Abstract
Decidualization denotes the transformation of endometrial stromal cells into specialized decidual cells. In pregnancy, decidual cells form a protective matrix around the implanting embryo, enabling coordinated trophoblast invasion and formation of a functional placenta. Continuous progesterone (P4) signaling renders decidual cells resistant to various environmental stressors, whereas withdrawal inevitably triggers tissue breakdown and menstruation or miscarriage. Here, we show that PLCL1, coding phospholipase C (PLC)-related catalytically inactive protein 1 (PRIP-1), is highly induced in response to P4 signaling in decidualizing human endometrial stromal cells (HESCs). Knockdown experiments in undifferentiated HESCs revealed that PRIP-1 maintains basal phosphoinositide 3-kinase/Protein kinase B activity, which in turn prevents illicit nuclear translocation of the transcription factor forkhead box protein O1 and induction of the apoptotic activator BIM. By contrast, loss of this scaffold protein did not compromise survival of decidual cells. PRIP-1 knockdown did also not interfere with the responsiveness of HESCs to deciduogenic cues, although the overall expression of differentiation markers, such as PRL, IGFBP1, and WNT4, was blunted. Finally, we show that PRIP-1 in decidual cells uncouples PLC activation from intracellular Ca2+ release by attenuating inositol 1,4,5-trisphosphate signaling. In summary, PRIP-1 is a multifaceted P4-inducible scaffold protein that gates the activity of major signal transduction pathways in the endometrium. It prevents apoptosis of proliferating stromal cells and contributes to the relative autonomy of decidual cells by silencing PLC signaling downstream of Gq protein-coupled receptors.
Decidualization of the endometrium is indispensible for pregnancy (1). The postovulatory surge in progesterone (P4) and rising cellular cAMP levels during the midluteal phase of the cycle initiate this transformational process (2, 3), characterized by the differentiation of stromal cells in the superficial endometrial layer into specialist decidual cells. Continuous P4 signaling is critical for maintaining and enhancing the decidual phenotype throughout pregnancy (4). In the absence of a viable pregnancy, falling P4 levels trigger proteolytic breakdown of the superficial endometrial layer, focal bleeding and menstruation or menstruation-like bleeding in the case of miscarriage (5–8). Aberrant decidualization has been implicated in a range of reproductive disorders including endometriosis (9, 10), infertility, and recurrent pregnancy loss (11–14).
Once the luminal endometrial epithelium is breached, migratory decidualizing stromal cells rapidly encapsulate the invading blastocyst (1, 15, 16). Emerging evidence indicates that decidual cells play a critical role in embryo biosensoring and selection (17–19). As pregnancy unfolds, decidual cells safeguard the conceptus against various stressors. For example, stress-induced signaling through c-Jun N-terminal kinase and p38 pathways is selectively inactivated upon decidualization of human endometrial stromal cells (HESCs) (20). Combined with the induction of various free radical scavengers, silencing of these stress-signaling pathways renders decidual cells extraordinarily resistant to oxidative cell death (21–24). Moreover, circadian oscillations within the endometrium are firmly disabled upon decidualization, further isolating the implanting blastocyst from the maternal environment (25).
This study investigates the role of phospholipase C (PLC)-related catalytically inactive protein 1 (PRIP-1) in decidualizing HESCs. PRIP-1, coded by PLCL1, is structurally homologous to members of the PLC family although it lacks catalytic activity (26, 27). Like PLC-enzymes, PRIP-1 contains a pleckstrin homology domain enabling binding of phosphatidylinositol 4,5-bisphosphate (PIP2) and other phosphoinositides. However, 2 key amino acid mutations within the catalytic domain of PRIP-1 ensure that it cannot convert PIP2 to inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (28). Consequently, PRIP-1 attenuates IP3-dependent Ca2+ release from the endoplasmic reticulum (ER) (29). PRIP-1 also acts as a scaffold protein capable of binding protein phosphatase (PP)1 and PP2A as well as Protein kinase B (AKT), a serine/threonine kinase that relays growth factor signaling downstream of phosphoinositide 3-kinase (PI3K) (30, 31). The forkhead box protein O1 (FOXO1) is a key decidual transcription factor downstream of the PI3K/AKT pathway (32–34). By binding to the P4 receptor (PGR) and other decidual transcription factors, FOXO1 drives the expression of several decidual marker genes, including PRL, IGFBP1, and WNT4 (34–36). However, FOXO1 is also important for cell fate decisions and up-regulates the proapototic B-cell lymphoma 2 family member BIM, coded by BCL2L11, upon withdrawal of P4 from decidualizing cultures, triggering cell death (33).
Gene deletions in mice highlighted the importance of both Prip-1 and its analog Prip-2 in reproduction. Double Prip-1/2-deficient mice display reduced litter sizes and exhibit prolonged intervals between litters. Furthermore, mutant female mice have smaller uteri at puberty, spend more time in estrous, and have higher gonadotrophin levels (37). PRIP-1 has also been identified as a P4-responsive gene in the human myometrium (38). Taken together, these findings suggest that PRIP proteins are not only essential for optimal regulation of the hypothalamic-pituitary-gonadal axis but may also play a role in modulating steroid hormone responses in target tissues, such as the uterus.
Here, we report that PRIP-1 is strongly induced in decidualizing HESCs in response to P4 signaling. We show that this scaffold protein not only modulates the activity of the PI3K/AKT/FOXO1 pathway in undifferentiated HESCs but also acts as a chelator of IP3 signaling in decidual cells.
Materials and Methods
Patient selection and endometrial sampling
The study was approved by the National Health Service National Research Ethics-Hammersmith and Queen Charlotte's and Chelsea Research Ethics Committee (1997/5065). Subjects were recruited from the Implantation Clinic, a dedicated research clinic at University Hospitals Coventry and Warwickshire National Health Service Trust. Written informed consent was obtained from all participants in accordance with the guidelines in The Declaration of Helsinki 2000. Samples were obtained using a Wallach Endocell sampler (Wallach), starting from the uterine fundus and moving downward to the internal cervical ostium. A total of 43 endometrial biopsies were processed for primary cultures in this study. The average age (±SD) of the participants was 35.9 ± 4.7 years. In addition, 98 biopsies were used to measure PRIP-1 expression in vivo at mRNA and protein level. All endometrial biopsies were timed between 6 and 10 days after the preovulatory LH surge. None of the subjects were on hormonal treatments for at least 3 months before the procedure.
Primary cell culture
HESCs were isolated from endometrial tissues as previously described (4). Purified HESCs were expanded in culture medium of DMEM/F-12 containing 10% dextran-coated charcoal-treated fetal bovine serum (DCC-FBS), L-glutamine (1%), 1% antibiotic-antimycotic solution, 2-μg/mL recombinant human insulin, and 1nM estradiol. Confluent monolayers were decidualized in DMEM/F-12 containing 2% DCC-FBS (with L-glutamine, excluding insulin and estradiol) with 0.5mM 8-bromo-cAMP (8-br-cAMP) (Sigma-Aldrich) alone or in combination with 1μM medroxyprogesterone acetate (MPA) to induce a decidual phenotype. Some cultures were also treated with dexamethasone (DEX) (0.1μM), dihydrotestosterone (DHT) (1μM), or P4 (1μM). To determine half-life time of PRIP-1 transcripts, actinomycin D (Sigma-Aldrich) was used at a final concentration of 2μM in dimethyl sulfoxide. All experiments were carried out before the third passage.
Transient transfection
Primary HESCs were transfected using a jetPRIME Polyplus transfection kit (VWR International). Undifferentiated HESCs were transiently transfected with 50nM PRIP-1-siGENOME SMARTpool or siGENOME nontargeting (NT) small interfering RNA (siRNA) Pool 1 (Dharmacon, GE Healthcare) for gene knockdown. Transfection studies were performed in triplicate and repeated on primary cultures from 3 or more different biopsies.
Immunohistochemistry and immunofluorescence
Paraffin-embedded, formalin-fixed endometrial specimens were immunostained for PRIP-1 using the Novolink polymer detection system (Leica) as per manufacturer's instructions. Universal LSAB Plus kits (DAKO) were used as described previously (39) using primary antibodies against PRIP-1 (1:750 dilution; Sigma-Aldrich). As a negative control, the primary antibody was omitted and replaced by the corresponding IgG isotype. For confocal microscopy, primary HESCs were cultured on glass chamber slides (Thermo Scientific) and transfected with either NT or PRIP-1 siRNA. HESCs were fixed in 4% paraformaldehyde for 10 minutes, permeabilized with 0.1% Triton X-100 in Tris-buffered saline with 0.05% Tween (TBS-T) for 30 minutes and blocked in 1% BSA in TBS-T for 1 hour. Endogenous proteins were stained with rabbit anti-FOXO1 (2880S, 1:100; Cell Signaling Technology), followed by antirabbit fluorescein isothiocyanate (F0205, 1:500; DAKO). Cells were mounted in Vectashield with 4′,6-diamidino-2-phenylindole (Vector Labs) and visualized under a Zeiss LSM 510 META confocal microscope.
Enzyme-linked immunosorbent assay
HESCs were decidualized and lysed in radioimmunoprecipitation buffer with protease inhibitors (cOmplete, Mini, EDTA-free; Roche). PRIP-1 levels in whole-cell lysates were determined using a quantitative sandwich ELISA (CusaBio) according to the manufacturer's protocol. The ELISA was validated by measuring PRIP-1 levels after knockdown and overexpression in cultured human myometrial cells. In spike and recovery experiments, recovered recombinant PRIP-1 within denatured samples was nonsignificant when compared with spiked concentrations (Supplemental Figure 1). Furthermore, inter- and intraassay data variance analysis revealed consistent coefficients of variance below 5%. Results were derived using a 4-parameter logistic regression analysis and normalized to total protein concentration as determined by Bio-Rad protein assay (Bio-Rad).
Real-time quantitative (qRT)-PCR
Total RNA was extracted from HESC cultures using RNA STAT-60 (AMS Biotechnology). Equal amounts of total RNA (1 μg) were treated with deoxyribonuclease and reverse transcribed using the QuantiTect Reverse Transcription kit (QIAGEN). Resulting cDNA was used as a template in qRT-PCR analysis. Detection of gene expression was performed with Power SYBR Green Master Mix (Life Technologies) using the 7500 Real Time PCR System (Applied Biosystems). The expression levels of the samples were calculated using the ΔΔCT method, incorporating the efficiencies of each primer pair. Reaction specificity was confirmed by dissociation curve analysis. L19 was used as a reference gene for normalization. All measurements were performed in triplicate. Primer sequences used were as follows: IGFBP1 forward, 5′-cga agg ctc tcc atg tca cca-3′ and IGFBP1 reverse, 5′-tgt ctc ctg cct tgg cta aac-3′; L19 forward, 5′-gcg gaa ggg tac agc caa-3′ and L19 reverse, 5′-gca gcc ggg cgc aaa-3′; PRIP-1 forward, 5′-gca gca gca tca tca agg-3′ and PRIP-1 reverse, 5′-gct gct gaa aga cac ggt tt-3′; PRL forward, 5′-aag ctg tag aga ttg agg agc aaa c-3′ and PRL reverse, 5′-tca gga tga acc tgg ctg act a-3′; and WNT4 forward, 5′- gca gag ccc tca tga acc t-3′ and WNT4 reverse, 5′-cac cgc atg tgt gtc ag-3′.
Western blot analysis and proteome array
Whole-cell protein extracts were prepared by lysing cells in radioimmunoprecipitation buffer containing protease inhibitors (cOmplete, Mini, EDTA-free; Roche). For nuclear and cytoplasmic protein fractionation, cells were lysed in Buffer A (10mM HEPES, 10mM KCl, and 0.1mM EDTA), centrifuged at 100g, and the supernatant containing the cytoplasmic fraction collected. The remaining pellet was then lysed on ice in high salt buffer (5mM HEPES, 1.5mM MgCl2, 0.2mM EDTA, and 300mM NaCl) for 10 minutes, centrifuged, and the supernatant, containing the nuclear fraction, retained. Protein yield was measured using the Bradford assay. Equal amounts of protein were separated by SDS-PAGE and wet transfer onto polyvinylidene difluoride (PVDF) membrane (GE Healthcare UK). Nonspecific binding sites were blocked by incubation with 5% nonfat dry milk in TBS-T (130 mmol/L NaCl, 20 mmol Tris [pH 7.6], and 0.05% Tween). The primary antibodies used are tabulated in Table 1. Protein complexes were visualized with ECL Plus chemiluminescence (GE Healthcare). Relative phosphorylation of 26 phospho-kinases was determined by Proteome Profiler Human Phospho-MAPK array kit (R&D Systems). The array was performed according to manufacturer's specifications using 250-μg total protein lysates. Blots were visualized after exposure to chemiluminescent reagents and densitometry performed with individual phospho-proteins expressed as a percentage of reference dots.
Table 1.
Antibody Table
| Protein Target | Manufacturer | Dilution |
|---|---|---|
| PRIP-1 | Sigma-Aldrich (HPA031849) | WB 1:500 immunohistochemistry 1:750 |
| AKT | Cell Signaling Technology (4691) | WB 1:1000 |
| p-AKT (Ser473) | Cell Signaling Technology (4060) | WB 1:1000 |
| FOXO1A | Cell Signaling Technology (2880) | WB 1:1000 |
| BIM | Cell Signaling Technology (2933) | WB 1:1000 |
| VINCULIN | Abcam (ab18058) | WB 1:1000 |
| LAMIN A/C | Santa Cruz Biotechnology, Inc (sc-7292) | WB 1:500 |
| β-ACTIN | Abcam (ab8226) | WB 1:10 000 |
Confocal imaging of intracellular Ca2+ ([Ca2+]i) oscillations
Decidualizing HESCs were washed in modified Krebs'-Henseleit buffer (133 mmol/L NaCl, 4.7 mmol/L KCl, 11.1 mmol/L glucose, 1.2 mmol/L MgSO4, 1.2 mmol/L KH2PO4, 2.5 mmol/L CaCl, and 10 mmol/L TES; pH 7.4 at 37°C) and loaded with 5μM Fluo-4-AM for 1 hour at room temperature. Cells were washed and incubated in 2-mL Krebs'-Henseleit buffer. The glass-bottomed Petri dish containing Fluo-4-loaded cells was secured in a spring-loaded holder in a temperature-controlled environmental chamber on the stage of an inverted microscope (Axiovert 200M) equipped with an LSM 510 META confocal scanner (Carl Zeiss). Cells were excited with a krypton/argon laser at 488 nm and emitted light collected above 510 nm through a ×40 oil immersion objective lens. Decidualizing cells transfected with PRIP-1 or NT siRNA were challenged with 5μM m-3M3FBS or vehicle by direct addition into the cell-containing Petri dish. Image sequences of Fluo-4 fluorescence were recorded for 10 minutes at a rate of approximately one frame per second and used as an indication of changes in [Ca2+]i. Videos were analyzed using LSM image analysis software. Averaged fluorescence intensity was measured in regions of interest placed over individual cells and expressed as a fold increase from time 0 (F/F0). Data traces were plotted and peak response, integral (area under the curve; baseline y = 1) and oscillatory frequency (Hz) were measured.
Viability and proliferation assays
The number of viable cells was assessed by trypan blue exclusion. Cells were counted on the Luna cell counter (Logosbio), and percentage viability calculated. Apoptosis was assessed by measurement of the activities of caspase 3/7 using Apo-One Homogenous kit (Promega) according to manufacturer's guidelines. Cleavage of a nonfluorescent substrate by caspase 3/7 resulted in fluorescence, measured at 530-nm emission and 490-nm excitation on the PHERAStar FS microplate reader (BMG Labtect). Real-time adherent cell proliferation was determined by the label-free xCELLigence Real-Time Cell Analyzer (RTCA) DP instrument (Roche Diagnostics GmbH). HESCs were seeded into 16-well plates (E-plate-16; Roche Diagnostics GmbH) at a density of 10 000 cells per well and cultured in 10% DCC-FBS until reaching approximately 80% confluency. The RTCA DP instrument was placed at 37°C in a humidified environment with 95% air and 5% CO2. Individual wells within the E-plate-16 were referenced immediately and measured first every 15 minutes for 3 hours and then hourly for 100 hours. Changes in cell index were analyzed using RTCA Software v1.2.
Statistical analysis
Data were analyzed with the statistical package GraphPad Prism v6 (GraphPad Software, Inc). Unpaired Student's t test, Mann-Whitney U test and Pearson's correlation were used when appropriate. Statistical significance was assumed at P < .05. In the actinomycin D experiments, PRIP-1 mRNA half-life was calculated using t1/2 = 0.693/k, where k is the slope derived from a linear equation lnC = lnC0 − kt, and where C is the relative level of PRIP-1 mRNA in HESCs (40).
Results
Induction of PRIP-1 in response to P4 signaling
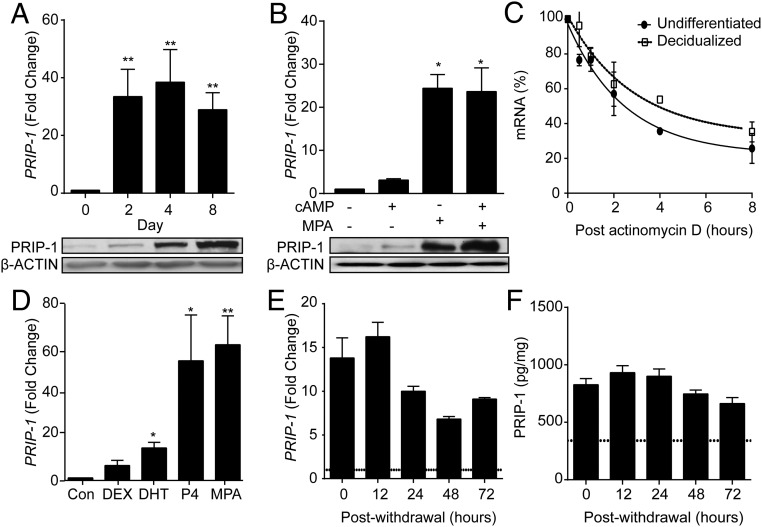
PRIP-1 mRNA and protein levels were measured in undifferentiated HESCs and cells decidualized for 2, 4, or 8 days (Figure 1A). Decidualization was associated with marked up-regulation of PRIP-1 transcripts with levels rising more than 30-fold within 48 hours of differentiation. The induction of PRIP-1 mRNA was then maintained over the 8-day time course. By contrast, induction of PRIP-1 protein was more gradual and expression peaked on day 8 of decidualization (Figure 1A, lower panel). To define the signaling pathway that drives PRIP-1 in differentiating HESCs, 3 independent primary cultures were treated with either 8-br-cAMP, MPA, or a combination (Figure 1B). Although 8-br-cAMP up-regulated PRIP-1 mRNA levels modestly (∼3-fold) after 4 days of treatment, MPA triggered a robust induction (∼25-fold). Combined treatment did not yield an additive effect (Figure 1B, upper panel), indicating that induction of PRIP-1 is primarily dependent on P4 signaling. Regulation at protein level was somewhat divergent as 8-br-cAMP augmented the induction of PRIP-1 in response to MPA treatment (Figure 1B, lower panel). We speculated that the rise in PRIP-1 transcripts in decidualizing cells could, at least partly, reflect increased RNA stability. To test this hypothesis, undifferentiated and decidualized HESCs were treated with actinomycin D, a potent transcription inhibitor, for 0.5, 1, 2, 4, or 8 hours. The half-life of PRIP-1 mRNA in decidualizing cells was nonsignificantly higher when compared with undifferentiated cells (4.9 vs 3.6 h, respectively; P > .05), indicating that the rise in PRIP-1 is primarily accounted for by P4-dependent transcription. MPA is not only a potent activator of the PGR but also exhibits glucocorticoid- and androgen-like activities in HESCs (41). To determine whether PRIP-1 is indeed a P4-responsive gene, primary HESC cultures were treated either with DEX, DHT, P4, or MPA. As show in Figure 1D, the induction of PRIP-1 transcripts in cultures treated with P4 was comparable with the response with MPA. Notably, DHT but not DEX triggered a small but significant increase in PRIP-1 expression (P < .05).
Figure 1. P4 regulates PRIP-1 expression in HESCs.
A, PRIP-1 transcript and protein levels were measured in undifferentiated HESCs and cells decidualized with 8-br-cAMP and MPA for 2, 4, or 8 days. The upper panel shows fold induction of PRIP-1 mRNA (mean ± SEM) in 3 independent primary cultures relative to expression in undifferentiated cells. Total protein lysates from parallel cultures were subjected to Western blotting (lower panel). β-Actin served as a loading control. B, Primary HESC cultures were treated with either 8-br-cAMP, MPA, or a combination for 96 hours. The upper panel shows the relative increase in PRIP-1 mRNA levels (mean ± SEM) compared with vehicle-treated undifferentiated HESC cultures established from 3 biopsies. The lower panel shows the corresponding protein levels, determined by Western blotting. β-Actin served as a loading control. C, Primary cultures (n = 3) remained undifferentiated or were decidualized for 96 hours before treatment with 2μM actinomycin D. RNA was extracted at the indicated time points and subjected to qRT-PCR analysis. D, Primary HESCs isolated from 3 different biopsies were treated with DEX, DHT, P4, MPA, or vehicle (control). Total RNA was harvested 96 hours later and subjected to qRT-PCR analysis. The data show induction of PRIP-1 transcripts (mean ± SEM) relative to control cells. E, PRIP-1 transcripts were measured after withdrawal of MPA for the indicated time points in 3 separate cultures first decidualized with 8-br-cAMP and MPA for 96 hours. PRIP-1 mRNA levels were normalized to the level of expression in undifferentiated cells (dotted line). F, Total protein lysates were harvested from parallel cultures PRIP-1 protein levels determined by ELISA; *, P < .05; **, P < .01.
Finally, we investigated the expression of PRIP-1 in decidualizing cells upon withdrawal of deciduogenic signals. Parallel cultures were differentiated with 8-br-cAMP and MPA for 4 days. The decidualization stimulus was then withdrawn for 12, 24, 48, or 72 hours and cultures harvested for mRNA and protein analyses. Interestingly, PRIP-1 transcript levels fell by 50% within 24 hours after withdrawal of 8-br-CAMP and MPA but then remained stable over the remainder of the time course (Figure 1E). By contrast, PRIP-1 protein levels, measured by ELISA, declined only modestly (20%) 72 hours after withdrawal of 8-br-cAMP and MPA (Figure 1F). Taken together, the data reveal a significant lag period between the rapid induction of PRIP-1 transcript levels in response to progestin signaling and the gradual rise in protein levels. Once induced, PRIP-1 expression is stable and relatively resistant to P4 withdrawal.
PRIP-1 expression in luteal phase endometrium
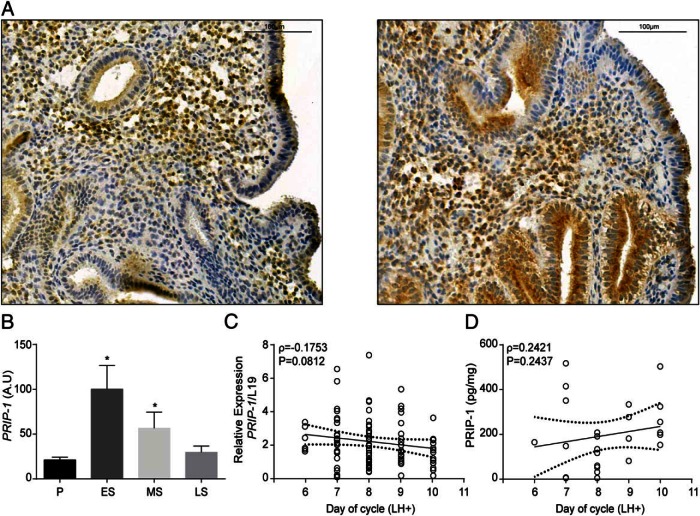
Immunohistochemical analysis of midluteal endometrial biopsies revealed that PRIP-1 is widely expressed in both the epithelial and stromal compartments (Figure 2A). PRIP-1 immunoreactivity, however, was heterogeneous, especially in the stromal compartment with some cells staining intensely, whereas others showed little expression (Figure 2A). Mining of the GEO database (GDS2052) revealed that endometrial PRIP-1 mRNA levels increase markedly during the early-luteal phase, presumably in response to the rapid rise in postovulatory P4 levels. Transcript levels then decline and by the late luteal phase are comparable with proliferative phase endometrium (Figure 2B). Detailed analysis of timed endometrial biopsies (LH+6 to LH+10) indicated that PRIP-1 expression is relatively stable over the periimplantation window. Notably, although transcript levels appeared to decline as the cycle progresses to the late luteal phase (Spearman's ρ = −0.1753, P = .0812) (Figure 2C), PRIP-1 protein levels, measured by ELISA, remained stable (Spearman's ρ = 0.2421, P = .2437) (Figure 2D).
Figure 2. PRIP-1 expression in midluteal endometrium.
A, Immunohistochemistry of midluteal endometrial biopsies demonstrates heterogeneous expression of PRIP-1 in both stromal and epithelial cells. B, Comparison of endometrial PRIP-1 transcripts, expressed in arbitrary units (A.U.), in proliferative, early-, mid-, and late-secretory endometrium. The data were derived from in silico analysis of publicly available microarray data (GEO Profiles; ID, GDS2052); *, P < .05. C, PRIP-1 transcript levels were measured by qRT-PCR in 73 endometrial biopsies obtained between 6 and 10 days after the LH surge (LH+). D, PRIP-1 protein levels were measured, in triplicate, in 25 whole endometrial samples by ELISA. Dotted lines represent 95% confidence intervals.
Survival function of PRIP-1 in undifferentiated HESCs
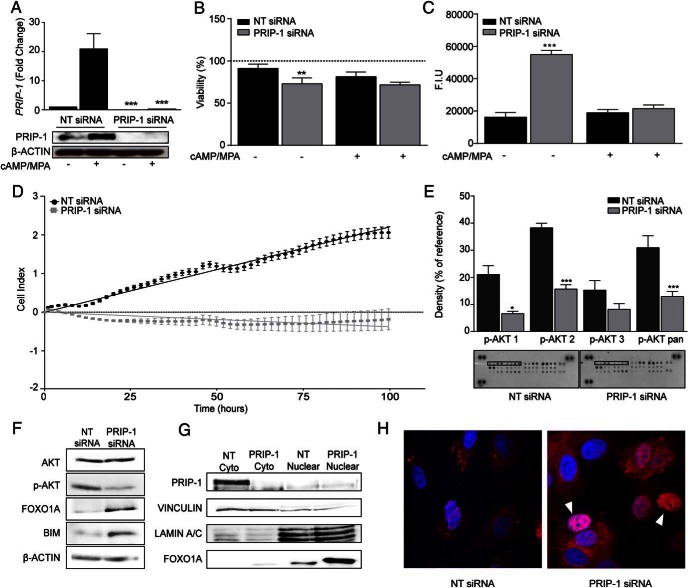
siRNA-mediated knockdown experiments were performed to examine the function of PRIP-1 in HESCs. Knockdown of this scaffold protein was highly effective; it not only abolished the induction of PRIP-1 in cells decidualized for 4 days but also lowered basal expression in undifferentiated HESCs (Figure 3A). A trypan blue exclusion assay revealed a 28% reduction in numbers of live cells in undifferentiated HESCs transfected with PRIP-1 siRNA (Figure 3B). In parallel, caspase-3 and caspase-7 activities increased by more than 3-fold (Figure 3C). Notably, loss of PRIP-1 did not compromise the viability of differentiating cells treated with MPA and 8-br-cAMP (Figure 3, B and C). Real-time monitoring of cell proliferation over 100 hours using microelectronic sensor technology (xCELLigence) confirmed that PRIP-1 knockdown completely abolished expansion of undifferentiated HESCs in culture (Figure 3D). To determine how PRIP-1 exerts its survival function, total protein lysates from HESCs first transfected with NT or PRIP-1 siRNA were hybridized to a proteome array. Analysis of the relative phosphorylation levels of 26 kinases revealed that PRIP-1 knockdown selectively inhibits AKT activation (Figure 3E). Phosphorylation levels of AKT1 (S473), AKT2 (S474), AKT3 (S472), and pan-AKT (S473, S474, and S472) were reduced by 69%, 59%, 46%, and 58%, respectively. We speculated that loss of AKT activity upon PRIP-1 knockdown would attenuate FOXO1 turnover by promoting nuclear translocation of this transcription factor (42). Western blot analysis of total protein lysates provided support for this conjecture and further revealed that the increase in FOXO1 levels in undifferentiated HESCs transfected with PRIP-1 coincided with induction of BIM, a well-characterized proapoptotic FOXO1 target (Figure 3F) (43). Analysis of fractionated cytoplasmic and nuclear proteins confirmed that PRIP-1 knockdown increases nuclear FOXO1 levels (Figure 3G). However, confocal analysis revealed cellular heterogeneity in this response with some stromal cells displaying intense nuclear FOXO1 staining, whereas other cells did not (Figure 3H).
Figure 3. PRIP-1 is a survival factor in undifferentiated HESCs.
A, Three independent primary cultures were transfected with either NT or PRIP-1 siRNA. After 48 hours, some cultures were decidualized for 96 hours, whereas others remained untreated. PRIP-1 mRNA and protein levels were measured in parallel cultures by qRT-PCR (upper panel) and Western blotting (lower panel), respectively. Transcript levels were normalized to expression in undifferentiated cells transfected with NT siRNA; ***, P < .001. B, Cell viability as measured by trypan blue exclusion assay in 3 independent primary cultures first transfected with either NT or PRIP-1 siRNA. After transfection, the cultures remained either untreated or were decidualized for 96 hours. Data normalized to untransfected control (dotted line); **, P < .01. C, In parallel experiments, caspase 3/7 activity was measured and expressed in fluorescent intensity units (F.I.U.). The data represent mean (±SEM) activity in 3 independent cultures; **, P < .01 and ***, P < .001. D, Real-time monitoring of the growth of undifferentiated HESCs over 100 hours after transfection with NT or PRIP-1 siRNA. E, Protein lysates from undifferentiated HESC transfected with either NT or PRIP-1 siRNA were subjected to proteome profiler MAPK array membranes and analyzed by densitometry; *, P < .05 and ***, P < .01. F, Western blot analysis of total protein lysates from undifferentiated HESCs 48 hours after transfection with either NT or PRIP-1 siRNA. G, Nuclear accumulation of FOXO1 was confirmed by Western blot analysis of cytoplasmic and nuclear cell fractions. VINCULIN and LAMIN A/C confirmed enrichment of the cytoplasmic and nuclear proteins, respectively. H, Confocal microscopy showing FOXO1 immunoreactivity in primary HESCs transfected with either NT or PRIP-1 siRNA. Arrowheads indicate cells characterized by marked nuclear FOXO1 accumulation.
Expression of decidual markers in response to PRIP-1 knockdown
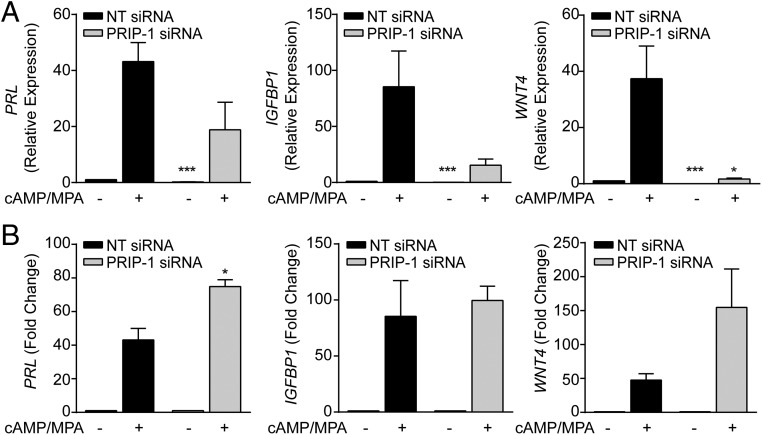
We next examined whether PRIP-1 is important for the expression of decidual marker genes. When compared with cell transfected NT siRNA, knockdown of PRIP-1 before decidualization reduced the overall levels of PRL, IGFBP1, and WNT4 transcripts after 4 days of treatment with 8-br-cAMP and MPA. However, only the reduction in WNT4 mRNA was statistically significant (P < .05). Furthermore, basal expression of these 3 genes in undifferentiated cells was significantly lower (Figure 4A). Consequently, the overall responsiveness to deciduogenic signals, determined by fold induction, was unaffected upon PRIP-1 knockdown; and in case of PRL even significantly enhanced (Figure 4B).
Figure 4. PRIP-1 blunts the overall expression of differentiation markers in decidualizing HESCs.
A, Three independent primary HESCs were first transfected with NT or PRIP-1 siRNA. The cultures then remained untreated or were decidualized with 8-br-cAMP and MPA. The data shows relative expression (mean ± SEM) of decidual marker genes. B, The same data expressed as fold induction in transcript level relative to the basal levels in undifferentiated cells transfected with NT or PRIP-1 siRNA; *, P < .05 and ***, P < .01.
PRIP-1 inhibits PLC-dependent Ca2+ signaling
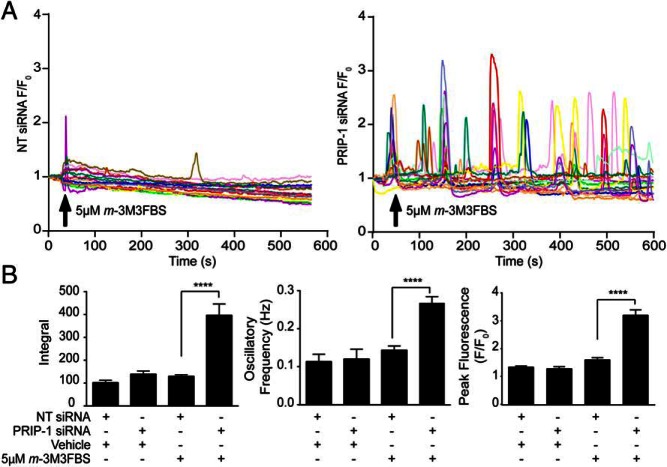
We hypothesized that induction of PRIP-1 in decidualizing cells could serve to silence PLC-dependent Ca2+ signaling through PIP2/IP3 chelation. To test this conjecture, primary HESCs were transfected with either NT or PRIP-1 siRNA before decidualization for 4 days. The cultures were then loaded with the fluorescent Ca2+ indicator Fluo-4 and challenged with the PLC activator m-3M3FBS or vehicle (dimethyl sulfoxide). Decidualizing cultures transfected with NT siRNA displayed minimal [Ca2+]i oscillations in response to PLC activation. By contrast, cells transfected with PRIP-1 siRNA exhibited robust and sustained [Ca2+]i fluxes over the entire recording (Figure 5A). Analysis of 4 independent cultures revealed PRIP-1 knockdown resulted in 2-fold increase in oscillation frequency, 2-fold increase in peak fluorescence, and 3-fold increase in the area under the curve (Figure 5B) in response to PLC activation. These results support the conjecture that the sustained induction of PRIP-1 in decidualizing HESCs acts to sequester phosphoinositides and to limit [Ca2+]i release.
Figure 5. PRIP-1 blocks PLC-dependent Ca2+ signaling in decidual cells.
A, HESCs cultured in glass bottomed Petri dishes were transfected with NT (left panel) or PRIP-1 (right panel) siRNA and decidualized for 4 days. Cells were then loaded with 5μM Fluo-4-AM and challenged with 5μM m-3M3FBS or vehicle (data not shown) at the indicated time point. Cytosolic fluorescence recorded by confocal microscopy over 10 minutes was used as an index of [Ca2+]i. Traces showing fluorescence within individual cells, transfected either with NT (left panel) or PRIP-1 siRNA (right panel) are expressed as a fold increase over fluorescence at time 0 (F/F0). Data were obtained from 4 independent cultures. B, Traces were analyzed to assess the peak changes in fluorescence, the integral, and oscillation frequency (Hz). Data show mean ± SEM; ****, P < .0001.
Discussion
By activating its cognate nuclear receptor, P4 controls the expression of numerous genes that encode membrane-bound receptors and intermediates in various signal transduction pathways in differentiating HESCs (1, 44). Consequently, P4 transforms the processing of cellular signals and environmental cues upon decidualization. For example, knockdown of PGR is sufficient to inhibit activation of the WNT/β-catenin, TGFβ/SMAD, and signal transducer and activator of transcription pathways in decidualizing cells (44). P4 also induces MAPK phosphatase 1 (DUSP1) (45), which in turn disables c-Jun N-terminal kinase and p38 stress-responsive pathways in decidualizing HESCs (46). We now report that P4 strongly up-regulates PRIP-1 expression in HESCs. Once induced, this scaffold protein accumulates in decidualizing HESCs, and levels remain relatively stable even upon withdrawal of progestins.
Unexpectedly, knockdown experiments demonstrated that basal PRIP-1 levels are critical for survival of undifferentiated HESCs. This antiapoptotic function of PRIP-1 is predicated on its ability to regulate the activity of AKT, either by facilitating interaction with upstream kinases or, conversely, by sequestering AKT phosphatases such as PP2A (31). AKT-dependent phosphorylation of FOXO1 leads to its nuclear export, ubiquitination mediated by E3 ligases such as S-phase kinase-associated protein 2 and mouse double minute 2 homolog, and proteasomal degradation (47, 48). PRIP-1 deficiency in HESCs selectively reduced AKT activity by more than 50%, which was sufficient to increase nuclear FOXO1 levels and activate the proapoptotic machinery. Notably, loss of cell viability upon PRIP-1 knockdown was only partial. It is increasingly appreciated that primary HESC cultures consist of distinct communities of cells, including clonogenic mesemchymal, mature progeny, perivascular SUSD2-positive cells, and senescent fibroblasts (11, 51). Hence, it is not inconceivable that some but not all subpopulations of HESCs are dependent on PRIP-1 for survival, although this conjecture requires further testing.
PRIP-1 knockdown did not interfere with the responsiveness of the HESCs to deciduogenic cues. This is not surprising as FOXO1 accumulates in the nuclei of differentiating HESCs where it binds other decidual transcription factors, including PGR, homeobox A11, and CCAAT/Enhancer binding protein beta resulting in formation of transcriptional complexes that activate differentiation genes, including WNT4, PRL, and IGFBP1 (32, 34, 35, 52). Unexpectedly, basal expression levels of WNT4, PRL, and IGFBP1 were markedly lower in undifferentiated HESCs transfected with PRIP-1 siRNA. A parsimonious explanation for this intriguing observation is that loss of PRIP-1 triggers apoptosis in a subpopulation of HESCs, characterized by relatively high basal expression of decidual markers. Notably, knockdown of FOXO1 in decidualizing HESC cultures completely eliminates apoptosis upon progestin withdrawal (33, 42), indicating that the ability of FOXO1 to switch between apoptosis and differentiation targets is dependent on P4 signaling.
Up-regulation of PRIP-1 in decidual cells may primarily serve to silence PLC signaling downstream of Gq protein-coupled receptors. Upon implantation, decidual cells form a nutritive matrix for trophoblast invasion that is relatively autonomous and resistant to potentially harmful maternal inputs. Various mechanisms underpinning decidual resistance have been described, including silencing of circadian oscillations (25), massive increase in cellular ROS-scavenging potential (21, 23), global cellular hypo-SUMOylation (3, 22), and the aforementioned inhibition of stress-responsive pathways (20, 22). These adaptations ensure unimpeded P4 signaling even under conditions of intense tissue remodeling and changing oxygen tension at the feto-maternal interface (22). The trophic function of decidual cells depends on acquisition of a secretory phenotype, which in turn requires rapid expansion of the ER in differentiating HESCs. Ergo, differentiating HESCs mount a physiological unfolded protein response characterized by up-regulation of various molecular chaperones, including protein disulfide isomerase, binding immunoglobulin protein, endoplasmic oxidoreductin-1α, and calnexin (17). We now show that, by chelating IP3, PRIP-1 limits Ca2+ release from the expanding ER, which arguably safeguards decidual cells against excessive Ca2+ accumulation in the mitochondrial matrix, permeabilization of the mitochondrial outer membrane and subsequent apoptosis (53). Interestingly, PRIP-1 also inhibits autophagosome formation by binding to microtubule-associated protein 1 light chain 3, a key initiator of the autophagy pathway (54). The importance of these pathways in the endometrium is underscored by recent observations demonstrating that ER stress and autophagy in decidual cells mediate recognition and rejection of developmentally compromised human embryos (17). Arguably, silencing PLC signaling downstream of Gq protein-coupled receptors may also enhance the biosensoring function of decidual cells by restricting signaling to discrete embryonic cues.
In conclusion, PRIP-1 is a versatile P4-inducible scaffold protein in the endometrium. Our data suggest that the function of PRIP-1 switches from amplifying AKT activity in proliferating HESCs to inhibiting PLC signaling downstream of Gq protein-coupled receptors in decidual cells. The role of PRIP-1 in regulating the responses of decidual cells to embryonic and trophoblast signals warrants further investigation.
Acknowledgments
This work was supported by the Biomedical Research Unit in Reproductive Health, a joint initiative between the University Hospitals Coventry and Warwickshire National Health Service Trust and Warwick Medical School.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- 8-br-cAMP
- 8-bromo-cAMP
- [Ca2+]i
- intracellular Ca2+
- Akt
- Protein kinase B
- DCC-FBS
- dextran-coated charcoal-treated fetal bovine serum
- DEX
- dexamethasone
- DHT
- dihydrotestosterone
- ER
- endoplasmic reticulum
- FOXO1
- forkhead box protein O1
- HESC
- human endometrial stromal cell
- IP3
- inositol 1,4,5-trisphosphate
- MPA
- medroxyprogesterone acetate
- NT
- nontargeting
- P4
- progesterone
- PGR
- P4 receptor
- PI3K
- phosphoinositide 3-kinase
- PIP2
- phosphatidylinositol 4,5-bisphosphate
- PLC
- phospholipase C
- PP
- protein phosphatase
- PRIP-1
- PLC-related catalytically inactive protein 1
- qRT
- real-time quantitative
- RTCA
- Real-Time Cell Analyzer
- siRNA
- small interfering RNA
- TBS-T
- Tris-buffered saline with 0.05% Tween.
References
- 1. Gellersen B, Brosens JJ. Cyclic decidualization of the human endometrium in reproductive health and failure. Endocr Rev. 2014;35:851–905. [DOI] [PubMed] [Google Scholar]
- 2. Bernardini L, Moretti-Rojas I, Brush M, Rojas FJ, Balmaceda JP. Changes in expression of adenyl cyclase activity in human endometrium during hormone replacement therapy and ovarian stimulation. Mol Hum Reprod. 1999;5:955–960. [DOI] [PubMed] [Google Scholar]
- 3. Jones MC, Fusi L, Higham JH, et al. Regulation of the SUMO pathway sensitizes differentiating human endometrial stromal cells to progesterone. Proc Natl Acad Sci USA. 2006;103:16272–16277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brosens JJ, Hayashi N, White JO. Progesterone receptor regulates decidual prolactin expression in differentiating human endometrial stromal cells. Endocrinology. 1999;140:4809–4820. [DOI] [PubMed] [Google Scholar]
- 5. Evans J, Salamonsen LA. Inflammation, leukocytes and menstruation. Rev Endocr Metab Disord. 2012;13:277–288. [DOI] [PubMed] [Google Scholar]
- 6. Evans J, Salamonsen LA. Decidualized human endometrial stromal cells are sensors of hormone withdrawal in the menstrual inflammatory cascade. Biol Reprod. 2014;90:14. [DOI] [PubMed] [Google Scholar]
- 7. Henriet P, Gaide Chevronnay HP, Marbaix E. The endocrine and paracrine control of menstruation. Mol Cell Endocrinol. 2012;358:197–207. [DOI] [PubMed] [Google Scholar]
- 8. Jabbour HN, Kelly RW, Fraser HM, Critchley HO. Endocrine regulation of menstruation. Endocr Rev. 2006;27:17–46. [DOI] [PubMed] [Google Scholar]
- 9. Aghajanova L, Horcajadas JA, Weeks JL, et al. The protein kinase A pathway-regulated transcriptome of endometrial stromal fibroblasts reveals compromised differentiation and persistent proliferative potential in endometriosis. Endocrinology. 2010;151:1341–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Klemmt PA, Carver JG, Kennedy SH, Koninckx PR, Mardon HJ. Stromal cells from endometriotic lesions and endometrium from women with endometriosis have reduced decidualization capacity. Fertil Steril. 2006;85:564–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lucas ES, Dyer NP, Murakami K, et al. Loss of endometrial plasticity in recurrent pregnancy loss. Stem Cells. 2016;34:346–356. [DOI] [PubMed] [Google Scholar]
- 12. Salker M, Teklenburg G, Molokhia M, et al. Natural selection of human embryos: impaired decidualization of the endometrium disables embryo-maternal interactieons and causes recurrent pregnant loss. PLoS One. 2010;5:e10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Salker MS, Christian M, Steel JH, et al. Deregulation of the serum- and glucocorticoid-inducible kinase SGK1 in the endometrium causes reproductive failure. Nat Med. 2011;17:1509–1513. [DOI] [PubMed] [Google Scholar]
- 14. Salker MS, Nautiyal J, Steel JH, et al. Disordered IL-33/ST2 activation in decidualizing stromal cells prolongs uterine receptivity in women with recurrent pregnancy loss. PLoS One. 2012;7:e52252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Weimar CH, Kavelaars A, Brosens JJ, et al. Endometrial stromal cells of women with recurrent miscarriage fail to discriminate between high- and low-quality human embryos. PLoS One. 2012;7:e41424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Weimar CH, Macklon NS, Post Uiterweer ED, Brosens JJ, Gellersen B. The motile and invasive capacity of human endometrial stromal cells: implications for normal and impaired reproductive function. Hum Reprod Update. 2013;19:542–557. [DOI] [PubMed] [Google Scholar]
- 17. Brosens JJ, Salker MS, Teklenburg G, et al. Uterine selection of human embryos at implantation. Sci Rep. 2014;4:3894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Macklon NS, Brosens JJ. The human endometrium as a sensor of embryo quality. Biol Reprod. 2014;91:98. [DOI] [PubMed] [Google Scholar]
- 19. Teklenburg G, Salker M, Molokhia M, et al. Natural selection of human embryos: decidualizing endometrial stromal cells serve as sensors of embryo quality upon implantation. PLoS One. 2010;5:e10258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Leitao B, Jones MC, Fusi L, et al. Silencing of the JNK pathway maintains progesterone receptor activity in decidualizing human endometrial stromal cells exposed to oxidative stress signals. FASEB J. 2010;24:1541–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kajihara T, Jones M, Fusi L, et al. Differential expression of FOXO1 and FOXO3a confers resistance to oxidative cell death upon endometrial decidualization. Mol Endocrinol. 2006;20:2444–2455. [DOI] [PubMed] [Google Scholar]
- 22. Leitao BB, Jones MC, Brosens JJ. The SUMO E3-ligase PIAS1 couples reactive oxygen species-dependent JNK activation to oxidative cell death. FASEB J. 2011;25:3416–3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sugino N, Karube-Harada A, Kashida S, Takiguchi S, Kato H. Differential regulation of copper-zinc superoxide dismutase and manganese superoxide dismutase by progesterone withdrawal in human endometrial stromal cells. Mol Hum Reprod. 2002;8:68–74. [DOI] [PubMed] [Google Scholar]
- 24. Sugino N, Karube-Harada A, Sakata A, Takiguchi S, Kato H. Nuclear factor-κ B is required for tumor necrosis factor-α-induced manganese superoxide dismutase expression in human endometrial stromal cells. J Clin Endocrinol Metab. 2002;87:3845–3850. [DOI] [PubMed] [Google Scholar]
- 25. Muter J, Lucas ES, Chan YW, et al. The clock protein period 2 synchronizes mitotic expansion and decidual transformation of human endometrial stromal cells. FASEB J. 2015;29:1603–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Matsuda M, Kanematsu T, Takeuchi H, Kukita T, Hirata M. Localization of a novel inositol 1,4,5-trisphosphate binding protein, p130 in rat brain. Neurosci Lett. 1998;257:97–100. [DOI] [PubMed] [Google Scholar]
- 27. Uji A, Matsuda M, Kukita T, Maeda K, Kanematsu T, Hirata M. Molecules interacting with PRIP-2, a novel Ins(1,4,5)P3 binding protein type 2: comparison with PRIP-1. Life Sci. 2002;72:443–453. [DOI] [PubMed] [Google Scholar]
- 28. Yoshida M, Kanematsu T, Watanabe Y, et al. D-myo-inositol 1,4,5-trisphosphate-binding proteins in rat brain membranes. J Biochem. 1994;115:973–980. [DOI] [PubMed] [Google Scholar]
- 29. Harada K, Takeuchi H, Oike M, et al. Role of PRIP-1, a novel Ins(1,4,5)P3 binding protein, in Ins(1,4,5)P3-mediated Ca2+ signaling. J Cell Physiol. 2005;202:422–433. [DOI] [PubMed] [Google Scholar]
- 30. Fujii M, Kanematsu T, Ishibashi H, et al. Phospholipase C-related but catalytically inactive protein is required for insulin-induced cell surface expression of γ-aminobutyric acid type A receptors. J Biol Chem. 2010;285:4837–4846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sugiyama G, Takeuchi H, Kanematsu T, Gao J, Matsuda M, Hirata M. Phospholipase C-related but catalytically inactive protein, PRIP as a scaffolding protein for phospho-regulation. Adv Biol Regul. 2013;53:331–340. [DOI] [PubMed] [Google Scholar]
- 32. Christian M, Zhang X, Schneider-Merck T, et al. Cyclic AMP-induced forkhead transcription factor, FKHR, cooperates with CCAAT/enhancer-binding protein β in differentiating human endometrial stromal cells. J Biol Chem. 2002;277:20825–20832. [DOI] [PubMed] [Google Scholar]
- 33. Labied S, Kajihara T, Madureira PA, et al. Progestins regulate the expression and activity of the forkhead transcription factor FOXO1 in differentiating human endometrium. Mol Endocrinol. 2006;20:35–44. [DOI] [PubMed] [Google Scholar]
- 34. Takano M, Lu Z, Goto T, et al. Transcriptional cross talk between the forkhead transcription factor forkhead box O1A and the progesterone receptor coordinates cell cycle regulation and differentiation in human endometrial stromal cells. Mol Endocrinol. 2007;21:2334–2349. [DOI] [PubMed] [Google Scholar]
- 35. Lynch VJ, Brayer K, Gellersen B, Wagner GP. HoxA-11 and FOXO1A cooperate to regulate decidual prolactin expression: towards inferring the core transcriptional regulators of decidual genes. PLoS One. 2009;4:e6845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lynch VJ, Leclerc RD, May G, Wagner GP. Transposon-mediated rewiring of gene regulatory networks contributed to the evolution of pregnancy in mammals. Nat Genet. 2011;43:1154–1159. [DOI] [PubMed] [Google Scholar]
- 37. Matsuda M, Tsutsumi K, Kanematsu T, et al. Involvement of phospholipase C-related inactive protein in the mouse reproductive system through the regulation of gonadotropin levels. Biol Reprod. 2009;81:681–689. [DOI] [PubMed] [Google Scholar]
- 38. Chan YW, van den Berg HA, Moore JD, Quenby S, Blanks AM. Assessment of myometrial transcriptome changes associated with spontaneous human labour by high-throughput RNA-seq. Exp Physiol. 2014;99:510–524. [DOI] [PubMed] [Google Scholar]
- 39. Feroze-Zaidi F, Fusi L, Takano M, et al. Role and regulation of the serum- and glucocorticoid-regulated kinase 1 in fertile and infertile human endometrium. Endocrinology. 2007;148:5020–5029. [DOI] [PubMed] [Google Scholar]
- 40. Ross J. mRNA stability in mammalian cells. Microbiol Rev. 1995;59:423–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Africander D, Verhoog N, Hapgood JP. Molecular mechanisms of steroid receptor-mediated actions by synthetic progestins used in HRT and contraception. Steroids. 2011;76:636–652. [DOI] [PubMed] [Google Scholar]
- 42. Brosens JJ, Gellersen B. Death or survival–progesterone-dependent cell fate decisions in the human endometrial stroma. J Mol Endocrinol. 2006;36:389–398. [DOI] [PubMed] [Google Scholar]
- 43. Dijkers PF, Medema RH, Lammers JW, Koenderman L, Coffer PJ. Expression of the pro-apoptotic Bcl-2 family member Bim is regulated by the forkhead transcription factor FKHR-L1. Curr Biol. 2000;10:1201–1204. [DOI] [PubMed] [Google Scholar]
- 44. Cloke B, Huhtinen K, Fusi L, et al. The androgen and progesterone receptors regulate distinct gene networks and cellular functions in decidualizing endometrium. Endocrinology. 2008;149:4462–4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chen CC, Hardy DB, Mendelson CR. Progesterone receptor inhibits proliferation of human breast cancer cells via induction of MAPK phosphatase 1 (MKP-1/DUSP1). J Biol Chem. 2011;286:43091–43102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Leitao B, Jones MC, Fusi L, et al. Silencing of the JNK pathway maintains progesterone receptor activity in decidualizing human endometrial stromal cells exposed to oxidative stress signals. FASEB J. 2010;24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Huang H, Tindall DJ. Regulation of FOXO protein stability via ubiquitination and proteasome degradation. Biochim Biophys Acta. 2011;1813:1961–1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Huang H, Regan KM, Wang F, et al. Skp2 inhibits FOXO1 in tumor suppression through ubiquitin-mediated degradation. Proc Natl Acad Sci USA. 2005;102:1649–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Murakami K, Lee YH, Lucas ES, et al. Decidualization induces a secretome switch in perivascular niche cells of the human endometrium. Endocrinology. 2014;155:4542–4553. [DOI] [PubMed] [Google Scholar]
- 50. Kusama K, Yoshie M, Tamura K, et al. Regulation of decidualization in human endometrial stromal cells through exchange protein directly activated by cyclic AMP (Epac). Placenta. 2013;34:212–221. [DOI] [PubMed] [Google Scholar]
- 51. Gargett CE, Schwab KE, Deane JA. Endometrial stem/progenitor cells: the first 10 years. Hum Reprod Update. 2015:dmv051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Vasquez YM, Mazur EC, Li X, et al. FOXO1 is required for binding of PR on IRF4, novel transcriptional regulator of endometrial stromal decidualization. Mol Endocrinol. 2015;29:421–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Deniaud A, Sharaf el dein O, Maillier E, et al. Endoplasmic reticulum stress induces calcium-dependent permeability transition, mitochondrial outer membrane permeabilization and apoptosis. Oncogene. 2008;27:285–299. [DOI] [PubMed] [Google Scholar]
- 54. Umebayashi H, Mizokami A, Matsuda M, et al. Phospholipase C-related catalytically inactive protein, a novel microtubule-associated protein 1 light chain 3-binding protein, negatively regulates autophagosome formation. Biochem Biophys Res Commun. 2013;432:268–274. [DOI] [PubMed] [Google Scholar]