Abstract
Kynurenines are a wide range of catabolites which derive from tryptophan through the “Kynurenine Pathway” (KP). In addition to its peripheral role, increasing evidence shows a role of the KP in the central nervous system (CNS), mediating both physiological and pathological functions. Indeed, an imbalance in this route has been associated with several neurodegenerative disorders such as Alzheimer’s and Huntington’s diseases. Altered KP catabolism has also been described during both acute and chronic phases of stroke; however the contribution of the KP to the pathophysiology of acute ischemic damage and of post-stroke disorders during the chronic phase including depression and vascular dementia, and the exact mechanisms implicated in the regulation of the KP after stroke are not well established yet. A better understanding of the regulation and activity of the KP after stroke could provide new pharmacological tools in both acute and chronic phases of stroke. In this review, we will make an overview of CNS modulation by the KP. We will detail the KP contribution in the ischemic damage, how the unbalance of the KP might trigger an alteration of the cognitive function after stroke as well as potential targets for the development of new drugs.
Keywords: Tryptophan, kynurenine, ischemia, stroke, neurodegeneration, depression, cognitive impairment, dementia
1. INTRODUCTION
Ischemic stroke, which results from cerebral arterial occlusion, is a major cause of morbidity and mortality in today’s society that affects millions of lives every year [1]. Due to the impact around the world, mainly in industrialized countries, stroke consumes over 4–5% of total health care costs [2]. Despite this, little therapeutic options are available for stroke treatment. Until recently, the only approved treatment for the acute ischemic stroke was recombinant thrombolytic tissue plasminogen activator (rtPA) [3], but due to strict eligibility criteria and narrow therapeutic window, only 2%–5% of stroke patients receive rtPA treatment. In addition, with the publication of several randomized clinical trials of endovascular treatment for acute ischemic stroke (MR CLEAN, ESCAPE, EXTEND-IA, SWIFT PRIME and REVASCAT) [4–8], intra-arterial thrombectomy represents an alternative effective option for a subset of patients with acute stroke, for example, those patients with large artery occlusions.
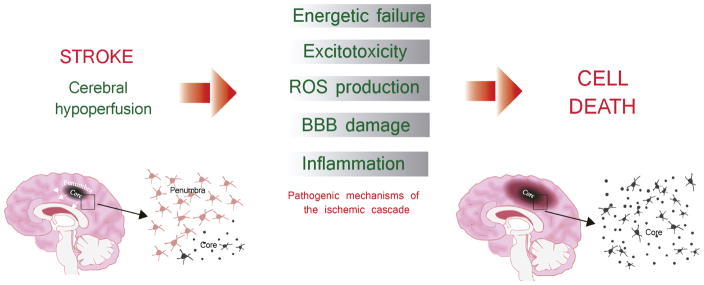
In the acute phase of stroke, ischemic injury results from a series of cellular and molecular events caused by a sudden decrease or loss of blood flow and subsequent reperfusion of the ischemic territory. This succession of events is the so called ischemic cascade, consisting of a cellular bioenergetics failure, followed by excitotoxicity, oxidative stress, blood-brain barrier (BBB) dysfunction and post-ischemic inflammation, which collectively contribute to cell death in the ischemic territory [9, 10] Fig. 1. These phenomena are critical events to determine the fate of the ischemic brain and the survival of stroke patients. Thus, stroke treatment has focused on reducing ischemic cell death with neuroprotective drugs, by targeting different points of the ischemic cascade. For instance, among the most effective candidates used in preclinical stroke studies are the NMDA receptor antagonists [11–14], which target excitotoxicity, a process caused by pathological activation and calcium uptake by neurons due to abnormal release of excitatory neurotransmitters from dying cells, especially glutamate [15]. Other neuroprotective drugs include sodium-channel and calcium-channel blockers, growth factors (such as basic fibroblast growth factor), free radical scavengers (such as tirilazad), and anti-inflammatory compounds (such as enlimomab) [11–13].
Fig. 1. The ischemic cascade.
Ischemic injury results from a series of cellular and molecular events caused by a sudden decrease or loss of blood flow and subsequent reperfusion of the ischemic territory. This succession of events is the so called ischemic cascade, consisting of a cellular bioenergetics failure, followed by excitotoxicity, oxidative stress, blood-brain barrier (BBB) dysfunction and post-ischemic inflammation, which collectively contribute to cell death in the ischemic. Within the first minutes, the lack of blood flow produces the energy failure in brain cells, triggering the release of the neurotransmitter glutamate. The postsynaptic overactivated NMDA (N-metil-D-aspartate) receptors will lead the excitotoxicity processes (mainly due to the influx of Ca2+) and then, the subsequent cellular damage due to the production of reactive oxygen species (ROS). Afterwards, the lack of blood flow and ROS will also damage the microvasculature producing the breakdown of the brain-blood-barrier (BBB), allowing the infiltration of leukocytes into the brain parenchyma. Resident brain cells (mainly microglial cells), but also astrocytes, together with infiltrated cells will release pro-inflammatory citokines triggering neuroinflammation, and therefore enhancing the brain damage. Two different regions can be distingued during the progression of the ischemic injury, that is, core and penumbra. The infarct core is the region in which the severe decrease of blood flow caused an energy failure and therefore cells rapidly die by necrosis. By contrary, the penumbra is the region which a preserved energy state. Without any intervention, the infarct core finally expands into the penumbra.
Unfortunately, so far, translational research with neuroprotectants from animal studies to the clinic has failed [11–13]. The ischemic cascade seems to be so complex that targeting a single pathway may be ineffective, or even have adverse side effects. In addition, the presence of comorbidities in patients such as hypertension, aging, obesity or bacterial infections [16–20] can modify stroke outcome and survival.
Despite the reduced therapeutic opportunities for acute stroke treatment, advances in prevention and healthcare have increased life expectancy and produced a progressive decrease in stroke mortality in the last few decades [21]. However, a direct consequence is the high prevalence stroke survivors suffering a range of motor and cognitive impairments. Even with effective thrombolysis, the prevalence of disability is estimated in 24–54% [22]. Beyond the motor deficits, vascular cognitive impairment (VCI) and post-stroke depression (PSD) are among the most common long-term sequelae afflicting stroke survivors during the chronic phase [23–25]. The increase in the number of survivors with residual impairments and disabilities has been accompanied by a growing interest in the factors that could interfere with functional outcome and quality of life during this phase. This chronic brain damage occurring after stroke injury could be mediated, at least in part, by a delayed neurodegeneration which is caused, not by the initial insult itself, but by a secondary process in which several factors such as immune activation, neurotoxic/neuroinhibitory factors and oxidative stress among others have been suggested to play a pivotal role [26]. Because these processes occur weeks after primary damage, the development of therapies targeting delayed neurodegeneration offers new opportunities to enhance brain recovery and improve functional outcome of stroke survivors.
As we will describe in detail below, the kynurenine pathway (KP) mediates important physiological functions in the CNS and has been also implicated in different brain pathologies such as Alzheimer’s, Parkinson’s and Huntington’s diseases. Several tryptophan catabolites of this route present the ability to modulate glutamatergic and nicotinic receptors, to regulate the response of the immune system after inflammation and/or infection and even to modify the generation of reactive oxygen species. Since all these mechanisms have been previously demonstrated to play a key role in both acute and chronic pathogenic phases of stroke, interfering KP catabolites could represent a valuable tool for ameliorating stroke deficits.
In this sense, the main focus of this review is to summarize the present level of understanding of the involvement of the kynurenine system in the pathomechanisms of brain injury after cerebral ischemia and to evaluate the therapeutic options for manipulating the KP in the acute phase of stroke. We will also discuss the possible role of altered KP as a predisposing factor for stroke. Finally, we will try to evaluate the role of kynurenine and tryptophan catabolism during the chronic phase of stroke with special emphasis in VCI and PSD.
2. THE KYNURENINE PATHWAY IN THE BRAIN
Tryptophan (L-Trp) is one of the essential amino acids which is used for protein synthesis but also is the metabolic precursor of serotonin and the KP, being the latter the major metabolic pathway of dietary L-Trp (more than 95% of tryptophan catabolism) [27, 28]. The initial interest in the KP was based on the fact that this route ends with the generation of nicotinamide and the important enzyme co-factor nicotinamide adenine dinucleotide (NAD), which plays a critical role in many fundamental biological processes. Nowadays, the KP is becoming recognized as a key player in the mechanisms of neuronal damage in several neurodegenerative disorders and acute brain damage [27–30]. This point of view is supported by data which demonstrated that kynurenine catabolism is altered in such brain pathologies and by the fact that several of its catabolites could exert a regulatory role in a different plethora of biological functions which including neuroactive and neurotoxic properties.
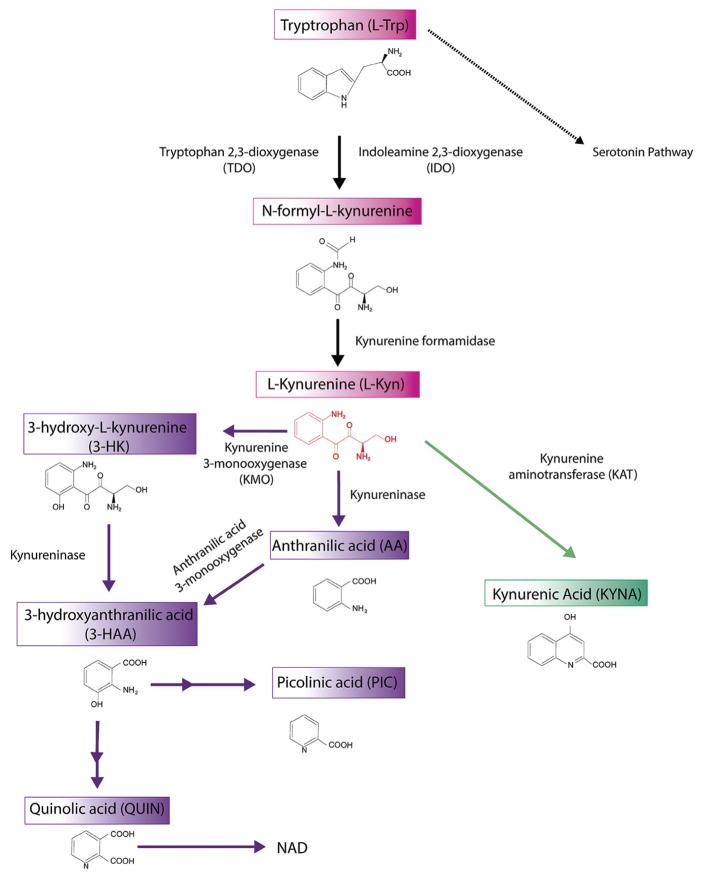
L-Trp is transported into brain across the BBB through a neutral amino acid transporter [31]. The initial and rate-limiting step of the KP in the brain is carried out by two key enzymes, indoleamine-2,3-dioxygenase (IDO-1 and IDO-2) and tryptophan-2,3-dioxygenase (TDO), that metabolize L-Trp into N-formyl-L-kynurenine [32, 33], an unstable compound which is further converted by kynurenine formidase into L-kynurenine (L-Kyn). L-Kyn has a central role on this pathway since its degradation could be produced by three different routes. In the first route, L-Kyn is converted in one of the final products, kynurenic acid (KYNA) after an irreversible transamination reaction by kynurenine aminotransferase (KAT) family enzymes [34]. In the second branch of L-Kyn degradation, L-Kyn is transformed into anthranilic acid (ANA) by the action of kynureninase (KYNU). Finally, L-Kyn can also be hydroxylated by kynurenine 3-monooxygenase (KMO) to produce 3-hydroxykynurenine (3-HK) [35]. At this point, both 3-HK and ANA may be enzymatically converted into 3-hydroxyanthranilic acid (3-HAA) by kynurenine 3-hydroxylase (K3H) and KYNU, respectively. Then, 3-HAA is metabolized by 3-hydroxyanthranilate 3,4-dioxygenase (3-HAO) producing 2-amino-3-carboxymuconate semialdehyde (ACMS), a very unstable compound which could be further metabolized to generate picolinic acid (PIC) by 2-amino-3-carboxymuconate-6-semialdehyde decarboxylase or non-enzimatically transformed into quinolinic acid (QUIN). Finally, QUIN generates nicotinamide, and ultimately NAD+/NADP+ through a transamination reaction driven by quinolate phosphoribisyltransferase (QPRT) (Fig. 2). All these catabolites are altogether commonly called kynurenines.
Fig. 2. The Kynurenine pathway of tryptophan degradation.
One of the main metabolic pathways of dietary L-tryptophan (L-Trp) in the brain is the kynurenine pathway (KP). The rate-limiting step of this pathway is the conversion of L-Trp to L-Kynurenine (L-Kyn) by indoleamine 2,3-dioxygenase (IDO) and tryptophan 2,3-dioxygenase (TDO). Through three pathways L-kynurenine is converted into kynurenic acid (KYNA), 3-hydroxykynurenine (3-HK) and anthranilic acid (ANA). The next step of the pathway is the conversion of 3-HK and ANA into 3-hydroxyanthranilic acid (3-HAA). 3-HAA, through several enzimatic reactions is converted into the final kynurenine pathway products picolinic acid (PIC) and quinolinic acid (QUIN). Finally, QUIN generates nicotinamide, and ultimately NAD+/NADP+
3. DIFFERENT ACTIONS OF KYNURENINE CATABO-LITES IN THE BRAIN
3.1. L-Kynurenine
Until very recently, research attention on L-Kyn was only because of its central role in the KP not by its properties itself, being considered an inert catabolite of this pathway. In fact, changes in L-Kyn levels and L-Kyn/L-Trp ratio are commonly used as indicators of altered KP catabolism after brain pathology. One of the main properties of L-Kyn is to be readily transported across the BBB. In fact, 60% of L-Kyn is taken up from the blood and 40% is generated locally from cerebral L-Trp [36]. In brain, L-Kyn availability is determinant for the synthesis of the rest of KP catabolites, which are well known to exert neuroactive actions within brain as we will discuss later. For that, L-Kyn and its halogenated derivatives such as 4-chlorokynurenine [37] (that acts as prodrugs) are used as indirect pharmacological strategies to exploit the therapeutic potential of KP to increase catabolites with neuroprotective actions, as KYNA, given that peripheral KYNA crosses poorly the BBB.
However, it is necessary to keep in mind that recent studies have shown specific actions for L-Kyn apart from its role as catabolic precursor. A recent study demonstrated that catabolism of L-Trp to L-Kyn by endothelial IDO contributes to arterial vessel relaxation and therefore, L-Kyn is positioned as an endothelium-derived vasodilator during inflammation [38]. Furthermore, different pieces of evidence strongly suggest that L-Kyn can be considered as a potential endogenous antioxidant, which can protect macromolecules against oxidative modifications. In contrast, L- Kyn has also shown pro-oxidant effects [39]. Finally, L-Kyn has been identified as an endogenous ligand of the aryl hydrocarbon receptor (AhR) in different contexts including brain [40–43]. AhR is a ligand-activated transcription factor [44] mainly known to participate in the metabolism of xenobiotics from environmental pollutants such as polyaromatic hydrocarbons, polychlorinated biphenyls, and dioxins [45]. AhR activation by xenobiotics leads to a wide variety of toxic responses including severe thymic involution, wasting syndrome, chloracne, immune suppression, inflammation, reduced fertility, hepatotoxicity, tumour promotion and death[46, 47]. In addition, several metabolites of Trp such as 6-formylindolo-[3,2-b]carbazole (FICZ) and bacterial products such as indole-3-aldehyde have been identified as AhR endogenous ligands supporting that this receptor also plays important roles in normal cell physiology and function [43, 48–53]. Some of the best-characterized actions of the L-Kyn-AhR axis are related to autoimmunity, tumour immunity and disease tolerance. Regarding autoinmmunity, preclinical studies demonstrated that AhR activation by L-Kyn promotes the differentiation of naïve CD4+ T-helper (Th) cells into a regulatory T cell phenotype (Treg) while suppressing the differentiation into interleukin (IL)-17)-producing Th (Th17) cells [42]. Moreover, a regulatory pathway has recently been described in which AhR activation by L-Kyn produced by innate immune cells limits endotoxin-triggered inflammation [54]. In addition, the study by Opitz and cols. in human gliomas demonstrated that AhR activation by L-Kyn is able to suppress antitumor immune responses and promotes tumor-cell survival, being therefore associated with malignant progression and poor survival [43]. Interestingly, AhR is also expressed in the brain suggesting a role of the L-Kyn-AhR axis in neuronal damage in neurodegeneration and brain injury [27–30]. As we will discuss later, results of our group support this idea in the context of stroke [40].
3.2. Kynurenic Acid (KYNA)
Kynurenic acid (KYNA) is present in low nanomolar concentrations in mammals [55] and is mainly known to be an endogenous antagonist of glutamate receptors inhibiting all three ionotropic glutamatergic receptors; that is, AMPA- (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid), NMDA- (N-methyl-D-aspartate) and kainate- receptors [56, 57]. However, the strongest inhibitory effects of KYNA in glutamatergic transmission are mainly mediated by acting as an inhibitor at the strychnine-insensitive glycine-binding site of the NMDA receptor [58] with a comparingly weak antagonistic properties on AMPA and kainate receptors. As we will see below, the potent inhibitory features of KYNA on NMDA receptors raise the possibility of its neuroprotective efficacy against NMDA receptor- mediated excitotoxicity, one of critical events in the neuronal death after cerebral ischemia [59].
In addition to its established role in NMDA receptor blockade, several actions have also been ascribed to KYNA [60]. For instance, KYNA is able to regulate cholinergic transmission due to its activity as a non-competitive modulator of the α7-nicotinic acetylcholine receptor (α7nAChR) [61]. Recently, KYNA has been identified as a ligand for the orphan G protein-coupled receptor GPR35 [62]. Acting on GPR35, KYNA may induce the production of inositol triphosphate and promote Ca2+ mobilization. However, GPR35 is predominantly expressed in the intestine and immune cells including neutrophils, monocytes, T cells and dendritic cells with very low levels in the brain. For this reason, its role in the CNS is still under debate, although some forms of mental retardation occur with loss of GPR35 [63] suggesting a possible function of this receptor in CNS. Moreover, KYNA has also been described to be a potent agonist for the AhR, although the effects of this activation are not well established yet [64]. Independently of its actions over receptors, KYNA could also modulate the expression and/or release of different growth factors such as nerve growth factor (NGF) [65] or fibroblast growth factor-1 (FGF-1) [66] and, furthermore, KYNA is an endogenous antioxidant; its protective effect in diverse toxicity models may be due to its redox character in addition to its activity on receptors [39].
3.3. Quinolinic Acid (QUIN)
QUIN is a weak but competitive agonist of NMDA receptors, specifically, of NMDA receptor subtypes containing NR2A and NR2B subunits [67]. Therefore, QUIN exerts the greatest damage to neurons where these receptor subtypes are present. NMDA receptor-dependent activation by QUIN produces ROS due to intracellular Ca2+ influx enhancing peroxynitrite levels and damaging the cell [68]. However, its well established neurotoxic capacity may also be attributed to different mechanisms, such as removal of endogenous antioxidants, inhibition of glutamate uptake by astrocytes, generation of ROS and even metabolic impairment [69, 70]. In addition to modulate neuronal activity, QUIN can also act as an initiator and promoter of local inflammation within the CNS, increasing the expression and secretion of chemokines such as monocyte chemoattractant protein-1 (MCP-1) and regulated on activation normal T cell expressed and secreted (RANTES) [71].
3.4. 3-Hydroxykynurenine (3-HK)
3-HK is other neuroactive KP catabolite with cytotoxic properties. 3-HK is able to cross the BBB due to its hydrophobic nature, increasing its bioavailability in the brain [36]. In addition, after tissue damage or inflammation, KMO expression and 3-HK production also increase, shuttling L-Kyn catabolism toward production of QA. Regarding its activity, most studies have demonstrated that 3-HK mediates its neurotoxic effects through ROS generation [72–74], in part due to superoxide anions from the autooxidation of its metabolite 3-HAA [75]. However, more recent studies have demonstrated that 3-HK has the ability to be both antioxidant as well as pro-oxidant depending on the circumstances [76].
3.5. Functions of other Kynurenines
3.5.1. 3-hydroxy-Anthranilic Acid (3-HAA)
3-HAA is known as one of the main free radicals generators due to is able to generate ROS throughout its autooxidation [77, 78]. This autooxidation process first consists in the production of anthraniloyl radical, which is oxidized to the quinoneimine. Next, a condensation and an oxidative reaction on quinoneimine yields cinnabarinic acid. Some reports also suggest that 3-HAA modulates the survival of immune cells, specifically differentiated T helper cells 1, through a caspase-8 and cytochrome c dependent apoptosis pathway [79]. 3-HAA modulates autoimmune neuroinflammation [80] and regulates innate immunity, at least in part, by inducing hemeoxygenase-1 (HO-1) [81], an enzyme well known for its anti-inflammatory and protective actions. In addition, as well as other KP catabolites, 3-HAA seems to exert several antioxidant actions. A role of 3-HAA as a potent antioxidant preventing LDL lipid peroxidation [82] and protecting against oxidative stress in the brain [83] has been described. Furthermore, 3-HAA has been recently demonstrated to inhibit atherosclerosis by regulating lipid metabolism and reducing vascular inflammation [84, 85].
3.5.2. Picolinic Acid (PIC)
PIC is known to be one of the best chelating agents, with capacity to chelate a wide range of bivalent metals as Cu2+, Fe2+, Ni2+, Zn2+, Cd2+ and Pb2+ [86]. Beyond its chelator attributes, PIC also plays a role over the immune system. Specifically, in the presence of IFN-γ, PIC activates macrophages through macrophage inhibitory protein (MIP)-1α and -1β [87], and also induces the expression of inducible nitric oxide synthase [88]. In brain, PIC presented a neuroprotective effect in cholinergic neurons of nucleus basalis after a neurotoxic context evoked by overstimulation with QUIN [89, 90]. In addition, PIC also has agonistic activity on the ionotropic amino acid neurotransmitter glycine receptor [91]. Finally, it has been described that PIC causes several hippocampal, substantia nigra and striatal cellular toxicity [92], probably due to its capacity to generate hydroxyl radicals.
3.5.3. Anthranilic acid (ANA)
Although its exact role is not very clear, some anti-inflammatory actions have been attributed to ANA. In fact, ANA is able to bind to copper forming an anti-inflammatory complex ANA-Cu2+ which decreases the levels of hydroxyl radicals after inflammation [93, 94].
4. CATABOLISM AND REGULATION OF THE KP
Brain kynurenines are linked and influenced by the peripheral KP. Therefore, fluctuations in the blood levels of KP catabolites directly affect the KP in the brain. In fact, only 40% of brain L-Kyn is generated locally and most L-Kyn comes from circulation after being generated by hepatic conversion of L-Trp mainly by TDO [95]. As we previously delineated, local synthesis of L-Kyn starts with enzymatic reactions driven by IDO and TDO. Although they are implicated in the same reaction (conversion of L-Trp into N-formyl-L-kynurenine), they present different localization, structure and regulation [96]. TDO is mainly expressed in liver, but its location in glial cells and neurons [97] has also been described. TDO may be activated by several inducers including L-Kyn, amino acids (hystidine, tyrosine and phenylalanine), increased tryptophan levels, glucocorticoids, corticosteroids, or its expression may be indirectly induced by inflammation [98, 99].
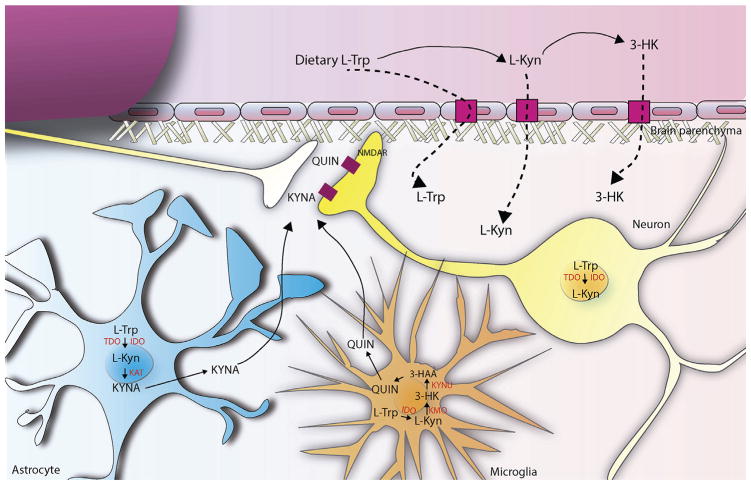
On the other hand, IDO presents a closed relationship with the immune system. In fact, the two described IDO isoforms, IDO1 and IDO2 are mainly expressed in monocytes, macrophages, dendritic cells and importantly in microglia, the tissue-resident macrophages of the brain [100–102]. Moreover, the regulation of IDO is mainly mediated by pro-inflammatory mediators being interferon-gamma (IFN-γ) one of its main activators during the immune response [99, 103]. Due to the low activity of both brain IDO and TDO under physiological conditions, brain KP is in part driven by L-Trp peripheral conversion to L-Kyn and also 3-HK, and the subsequent entry of these catabolites into the brain across the BBB. However, both brain IDO and TDO may be activated by brain injury, also allowing an increased local production of L-Kyn [104]. Independently of its origin, L-Kyn is next metabolized through the previously described branches of the pathway. Interestingly, at physiological level, although L-Kyn degradation occurs in all brain cells, there is a segregation of the 2 main pathways into specific cell types, mainly glial cells (Fig. 3). In fact, KMO is mainly expressed in the outer mitochondrial membrane of microglial and monocytes [105, 106] where it oxidates L-Kyn in the presence of NADPH to produce 3-HK, which will be subsequently transformed into its major downstream metabolites being its production clearly regulated by inflammation [107]. Meanwhile, astrocytes, which predominantly contain KATs but do not contain KMO, account for most KYNA biosynthesis, which appears to be regulated by intracellular metabolic events [108, 109].
Fig. 3. The Kynurenine pathway in the brain.
Brain kynurenines are linked and influenced by the peripheral KP. Fluctuations in the blood levels of L-Trp, L-Kyn and 3-HK directly affect metabolism of KP in the brain, since these metabolites readily cross the BBB using the large neutral amino acid transporter. Under physiological conditions, kynurenine pathway enzymes in the mammalian brain are preferentially, although not exclusively, localized in non-neuronal cells (see below) and the two routes of L-Kyn degradation are physically segregated in the brain. In fact, astrocytes, which contain KAT, are the main responsible of kynurenic acid (KYNA) biosynthesis, a well established NMDA receptor antagonist. On the contrary, microglial cells generate 3-HK and its major downstream metabolites, including quinolinic acid (QUIN), which present neurotoxic properties, at least in part, by being an NMDAR agonist. Both QUIN and KYNA are released into the extracellular space and act over NMDA receptors in the postsynaptic compartment of the neurons. In addition, under several conditions, neurons can also express different kynurenine pathway enzymes, such as TDO and IDO.
Because of their polar nature and the lack of an active transport process, QUIN and KYNA are not able to cross BBB and they are produced only locally within the brain [36]. Once synthesized, QUIN and KYNA are released into the extracellular space to exert their neurotoxic and neuroactive actions, respectively, in the pre- and post-synaptic membranes of neurons.
5. THE KYNURENINE PATHWAY IN THE ACUTE STROKE PHASE
5.1. Evidence of Altered KP After Stroke
The first data on abnormal KP and tryptophan catabolites after ischemic stroke were provided by Saito and collaborators [110–112] who demonstrated a delayed increase in the levels of brain QUIN after transient ischemia in gerbils. This increase was mediated by the activation of IDO, KYNU, 3-HK, and 3-hydroxyanthranilate-3,4-dioxygenase and eventually produced an abnormal increase in the QUIN/KYNA ratio. While these results do not support a role for increased QUIN concentrations in early excitotoxic neuronal damage, delayed increases in brain QUIN could play a role in the progression of post-ischemic injury. In fact, QUIN levels were also found to be correlated with lesion severity, supporting the idea of its secondary cytotoxic role after cerebral ischemia.
Interestingly, while QUIN remained unchanged during the first 24h after the ischemic insult, authors found significant increases at 2, 4 or even 7 days after injury coinciding with the peak of immune infiltration, glial activation and inflammation, which could suggest a direct contribution of these inflammatory cells in the delayed increase of QUIN levels. Supporting this, after global ischemia in gerbils, most QUIN is detected in microglial and infiltrated macrophages [113]. Different studies after stroke have focused their attention in evaluating KYNA levels and enzymes related to KYNA production [101,114], without detecting any change in the endogenous levels of KYNA and the activity of its biosynthetic enzymes. Data from our group after permanent middle cerebral artery occlusion (MCAO) in mice [40] also demonstrated an altered KP after cerebral ischemia. Interestingly, an increase in brain L-Kyn levels were found as early as 3 hours after MCAO and remained elevated 24h (detecting also a reduction of L-Trp from 3 to 24h). On the contrary, minor changes were detected in plasma L-Kyn or L-Trp. All these previous data seem to suggest an altered KP after experimental stroke, in spite of differences observed in the timing, location and metabolites increased after ischemia.
Several clinical studies in patients hospitalized after stroke also support the idea of an increased KP and tryptophan catabolism, and suggest a causal relation between the KP and stroke outcome. In this sense, Darlington and colaborators [115] found a significant reduction in L-Trp levels compared to healthy controls at several days after stroke onset. In addition, the L-Kyn/L-Trp ratio was much higher in stroke patients than in control population hereby confirming the increased activity of the KP in acute ischemic stroke. This activation was also accompanied with a high reduction in the 3-HAA/AA ratio which positively correlates with infarct volume. This reversed ratio could produce a ‘cleaning up’ effect after ischemic injury, and could protect against primary and secondary damage after ischemic insult [116]. Surprisingly, KYNA levels were higher in patients who died within 21 days after stroke compared to survivors. Since KYNA is an antagonist of NMDA receptors, this increase could be a compensatory protective response to limit brain damage caused by QUIN after stroke.
Applying similar techniques to determine KP and tryptophan catabolites, a report by Brouns [117] and collaborators confirmed the increased activity of plasmatic KP after stroke in a much larger population. Moreover, authors found a strong association between activity of the KP, stroke severity and long-term outcome. On the contrary, no differences were observed in 3-HAA concentration or the 3-HAA/AA ratio. Possible explanations to reconcile these different results could be that differences in the study population, exclusion criteria for concomitant conditions or medication known to influence L-Trp catabolism were not formulated. Differences could also be due to different times of blood sampling after stroke onset.
Finally, a recent report [118] also demonstrated lower blood L-Trp and KYNA levels in stroke patients which were accompanied by increased activity of IDO and KAT, calculated as the L-Kyn/L-Trp and KYNA/L-Kyn ratios, respectively.
A common interesting result of all these clinical studies is the relationship between L-Trp oxidation and stroke-induced inflammatory response determined by using different inflammatory markers. In this sense, the report by Darlington and cols. showed elevations of plasma S100B and peroxidation markers which were correlated with lesion extent by computed tomography, supporting the inflammatory component of acute ischemic damage [119,120]. Moreover, they also demonstrated a strong association between the inflammatory marker neopterin and the L-Kyn/L-Trp ratio, which persists from day 1 to 14 after stroke. Supporting these results, the clinical study performed by Brouns et al. also found a positive correlation between post-stroke inflammation and L-Kyn/L-Trp ratio by using, in this case, C-reactive protein (CRP), erythrocyte sedimentation rate (ESR) and neutrophil/lymphocyte ratio (NLR) as indicators of inflammation.
5.2. Targeting KP After Stroke in Experimental Models
As previously mentioned, glutamate-induced excitotoxicity is among the crucial factors of cell death in brain ischemia by finally promoting cellular necrosis or apoptosis [121]. Given the importance of excitotoxicity after stroke, synthetic antagonists of glutamate receptors as neuroprotective agents has been extensively employed to prevent excitotoxic neuronal loss in the stroke field [11–14]. However, these synthetic drugs also blocked normal neuronal function and consequently had severe side effects. In this sense, the KP provides an opportunity to block NMDA receptors after stroke by using KYNA as an endogenous NMDA receptor inhibitor, which could diminish the side effects of NMDA receptor blocking caused by synthetic antagonists. The neuroprotective actions of KYNA in the stroke field are supported by different types of experimental evidence, such as those that associate neuroprotection after in vivo and in vitro cerebral ischemia with a moderate increase in brain KYNA after its systemic administration at very high doses, or after KP manipulation [122–126].
Nevertheless, the therapeutic value of KYNA per se is clearly restricted by its low capacity to cross BBB [36]. Therefore, an increase in brain KYNA levels may be obtained by administering analogues, direct or indirect precursors, transport inhibitors, or inhibitors of KMO [127]. For instance, the KYNA amide analogue, N-(2-N,N-dimethylaminoethyl)-4-oxo-1H-quinoline-2-carboxamide hydrochloride, was demonstrated to exert beneficial effects against ischemia-induced neuronal loss [128]. In addition, another option is the use of KMO inhibitors (such as m-nitrobenzoyl)-alanine (mNBA) or 3,4-dimethoxy-[-N-4-(nitrophenyl)thiazol-2yl]-benzenesulfonamide (Ro 61-8048)) to reduce 3-HK and QUIN synthesis and facilitate kynurenine and tryptophan catabolism towards KYNA formation. In this sense, administration of KMO inhibitors has been shown to reduce ischemic injury after both in vitro and in vivo stroke models [122–124].
Moreover, systemic administration of its precursor L-Kyn has been widely used in different pathologies to increase brain KYNA concentration [28]. Under physiological conditions, systemic administration of L-Kyn may increase several downstream catabolites of the KP; however, the most profound change occurs in brain KYNA concentration, which dose-dependently increases in different brain regions 2h after administration [129–132]. In the context of cerebral ischemia, studies on the effect of L-Kyn administration are controversial: L-Kyn, apparently by elevating brain KYNA levels, results in neuroprotection when administered before hypoxia/ischemia and NMDA lesions [133–136]; conversely, post-ischemic L-Kyn sulphate administration exacerbated acute neuronal damage after permanent and transient MCAO [40, 137]. This discrepancy could be due to direct actions of L-Kyn after stroke, without the involvement of its downstream products.
Effectively, studies from our group [40] support this assumption, demonstrating that L-Kyn accumulatesin the brain during acute ischemia where it functions as an endogenous activator of AhR, promoting its transcriptional activity. Furthermore, our results also shown that exogenous L-Kyn administration after occlusion exacerbates brain damage in an AhR-dependent fashion. Perhaps most intriguingly, our data also pointed toward L-Kyn synthesis by TDO as the foremost pathway for the increased L-Kyn concentration in the ischemic brain. In fact, inhibition of L-Kyn production by the TDO inhibitor 680C91I decreased AhR activation and reduced infarct volume in our experimental setting. Interestingly, pharmacological blockade of IDO by the IDO inhibitor 1-methyl-D-tryptophan (1-MT) did not produce any effect in the infarct volume. In fact, supporting our results, a previous study from Sobey and cols. [138] demonstrated that although IDO expression and activity (higher plasmatic L-Kyn/L-Trp ratio) are increased after transient MCAO in mice, genetic and pharmacological approaches for IDO inhibition after ischemia (IDO knockout mice and 1-MT treatment, respectively) had no beneficial effects on infarct volume and neurological outcome. Therefore, these results could suggest that brain TDO activity mainly accounts for L-Kyn biosynthesis, at least, at early times after stroke onset.
It is important to understand which cell types are responsible for producing L-Kyn during cerebral ischemia as well as the mechanisms that mediate their activation. Cerebral ischemia evokes a strong inflammatory response which is thought to contribute to the progression of ischemic brain injury [9]. After stroke, a cascade of signals leads to the activation of resident glial cells -mainly microglia-, perivascular macrophages, as well as to an influx of blood-derived cells recruited by cytokines, adhesion molecules, and chemokines [9, 120, 139–141]. Given that IDO activity in mainly regulated by inflammatory cytokines [98–99] and its expression is largely confined to immune cells [100–102], previous studies in the stroke field pointed to IDO as the main actor responsible for the increased L-Kyn/L-Trp ratio, by consuming L-Trp while increasing peripheral L-Kyn and therefore, the influx of L-Kyn into the brain. However, local L-Kyn production has also been demonstrated after stroke, although cell types responsible for its generation are not well defined yet. In this sense, several studies after global ischemia in mice reported that IDO is mainly up-regulated in neurons and not in inflammatory cells, and its activation is independent of its main inductor IFN-γ [142,143]. Furthermore, our work also provides evidence of neuronal TDO after stroke, which is, at least in part, responsible for brain L-Kyn synthesis. TDO has always been thought to contribute mainly to systemic L-Trp catabolism. However, recent reports have suggested that TDO may also play important roles in both mouse and human brain functions. Its expression in the brain is mainly confined to neurons and, to a lesser extent, to astrocytes, being absent in microglia [97, 144–147]. TDO activation is mainly regulated by its own substrate L-Trp and by hormones such as cortisol and prolactin [98, 99]. In the context of cerebral ischemia, an early and transient increase in L-Trp levels in brain has been clearly observed by several groups [40, 110]. Thus, the activation of TDO after stroke could be mediated by an increased availability of its substrate L-Trp. In addition, although TDO is not directly modulated by pro-inflammatory cytokines as IDO does, an inflammatory context, for instance produced by lipopolysaccharide (LPS) in a toll-like receptor (TLR)4-dependent manner, could induce its expression [54]. In this sense, it is interesting to note that very early after ischemic injury, TLR4 is activated by damage-associated molecular patterns (DAMPs) from necrotic and apoptotic cells to induce/amplify the inflammatory response and contribute to cerebral damage [9]. Given the well established role of TLR4 on exacerbating cerebral damage [148–151], TLR4-dependent regulation of TDO could also be a possible mechanism contributing to the increased KP after stroke.
Consequently, an interplay between the immune system and the KP could exist after stroke, but different inflammatory-independent mechanisms could also mediate a role in the initial regulation of this pathway, modulating the rate-limiting enzymes of tryptophan catabolism. An interesting possibility could be that AhR acts as a link between TDO and IDO expression and their activation after ischemia. In fact, AhR is able to regulate at the transcriptional level the expression of IDO1 [152]. Intriguingly, AhR-mediated IDO induction may act as a positive feedback mechanism further activating AhR. A pathway implicating this possibility has been recently demonstrated for endotoxin tolerance [54] in which TDO expression induced by LPS in a TLR4-dependent manner results in the production of L-Kyn and the activation of AhR in innate immune cells. AhR activation induces IDO1 expression resulting in the establishment of endotoxin tolerance. Thus, the current observations raise the possibility that a similar pathway may be involved in cerebral ischemia in which AhR activation by TDO-L-Kyn finally modulates the expression of IDO at later time points, and thus could contribute to delayed brain damage.
5.3. Kynurenines as a Predisposing Factor for Stroke Risk and Stroke Outcome?
Different studies support that the risk and even the outcome and mortality of stroke patients may be increased for different predisposing factors or comorbidities. Among these mechanisms, atherosclerosis [153], chronic inflammation [154], systemic infections [155], metabolic syndrome [156], acute coronary events [157] and aging [158] are the best characterized. Interestingly, all these conditions have been found to present common features related with the KP: an overactivation of some KP enzymes (mainly IDO), higher L-Kyn/L-Trp ratios and also higher levels of KP catabolites such as L-Kyn and QUIN. Therefore, KP alterations could be a novel significant predictor tool for avoiding the risk and an unfavourable outcome of stroke.
For instance, both extra- and intracranial atherosclerosis are not only leading causes for stroke but also are associated stroke risk and poor stroke outcome [159,160]. Atherosclerosis is an inflammatory initiated by the accumulation of Apolipoprotein B100 containing low density lipoprotein (LDL) in the artery wall leading to a chronic inflammatory response in which both vascular and immune cells are activated contributing in all phases of the atherosclerotic process and even finally, in its thrombotic complications [161, 162]. Abnormalities in the KP have been also detected in patients with stable coronary artery disease. In fact, increased plasmatic IDO activity (L-Kyn/-Trp ratio), L-Kyn and 3-HK levels were positive correlated with poor prognosis and risk of acute coronary events [163]. Furthermore, higher KYNA levels were associated with unstable human plaque phenotype and predict adverse cardiovascular outcomes [164]. However, most part of pre-clinical data in animal models does not support clinical observations. Actually, using different murine models of atherosclerosis (LDLr−/− and ApoE−/− mice) or graft arteriosclerosis, increased IDO1 expression in plasmacytoid dendritic, vascular smooth muscle- or B cells was demonstrated to regulate immune response, by dampening T cell activation and proliferation [165–167], by directly modulating adhesion molecules, such as VCAM-1 [85] or even regulate the production of IL-10 [168]. In agreement with these results, IDO inhibition by 1-MT in hypercholesterolemic mice [85] or IDO deficiency (IDO−/−) in ApoE−/− mice [168] increased lesion size. Administration of L-Trp catabolites such as 3-HAA [84, 85] decreased atherosclerosis lesion and reversed detrimental effects of 1-MT in ApoE−/−. In addition, a synthetic derivative of the anthranilic acid [3,4,-dimethoxycinnamoyl anthranilic acid (3,4- DAA)] [168] was also demonstrated to reduced lesion formation and inflammation after arterial injury in ApoE−/− mice. Taken together, IDO seems to be a key regulator of inflammation in atherosclerosis, probably due to the well established role of IDO1 as a homeostatic mechanism against excessive immune activation promoting immune tolerance.
However, the role of IDO in atherosclerosis is still controversial. For instance, the study of Nakajima and cols. did not find any difference in lesion size after the administration of 1-MT to LDLr deficient mice [167]. Supporting this observation, IDO−/−/ApoE−/− mice presented an accelerated formation of atherosclerotic lesion but no differences were observed at later time points [168]. Finally, a recent study found that IDO deficiency reduces the development of atherosclerosis in LDLr−/− mice through an enhanced IL-10 production and identifies KYNA as the main inhibitor of IDO-dependent IL-10 production [164]. Given this background, further studies are necessary to interpret and clarify these discrepancies regarding the effect of KP in the context of atherosclerosis.
Among non-modifiable risk factors, age is one the most important ones for cerebral ischemia, and recovery after stroke is significantly influenced by age. Aging is associated with altered KP catabolism. In fact, an increased L-Kyn/L-Trp ratio has been detected across aging models in different species [169] including humans [158]. Several studies focused in invertebrates indicated that reducing L-Kyn levels through pharmacological or genetic inhibition of TDO prolonged life span in Drosophila and C. elegans models [170,171] and, even most interestingly, TDO in these models acts as a metabolic regulator of age-related α-synuclein, amyloid-β and polyglutamine proteins toxicity linking the KP with aging-associated neurodegeneration. Multiple mechanisms may explain aging-associated up-regulation of L-Kyn/L-Trp ratio. For instance, aging increases cortisol production, which may in turn induce TDO expression [172]. In addition, as a consequence of the “inflammaging” process (or aging-associated chronic inflammation) [173], IDO could also be activated by pro-inflammatory cytokines as previously described. Besides IDO activation could interfere with T cell functions contributing therefore to aging immunosenescence [174].
6. BEHAVIORAL AND COGNITIVE EFFECTS OF KYNURENINES
6.1. Effect of KP Manipulation in Animal Models
The analysis of the subsequent behaviour after manipulation of kynurenines in experimental animal models has allowed to establish that this pathway plays a key role in several aspects of cognitive function [175]. Increasing cerebral KYNA levels produces long-term impairment of different cognitive domains which include deficits of spatial working memory [176–179], changes in pre-pulse inhibition of auditory startle stimuli [178], alterations in the freezing response after contextual fear conditioning [180], impaired context discrimination or even deficits in the acquisition of the extra-dimensional shift task [179]. On the contrary, after decreasing KYNA levels by using a genetic model in which KAT II is deleted, an improved cognitive performance was observed in behavioural tasks including exploration, object recognition and passive avoidance learning [181]. Therefore, imbalance in the KP could affect different cognitive domains. Most of these deficits commonly occur in a number of psychiatric conditions and neurodegenerative process. All evidence supports that changes in the KP could be involved in cognitive dysfunction after pathological conditions. Moreover, its role in depressive-like behavior in animal models has also been described. While administration of L-Kyn, QUIN and 3-HK exerted an anxiogenic effect, KYNA administration produced an anxiolytic activity in different animal models of anxiety. Supporting the role of the KP in depression, TDO knockout mice displayed an anxiety-related behavior [146].
6.2. A Role of KP in Cognitive Impairment After Stroke?: Focus on Dementia and Depression
Despite efforts to delineate the effects of KP after the acute phase of stroke, little attention has been focused in evaluating the role of altered KP in the chronic phase of this pathology in which stroke survivors present a high incidence of disability with a plethora of cognitive deficits. Interestingly, a study from Mackay and cols. [182] found that alterations in the KP catabolism persist at least one year after stroke onset suggesting that KP activation possibly contributes to the continuing cerebral dysfunction in these patients. Common disabilities observed in stroke survivors include dementia (also known as vascular dementia) and depression.
6.3. Vascular Cognitive Impairment
Dementia could be defined like an acquired cognitive decline beyond what might be attributed to normal aging. The main pathogenic mechanisms involved in the development of dementia are neurodegeneration but also cerebrovascular dysfunction (as the case of stroke). In fact, both factors have been observed to be closely associated [24]. Among patients with stroke, 10% of them develop a new dementia after a first stroke, and after recurrent stroke, >30% of the patients have dementia. Given the high prevalence of this type of dementia, the concept of VCI has gained wide acceptance and is defined as “a syndrome with evidence of clinical stroke or subclinical vascular brain injury and cognitive impairment affecting at least one cognitive domain” [183]. VCI may progress to a most severe form of dementia called vascular dementia (VD). The main cognitive domains affected in patients with VCI/VD include attention, memory, language, orientation, visuospatial skills and abstract reasoning [184,185]. In addition, it is interesting to note that mixed lesions overlapping AD neuropathology and cerebrovascular deficits are observed in more than 50% of dementia patients [186]. Interestingly, vascular lesions may also be found in other neurodegenerative diseases, such as hippocampal sclerosis, synucleinopathies or frontotemporal lobar degeneration, but the mixed vascular-AD pathology is the most frequent [24].
Despite the growing body of evidence that supports the notion that KP alterations could play an important role in the pathogenic mechanism of AD [175, 187], such as the increased ratio of 3-hydroxykynurenine to L-Trp in serum [188, 189] and the accumulation of QUIN in the brain of AD patients [190], its role in VCI has only been briefly explored. Only one study demonstrated an association between elevated L-Kyn/L-Trp ratios and the extent of cognitive impairment among acute ischemic stroke patients. In fact, the L-Kyn/L-Trp ratio was able to identify subjects at risk of significant VCI. So far, this study is the only evidence which suggests a relationship between cognitive impairment and activation of the post-stroke kynurenine pathway [191]. However, it remains to be demonstrated whether a similar correlation between KP levels and VCI persists in the chronic phase of stroke and also which are the specific domains affected by the altered KP after stroke.
6.4. Post-Stroke Depression
Depression is commonly characterized by anhedonia (loss of interest or pleasure) for 2 weeks or longer and the presence of at least four of the following persistent symptoms: weight loss or gain, sleep disturbances, psychomotor agitation or retardation, fatigue, worthlessness or inappropriate guilt, diminished concentration, or indecisiveness [192]. One of the most common disorders affecting stroke survivors is PSD. In fact, depression occurs more frequently in patients after stroke than in the general population [193, 194], reporting an incidence from 18% to 61%, depending on patient selection and criteria used. In addition, PSD is associated with cognitive impairment, increased mortality and risk of falls, increased disability, and worse rehabilitation outcome.
Several neuroendocrine alterations have been reported in depression, including changes in tryptophan catabolism. In fact, a reduction in 5-HT production and an increased KP catabolism is clearly observed after depression. In this context, several studies reported an increase activation of both TDO/IDO and also KATs which lead to an augmentation of 3-HK, QUIN, L- Kyn and KYNA production [195], that finally induce ROS generation and alterations in glutamatergic neurotransmission [196–198]. Finally, all these changes would mediate damage in several brain regions including hippocampus, inhibiting neurogenesis and increasing apoptosis signaling pathways [199]. This is known as the “kynurenine hypothesis of depression” [200]. Moreover, the high levels of KYNA found in depression could also modulate, not only the glutamatergic but also the cholinergic hippocampal neurotransmission since KYNA acts as an antagonist of the α7nAChR [201]. Several clinical studies in the stroke field have tried to evaluate the role of KP in post-stroke depression and its relation to pro-inflammatory markers, given that inflammation is also commonly observed in depressive patients. Unfortunately, aforementioned studies did not find a correlation between peripheral L-Kyn/L-Trp ratios and depressive symptoms in the post-stroke population [202, 203]. However, several interesting results were observed in these studies. First, exploratory analyses suggested that inflammatory activity was associated with mild–moderate but not with severe depressive symptoms. Second, stroke patients with post-stroke fatigue (PSF) have a lower bioavailability of L-Trp for 5-HT synthesis in the brain in the acute stroke phase. Therefore, further studies are necessary to clarify the role of the KP in PSD.
CONCLUSION
The tryptophan catabolism has been reported to be altered in different pathologies affecting the brain, such is the case of stroke, a devastating disease which is one of the main causes of death and disability worldwide but treatment options are still limited including rtPA administration and mechanical thrombectomy. The activation of the KP in the acute phase of stroke may participate in the ischemic damage by direct mechanisms which include excitotoxicity and oxidative stress among others, since inhibition of the KP decreases brain injury in animal models of stroke. However, further studies are necessary to determine the mechanism which modulates the activation of KP after cerebral ischemia. Probably, an interplay between the immune system and the KP could exist after stroke, but also different inflammatory-independent mechanisms could mediate a role in the regulation of this pathway, modulating the rate-limiting enzymes of tryptophan catabolism. Interestingly, the kynurenine pathway after cerebral ischemia could also play a role during the chronic phase of this pathology in which stroke survivors present a high incidence of disabilities such as dementia and depression or even being a risk factor for stroke outcome and mortality. All together the kynurenine and tryptophan catabolism could have a significant role in after cerebral ischemia. A deepest knowledge about the regulation and activity of the KP after stroke could provide new pharmacological tools in both acute and chronic phases of stroke.
Acknowledgments
This work was supported by grants from Spanish Ministry of Economy and Competitiveness SAF2012-33216 (MAM), CSD2010-00045 (MAM), and from Regional Madrid Government S2010/BMD-2336 (MAM).
LIST OF ABBREVIATIONS
- 1-MT
1-methyl-D-tryptophan
- 3-HAA
3-hydroxy-anthranilic acid
- 3-HAO
3-hydroxyanthranilate 3,4-dioxygenase
- 3-HK
3-hydroxykynurenine
- α7nAChR
α7-nicotinic acetylcholine receptor
- ACMS
2-amino-3-carboxymuconate semialdehyde
- AhR
aryl hydrocarbon receptor
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- ANA
anthranilic acid
- BBB
blood-brain barrier
- CNS
central nervous system
- IDO
indoleamine-2,3-dioxygenase
- IFN-γ
interferon-gamma
- IL
interleukin
- K3H
kynurenine 3-hydroxylase
- KAT
kynurenine aminotransferase
- KMO
kynurenine 3-monooxygenase
- KP
kynurenine pathway
- KYNA
kynurenic acid
- KYNU
kynureninase
- LDL
low density lipoprotein
- L-Kyn
L-kynurenine
- LPS
lipopolysaccharide
- L-Tryp
tryptophan
- MCAO
middle cerebral artery occlusion
- NAD
nicotinamide adenine dinucleotide
- NMDA
N-methyl-D-aspartate
- PIC
picolinic acid
- PSD
post-stroke depression
- QPRT
quinolate phosphoribisyltransferase
- QUIN
quinolinic acid
- rtPA
recombinant thrombolytic tissue plasminogen activator
- TDO
tryptophan-2,3-dioxygenase
- Th
T-helper
- TLR
toll-like receptor
- Treg
regulatory T cell
- VCI
vascular cognitive impairment
- VD
vascular dementia
Footnotes
CONFLICT OF INTEREST
Funding Sources: This work was supported by grants from Spanish Ministry of Economy and Competitiveness SAF2012-33216 (MAM), CSD2010-00045 (MAM), and from Regional Madrid Government S2010/BMD-2336 (MAM). JdlP is a fellow of the Spanish Ministry of Economy and Competitiveness.
References
- 1.Murray CJ, Lopez AD. Mortality by cause for eight regions of the world: Global Burden of Disease Study. Lancet. 1997;349:1269–76. doi: 10.1016/S0140-6736(96)07493-4. [DOI] [PubMed] [Google Scholar]
- 2.Mozaffarian D, Benjamin EJ, Go AS, et al. Subcommittee AHAS-CaSS. Heart disease and stroke statistics--2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 3.The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333:1581–7. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 4.Berkhemer OA, Fransen PS, Beumer D, et al. A randomized trial of intraarterial treatment for 786 acute ischemic stroke. N Engl J Med. 2015;372:11–20. doi: 10.1056/NEJMoa1411587. [DOI] [PubMed] [Google Scholar]
- 5.Campbell BC, Mitchell PJ, Kleinig TJ, et al. Endovascular therapy for ischemic stroke with perfusion-imaging 794 selection. N Engl J Med. 2015;372:1009–18. doi: 10.1056/NEJMoa1414792. [DOI] [PubMed] [Google Scholar]
- 6.Saver JL, Goyal M, Bonafe A, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. 2015;372:2285–95. doi: 10.1056/NEJMoa1415061. [DOI] [PubMed] [Google Scholar]
- 7.Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372:1019–30. doi: 10.1056/NEJMoa1414905. [DOI] [PubMed] [Google Scholar]
- 8.Jovin TG, Chamorro A, Cobo E, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015;372:2296–306. doi: 10.1056/NEJMoa1503780. [DOI] [PubMed] [Google Scholar]
- 9.Iadecola C, Anrather J. The immunology of stroke: from mechanisms to translation. Nat Med. 2011;17:796–808. doi: 10.1038/nm.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999;22:391–7. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- 11.Minnerup J, Sutherland BA, Buchan AM, Kleinschnitz C. Neuroprotection for stroke: current status and future perspectives. Int J Mol Sci. 2012;13:11753–72. doi: 10.3390/ijms130911753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sutherland BA, Minnerup J, Balami JS, Arba F, Buchan AM, Kleinschnitz C. Neuroprotection for ischaemic stroke: translation from the bench to the bedside. Int J Stroke. 2012;7:407–18. doi: 10.1111/j.1747-4949.2012.00770.x. [DOI] [PubMed] [Google Scholar]
- 13.Saver JL. Targeting the brain: neuroprotection and neurorestoration in ischemic stroke. Pharmacotherapy. 2010;30:62S–9S. doi: 10.1592/phco.30.pt2.62S. [DOI] [PubMed] [Google Scholar]
- 14.Mehta A, Prabhakar M, Kumar P, Deshmukh R, Sharma PL. Excitotoxicity: bridge to various triggers in neurodegenerative disorders. 832. Eur J Pharmacol. 2013;698:6–18. doi: 10.1016/j.ejphar.2012.10.032. [DOI] [PubMed] [Google Scholar]
- 15.Choi DW. Ionic dependence of glutamate neurotoxicity. J Neurosci. 1987;7:369–79. doi: 10.1523/JNEUROSCI.07-02-00369.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moller K, Boltze J, Posel C, Seeger J, Stahl T, Wagner DC. Sterile inflammation after permanent distal MCA occlusion in hypertensive rats. J Cereb Blood Flow Metab. 2014;34:307–15. doi: 10.1038/jcbfm.2013.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murray KN, Buggey HF, Denes A, Allan SM. Systemic immune activation shapes stroke outcome. Mol Cell Neurosci. 2013;53:14–25. doi: 10.1016/j.mcn.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 18.Dénes Á, Pradillo JM, Drake C, et al. Streptococcus pneumoniae worsens cerebral ischemia via interleukin 1 and platelet glycoprotein Ibα. Ann Neurol. 2014;75:670–83. doi: 10.1002/ana.24146. [DOI] [PubMed] [Google Scholar]
- 19.Pradillo JM, Denes A, Greenhalgh AD, et al. Delayed administration of interleukin-1 receptor antagonist reduces ischemic brain damage and inflammation in comorbid rats. J Cereb Blood Flow Metab. 2012;32:1810–9. doi: 10.1038/jcbfm.2012.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manwani B, Liu F, Scranton V, Hammond MD, Sansing LH, McCullough LD. Differential effects of aging and sex on stroke induced inflammation across the lifespan. Exp Neurol. 2013;249:120–31. doi: 10.1016/j.expneurol.2013.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sarti C, Rastenyte D, Cepaitis Z, Tuomilehto J. International trends in mortality from stroke 1968 to 1994. Stroke. 2000;31:1588–601. doi: 10.1161/01.str.31.7.1588. [DOI] [PubMed] [Google Scholar]
- 22.Sacco RL. Risk factors, outcomes, and stroke subtypes for ischemic stroke. Neurology. 1997;49:S39–44. doi: 10.1212/wnl.49.5_suppl_4.s39. [DOI] [PubMed] [Google Scholar]
- 23.House A. Mood disorders in the first year after stroke. Nurs Times. 1991;87:53–4. [PubMed] [Google Scholar]
- 24.Iadecola C. The pathobiology of vascular dementia. Neuron. 2013;80:844–66. doi: 10.1016/j.neuron.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paolucci S. Epidemiology and treatment of post-stroke depression. Neuropsychiatr Dis Treat. 2008;4:145–54. doi: 10.2147/ndt.s2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang J, Zhang Y, Xing S, Liang Z, Zeng J. Secondary neurodegeneration in remote regions after focal cerebral infarction: a new target for stroke management? Stroke. 2012;43:1700–5. doi: 10.1161/STROKEAHA.111.632448. [DOI] [PubMed] [Google Scholar]
- 27.Schwarcz R, Bruno JP, Muchowski PJ, Wu HQ. Kynurenines in the mammalian brain: when physiology meets pathology. Nat Rev Neurosci. 2012;13:465–77. doi: 10.1038/nrn3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vécsei L, Szalárdy L, Fülöp F, Toldi J. Kynurenines in the CNS: recent advances and new questions. Nat Rev Drug Discov. 2013;12:64–82. doi: 10.1038/nrd3793. [DOI] [PubMed] [Google Scholar]
- 29.Vamos E, Pardutz A, Klivenyi P, Toldi J, Vecsei L. The role of kynurenines in disorders of the central nervous system: possibilities for 875 neuroprotection. J Neurol Sci. 2009;283:21–7. doi: 10.1016/j.jns.2009.02.326. [DOI] [PubMed] [Google Scholar]
- 30.Zádori D, Klivényi P, Vámos E, Fülöp F, Toldi J, Vécsei L. Kynurenines in chronic neurodegenerative disorders: future therapeutic strategies. J Neural Transm. 2009;116:1403–9. doi: 10.1007/s00702-009-0263-4. [DOI] [PubMed] [Google Scholar]
- 31.Pardridge WM. Blood-brain barrier carrier-mediated transport and brain metabolism of amino acids. Neurochem Res. 1998;23:635–44. doi: 10.1023/a:1022482604276. [DOI] [PubMed] [Google Scholar]
- 32.Saito Y, Hayaishi O, Rothberg S. Studies on oxygenases; enzymatic formation of 3-hydroxy-L-kynurenine from L-kynurenine. J Biol Chem. 1957;229:921–34. [PubMed] [Google Scholar]
- 33.Shimizu T, Nomiyama S, Hirata F, Hayaishi O. Indoleamine 2,3-dioxygenase. Purification and some properties. J Biol Chem. 1978;253:4700–6. [PubMed] [Google Scholar]
- 34.Guillemin GJ, Smith DG, Kerr SJ, et al. Characterisation of kynurenine pathway metabolism in human astrocytes and implications in neuropathogenesis. Redox Rep. 2000;5:108–11. doi: 10.1179/135100000101535375. [DOI] [PubMed] [Google Scholar]
- 35.Guillemin GJ, Smith DG, Smythe GA, Armati PJ, Brew BJ. Expression of the kynurenine pathway enzymes in human microglia and macrophages. Adv Exp Med Biol. 2003;527:105–12. doi: 10.1007/978-1-4615-0135-0_12. [DOI] [PubMed] [Google Scholar]
- 36.Fukui S, Schwarcz R, Rapoport SI, Takada Y, Smith QR. Blood-brain barrier transport of kynurenines: implications for brain synthesis and metabolism. J Neurochem. 1991;56:2007–17. doi: 10.1111/j.1471-4159.1991.tb03460.x. [DOI] [PubMed] [Google Scholar]
- 37.Hokari M, Wu HQ, Schwarcz R, Smith QR. Facilitated brain uptake of 4- chlorokynurenine and conversion to 7-chlorokynurenic acid. Neuroreport. 1996;8:15–8. doi: 10.1097/00001756-199612200-00004. [DOI] [PubMed] [Google Scholar]
- 38.Wang Y, Liu H, McKenzie G, et al. Kynurenine 903 is an endothelium-derived relaxing factor produced during inflammation. 904. Nat Med. 2010;16:279–85. doi: 10.1038/nm.2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reyes Ocampo J, Lugo Huitrón R, González-Esquivel D, et al. Kynurenines with neuroactive and redox properties: relevance to aging and brain diseases. Oxid Med Cell Longev. 2014;2014:646909. doi: 10.1155/2014/646909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cuartero MI, Ballesteros I, de la Parra J, et al. L-kynurenine/aryl hydrocarbon receptor pathway mediates brain damage after experimental stroke. Circulation. 2014;130:2040–51. doi: 10.1161/CIRCULATIONAHA.114.011394. [DOI] [PubMed] [Google Scholar]
- 41.Kawasaki H, Chang HW, Tseng HC, et al. A tryptophan metabolite, kynurenine, promotes mast cell activation through aryl hydrocarbon receptor. Allergy. 2014;69:445–52. doi: 10.1111/all.12346. [DOI] [PubMed] [Google Scholar]
- 42.Mezrich JD, Fechner JH, Zhang X, Johnson BP, Burlingham WJ, Bradfield CA. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J Immunol. 2010;185:3190–8. doi: 10.4049/jimmunol.0903670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Opitz CA, Litzenburger UM, Sahm F, et al. An endogenous tumour-promoting ligand of the human aryl 924 hydrocarbon receptor. Nature. 2011;478:197–203. doi: 10.1038/nature10491. [DOI] [PubMed] [Google Scholar]
- 44.Kewley RJ, Whitelaw ML, Chapman-Smith A. The mammalian basic helix-loop-helix/PAS family of transcriptional regulators. Int J Biochem 927 Cell Biol. 2004;36:189–204. doi: 10.1016/s1357-2725(03)00211-5. [DOI] [PubMed] [Google Scholar]
- 45.Denison MS, Nagy SR. Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu Rev Pharmacol Toxicol. 2003;43:309–34. doi: 10.1146/annurev.pharmtox.43.100901.135828. [DOI] [PubMed] [Google Scholar]
- 46.Bock KW, Köhle C. Ah receptor: dioxin-mediated toxic responses as hints to deregulated physiologic functions. Biochem Pharmacol. 2006;72:393–404. doi: 10.1016/j.bcp.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 47.Furness SG, Whelan F. The pleiotropy of dioxin toxicity--xenobiotic misappropriation of the aryl hydrocarbon receptor’s alternative physiological roles. Pharmacol Ther. 2009;124:336–53. doi: 10.1016/j.pharmthera.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 48.Barouki R, Coumoul X, Fernandez-Salguero PM. The aryl hydrocarbon receptor, more than a xenobiotic-interacting protein. FEBS Lett. 2007;581:3608–15. doi: 10.1016/j.febslet.2007.03.046. [DOI] [PubMed] [Google Scholar]
- 49.Fernandez-Salguero PM, Ward JM, Sundberg JP, Gonzalez FJ. Lesions of aryl-hydrocarbon receptor-deficient mice. Vet Pathol. 1997;34:605–14. doi: 10.1177/030098589703400609. [DOI] [PubMed] [Google Scholar]
- 50.Esser C. The immune phenotype of AhR null mouse mutants: not a simple mirror of xenobiotic receptor over-activation. Biochem Pharmacol. 2009;77:597–607. doi: 10.1016/j.bcp.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 51.Esser C, Rannug A, Stockinger B. The aryl hydrocarbon receptor in immunity. Trends Immunol. 2009;30:447–54. doi: 10.1016/j.it.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 52.Moennikes O, Loeppen S, Buchmann A, et al. A constitutively active dioxin/aryl hydrocarbon receptor promotes hepatocarcinogenesis in mice. Cancer Res. 2004;64:4707–10. doi: 10.1158/0008-5472.CAN-03-0875. [DOI] [PubMed] [Google Scholar]
- 53.Andersson P, McGuire J, Rubio C, et al. A constitutively active dioxin/aryl hydrocarbon receptor induces stomach tumors. Proc Natl Acad Sci USA. 2002;99:955 9990–5. doi: 10.1073/pnas.152706299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bessede A, Gargaro M, Pallotta MT, et al. Aryl hydrocarbon receptor control of a disease tolerance defence pathway. Nature. 2014;511:184–90. doi: 10.1038/nature13323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Turski WA, Nakamura M, Todd WP, Carpenter BK, Whetsell WO, Schwarcz R. Identification and quantification of kynurenic acid in human brain tissue. Brain Res. 1988;454:164–9. doi: 10.1016/0006-8993(88)90815-3. [DOI] [PubMed] [Google Scholar]
- 56.Birch PJ, Grossman CJ, Hayes AG. Kynurenic acid antagonises responses to NMDA via an action at the strychnine-insensitive glycine receptor. Eur J Pharmacol. 1988;154:85–7. doi: 10.1016/0014-2999(88)90367-6. [DOI] [PubMed] [Google Scholar]
- 57.Perkins MN, Stone TW. An iontophoretic investigation of the actions of convulsant kynurenines and their interaction with the endogenous excitant quinolinic acid. Brain Res. 1982;247:184–7. doi: 10.1016/0006-8993(82)91048-4. [DOI] [PubMed] [Google Scholar]
- 58.Kessler M, Terramani T, Lynch G, Baudry M. A glycine site associated with N-methyl-D-aspartic acid receptors: characterization and identification of a new class of antagonists. J Neurochem. 1989;52:1319–28. doi: 10.1111/j.1471-4159.1989.tb01881.x. [DOI] [PubMed] [Google Scholar]
- 59.Carpenedo R, Pittaluga A, Cozzi A, et al. Presynaptic kynurenate-sensitive receptors inhibit glutamate release. 979. Eur J Neurosci. 2001;13:2141–7. doi: 10.1046/j.0953-816x.2001.01592.x. [DOI] [PubMed] [Google Scholar]
- 60.Stone TW, Stoy N, Darlington LG. An expanding range of targets for kynurenine metabolites of tryptophan. Trends Pharmacol Sci. 2013;34:136–43. doi: 10.1016/j.tips.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 61.Hilmas C, Pereira EF, Alkondon M, Rassoulpour A, Schwarcz R, Albuquerque EX. The brain metabolite kynurenic acid inhibits alpha7 nicotinic receptor activity and increases non-alpha7 nicotinic receptor expression: physiopathological implications. J Neurosci. 2001;21:7463–73. doi: 10.1523/JNEUROSCI.21-19-07463.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang J, Simonavicius N, Wu X, et al. Kynurenic acid as a ligand for orphan G protein-coupled receptor GPR35. J Biol Chem. 2006;281:22021–8. doi: 10.1074/jbc.M603503200. [DOI] [PubMed] [Google Scholar]
- 63.Chassaing N, De Mas P, Tauber M, et al. Molecular characterization of a cryptic 2q37 deletion in a patient with Albright hereditary osteodystrophy-like phenotype. Am J Med Genet A. 2004;128A:410–3. doi: 10.1002/ajmg.a.30199. [DOI] [PubMed] [Google Scholar]
- 64.DiNatale BC, Murray IA, Schroeder JC, et al. Kynurenic acid is a potent endogenous aryl hydrocarbon receptor ligand that synergistically induces interleukin-6 in the presence of inflammatory signaling. Toxicol Sci. 2010;115:89–97. doi: 10.1093/toxsci/kfq024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dong-Ryul L, Kondo H, Furukawa S, Nakano K. Stimulation of NGF production by tryptophan and its metabolites in cultured mouse astroglial cells. Brain Res. 1997;777:228–30. doi: 10.1016/s0006-8993(97)01164-5. [DOI] [PubMed] [Google Scholar]
- 66.Di Serio C, Cozzi A, Angeli I, et al. Kynurenic acid inhibits the release of the neurotrophic fibroblast growth factor (FGF)-1 and enhances proliferation of glia cells, in vitro. Cell Mol Neurobiol. 2005;25:981–93. doi: 10.1007/s10571-005-8469-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stone TW, Perkins MN. Quinolinic acid: a potent endogenous excitant at amino acid receptors in CNS. Eur J Pharmacol. 1981;72:411–2. doi: 10.1016/0014-2999(81)90587-2. [DOI] [PubMed] [Google Scholar]
- 68.Santiago-López D, Vázquez-Román B, Pérez-de La Cruz V, et al. Peroxynitrite decomposition catalyst, iron metalloporphyrin, reduces quinolinate-induced neurotoxicity in rats. Synapse. 2004;54:233–8. doi: 10.1002/syn.20084. [DOI] [PubMed] [Google Scholar]
- 69.Guillemin GJ. Quinolinic acid, the inescapable neurotoxin. FEBS J. 2012;279:1356–65. doi: 10.1111/j.1742-4658.2012.08485.x. [DOI] [PubMed] [Google Scholar]
- 70.Lugo-Huitrón R, Ugalde Muñiz P, Pineda B, Pedraza-Chaverrí J, Ríos C, Pérez-de la Cruz V. Quinolinic acid: an endogenous neurotoxin with multiple targets. Oxid Med Cell Longev. 2013;2013:104024. doi: 10.1155/2013/104024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Guillemin GJ, Croitoru-Lamoury J, Dormont D, Armati PJ, Brew BJ. Quinolinic acid upregulates chemokine production and chemokine receptor expression in astrocytes. Glia. 2003;41:371–81. doi: 10.1002/glia.10175. [DOI] [PubMed] [Google Scholar]
- 72.Eastman CL, Guilarte TR. Cytotoxicity of 3-hydroxykynurenine in a neuronal hybrid cell line. Brain Res. 1989;495:225–31. doi: 10.1016/0006-8993(89)90216-3. [DOI] [PubMed] [Google Scholar]
- 73.Eastman CL, Guilarte TR. The role of hydrogen peroxide in the in vitro cytotoxicity of 3-hydroxykynurenine. Neurochem Res. 1990;15:1101–7. doi: 10.1007/BF01101711. [DOI] [PubMed] [Google Scholar]
- 74.Okuda S, Nishiyama N, Saito H, Katsuki H. Hydrogen peroxide-mediated neuronal cell death induced by an endogenous neurotoxin, 3-hydroxykynurenine. Proc Natl Acad Sci USA. 1996;93:12553–8. doi: 10.1073/pnas.93.22.12553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Szalardy L, Klivenyi P, Zadori D, Fulop F, Toldi J, Vecsei L. Mitochondrial disturbances, tryptophan metabolites and neurodegeneration: medicinal chemistry aspects. Curr Med Chem. 2012;19:1899–920. doi: 10.2174/092986712800167365. [DOI] [PubMed] [Google Scholar]
- 76.Colín-González AL, Maldonado PD, Santamaría A. 3-Hydroxykynurenine: an intriguing molecule exerting dual actions in the central nervous system. Neurotoxicology. 2013;34:189–204. doi: 10.1016/j.neuro.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 77.Breton J, Avanzi N, Magagnin S, et al. Functional characterization and mechanism of action of recombinant human kynurenine 3-hydroxylase. Eur J Biochem. 2000;267:1092–9. doi: 10.1046/j.1432-1327.2000.01104.x. [DOI] [PubMed] [Google Scholar]
- 78.Bender DA, McCreanor GM. The preferred route of kynurenine metabolism in the rat. Biochim Biophys Acta. 1982;717:56–60. doi: 10.1016/0304-4165(82)90379-8. [DOI] [PubMed] [Google Scholar]
- 79.Fallarino F, Grohmann U, Vacca C, et al. T cell apoptosis by tryptophan catabolism. Cell Death Differ. 2002;9:1069–77. doi: 10.1038/sj.cdd.4401073. [DOI] [PubMed] [Google Scholar]
- 80.Platten M, Ho PP, Youssef S, et al. Treatment of autoimmune neuroinflammation with a synthetic tryptophan metabolite. Science. 2005;310:850–5. doi: 10.1126/science.1117634. [DOI] [PubMed] [Google Scholar]
- 81.Krause D, Suh HS, Tarassishin L, et al. The tryptophan metabolite 3-hydroxyanthranilic acid plays anti-inflammatory and neuroprotective roles during inflammation: role of hemeoxygenase-1. Am J Pathol. 2011;179:1360–72. doi: 10.1016/j.ajpath.2011.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Thomas SR, Witting PK, Stocker R. 3-Hydroxyanthranilic acid is an efficient, cell-derived co-antioxidant for alpha-tocopherol, inhibiting human low density lipoprotein and plasma lipid peroxidation. J Biol Chem. 1996;271:32714–21. doi: 10.1074/jbc.271.51.32714. [DOI] [PubMed] [Google Scholar]
- 83.Latini A, Rodriguez M, Borba Rosa R, et al. 3-Hydroxyglutaric acid moderately impairs energy metabolism in brain of young rats. Neuroscience. 2005;135:111–20. doi: 10.1016/j.neuroscience.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 84.Zhang L, Ovchinnikova O, Jönsson A, et al. The tryptophan metabolite 3-hydroxyanthranilic acid lowers plasma lipids and decreases atherosclerosis in hypercholesterolaemic mice. Eur Heart J. 2012;33:2025–34. doi: 10.1093/eurheartj/ehs175. [DOI] [PubMed] [Google Scholar]
- 85.Polyzos KA, Ovchinnikova O, Berg M, et al. Inhibition of indoleamine 2,3-dioxygenase promotes 1068 vascular inflammation and increases atherosclerosis in Apoe−/− mice. Cardiovasc Res. 2015;106:295–302. doi: 10.1093/cvr/cvv100. [DOI] [PubMed] [Google Scholar]
- 86.Takaki F, Suzuki T, Yasuda H, Tomi M, Mikuni K, Yamaguchi H. Photo- and electron-microscopical studies of inclusion body-like structures seen in malignant tumor cells I. Gan. 1957;48:324–6. [PubMed] [Google Scholar]
- 87.Bosco MC, Rapisarda A, Massazza S, Melillo G, Young H, Varesio L. The tryptophan catabolite picolinic acid selectively induces the chemokines macrophage inflammatory protein-1 alpha and -1 beta in macrophages. J Immunol. 2000;164:3283–91. doi: 10.4049/jimmunol.164.6.3283. [DOI] [PubMed] [Google Scholar]
- 88.Melillo G, Cox GW, Biragyn A, Sheffler LA, Varesio L. Regulation of nitric-oxide synthase mRNA expression by interferon-gamma and picolinic acid. J Biol Chem. 1994;269:8128–33. [PubMed] [Google Scholar]
- 89.Cockhill J, Jhamandas K, Boegman RJ, Beninger RJ. Action of picolinic acid and structurally related pyridine carboxylic acids on quinolinic acid-induced cortical cholinergic damage. Brain Res. 1992;599:57–63. doi: 10.1016/0006-8993(92)90852-z. [DOI] [PubMed] [Google Scholar]
- 90.Kalisch BE, Jhamandas K, Boegman RJ, Beninger RJ. Picolinic acid protects against quinolinic acid-induced depletion of NADPH diaphorase containing neurons in the rat striatum. Brain Res. 1994;668:1–8. doi: 10.1016/0006-8993(94)90504-5. [DOI] [PubMed] [Google Scholar]
- 91.Tonohiro T, Tanabe M, Kaneko T, Iwata N. Is picolinic acid a glycine agonist at strychnine-sensitive receptors? Brain Res. 1990;516:332–4. doi: 10.1016/0006-8993(90)90937-7. [DOI] [PubMed] [Google Scholar]
- 92.Beskid M, Jachimowicz J, Taraszewska A, Kukulska D. Histological and ultrastructural changes in the rat brain following systemic administration of picolinic acid. Exp Toxicol Pathol. 1995;47:25–30. doi: 10.1016/S0940-2993(11)80278-2. [DOI] [PubMed] [Google Scholar]
- 93.Miche H, Brumas V, Berthon G. Copper(II) interactions with non-steroidal antiinflammatory agents. II. Anthranilic acid as a potential. OH-inactivating ligand. J Inorg Biochem. 1997;68:27–38. doi: 10.1016/s0162-0134(97)00005-6. [DOI] [PubMed] [Google Scholar]
- 94.Gaubert S, Bouchaut M, Brumas V, Berthon G. Copper--ligand interactions and the physiological free radical processes. Part 3. Influence of histidine, salicylic acid and anthranilic acid on copper-driven Fenton chemistry in vitro. Free Radic Res. 2000;32:451–61. doi: 10.1080/10715760000300451. [DOI] [PubMed] [Google Scholar]
- 95.Gál EM, Sherman AD. Synthesis and metabolism of L-kynurenine in rat brain. J Neurochem. 1978;30:607–13. doi: 10.1111/j.1471-4159.1978.tb07815.x. [DOI] [PubMed] [Google Scholar]
- 96.Yoshida R, Hayaishi O. Indoleamine 2,3-dioxygenase. Methods Enzymol. 1987;142:188–95. doi: 10.1016/s0076-6879(87)42028-4. [DOI] [PubMed] [Google Scholar]
- 97.Kanai M, Nakamura T, Funakoshi H. Identification and characterization of novel variants of the tryptophan 2,3-dioxygenase gene: differential regulation in the mouse nervous system during development. Neurosci Res. 2009;64:111–7. doi: 10.1016/j.neures.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 98.Walker AK, Budac DP, Bisulco S, et al. NMDA receptor blockade by ketamine abrogates 1108 lipopolysaccharide-induced depressive-like behavior in C57BL/6J mice. Neuropsychopharmacology. 2013;38:1609–16. doi: 10.1038/npp.2013.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lestage J, Verrier D, Palin K, Dantzer R. The enzyme indoleamine 2,3-dioxygenase is induced in the mouse brain in response to peripheral administration of lipopolysaccharide and superantigen. Brain Behav Immun. 2002;16:596–601. doi: 10.1016/s0889-1591(02)00014-4. [DOI] [PubMed] [Google Scholar]
- 100.Wirleitner B, Neurauter G, Schröcksnadel K, Frick B, Fuchs D. Interferon- gamma-induced conversion of tryptophan: immunologic and neuropsychiatric aspects. Curr Med Chem. 2003;10:1581–91. doi: 10.2174/0929867033457179. [DOI] [PubMed] [Google Scholar]
- 101.Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol. 2004;4:762–74. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- 102.Mándi Y, Vécsei L. The kynurenine system and immunoregulation. J Neural Transm. 2012;119:197–209. doi: 10.1007/s00702-011-0681-y. [DOI] [PubMed] [Google Scholar]
- 103.Heyes MP, Chen CY, Major EO, Saito K. Different kynurenine pathway enzymes limit quinolinic acid formation by various human cell types. Biochem J. 1997;326(Pt 2):351–6. doi: 10.1042/bj3260351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kita T, Morrison PF, Heyes MP, Markey SP. Effects of systemic and central nervous system localized inflammation on the contributions of metabolic precursors to the L-kynurenine and quinolinic acid pools in brain. J Neurochem. 2002;82:258–68. doi: 10.1046/j.1471-4159.2002.00955.x. [DOI] [PubMed] [Google Scholar]
- 105.Thevandavakkam MA, Schwarcz R, Muchowski PJ, Giorgini F. Targeting kynurenine 3-monooxygenase (KMO): implications for therapy in Huntington’s disease. CNS Neurol Disord Drug Targets. 2010;9:791–800. doi: 10.2174/187152710793237430. [DOI] [PubMed] [Google Scholar]
- 106.Giorgini F, Möller T, Kwan W, et al. Histone deacetylase inhibition modulates kynurenine pathway activation in yeast, microglia, and mice expressing a mutant huntingtin fragment. J Biol Chem. 2008;283:7390–400. doi: 10.1074/jbc.M708192200. [DOI] [PubMed] [Google Scholar]
- 107.Saito K, Chen CY, Masana M, Crowley JS, Markey SP, Heyes MP. 4-Chloro-3-hydroxyanthranilate, 6-chlorotryptophan and norharmane attenuate quinolinic acid formation by interferon-gamma-stimulated monocytes (THP-1 cells) Biochem J. 1993;291(Pt 1):11–4. doi: 10.1042/bj2910011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Schwarcz R, Poeggeler B, Rassoulpour A, Ceresoli-Borroni G, Hodgkins PS. Regulation of kynurenic acid levels in the developing rat brain. Amino Acids. 1998;14:243–9. doi: 10.1007/BF01345270. [DOI] [PubMed] [Google Scholar]
- 109.Gramsbergen JB, Hodgkins PS, Rassoulpour A, Turski WA, Guidetti P, Schwarcz R. Brain-specific modulation of kynurenic acid synthesis in the rat. J Neurochem. 1997;69:290–8. doi: 10.1046/j.1471-4159.1997.69010290.x. [DOI] [PubMed] [Google Scholar]
- 110.Saito K, Crowley JS, Markey SP, Heyes MP. A mechanism for increased 1148 quinolinic acid formation following acute systemic immune stimulation. J Biol Chem. 1993;268:15496–503. [PubMed] [Google Scholar]
- 111.Saito K, Nowak TS, Suyama K, et al. Kynurenine pathway enzymes in brain: responses to ischemic brain injury versus systemic immune activation. J Neurochem. 1993;61:2061–70. doi: 10.1111/j.1471-4159.1993.tb07443.x. [DOI] [PubMed] [Google Scholar]
- 112.Heyes MP, Nowak TS. Delayed increases in regional brain quinolinic acid follow transient ischemia in the gerbil. J Cereb Blood Flow Metab. 1990;10:660–7. doi: 10.1038/jcbfm.1990.119. [DOI] [PubMed] [Google Scholar]
- 113.Barattè S, Molinari A, Veneroni O, Speciale C, Benatti L, Salvati P. Temporal and spatial changes of quinolinic acid immunoreactivity in the gerbil hippocampus following transient cerebral ischemia. Brain Res Mol Brain Res. 1998;59:50–7. doi: 10.1016/s0169-328x(98)00136-3. [DOI] [PubMed] [Google Scholar]
- 114.Luchowska E, Luchowski P, Sarnowska A, Wielosz M, Turski WA, Urbańska EM. Endogenous level of kynurenic acid and activities of kynurenine aminotransferases following transient global ischemia in the gerbil hippocampus. Pol J Pharmacol. 2003;55:443–7. [PubMed] [Google Scholar]
- 115.Darlington LG, Mackay GM, Forrest CM, Stoy N, George C, Stone TW. Altered kynurenine metabolism correlates with infarct volume in stroke. Eur J Neurosci. 2007;26:2211–21. doi: 10.1111/j.1460-9568.2007.05838.x. [DOI] [PubMed] [Google Scholar]
- 116.Darlington LG, Forrest CM, Mackay GM, et al. On the Biological Importance of the 3-hydroxyanthranilic Acid: Anthranilic Acid Ratio. Int J Tryptophan Res. 2010;3:51–9. doi: 10.4137/ijtr.s4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Brouns R, Verkerk R, Aerts T, et al. The role of tryptophan catabolism along the kynurenine pathway in acute ischemic stroke. Neurochem Res. 2010;35:1315–22. doi: 10.1007/s11064-010-0187-2. [DOI] [PubMed] [Google Scholar]
- 118.Mo X, Pi L, Yang J, Xiang Z, Tang A. Serum indoleamine 2,3-dioxygenase and kynurenine aminotransferase enzyme activity in patients with ischemic stroke. J Clin Neurosci. 2014;21:482–6. doi: 10.1016/j.jocn.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 119.Amantea D, Micieli G, Tassorelli C, et al. Rational modulation of the innate immune system for neuroprotection in ischemic stroke. Front Neurosci. 2015;9:147. doi: 10.3389/fnins.2015.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cuartero MI, Ballesteros I, Lizasoain I, Moro MA. Complexity of the cell-cell interactions in the innate immune response after cerebral ischemia. Brain Res. 2015 doi: 10.1016/j.brainres.2015.04.047. [DOI] [PubMed] [Google Scholar]
- 121.Dohmen C, Kumura E, Rosner G, Heiss WD, Graf R. Extracellular correlates of glutamate toxicity in short-term cerebral ischemia and reperfusion: a direct in vivo comparison between white and gray matter. Brain Res. 2005;1037:43–51. doi: 10.1016/j.brainres.2004.12.046. [DOI] [PubMed] [Google Scholar]
- 122.Moroni F, Carpenedo R, Cozzi A, et al. Studies on the neuroprotective action of kynurenine mono-oxygenase inhibitors in post-ischemic brain damage. Adv Exp Med Biol. 2003;527:127–36. doi: 10.1007/978-1-4615-0135-0_15. [DOI] [PubMed] [Google Scholar]
- 123.Carpenedo R, Meli E, Peruginelli F, Pellegrini-Giampietro DE, Moroni F. Kynurenine 3-mono-oxygenase inhibitors attenuate post-ischemic neuronal death in organotypic hippocampal slice cultures. J Neurochem. 2002;82:1465–71. doi: 10.1046/j.1471-4159.2002.01090.x. [DOI] [PubMed] [Google Scholar]
- 124.Cozzi A, Carpenedo R, Moroni F. Kynurenine hydroxylase inhibitors reduce ischemic brain damage: studies with (m-nitrobenzoyl)-alanine (mNBA) and 3,4-dimethoxy-[-N-4-(nitrophenyl)thiazol-2yl]-benzenesulfonamide (Ro 61-8048) in models of focal or global brain ischemia. J Cereb Blood Flow Metab. 1999;19:771–7. doi: 10.1097/00004647-199907000-00007. [DOI] [PubMed] [Google Scholar]
- 125.Gill R, Woodruff GN. The neuroprotective actions of kynurenic acid and MK-801 in gerbils are synergistic and not related to hypothermia. Eur J Pharmacol. 1990;176:143–9. doi: 10.1016/0014-2999(90)90522-8. [DOI] [PubMed] [Google Scholar]
- 126.Salvati P, Ukmar G, Dho L, et al. Brain concentrations of kynurenic acid after a systemic neuroprotective dose in the gerbil model of global ischemia. Prog Neuropsychopharmacol Biol Psychiatry. 1999;23:741–52. doi: 10.1016/s0278-5846(99)00032-9. [DOI] [PubMed] [Google Scholar]
- 127.Moroni F, Cozzi A, Sili M, Mannaioni G. Kynurenic acid: a metabolite with multiple actions and multiple targets in brain and periphery. J Neural Transm. 2012;119:133–9. doi: 10.1007/s00702-011-0763-x. [DOI] [PubMed] [Google Scholar]
- 128.Gellért L, Fuzik J, Göblös A, et al. Neuroprotection with a new kynurenic acid analog in the four-vessel occlusion model of ischemia. Eur J Pharmacol. 2011;667:182–7. doi: 10.1016/j.ejphar.2011.05.069. [DOI] [PubMed] [Google Scholar]
- 129.Swartz KJ, During MJ, Freese A, Beal MF. Cerebral synthesis and release of kynurenic acid: an endogenous antagonist of excitatory amino acid receptors. J Neurosci. 1990;10:2965–73. doi: 10.1523/JNEUROSCI.10-09-02965.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Swartz KJ, Matson WR, MacGarvey U, Ryan EA, Beal MF. Measurement of kynurenic acid in mammalian brain extracts and cerebrospinal fluid by high-performance liquid chromatography with fluorometric and coulometric electrode array detection. Anal Biochem. 1990;185:363–76. doi: 10.1016/0003-2697(90)90309-w. [DOI] [PubMed] [Google Scholar]
- 131.Zmarowski A, Wu HQ, Brooks JM, et al. Astrocyte-derived kynurenic acid modulates basal and evoked cortical acetylcholine release. Eur J Neurosci. 2009;29:529–38. doi: 10.1111/j.1460-9568.2008.06594.x. [DOI] [PubMed] [Google Scholar]
- 132.Scharfman HE, Goodman JH, Schwarcz R. Electrophysiological effects of exogenous and endogenous kynurenic acid in the rat brain: studies in vivo and in vitro. Amino Acids. 2000;19:283–97. doi: 10.1007/s007260070060. [DOI] [PubMed] [Google Scholar]
- 133.Robotka H, Sas K, Agoston M, et al. Neuroprotection achieved in the ischaemic rat cortex with L-1230 kynurenine sulphate. Life Sci. 2008;82:915–9. doi: 10.1016/j.lfs.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 134.Sas K, Robotka H, Rózsa E, et al. Kynurenine diminishes the ischemia-induced histological and electrophysiological deficits in the rat hippocampus. Neurobiol Dis. 2008;32:302–8. doi: 10.1016/j.nbd.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 135.Gigler G, Szénási G, Simó A, et al. Neuroprotective effect of L-kynurenine sulfate administered before focal cerebral ischemia in mice and global cerebral ischemia in gerbils. Eur J Pharmacol. 2007;564:116–22. doi: 10.1016/j.ejphar.2007.02.029. [DOI] [PubMed] [Google Scholar]
- 136.Nozaki K, Beal MF. Neuroprotective effects of L-kynurenine on hypoxia-ischemia and NMDA lesions in neonatal rats. J Cereb Blood Flow Metab. 1992;12:400–7. doi: 10.1038/jcbfm.1992.57. [DOI] [PubMed] [Google Scholar]
- 137.Gellért L, Knapp L, Németh K, et al. Post-ischemic treatment with L-kynurenine sulfate exacerbates neuronal damage after transient middle cerebral artery occlusion. Neuroscience. 2013;247C:95–101. doi: 10.1016/j.neuroscience.2013.04.063. [DOI] [PubMed] [Google Scholar]
- 138.Jackman KA, Brait VH, Wang Y, et al. Vascular expression, activity and function of indoleamine 2,3-dioxygenase-1 following cerebral ischaemia-reperfusion in mice. Naunyn Schmiedebergs Arch Pharmacol. 2011;383:471–81. doi: 10.1007/s00210-011-0611-4. [DOI] [PubMed] [Google Scholar]
- 139.Kriz J. Inflammation in ischemic brain injury: timing is important. Crit Rev Neurobiol. 2006;18:145–57. doi: 10.1615/critrevneurobiol.v18.i1-2.150. [DOI] [PubMed] [Google Scholar]
- 140.Chamorro A, Meisel A, Planas AM, Urra X, van de Beek D, Veltkamp R. The immunology of acute stroke. Nature reviews Neurology. 2012;8:401–10. doi: 10.1038/nrneurol.2012.98. [DOI] [PubMed] [Google Scholar]
- 141.Jin R, Yang G, Li G. Inflammatory mechanisms in ischemic stroke: role of inflammatory cells. J Leukoc Biol. 2010;87:779–789. doi: 10.1189/jlb.1109766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hoshi M, Saito K, Murakami Y, et al. Marked increases in hippocampal neuron indoleamine 2, 3-dioxygenase via IFN-gamma-independent pathway following transient global ischemia in mouse. Neurosci Res. 2009;63:194–8. doi: 10.1016/j.neures.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 143.Taguchi A, Hara A, Saito K, et al. Localization and spatiotemporal expression of IDO following transient forebrain ischemia in gerbils. Brain Res. 2008;1217:78–85. doi: 10.1016/j.brainres.2008.02.067. [DOI] [PubMed] [Google Scholar]
- 144.Wu W, Nicolazzo JA, Wen L, et al. Expression of tryptophan 2,3-dioxygenase and production of kynurenine pathway metabolites in triple transgenic mice and human Alzheimer’s disease brain. PLoS One. 2013;8:e59749. doi: 10.1371/journal.pone.0059749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Guillemin GJ, Cullen KM, Lim CK, et al. Characterization of the kynurenine pathway in human neurons. J Neurosci. 2007;27:12884–92. doi: 10.1523/JNEUROSCI.4101-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kanai M, Funakoshi H, Takahashi H, et al. Tryptophan 2,3-dioxygenase is a key modulator of physiological neurogenesis and anxiety-related behavior in mice. Mol Brain. 2009;2:8. doi: 10.1186/1756-6606-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Miller CL, Llenos IC, Dulay JR, Barillo MM, Yolken RH, Weis S. Expression of the kynurenine pathway enzyme tryptophan 2,3-dioxygenase is increased in the frontal cortex of individuals with schizophrenia. Neurobiol Dis. 2004;15:618–29. doi: 10.1016/j.nbd.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 148.Caso JR, Pradillo JM, Hurtado O, Lorenzo P, Moro MA, Lizasoain I. Toll-like receptor 4 is involved in brain damage and inflammation after experimental stroke. Circulation. 2007;115:1599–608. doi: 10.1161/CIRCULATIONAHA.106.603431. [DOI] [PubMed] [Google Scholar]
- 149.Cao CX, Yang QW, Lv FL, Cui J, Fu HB, Wang JZ. Reduced cerebral ischemia-reperfusion injury in Toll-like receptor 4 deficient mice. Biochem Biophys Res Commun. 2007;353:509–14. doi: 10.1016/j.bbrc.2006.12.057. [DOI] [PubMed] [Google Scholar]
- 150.Tang SC, Arumugam TV, Xu X, et al. Pivotal role for neuronal Toll-like receptors in ischemic brain injury and functional deficits. Proc Natl Acad Sci USA. 2007;104:13798–803. doi: 10.1073/pnas.0702553104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hua F, Ma J, Ha T, et al. Activation of Toll-like receptor 4 signaling contributes to hippocampal neuronal death following global cerebral ischemia/reperfusion. J Neuroimmunol. 2007;190:101–11. doi: 10.1016/j.jneuroim.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Nguyen NT, Kimura A, Nakahama T, et al. Aryl hydrocarbon receptor negatively regulates dendritic cell immunogenicity via a kynurenine-dependent mechanism. Proc Natl Acad Sci USA. 2010;107:19961–6. doi: 10.1073/pnas.1014465107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Pawlak K, Brzosko S, Mysliwiec M, Pawlak D. Kynurenine, quinolinic acid--the new factors linked to carotid atherosclerosis in patients with end-stage renal disease. Atherosclerosis. 2009;204:561–6. doi: 10.1016/j.atherosclerosis.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 154.Schiepers OJ, Wichers MC, Maes M. Cytokines and major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:201–17. doi: 10.1016/j.pnpbp.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 155.Wang Q, Liu D, Song P, Zou MH. Tryptophan-kynurenine pathway is dysregulated in inflammation, and immune activation. Front Biosci (Landmark Ed) 2015;20:1116–43. doi: 10.2741/4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Oxenkrug GF. Metabolic syndrome, age-associated neuroendocrine disorders, and dysregulation of tryptophan-kynurenine metabolism. Ann N Y Acad Sci. 2010;1199:1–14. doi: 10.1111/j.1749-6632.2009.05356.x. [DOI] [PubMed] [Google Scholar]
- 157.Eussen SJ, Ueland PM, Vollset SE, et al. Kynurenines as predictors of acute coronary events in the Hordaland Health Study. Int J Cardiol. 2015;189:18–24. doi: 10.1016/j.ijcard.2015.03.413. [DOI] [PubMed] [Google Scholar]
- 158.Pertovaara M, Raitala A, Lehtimäki T, et al. Indoleamine 2,3-dioxygenase activity in nonagenarians is markedly increased and predicts mortality. Mech Ageing Dev. 2006;127:497–9. doi: 10.1016/j.mad.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 159.Ritz K, Denswil NP, Stam OC, van Lieshout JJ, Daemen MJ. Cause and mechanisms of intracranial atherosclerosis. Circulation. 2014;130:1407–14. doi: 10.1161/CIRCULATIONAHA.114.011147. [DOI] [PubMed] [Google Scholar]
- 160.Wendorff C, Wendorff H, Pelisek J, et al. Carotid plaque morphology is significantly associated with sex, age, and history of neurological symptoms. Stroke. 2015;46(11):3213–9. doi: 10.1161/STROKEAHA.115.010558. [DOI] [PubMed] [Google Scholar]
- 161.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–43. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 162.Lucas AR, Korol R, Pepine CJ. Inflammation in atherosclerosis: some thoughts about acute coronary syndromes. Circulation. 2006;113:e728–32. doi: 10.1161/CIRCULATIONAHA.105.601492. [DOI] [PubMed] [Google Scholar]
- 163.Pedersen ER, Midttun Ø, Ueland PM, et al. Systemic markers of interferon-γ-mediated immune activation and long-term prognosis in patients with stable coronary artery disease. Arterioscler Thromb Vasc Biol. 2011;31:698–704. doi: 10.1161/ATVBAHA.110.219329. [DOI] [PubMed] [Google Scholar]
- 164.Metghalchi S, Ponnuswamy P, Simon T, et al. Indoleamine 2,3-dioxygenase fine-tunes immune homeostasis in atherosclerosis and colitis through repression of interleukin-10 production. Cell Metab. 2015;22:460–71. doi: 10.1016/j.cmet.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 165.Daissormont IT, Christ A, Temmerman L, et al. Plasmacytoid dendritic cells protect against atherosclerosis by tuning T-cell proliferation and activity. Circ Res. 2011;109:1387–95. doi: 10.1161/CIRCRESAHA.111.256529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Cuffy MC, Silverio AM, Qin L, et al. Induction of indoleamine 2,3-dioxygenase in vascular smooth muscle cells by interferon-gamma contributes to medial immunoprivilege. J Immunol. 2007;179:5246–54. doi: 10.4049/jimmunol.179.8.5246. [DOI] [PubMed] [Google Scholar]
- 167.Nakajima K, Yamashita T, Kita T, et al. Orally administered eicosapentaenoic acid induces rapid regression of atherosclerosis via modulating the phenotype of dendritic cells in LDL receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2011;31:1963–72. doi: 10.1161/ATVBAHA.111.229443. [DOI] [PubMed] [Google Scholar]
- 168.Cole JE, Astola N, Cribbs AP, et al. Indoleamine 2,3-dioxygenase-1 is protective in atherosclerosis and its metabolites provide new opportunities for drug development. Proc Natl Acad Sci USA. 2015;112(42):13033–8. doi: 10.1073/pnas.1517820112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Groebe K, Krause F, Kunstmann B, et al. Differential proteomic profiling of mitochondria from Podospora anserina, rat and human reveals distinct patterns of age-related oxidative changes. Exp Gerontol. 2007;42:887–98. doi: 10.1016/j.exger.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 170.van der Goot AT, Zhu W, Vázquez-Manrique RP, et al. Delaying aging and the aging-associated decline in protein homeostasis by inhibition of tryptophan degradation. Proc Natl Acad Sci USA. 2012;109:14912–7. doi: 10.1073/pnas.1203083109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Oxenkrug GF. The extended life span of Drosophila melanogaster eye-color (white and vermilion) mutants with impaired formation of kynurenine. J Neural Transm. 2010;117:23–6. doi: 10.1007/s00702-009-0341-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Dilman VM. Age-associated elevation of hypothalamic, threshold to feedback control, and its role in development, ageine, and disease. Lancet. 1971;1:1211–9. doi: 10.1016/s0140-6736(71)91721-1. [DOI] [PubMed] [Google Scholar]
- 173.Franceschi C, Bonafè M, Valensin S, et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–54. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- 174.Campbell BM, Charych E, Lee AW, Möller T. Kynurenines in CNS disease: regulation by inflammatory cytokines. Front Neurosci. 2014;8:12. doi: 10.3389/fnins.2014.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Stone TW, Darlington LG. The kynurenine pathway as a therapeutic target in cognitive and neurodegenerative disorders. Br J Pharmacol. 2013;169:1211–27. doi: 10.1111/bph.12230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Chess AC, Simoni MK, Alling TE, Bucci DJ. Elevations of endogenous kynurenic acid produce spatial working memory deficits. Schizophr Bull. 2007;33:797–804. doi: 10.1093/schbul/sbl033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Akagbosu CO, Evans GC, Gulick D, Suckow RF, Bucci DJ. Exposure to kynurenic acid during adolescence produces memory deficits in adulthood. Schizophr Bull. 2012;38:769–78. doi: 10.1093/schbul/sbq151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Nilsson LK, Linderholm KR, Erhardt S. Subchronic treatment with kynurenine and probenecid: effects on prepulse inhibition and firing of midbrain dopamine neurons. J Neural Transm. 2006;113:557–71. doi: 10.1007/s00702-005-0343-z. [DOI] [PubMed] [Google Scholar]
- 179.Pocivavsek A, Wu HQ, Elmer GI, Bruno JP, Schwarcz R. Pre- and postnatal exposure to kynurenine causes cognitive deficits in adulthood. Eur J Neurosci. 2012;35:1605–12. doi: 10.1111/j.1460-9568.2012.08064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Chess AC, Bucci DJ. Increased concentration of cerebral kynurenic acid alters stimulus processing and conditioned responding. Behav Brain Res. 2006;170:326–32. doi: 10.1016/j.bbr.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 181.Potter MC, Elmer GI, Bergeron R, et al. Reduction of endogenous kynurenic acid formation enhances extracellular glutamate, hippocampal plasticity, and cognitive behavior. Neuropsychopharmacology. 2010;35:1734–42. doi: 10.1038/npp.2010.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Mackay GM, Forrest CM, Stoy N, et al. Tryptophan metabolism and oxidative stress in patients with chronic brain injury. Eur J Neurol. 2006;13:30–42. doi: 10.1111/j.1468-1331.2006.01220.x. [DOI] [PubMed] [Google Scholar]
- 183.Gorelick PB, Scuteri A, Black SE, et al. American Heart Association Stroke Council CoEaP, Council on Cardiovascular Nursing, C.uncil on Cardiovascular Radiology and Intervention, and Council on Cardiovascular Surgery and Anesthesia. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke. 2011;42:2672–713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Cumming TB, Marshall RS, Lazar RM. Stroke, cognitive deficits, and rehabilitation: still an incomplete picture. Int J Stroke. 2013;8:38–45. doi: 10.1111/j.1747-4949.2012.00972.x. [DOI] [PubMed] [Google Scholar]
- 185.Tatemichi TK, Desmond DW, Stern Y, Paik M, Sano M, Bagiella E. Cognitive impairment after stroke: frequency, patterns, and relationship to functional abilities. J Neurol Neurosurg Psychiatry. 1994;57:202–7. doi: 10.1136/jnnp.57.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Jellinger KA. Pathology and pathogenesis of vascular cognitive impairment-a critical update. Front Aging Neurosci. 2013;5:17. doi: 10.3389/fnagi.2013.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Majláth Z, Tajti J, Vécsei L. Kynurenines and other novel therapeutic strategies in the treatment of dementia. Ther Adv Neurol Disord. 2013;6:386–97. doi: 10.1177/1756285613494989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Schwarz MJ, Guillemin GJ, Teipel SJ, Buerger K, Hampel H. Increased 3-Hydroxykynurenine serum concentrations differentiate Alzheimer’s disease patients from controls. Eur Arch Psychiatry Clin Neurosci. 2012 doi: 10.1007/s00406-012-0384-x. [DOI] [PubMed] [Google Scholar]
- 189.Widner B, Leblhuber F, Walli J, Tilz GP, Demel U, Fuchs D. Tryptophan degradation and immune activation in Alzheimer’s disease. J Neural Transm. 2000;107:343–53. doi: 10.1007/s007020050029. [DOI] [PubMed] [Google Scholar]
- 190.Guillemin GJ, Williams KR, Smith DG, Smythe GA, Croitoru-Lamoury J, Brew BJ. Quinolinic acid in the pathogenesis of Alzheimer’s disease. Adv Exp Med Biol. 2003;527:167–76. doi: 10.1007/978-1-4615-0135-0_19. [DOI] [PubMed] [Google Scholar]
- 191.Gold AB, Herrmann N, Swardfager W, et al. The relationship between indoleamine 2,3-dioxygenase activity and post-stroke cognitive impairment. J Neuroinflamm. 2011;8:17. doi: 10.1186/1742-2094-8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Battle DE. Diagnostic and Statistical Manual of Mental Disorders (DSM) CODAS. 2013;25:191–2. doi: 10.1590/s2317-17822013000200017. [DOI] [PubMed] [Google Scholar]
- 193.Ayerbe L, Ayis S, Wolfe CD, Rudd AG. Natural history, predictors and outcomes of depression after stroke: systematic review and meta-analysis. Br J Psychiatry. 2013;202:14–21. doi: 10.1192/bjp.bp.111.107664. [DOI] [PubMed] [Google Scholar]
- 194.Hackett ML, Yapa C, Parag V, Anderson CS. Frequency of depression after stroke: a systematic review of observational studies. Stroke. 2005;36:1330–40. doi: 10.1161/01.STR.0000165928.19135.35. [DOI] [PubMed] [Google Scholar]
- 195.Bender DA, McCreanor GM. Kynurenine hydroxylase: a potential rate-limiting enzyme in tryptophan metabolism. Biochem Soc Trans. 1985;13:441–3. doi: 10.1042/bst0130441. [DOI] [PubMed] [Google Scholar]
- 196.Okuda S, Nishiyama N, Saito H, Katsuki H. 3-Hydroxykynurenine, an endogenous oxidative stress generator, causes neuronal cell death with apoptotic features and region selectivity. J Neurochem. 1998;70:299–307. doi: 10.1046/j.1471-4159.1998.70010299.x. [DOI] [PubMed] [Google Scholar]
- 197.Braidy N, Grant R, Adams S, Guillemin GJ. Neuroprotective effects of naturally occurring polyphenols on quinolinic acid-induced excitotoxicity in human neurons. FEBS J. 2010;277:368–82. doi: 10.1111/j.1742-4658.2009.07487.x. [DOI] [PubMed] [Google Scholar]
- 198.Pariante CM. Depression, stress and the adrenal axis. J Neuroendocrinol. 2003;15:811–2. doi: 10.1046/j.1365-2826.2003.01058.x. [DOI] [PubMed] [Google Scholar]
- 199.Sheline YI, Gado MH, Kraemer HC. Untreated depression and hippocampal volume loss. Am J Psychiatry. 2003;160:1516–8. doi: 10.1176/appi.ajp.160.8.1516. [DOI] [PubMed] [Google Scholar]
- 200.Lapin IP. Kynurenines as probable participants of depression. Pharmakopsychiatr Neuropsychopharmakol. 1973;6:273–9. doi: 10.1055/s-0028-1094391. [DOI] [PubMed] [Google Scholar]
- 201.Alkondon M, Pereira EF, Yu P, et al. Targeted deletion of the kynurenine aminotransferase ii gene reveals a critical role of endogenous kynurenic acid in the regulation of synaptic transmission via alpha7 nicotinic receptors in the hippocampus. J Neurosci. 2004;24:4635–48. doi: 10.1523/JNEUROSCI.5631-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Ormstad H, Verkerk R, Amthor KF, Sandvik L. Activation of the kynurenine pathway in the acute phase of stroke and its role in fatigue and depression following stroke. J Mol Neurosci. 2014;54:181–7. doi: 10.1007/s12031-014-0272-0. [DOI] [PubMed] [Google Scholar]
- 203.Bensimon K, Herrmann N, Swardfager W, et al. Kynurenine and depressive symptoms in a poststroke population. Neuropsychiatr Dis Treat. 2014;10:1827–35. doi: 10.2147/NDT.S65740. [DOI] [PMC free article] [PubMed] [Google Scholar]