Abstract
Effective perioperative pain management techniques and accelerated rehabilitation programs can improve health-related quality of life and functional status of patients after total hip arthroplasty. Traditionally, postoperative analgesia following arthroplasty was provided by intravenous patient-controlled analgesia or epidural analgesia. Recently, peripheral nerve blockade has emerged alternative analgesic approach. Multimodal analgesia strategy combines analgesics with different mechanisms of action to improve pain management. Intraoperative periarticular injection of multimodal drugs is one of the most important procedures in perioperative pain control for total hip arthroplasty. The goal of this review article is to provide a concise overview of the principles of multimodal pain management regimens as a practical guide for the perioperative pain management for total hip arthroplasty.
Keywords: Pain control, Total hip arthroplasty, Analgesics
INTRODUCTION
Total hip arthroplasty (THA) is one of the most common major surgical procedures, and efficacious and cost-effective interventions as well as improving health-related quality of life and functional status of patients1,2). However, despite these advantages, THA can be associated with significant postoperative pain. Postoperative pain after THA can adversely affect early postoperative patient recovery. Moreover, pain can negatively impact postoperative mobility, increasing the risk of venous thromboembolic disease, and also may impair rehabilitation. As a result, these consequences of pain can prolong patient recovery and can increase hospital length of stay and cost. Therefore, adequate postoperative pain management after THA should be emphasized to enhance patient well-being and to minimize the physiologic consequences of pain3,4).
The preemptive use of multimodal approach is the most important concept of pain management, and has been widely accepted as a gold standard approach of pain management following THA5,6) Preemptive means that initial pain management should be performed before surgical stimuli, and multimodal approach means that multi-drugs or multi-modalities with different mechanisms or sites should be applied to get synergetic effect. Using two concepts, it has been known to be effective for postoperative pain management, reduction of the opioid consumption, and early initiation of rehabilitation eventually.
PAIN PERCEPTION
Pain is an unpleasant feeling that is conveyed to the brain by sensory neurons. The discomfort signals actual or potential injury to the body. The pain pathways form a complex, dynamic, sensory, cognitive, and behavioral system that evolved to detect, integrate, and coordinate a protective response to incoming noxious stimuli that threatens tissue injury or organism survival7).
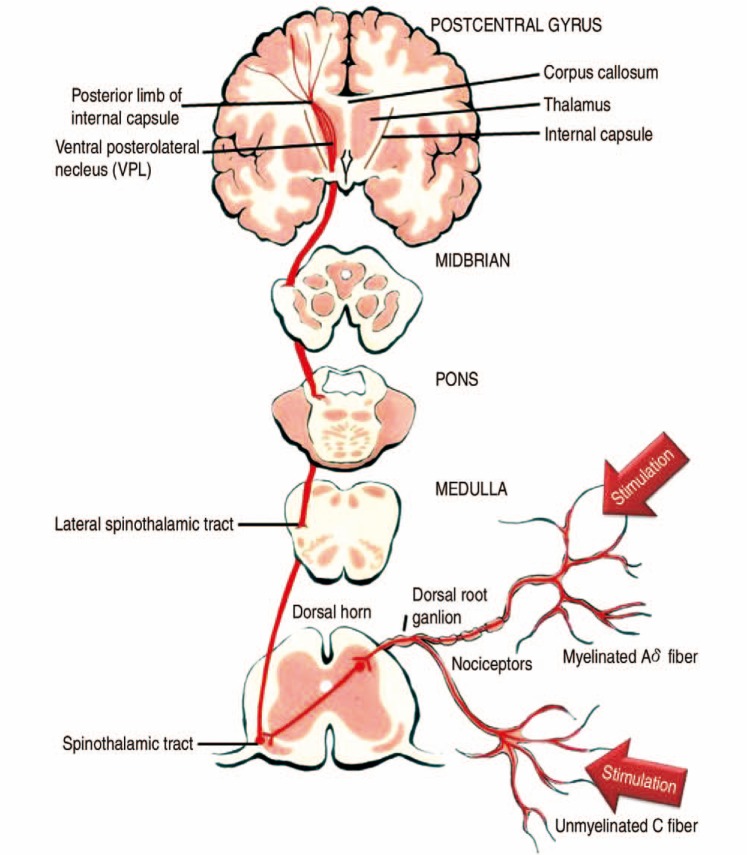
Nociceptors are the specialized sensory receptors responsible for the detection of noxious (unpleasant) stimuli, transforming the stimuli into electrical signals, which are then conducted to the central nervous system (CNS). They are the free nerve endings of primary afferent Aδ and C fibers. Distributed throughout the body (skin, viscera, muscles, joints, meninges), they can be stimulated by mechanical, thermal or chemical stimuli. Transmission is divided into two categories, fast and slow. A-delta fibers detect and transmit pain quickly. These fibers are relatively small (1-6 m), thinly myelinated neurons that can conduct at speeds of 6 to 30 m/sec. C fibers are small (<1.5 m) and unmyelinated, conducting pain at 0.5 to 2 m/sec8). Complex interactions occur in the dorsal horn between afferent neurons, interneurons and descending modulatory pathways. Sensory neuron cell bodies are located in the dorsal root ganglia (DRG). DRG neurons are classically pseudo unipolar; one process extends into the peripheral nerve and the other process extends centrally, transmitting information through the dorsal root into the spinal cord. Most sensory fibers project from the DRG through the dorsal root and into the dorsal root entry zone (DREZ). At the DREZ, most unmyelinated and small myelinated axons project laterally to enter. Lissauer tract9) fibers then extend vertically in this tract for several spinal segments before synapsing. There are two main pathways that carry nociceptive signals to higher centers in the brain. The spinothalamic tract: secondary afferent neurons decussate within a few segments of the level of entry into the spinal cord and ascend in the contralateral spinothalamic tract to nuclei within the thalamus. The spinothalamic tract transmits signals that are important for pain localization. The spinoreticular tract fibers also decussate and ascend the contralateral cord to reach the brainstem reticular formation, before projecting to the thalamus and hypothalamus. This pathway is involved in the emotional aspects of pain (Fig. 1).
Fig. 1. Spinothalamic tract. Pain transmission from receptors in the skin ascends in the spinal cord to the postcentral gyrus via the lateral spinothalamic tract. First-order neurons transmit this sensory information and enter the spinal cord. Second-order neurons from the dorsal horn then decussate at the ventral commissure and ascend in the lateral spinothalamic tract before ending in the ventral posterolateral nuclei of the thalamus. Third-order neurons then project to the postcentral gyrus.
Historically, osteoarthritis pain has been considered a nociceptive pain related to the degree of structural damage to the affected joint. Even though patients with osteoarthritis present structural anomalies, the severity of these changes is not always proportional to the degree of pain or disability. A significant proportion of these patients show signs of central sensitization, with pain modulation and processing altered at the CNS level. Central sensitization is defined as "an increased response of CNS neurons which inform of pain when faced with inputs coming from low threshold mechanoreceptors"10). One of the characteristics of central sensitization is that, once installed, it can persist in time despite the lack of new painful stimuli from the periphery. In clinical practice, it is not uncommon to find patients with osteoarthritis who show symptoms even after prosthetic substitution. It has been noted that patients suffering with osteoarthritis and a high degree of pain and low pain thresholds before surgery run a greater risk of continued pain after getting a prosthetic knee, which has been interpreted as an accurate reflection of central sensitization11).
PATIENT EDUCATION AND REHABILITATION
The purpose of arthroplasty of hip joint is to restore painless hip joint and provide early functional recovery. The rehabilitation protocol after THA is mandatory to improve range of motion and strengthen the muscle power around hip joint. This should include preoperative education, post-acute rehabilitation, such as early ambulation with walking aids and muscle strengthening exercise, and balancing exercise11). The structured postacute rehabilitation should be provided by trained professionals with knowledge at proper timing after THA. And for the early application of acute rehabilitation, well-designed multimodal pain management is necessary11). Also, the preoperative education can influence patient's perception of postoperative pain, walking and whole rehabilitation program. Consequently preoperative education can promote early discharge and reduce the total amount of analgesics usage. So, the preoperative education should be provided for patients and their family before THA11). The content should include the overall surgical procedure and rehabilitation protocol, expected benefits of THA, postoperative pain level and pattern, and pain management methods. Usually verbal format or small group sessions and is accompanied by a booklet as an adjunct to the verbal presentation12).
For the pain management after THA, it is more important to give information about postoperative pain than information about pathoanatomy, biomedical model of anatomy, and biomechanics of the disease and THA12). Educational sessions which aim to enhance patient knowledge of pain science and pain processing by the nervous system may help patients experience less fear and anxiety, and ultimately help alleviate postoperative pain12),13). Increasing a patient's knowledge of pain science may alter their perception of threat and they may then experience less fear and anxiety. Additionally, the increased knowledge and understanding of pain science may help modulate the pain experience. The patients also felt less pain perhaps because they were less stressed and better prepared to cope with pain. Anxiety has been reported to increase sensitivity to pain and to reduce anxiety decrease14).
CRYOTHERAPY
Cryotherapy involves the application of bag of ice or cooled water to the skin surrounding injury and operation area, and has been traditionally used in the post-operative recovery. The cold penetrates the soft tissues and, when applied over a joint, decrease in tissue metabolism associated with a reduction in enzymatic activity, and preventing tissue damage caused by injury15,16). Cryotherapy can reduce leukocyte migration and slow down nerve signal transmission, providing a reduction of inflammation and producing a short-term analgesic effect. Local hypothermia induces vasoconstriction and reduces extravasation of blood into surroundings tissues, local inflammation and edema production17,18). Many studies examining the benefits of cryotherapy on recovery after TKA have shown a reduction in blood loss19,20,21).
However, the clinical benefits on pain and range of motion have been equivocal, with some studies showing a benefit19,20,21),22,23) and others showing no difference in the treatment group19,24,25). Because most of the studies includes limited number of subjects and are nonrandomised, unblinded, cohort studies, and there are scanty of studies about the hip arthroplasty, further evaluation about the benefit of cryotherapy is needed.
MULTIMODAL ANALGESIA
Multimodal analgesia strategy combines analgesics with different mechanisms of action to improve postoperative pain management. This approach targets the various pathways and neurotransmitters involved in nociception and may allow a reduction in the dose of each individual analgesic. By using non-opioid adjuncts, perioperative opioid requirements and opioid-related side effects can be reduced such as nausea, vomiting, sedation, respiratory depression, urinary retention, and constipation26). In this section, we review the following multimodal analgesic adjuncts: acetaminophen, nonsteroidal anti-inflammatory drugs (NSAIDs), selective cyclo-oxygenase-2 (COX-2) inhibitors, and gabapentinoid.
1. Acetaminophen
Acetaminophen is a weak analgesic with minimal opioid-sparing ability but this agent is the most basic adjunct of a multimodal analgesia regimen due to its safety. According to literature review of meta-analyses and a Cochrane review, regular dosing significantly lowers visual analogue pain score (VAPS), decrease opioid requirements, reduces opioid-related adverse effects, and improve postoperative mobility27,28). A NSAIDs or COX-2 inhibitors can be added in the absence of contraindications. Hepatotoxicity can occur when ≥4 g are administered in 24 hours in healthy adults. Therefore, dose reduction is recommended in elderly patients, and its use should be limited in patients with compromised hepatic function.
2. Nonsteroidal Anti-inflammatory Drugs and Cyclo-oxygenase-2 Inhibitors
The NSAIDs has strong evidence supporting the efficacy for perioperative analgesia, and there are numerous NSAIDs with different onset, duration, route of administration, efficacy, and side-effect profile27). The Procedure-Specific Postoperative Pain Management Group summarized the relevant literature on perioperative analgesia specifically for patients undergoing THA3). They found that NSAIDs reduced morphine consumption and VAPS by 4 to 10 mm up to 32 hours postoperatively when compared with placebo3). The side-effects of NSIADs include gastrointestinal mucosal damage, renal dysfunction, and platelet dysfunction. Selective COX-2 inhibitors have minimal adverse gastrointestinal and hemostatic effects; consequently, these agents may be preferred in the perioperative setting. Several studies have shown that COX-2 inhibitors improve postoperative analgesia and reduce opioid consumption in patients undergoing THA29). Many COX-2 inhibitors have withdrawn from the market due to adverse cardiovascular effects. Celecoxib and meloxicam remain in use because the cardiovascular risk associated with these agents has been shown to be no higher than that associated with nonselective NSAIDs. However, widespread use of COX-2 inhibitiors still has been known to be associated to an increase of cardiovascular events, especially in elderly populations30). Recently, some authors have reported a cost-effectiveness and the safety of using a fixed-dose combination of NSAIDs and a proton pump inhibitor compared to COX-2 inhibitors31). Therefore, the use of NSAIDs in combination with a proton pump inhibitor could be an alternative treatment option in high risk of cardiovascular events. There are also concerns about inhibitory effects of NSAIDs and COX-2 inhibitors on bone healing because animal research showed that these agents may reduce new bone formation by inhibiting osteoblast and osteoclast function32), but the effect of small doses administration for short periods of time to human has yet to be determined conclusively.
3. Gabapentinoids
The gabapentinoids are effective postoperative analgesics that reduce opioid consumption by up to 50% compared with placebo33). Gabapentin and pregabalin are currently classified as a gabapentinoid. Pregabalin has been known to offer faster onset and more reliable, dose-dependent bioavailability than gabapentin due to improved absorption profile. The most common side effects of the gabapentinoids include somnolence and dizziness and can be minimized with dose reduction34). According to published data, the use of gabapentinoid alone for analgesia following major orthopedic surgery decreased opioid consumption but there were no differences in pain scores compared with placebo33). In addition to analgesia and reduced opioid consumption, gabapentinoids may confer other ancillary benefits throughout the early perioperative period.
ANESTHESIA AND NERVE BLOCK
1. Anesthesia
The most recent trend recommends the use of two or more analgesic modalities with different mechanisms of action that will provide analgesia while limiting side effects and adverse events to decrease pain in total joint arthroplasty35). It has been recommended that regional anesthesia offers significant advantages over general anesthesia with regard to intraoperative blood loss, deep vein thrombosis, and postoperative pain management11,35). Regional anesthesia during hip arthroplasty could be performed by spinal anesthesia, epidural anesthesia with or without indwelling catheters for 24 or 48 hours, intrathecal morphine, and epidural anesthesia in combination with spinal anesthesia or general anesthesia11,35),36,37).
Generally, single-shot spinal anesthesia is preferred typical method for hip arthroplasty11,35),38). Additionally, epidural anesthesia results in significantly less pain in perioperative period than other analgesic modalities for total joint arthroplasty. Compared with systemic analgesia, epidural analgesia results in excellent improvement for pain regulation and earlier mobilization, however higher rates of postoperative urinary retention, hypotension, and itchiness39,40). Uncorrected hypovolemia, increased intracranial pressure, infection or allergy to local anesthetic agent, and coagulopathy are absolute contraindications of regional anesthesia11,35,38,40). Furthermore, epidural hematoma, which is a rare but potentially serious complication of epidural analgesia, remains a concern among patients having hip arthroplasty who receive postoperative thromboprophylaxis38,39,40,41). When performing regional anesthesia, it should be considered that appropriate anesthesia level and safe analgesic dose with skillful procedure, to minimize complications related to anesthesia.
2. Nerve Block
Peripheral nerve blocks are effective adjuvant options for pain management for hip arthroplasty11,35,38,40,42).The use of nerve blocks has been proven to be very effective at controlling pain and minimizing narcotic requirements after hip arthroplasty11,43,44). Nerve block options include femoral block, sciatic block, posterior lumbar plexus block, fascia iliaca block, and periarticular local anesthesia infiltration41). Peripheral nerve block provides as good-quality analgesia as epidural anesthesia and it is superior to systemic opioids for pain relief38,43,45). Peripheral nerve blocks are associated with preserved contralateral limb strength that may facilitate postoperative rehabilitation when compared with the bilateral lower extremity sensorimotor block that can result with epidural analgesia38,45). Furthermore, peripheral nerve block has fewer complications, including hypotension and urinary retention with epidural anesthesia. The disadvantages of nerve blocks are increased time taking to place the blocks during perioperative period, and possibilities of injury associated motor blockade that limits functional recovery and delays rehabilitation44). Peripheral nerve blockade could be the assistant options regulating pain for postoperative analgesia after hip arthroplasty46,47). Both single-injection and continuous peripheral nerve block techniques are proven to reduce perioperative complications, reduce hospital length-of-stay, conserve hospital resources, and enhance patient satisfaction36,48,49,50,51).
PATIENT CONTROLLED ANALGESIA AND EPIDURAL INJECTION
Patient controlled analgesia (PCA) is a delivery system, based on the use of a sophisticated microprocessor-controlled infusion pump, with which patients self-administer small, predetermined dose of analgesic medication to relieve their pain. PCA was developed in the early 1980s52,53). Since its introduction, it has become widely used for the management of postoperative pain in major orthopedic surgery. Basic composition of PCA models include initial loading dose, bolus (demand) dose, lockout interval, and background infusion rate. The initial loading dose is titrated to achieve a minimal level of analgesia in the recovery room until the VAPS is ≤4. The bolus (demand) dose is the small amount of analgesia the patient receives each time. Optimal efficacy and safety of the analgesia depends on balance between the dose small enough to minimize adverse effect and the dose large enough to achieve analgesic satisfaction. The lockout period is defined as the length of time during which there will be no drug delivery. The lockout period is generally recommended between 5 and 10 minutes regardless of the opioid used. The background infusion rate is a constant rate of infusion administered to deliver the equivalent of the usual opioid dose, but it may cause respiratory depression.
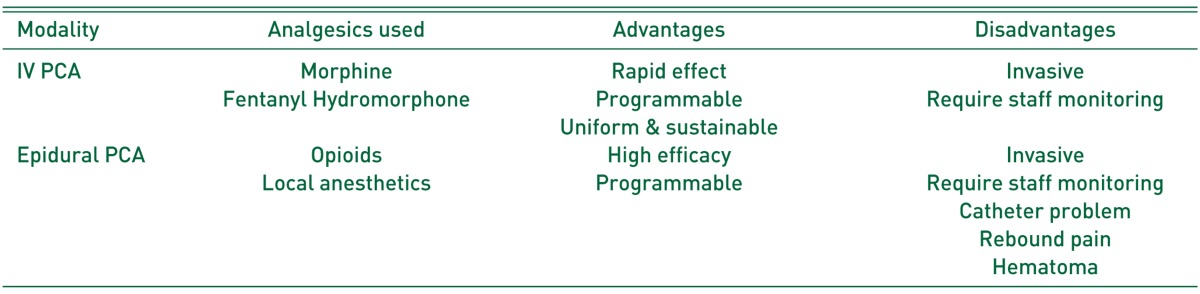
Baseline characteristics of common opioids for PCA are summarized in Table 1. The common adverse effects of PCA include nausea and vomiting, pruritus, respiratory depression, sedation and confusion, and urinary retention. In recent decades, alternative routes of PCA have extensively been developed, although the intravenous (IV) PCA is the most widely used method of PCA delivery. Variable modalities of PCA such as peripheral regional, intranasal, and transdermal PCA have been introduced54). The characteristics of IV and epidural PCA, which is the two major delivery methods of PCA, are summarized in Table 2.
Table 1. Common Opioids for Patient Controlled Analgesia.
Table 2. Characteristics of PCA Modalities.
PCA: patient controlled analgesia, IV: intravenous.
Epidural analgesia has been considered as one of the most effective methods for pain relief after surgery. Medical route of epidural blockage is directed to the epidural space of the spinal cord. Comparing to spinal block, a large dose of drug is typically necessary and the onset of analgesia is slower with epidural analgesia than with spinal anesthesia41). Contraindication of epidural block is uncorrected hypovolemia, increased intracranial pressure, coagulopathy, and prior spinal surgery. Adverse events include tachycardia, high blood pressure, light headache, metallic taste in mouth, ring in ears, and facial numbness.
PERIARTICULAR INJECTION
Intraoperative periarticular injection of multimodal drugs is one of the most important procedures in multimodal pain control protocol11,55,56). Injection using opioid and local anesthetics into injured or stretched nerves or tissues under the guidance of a surgeon can block axonal sodium channels and inhibit the conduction of pain messages. Wound infiltration with local anesthetic can act locally to reduce peripheral nociception with few systemic adverse effects38,56,57). Several authors reported its effectiveness on reducing postoperative pain and improving postoperative mobility after THA11,55,58,59,60,61). However, there is disagreement of the proper dosage and composition of injection drugs and techniques of injection and its efficacy on reduced opioid consumption is also unclear56,61). The most commonly used drugs for periarticular injections include local anesthetics (bupivacaine and ropivacaine), ketorolac, morphine, clonidine, and steroids (Table 3). These components can activate directly the mu-opioid receptor near the surgical site inhibiting the local inflammatory response and relieving the pain by preventing the production of pain transmitters61). Steroids are effective for lengthening the duration of action of the periarticular injection but care should be taken using in patients with high risk of infection, such as diabetes or immunocompromised patients11,56). Additional drugs can be used to prolong the effect (epinephrine) and to reduce the risk of infection (antibiotics). Authors also use periarticular injection as a part of multimodal pain control protocal since 200662). Components of authors' protocol are summarized in Table 4.
Table 3. Common Compositions and Sites of Intraoperative Periarticular Injection.
Table 4. Components of Periarthicular Injection in Authors' Protocol.
CONCLUSION
Although patient education and rehabilitation protocols are important in facilitating a patient's recovery after total joint arthroplasty, it cannot be overemphasized that the focus of rehabilitation protocol after joint replacement should be in controlling perioperative pain. Multimodal pain management has become an important part of the perioperative care of patients undergoing total joint arthroplasty. The principle of multimodal therapy is to use different techniques that target several different steps of the pain pathway, allowing more effective pain control with fewer side effects.
ACKNOWLEDGEMENTS
This clinical practice guideline was approved by Korean Hip Society on November 17, 2015. It is based on a systemic review of published articles on the management of perioperative pain control after total joint arthroplasty. This comprehensive review provided some suggestions of how orthopedic surgeons may serve as the ideal experts to deal with perioperative pain control after total joint arthroplasty. These suggestions will be revised regularly following further developments and innovations in this field.
Footnotes
CONFLICT OF INTEREST: The authors declare that there is no potential conflict of interest relevant to this article.
References
- 1.Cram P, Lu X, Kates SL, Singh JA, Li Y, Wolf BR. Total knee arthroplasty volume, utilization, and outcomes among Medicare beneficiaries, 1991-2010. JAMA. 2012;308:1227–1236. doi: 10.1001/2012.jama.11153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Larsen K, Hansen TB, Thomsen PB, Christiansen T, Soballe K. Cost-effectiveness of accelerated perioperative care and rehabilitation after total hip and knee arthroplasty. J Bone Joint Surg Am. 2009;91:761–772. doi: 10.2106/JBJS.G.01472. [DOI] [PubMed] [Google Scholar]
- 3.Fischer HB, Simanski CJ. A procedure-specific systematic review and consensus recommendations for analgesia after total hip replacement. Anaesthesia. 2005;60:1189–1202. doi: 10.1111/j.1365-2044.2005.04382.x. [DOI] [PubMed] [Google Scholar]
- 4.Buvanendran A, Kroin JS, Tuman KJ, et al. Effects of perioperative administration of a selective cyclooxygenase 2 inhibitor on pain management and recovery of function after knee replacement: a randomized controlled trial. JAMA. 2003;290:2411–2418. doi: 10.1001/jama.290.18.2411. [DOI] [PubMed] [Google Scholar]
- 5.Wall PD. The prevention of postoperative pain. Pain. 1988;33:289–290. doi: 10.1016/0304-3959(88)90286-2. [DOI] [PubMed] [Google Scholar]
- 6.Kehlet H, Dahl JB. The value of "multimodal" or "balanced analgesia" in postoperative pain treatment. Anesth Analg. 1993;77:1048–1056. doi: 10.1213/00000539-199311000-00030. [DOI] [PubMed] [Google Scholar]
- 7.Melzack R. From the gate to the neuromatrix. Pain. 1999;(Suppl 6):S121–S126. doi: 10.1016/S0304-3959(99)00145-1. [DOI] [PubMed] [Google Scholar]
- 8.Hall JE, Guyton AC. Guyton and hall textbook of medical physiology. 12th ed. Philadelphia, PA: Elsevier Saunders; 2011. pp. 563–564. [Google Scholar]
- 9.Traub RJ, Mendell LM. The spinal projection of individual identified A-delta- and C-fibers. J Neurophysiol. 1988;59:41–55. doi: 10.1152/jn.1988.59.1.41. [DOI] [PubMed] [Google Scholar]
- 10.Meyer RZ, Ringkamp M, Campbell JN, Raja SN. Peripheral mechanism of cutaneous nociception. In: McMahon SB, Koltzenburg M, Wall PD, Melzack R, editors. Wall and melzack's textbook of pain. 5th ed. Edinburgh, Scotland: Churchill Livingstone; 2006. p. 22. [Google Scholar]
- 11.Lundblad H, Kreicbergs A, Jansson KA. Prediction of persistent pain after total knee replacement for osteoarthritis. J Bone Joint Surg Br. 2008;90:166–171. doi: 10.1302/0301-620X.90B2.19640. [DOI] [PubMed] [Google Scholar]
- 12.Ranawat AS, Ranawat CS. Pain management and accelerated rehabilitation for total hip and total knee arthroplasty. J Arthroplasty. 2007;22(7 Suppl 3):12–15. doi: 10.1016/j.arth.2007.05.040. [DOI] [PubMed] [Google Scholar]
- 13.Louw A, Diener I, Butler DS, Puentedura EJ. Preoperative education addressing postoperative pain in total joint arthroplasty: review of content and educational delivery methods. Physiother Theory Pract. 2013;29:175–194. doi: 10.3109/09593985.2012.727527. [DOI] [PubMed] [Google Scholar]
- 14.Giraudet-Le Quintrec JS, Coste J, Vastel L, et al. Positive effect of patient education for hip surgery: a randomized trial. Clin Orthop Relat Res. 2003;(414):112–120. doi: 10.1097/01.blo.0000079268.91782.bc. [DOI] [PubMed] [Google Scholar]
- 15.Wright JG, Araki CT, Belkin M, Hobson RW., 2nd Postischemic hypothermia diminishes skeletal muscle reperfusion edema. J Surg Res. 1989;47:389–396. doi: 10.1016/0022-4804(89)90089-9. [DOI] [PubMed] [Google Scholar]
- 16.Ohkoshi Y, Ohkoshi M, Nagasaki S, Ono A, Hashimoto T, Yamane S. The effect of cryotherapy on intraarticular temperature and postoperative care after anterior cruciate ligament reconstruction. Am J Sports Med. 1999;27:357–362. doi: 10.1177/03635465990270031601. [DOI] [PubMed] [Google Scholar]
- 17.Ho SS, Coel MN, Kagawa R, Richardson AB. The effects of ice on blood flow and bone metabolism in knees. Am J Sports Med. 1994;22:537–540. doi: 10.1177/036354659402200417. [DOI] [PubMed] [Google Scholar]
- 18.McMaster WC, Liddle S. Cryotherapy influence on posttraumatic limb edema. Clin Orthop Relat Res. 1980;(150):283–287. [PubMed] [Google Scholar]
- 19.Gibbons CE, Solan MC, Ricketts DM, Patterson M. Cryotherapy compared with Robert Jones bandage after total knee replacement: a prospective randomized trial. Int Orthop. 2001;25:250–252. doi: 10.1007/s002640100227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levy AS, Marmar E. The role of cold compression dressings in the postoperative treatment of total knee arthroplasty. Clin Orthop Relat Res. 1993;(297):174–178. [PubMed] [Google Scholar]
- 21.Webb JM, Williams D, Ivory JP, Day S, Williamson DM. The use of cold compression dressings after total knee replacement: a randomized controlled trial. Orthopedics. 1998;21:59–61. doi: 10.3928/0147-7447-19980101-14. [DOI] [PubMed] [Google Scholar]
- 22.Kullenberg B, Ylipää S, Söderlund K, Resch S. Postoperative cryotherapy after total knee arthroplasty: a prospective study of 86 patients. J Arthroplasty. 2006;21:1175–1179. doi: 10.1016/j.arth.2006.02.159. [DOI] [PubMed] [Google Scholar]
- 23.Morsi E. Continuous-flow cold therapy after total knee arthroplasty. J Arthroplasty. 2002;17:718–722. doi: 10.1054/arth.2002.33562. [DOI] [PubMed] [Google Scholar]
- 24.Healy WL, Seidman J, Pfeifer BA, Brown DG. Cold compressive dressing after total knee arthroplasty. Clin Orthop Relat Res. 1994;(299):143–146. [PubMed] [Google Scholar]
- 25.Scarcella JB, Cohn BT. The effect of cold therapy on the postoperative course of total hip and knee arthroplasty patients. Am J Orthop (Belle Mead NJ) 1995;24:847–852. [PubMed] [Google Scholar]
- 26.Oshodi TO. The impact of preoperative education on postoperative pain. Part 1. Br J Nurs. 2007;16:706–710. doi: 10.12968/bjon.2007.16.12.23719. [DOI] [PubMed] [Google Scholar]
- 27.Guignard B, Bossard AE, Coste C, et al. Acute opioid tolerance: intraoperative remifentanil increases postoperative pain and morphine requirement. Anesthesiology. 2000;93:409–417. doi: 10.1097/00000542-200008000-00019. [DOI] [PubMed] [Google Scholar]
- 28.Elia N, Lysakowski C, Tramèr MR. Does multimodal analgesia with acetaminophen, nonsteroidal antiinflammatory drugs, or selective cyclooxygenase-2 inhibitors and patient-controlled analgesia morphine offer advantages over morphine alone? Meta-analyses of randomized trials. Anesthesiology. 2005;103:1296–1304. doi: 10.1097/00000542-200512000-00025. [DOI] [PubMed] [Google Scholar]
- 29.Toms L, McQuay HJ, Derry S, Moore RA. Single dose oral paracetamol (acetaminophen) for postoperative pain in adults. Cochrane Database Syst Rev. 2008;(4):CD004602. doi: 10.1002/14651858.CD004602.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kearney PM, Baigent C, Godwin J, Halls H, Emberson JR, Patrono C. Do selective cyclo-oxygenase-2 inhibitors and traditional non-steroidal anti-inflammatory drugs increase the risk of atherothrombosis? Meta-analysis of randomised trials. BMJ. 2006;332:1302–1308. doi: 10.1136/bmj.332.7553.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Capel M, Tornero J, Zamorano JL, et al. Efficiency of naproxen/esomeprazole in association for osteoarthrosis treatment in Spain. Reumatol Clin. 2014;10:210–217. doi: 10.1016/j.reuma.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 32.Malan TP, Jr, Marsh G, Hakki SI, Grossman E, Traylor L, Hubbard RC. Parecoxib sodium, a parenteral cyclooxygenase 2 selective inhibitor, improves morphine analgesia and is opioid-sparing following total hip arthroplasty. Anesthesiology. 2003;98:950–956. doi: 10.1097/00000542-200304000-00023. [DOI] [PubMed] [Google Scholar]
- 33.Harder AT, An YH. The mechanisms of the inhibitory effects of nonsteroidal anti-inflammatory drugs on bone healing: a concise review. J Clin Pharmacol. 2003;43:807–815. doi: 10.1177/0091270003256061. [DOI] [PubMed] [Google Scholar]
- 34.Mathiesen O, Jacobsen LS, Holm HE, et al. Pregabalin and dexamethasone for postoperative pain control: a randomized controlled study in hip arthroplasty. Br J Anaesth. 2008;101:535–541. doi: 10.1093/bja/aen215. [DOI] [PubMed] [Google Scholar]
- 35.American Society of Anesthesiologists Task Force on Acute Pain Management. Practice guidelines for acute pain management in the perioperative setting: an updated report by the American Society of Anesthesiologists Task Force on Acute Pain Management. Anesthesiology. 2012;116:248–273. doi: 10.1097/ALN.0b013e31823c1030. [DOI] [PubMed] [Google Scholar]
- 36.Indelli PF, Grant SA, Nielsen K, Vail TP. Regional anesthesia in hip surgery. Clin Orthop Relat Res. 2005;441:250–255. doi: 10.1097/01.blo.0000192355.71966.8e. [DOI] [PubMed] [Google Scholar]
- 37.Ilfeld BM, Ball ST, Gearen PF, et al. Ambulatory continuous posterior lumbar plexus nerve blocks after hip arthroplasty: a dual-center, randomized, triple-masked, placebo-controlled trial. Anesthesiology. 2008;109:491–501. doi: 10.1097/ALN.0b013e318182a4a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Viscusi ER, Parvizi J, Tarity TD. Developments in spinal and epidural anesthesia and nerve blocks for total joint arthroplasty: what is new and exciting in pain management. In: Marsh LJ, editor. Instructional course lectures. vol 56. Rosemont (IL): American Academy of Orthopaedic Surgeons; 2007. pp. 139–145. [PubMed] [Google Scholar]
- 39.Tang R, Evans H, Chaput A, Kim C. Multimodal analgesia for hip arthroplasty. Orthop Clin North Am. 2009;40:377–387. doi: 10.1016/j.ocl.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 40.Choi PT, Bhandari M, Scott J, Douketis J. Epidural analgesia for pain relief following hip or knee replacement. Cochrane Database Syst Rev. 2003;(3):CD003071. doi: 10.1002/14651858.CD003071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stowers MD, Lemanu DP, Coleman B, Hill AG, Munro JT. Review article: Perioperative care in enhanced recovery for total hip and knee arthroplasty. J Orthop Surg (Hong Kong) 2014;22:383–392. doi: 10.1177/230949901402200324. [DOI] [PubMed] [Google Scholar]
- 42.Johnson RL, Kopp SL. Optimizing perioperative management of total joint arthroplasty. Anesthesiol Clin. 2014;32:865–880. doi: 10.1016/j.anclin.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 43.Meding JB. Patient-controlled epidural analgesia after total hip arthroplasty: ready for prime time? J Bone Joint Surg Am. 2015;97:e46. doi: 10.2106/JBJS.O.00256. [DOI] [PubMed] [Google Scholar]
- 44.Tetsunaga T, Sato T, Shiota N, et al. Comparison of continuous epidural analgesia, patient-controlled analgesia with morphine, and continuous three-in-one femoral nerve block on postoperative outcomes after total hip arthroplasty. Clin Orthop Surg. 2015;7:164–170. doi: 10.4055/cios.2015.7.2.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pagnano MW, Hebl J, Horlocker T. Assuring a painless total hip arthroplasty: a multimodal approach emphasizing peripheral nerve blocks. J Arthroplasty. 2006;21(4 Suppl 1):80–84. doi: 10.1016/j.arth.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 46.Horlocker TT, Kopp SL, Pagnano MW, Hebl JR. Analgesia for total hip and knee arthroplasty: a multimodal pathway featuring peripheral nerve block. J Am Acad Orthop Surg. 2006;14:126–135. doi: 10.5435/00124635-200603000-00003. [DOI] [PubMed] [Google Scholar]
- 47.Hebl JR, Dilger JA, Byer DE, et al. A pre-emptive multimodal pathway featuring peripheral nerve block improves perioperative outcomes after major orthopedic surgery. Reg Anesth Pain Med. 2008;33:510–517. [PubMed] [Google Scholar]
- 48.Hebl JR, Kopp SL, Ali MH, et al. A comprehensive anesthesia protocol that emphasizes peripheral nerve blockade for total knee and total hip arthroplasty. J Bone Joint Surg Am. 2005;87(Suppl 2):63–70. doi: 10.2106/JBJS.E.00491. [DOI] [PubMed] [Google Scholar]
- 49.Biboulet P, Morau D, Aubas P, Bringuier-Branchereau S, Capdevila X. Postoperative analgesia after total-hip arthroplasty: Comparison of intravenous patient-controlled analgesia with morphine and single injection of femoral nerve or psoas compartment block. a prospective, randomized, double-blind study. Reg Anesth Pain Med. 2004;29:102–109. doi: 10.1016/j.rapm.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 50.Szczukowski MJ, Jr, Hines JA, Snell JA, Sisca TS. Femoral nerve block for total knee arthroplasty patients: a method to control postoperative pain. J Arthroplasty. 2004;19:720–725. doi: 10.1016/j.arth.2004.02.043. [DOI] [PubMed] [Google Scholar]
- 51.YaDeau JT, Cahill JB, Zawadsky MW, et al. The effects of femoral nerve blockade in conjunction with epidural analgesia after total knee arthroplasty. Anesth Analg. 2005;101:891–895. doi: 10.1213/01.ANE.0000159150.79908.21. table of contents. [DOI] [PubMed] [Google Scholar]
- 52.Momeni M, Crucitti M, De Kock M. Patient-controlled analgesia in the management of postoperative pain. Drugs. 2006;66:2321–2337. doi: 10.2165/00003495-200666180-00005. [DOI] [PubMed] [Google Scholar]
- 53.Bollish SJ, Collins CL, Kirking DM, Bartlett RH. Efficacy of patient-controlled versus conventional analgesia for postoperative pain. Clin Pharm. 1985;4:48–52. [PubMed] [Google Scholar]
- 54.Palmer PP, Miller RD. Current and developing methods of patient-controlled analgesia. Anesthesiol Clin. 2010;28:587–599. doi: 10.1016/j.anclin.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 55.Viscusi ER. Patient-controlled drug delivery for acute postoperative pain management: a review of current and emerging technologies. Reg Anesth Pain Med. 2008;33:146–158. doi: 10.1016/j.rapm.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 56.Parvataneni HK, Ranawat AS, Ranawat CS. The use of local periarticular injections in the management of postoperative pain after totalhip and knee replacement: a multimodal approach. Instr Course Lect. 2007;56:125–131. [PubMed] [Google Scholar]
- 57.Korean Knee Society. Guidelines for the management of postoperative pain after total knee arthroplasty. Knee Surg Relat Res. 2012;24:201–207. doi: 10.5792/ksrr.2012.24.4.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vendittoli PA, Makinen P, Drolet P, et al. A multimodal analgesia protocol for total knee arthroplasty. A randomized, controlled study. J Bone Joint Surg Am. 2006;88:282–289. doi: 10.2106/JBJS.E.00173. [DOI] [PubMed] [Google Scholar]
- 59.Parvataneni HK, Shah VP, Howard H, Cole N, Ranawat AS, Ranawat CS. Controlling pain after total hip and knee arthroplasty using a multimodal protocol with local periarticular injections: a prospective randomized study. J Arthroplasty. 2007;22(6 Suppl 2):33–38. doi: 10.1016/j.arth.2007.03.034. [DOI] [PubMed] [Google Scholar]
- 60.Bottros J, Klika AK, Milidonis MK, Toetz A, Fehribach A, Barsoum WK. A rapid recovery program after total hip arthroplasty. Curr Orthop Pract. 2010;21:381–384. [Google Scholar]
- 61.Wellman SS, Murphy AC, Gulcynski D, Murphy SB. Implementation of an accelerated mobilization protocol following primary total hip arthroplasty: impact on length of stay and disposition. Curr Rev Musculoskelet Med. 2011;4:84–90. doi: 10.1007/s12178-011-9091-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee KJ, Min BW, Bae KC, Cho CH, Kwon DH. Efficacy of multimodal pain control protocol in the setting of total hip arthroplasty. Clin Orthop Surg. 2009;1:155–160. doi: 10.4055/cios.2009.1.3.155. [DOI] [PMC free article] [PubMed] [Google Scholar]