesthesiology. 1942;9:603–607. [Google Scholar]
- 13.Walsh M, Preston C, Bong M, Patel V, Di Cesare PE. Relative risk factors for requirement of blood transfusion after total hip arthroplasty. J Arthroplasty. 2007;22:1162–1167. doi: 10.1016/j.arth.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 14.Salido JA, Marín LA, Gómez LA, Zorrilla P, Martínez C. Preoperative hemoglobin levels and the need for transfusion after prosthetic hip and knee surgery: analysis of predictive factors. J Bone Joint Surg Am. 2002;84-A:216–220. doi: 10.2106/00004623-200202000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Aderinto J, Brenkel IJ. Pre-operative predictors of the requirement for blood transfusion following total hip replacement. J Bone Joint Surg Br. 2004;86:970–973. doi: 10.1302/0301-620x.86b7.14682. [DOI] [PubMed] [Google Scholar]
- 16.Goodnough LT, Maniatis A, Earnshaw P, et al. Detection, evaluation, and management of preoperative anaemia in the elective orthopaedic surgical patient: NATA guidelines. Br J Anaesth. 2011;106:13–22. doi: 10.1093/bja/aeq361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.So-Osman C, Nelissen RG, Koopman-van Gemert AW, et al. Patient blood management in elective total hip- and knee-replacement surgery (Part 1): a randomized controlled trial on erythropoietin and blood salvage as transfusion alternatives using a restrictive transfusion policy in erythropoietin-eligible patients. Anesthesiology. 2014;120:839–851. doi: 10.1097/ALN.0000000000000134. [DOI] [PubMed] [Google Scholar]
- 18.Moonen AF, Thomassen BJ, Knoors NT, van Os JJ, Verburg AD, Pilot P. Pre-operative injections of epoetinalpha versus post-operative retransfusion of autologous shed blood in total hip and knee replacement: a prospective randomised clinical trial. J Bone Joint Surg Br. 2008;90:1079–1083. doi: 10.1302/0301-620X.90B8.20595. [DOI] [PubMed] [Google Scholar]
- 19.Cuenca J, García-Erce JA, Martínez F, Cardona R, Pérez-Serrano L, Muñoz M. Preoperative haematinics and transfusion protocol reduce the need for transfusion after total knee replacement. Int J Surg. 2007;5:89–94. doi: 10.1016/j.ijsu.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 20.Yoo YC, Shim JK, Kim JC, Jo YY, Lee JH, Kwak YL. Effect of single recombinant human erythropoietin injection on transfusion requirements in preoperatively anemic patients undergoing valvular heart surgery. Anesthesiology. 2011;115:929–937. doi: 10.1097/ALN.0b013e318232004b. [DOI] [PubMed] [Google Scholar]
- 21.Na HS, Shin SY, Hwang JY, Jeon YT, Kim CS, Do SH. Effects of intravenous iron combined with low-dose recombinant human erythropoietin on transfusion requirements in iron-deficient patients undergoing bilateral total knee replacement arthroplasty. Transfusion. 2011;51:118–124. doi: 10.1111/j.1537-2995.2010.02783.x. [DOI] [PubMed] [Google Scholar]
- 22.Kotzé A, Carter LA, Scally AJ. Effect of a patient blood management programme on preoperative anaemia, transfusion rate, and outcome after primary hip or knee arthroplasty: a quality improvement cycle. Br J Anaesth. 2012;108:943–952. doi: 10.1093/bja/aes135. [DOI] [PubMed] [Google Scholar]
- 23.Schmied H, Kurz A, Sessler DI, Kozek S, Reiter A. Mild hypothermia increases blood loss and transfusion requirements during total hip arthroplasty. Lancet. 1996;347:289–292. doi: 10.1016/s0140-6736(96)90466-3. [DOI] [PubMed] [Google Scholar]
- 24.Parker MJ, Livingstone V, Clifton R, McKee A. Closed suction surgical wound drainage after orthopaedic surgery. Cochrane Database Syst Rev. 2007;(3):CD001825. doi: 10.1002/14651858.CD001825.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Slaughter TF, Greenberg CS. Antifibrinolytic drugs and perioperative hemostasis. Am J Hematol. 1997;56:32–36. doi: 10.1002/(sici)1096-8652(199709)56:1<32::aid-ajh7>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 26.Ho KM, Ismail H. Use of intravenous tranexamic acid to reduce allogeneic blood transfusion in total hip and knee arthroplasty: a meta-analysis. Anaesth Intensive Care. 2003;31:529–537. doi: 10.1177/0310057X0303100507. [DOI] [PubMed] [Google Scholar]
- 27.Yang ZG, Chen WP, Wu LD. Effectiveness and safety of tranexamic acid in reducing blood loss in total knee arthroplasty: a meta-analysis. J Bone Joint Surg Am. 2012;94:1153–1159. doi: 10.2106/JBJS.K.00873. [DOI] [PubMed] [Google Scholar]
- 28.Chang CH, Chang Y, Chen DW, Ueng SW, Lee MS. Topical tranexamic acid reduces blood loss and transfusion rates associated with primary total hip arthroplasty. Clin Orthop Relat Res. 2014;472:1552–1557. doi: 10.1007/s11999-013-3446-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Selvaratnam V. Does pre-operative tranexamic acid increase the incidence of thromboembolism in primary lower limb arthroplasty? Open J Orthoped. 2013;3:249–252. [Google Scholar]
- 30.Duncan CM, Gillette BP, Jacob AK, Sierra RJ, Sanchez-Sotelo J, Smith HM. Venous thromboembolism and mortality associated with tranexamic acid use during total hip and knee arthroplasty. J Arthroplasty. 2015;30:272–276. doi: 10.1016/j.arth.2014.08.022. [DOI] [PubMed] [Google Scholar]
- 31.Kim TK, Chang CB, Koh IJ. Practical issues for the use of tranexamic acid in total knee arthroplasty: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2014;22:1849–1858. doi: 10.1007/s00167-013-2487-y. [DOI] [PubMed] [Google Scholar]
- 32.Gill JB, Rosenstein A. The use of antifibrinolytic agents in total hip arthroplasty: a meta-analysis. J Arthroplasty. 2006;21:869–873. doi: 10.1016/j.arth.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 33.Greenky M, Shaner J, Rasouli MR, Han SB, Parvizi J, Hozack WJ. Intraoperative blood salvage in revision total hip arthroplasty: who benefits most? J Arthroplasty. 2014;29:1298–1300. doi: 10.1016/j.arth.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 34.Amin A, Watson A, Mangwani J, Nawabi DH, Ahluwalia R, Loeffler M. A prospective randomised controlled trial of autologous retransfusion in total knee replacement. J Bone Joint Surg Br. 2008;90:451–454. doi: 10.1302/0301-620X.90B4.20044. [DOI] [PubMed] [Google Scholar]
- 35.Smith LK, Williams DH, Langkamer VG. Post-operative blood salvage with autologous retransfusion in primary total hip replacement. J Bone Joint Surg Br. 2007;89:1092–1097. doi: 10.1302/0301-620X.89B8.18736. [DOI] [PubMed] [Google Scholar]
- 36.Sinclair KC, Clarke HD, Noble BN. Blood management in total knee arthroplasty: a comparison of techniques. Orthopedics. 2009;32:19. doi: 10.3928/01477447-20090101-21. [DOI] [PubMed] [Google Scholar]
- 37.Gonzalez-Porras JR, Colado E, Conde MP, Lopez T, Nieto MJ, Corral M. An individualized pre-operative blood saving protocol can increase pre-operative haemoglobin levels and reduce the need for transfusion in elective total hip or knee arthroplasty. Transfus Med. 2009;19:35–42. doi: 10.1111/j.1365-3148.2009.00908.x. [DOI] [PubMed] [Google Scholar]
- 38.del Trujillo MM, Carrero A, Muñoz M. The utility of the perioperative autologous transfusion system OrthoPAT in total hip replacement surgery: a prospective study. Arch Orthop Trauma Surg. 2008;128:1031–1038. doi: 10.1007/s00402-007-0440-6. [DOI] [PubMed] [Google Scholar]
- 39.Moonen AF, Knoors NT, van Os JJ, Verburg AD, Pilot P. Retransfusion of filtered shed blood in primary total hip and knee arthroplasty: a prospective randomized clinical trial. Transfusion. 2007;47:379–384. doi: 10.1111/j.1537-2995.2007.01127.x. [DOI] [PubMed] [Google Scholar]
- 40.Bierbaum BE, Callaghan JJ, Galante JO, Rubash HE, Tooms RE, Welch RB. An analysis of blood management in patients having a total hip or knee arthroplasty. J Bone Joint Surg Am. 1999;81:2–10. doi: 10.2106/00004623-199901000-00002. [DOI] [PubMed] [Google Scholar]
- 41.Liu D, Dan M, Adivi N. Blood conservation strategies in total hip and knee arthroplasty. Reconstr Rev. 2014;4:39. [Google Scholar]