Abstract
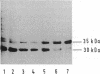
We have studied the refolding and membrane insertion of the outer membrane protein OmpA of Escherichia coli. The protein was extracted from its native membrane by sonication in the presence of urea and dissolved in the urea/water mixture in unfolded form. In this form it was purified. Upon addition of preformed lipid vesicles, the protein spontaneously refolded and inserted into the vesicle membranes. The vesicles had to be small and the lipids had to be in the fluid state. The insertion occurred in an oriented manner.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dill K. A. Dominant forces in protein folding. Biochemistry. 1990 Aug 7;29(31):7133–7155. doi: 10.1021/bi00483a001. [DOI] [PubMed] [Google Scholar]
- Dornmair K., Kiefer H., Jähnig F. Refolding of an integral membrane protein. OmpA of Escherichia coli. J Biol Chem. 1990 Nov 5;265(31):18907–18911. [PubMed] [Google Scholar]
- Driessen A. J., Wickner W. Proton transfer is rate-limiting for translocation of precursor proteins by the Escherichia coli translocase. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2471–2475. doi: 10.1073/pnas.88.6.2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisele J. L., Rosenbusch J. P. In vitro folding and oligomerization of a membrane protein. Transition of bacterial porin from random coil to native conformation. J Biol Chem. 1990 Jun 25;265(18):10217–10220. [PubMed] [Google Scholar]
- Engelman D. M., Steitz T. A. The spontaneous insertion of proteins into and across membranes: the helical hairpin hypothesis. Cell. 1981 Feb;23(2):411–422. doi: 10.1016/0092-8674(81)90136-7. [DOI] [PubMed] [Google Scholar]
- Freudl R., MacIntyre S., Degen M., Henning U. Alterations to the signal peptide of an outer membrane protein (OmpA) of Escherichia coli K-12 can promote either the cotranslational or the posttranslational mode of processing. J Biol Chem. 1988 Jan 5;263(1):344–349. [PubMed] [Google Scholar]
- Freudl R., Schwarz H., Stierhof Y. D., Gamon K., Hindennach I., Henning U. An outer membrane protein (OmpA) of Escherichia coli K-12 undergoes a conformational change during export. J Biol Chem. 1986 Aug 25;261(24):11355–11361. [PubMed] [Google Scholar]
- Huang K. S., Bayley H., Liao M. J., London E., Khorana H. G. Refolding of an integral membrane protein. Denaturation, renaturation, and reconstitution of intact bacteriorhodopsin and two proteolytic fragments. J Biol Chem. 1981 Apr 25;256(8):3802–3809. [PubMed] [Google Scholar]
- Jaenicke R. Protein folding: local structures, domains, subunits, and assemblies. Biochemistry. 1991 Apr 2;30(13):3147–3161. doi: 10.1021/bi00227a001. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lecker S. H., Driessen A. J., Wickner W. ProOmpA contains secondary and tertiary structure prior to translocation and is shielded from aggregation by association with SecB protein. EMBO J. 1990 Jul;9(7):2309–2314. doi: 10.1002/j.1460-2075.1990.tb07402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morona R., Klose M., Henning U. Escherichia coli K-12 outer membrane protein (OmpA) as a bacteriophage receptor: analysis of mutant genes expressing altered proteins. J Bacteriol. 1984 Aug;159(2):570–578. doi: 10.1128/jb.159.2.570-578.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaller R., Freitag H., Harmey M. A., Benz R., Neupert W. A water-soluble form of porin from the mitochondrial outer membrane of Neurospora crassa. Properties and relationship to the biosynthetic precursor form. J Biol Chem. 1985 Jul 5;260(13):8188–8193. [PubMed] [Google Scholar]
- Popot J. L., Engelman D. M. Membrane protein folding and oligomerization: the two-stage model. Biochemistry. 1990 May 1;29(17):4031–4037. doi: 10.1021/bi00469a001. [DOI] [PubMed] [Google Scholar]
- Schweizer M., Hindennach I., Garten W., Henning U. Major proteins of the Escherichia coli outer cell envelope membrane. Interaction of protein II with lipopolysaccharide. Eur J Biochem. 1978 Jan 2;82(1):211–217. doi: 10.1111/j.1432-1033.1978.tb12013.x. [DOI] [PubMed] [Google Scholar]
- Scotto A. W., Gompper M. E. Spontaneous incorporation of bacteriorhodopsin into large preformed vesicles. Biochemistry. 1990 Aug 7;29(31):7244–7251. doi: 10.1021/bi00483a012. [DOI] [PubMed] [Google Scholar]
- Scotto A. W., Zakim D. Reconstitution of membrane proteins. Spontaneous incorporation of integral membrane proteins into preformed bilayers of pure phospholipid. J Biol Chem. 1988 Dec 5;263(34):18500–18506. [PubMed] [Google Scholar]
- Sen K., Nikaido H. In vitro trimerization of OmpF porin secreted by spheroplasts of Escherichia coli. Proc Natl Acad Sci U S A. 1990 Jan;87(2):743–747. doi: 10.1073/pnas.87.2.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvius J. R., Allen T. M. Reconstitution of membrane proteins: a selected bibliography from Biophysical Society workshop on membrane protein reconstitution, 2 March 1988. Biophys J. 1989 Jan;55(1):207–208. doi: 10.1016/S0006-3495(89)82794-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tani K., Shiozuka K., Tokuda H., Mizushima S. In vitro analysis of the process of translocation of OmpA across the Escherichia coli cytoplasmic membrane. A translocation intermediate accumulates transiently in the absence of the proton motive force. J Biol Chem. 1989 Nov 5;264(31):18582–18588. [PubMed] [Google Scholar]
- Teather R. M., Bramhall J., Riede I., Wright J. K., Fürst M., Aichele G., Wilhelm U., Overath P. Lactose carrier protein of Escherichia coli. Structure and expression of plasmids carrying the Y gene of the lac operon. Eur J Biochem. 1980;108(1):223–231. doi: 10.1111/j.1432-1033.1980.tb04715.x. [DOI] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Van Alphen L., Havekes L., Lugtenberg B. Major outer membrane protein d of Escherichia coli K12. Purification and in vitro activity of bacteriophages k3 and f-pilus mediated conjugation. FEBS Lett. 1977 Mar 15;75(1):285–290. doi: 10.1016/0014-5793(77)80104-x. [DOI] [PubMed] [Google Scholar]
- Vogel H., Jähnig F. Models for the structure of outer-membrane proteins of Escherichia coli derived from raman spectroscopy and prediction methods. J Mol Biol. 1986 Jul 20;190(2):191–199. doi: 10.1016/0022-2836(86)90292-5. [DOI] [PubMed] [Google Scholar]
- Weiss M. S., Kreusch A., Schiltz E., Nestel U., Welte W., Weckesser J., Schulz G. E. The structure of porin from Rhodobacter capsulatus at 1.8 A resolution. FEBS Lett. 1991 Mar 25;280(2):379–382. doi: 10.1016/0014-5793(91)80336-2. [DOI] [PubMed] [Google Scholar]