Abstract
Background
Oral squamous cell carcinoma is a prevalent and frequently lethal malignancy worldwide. Existence of treatment-resistant cancer stem cells is considered to be associated with tumor formation, recurrence and metastasis. Wnt/beta-catenin signal is one of the crucial signaling pathways for cancer stem cells. Wnt/beta-catenin signal inhibitor may reduce the population of cancer stem cells and improve therapeutic effects on the cancers.
Methods
The effects of three derivatives of Wnt/beta-catenin signal inhibitors, HC-1, IC-2 and PN3-13, which we recently developed, on oral squamous cell carcinoma cell line HSC2, were examined by luciferase reporter assay, WST assay, cell sorting assay and apoptosis assay.
Results
The reporter assay showed that these small molecule compounds reduced Wnt/beta-catenin transcriptional activity in HSC2 cells. Of these compounds, IC-2 and PN3-13 inhibited cell viability in a dose-dependent manner, whereas HC-1 did not at even higher concentrations. Notably, however, the cell-sorting assay revealed that HC-1 significantly reduces the CD44-positive population of oral squamous cell carcinoma cells, compared to other compounds without affecting cell viability. In addition, HC-1 increases the cytotoxicity of HSC2 cells to 5-fluorouracil. The combination treatment of HC-1 with 5-fluorouracil significantly increased the apoptotic cells whereas treatment by either compound did not.
Conclusion
These data suggest that HC-1 is an effective compound to target cancer stem cells, and the combination treatment of HC-1 and 5-fluorouracil can stimulate the tumor suppressive effect on oral squamous cell carcinoma cells.
Keywords: 5-fluorouracil, cancer stem cells, oral squamous cell carcinoma, small molecule compound, Wnt/beta-catenin signal inhibitor
Oral squamous cell carcinoma (OSCC) is a major prevalent malignancy throughout the world, and its mortality rate is approximately 50%.1–3 Despite advances in conventional treatments including surgery, radiotherapy and chemotherapy, the survival rate for OSCC has not improved significantly over the past few decades.4 High mortality seems to be induced by the emergence of therapy-resistant local recurrences and the development of distant metastases.5, 6 Thus, more effective therapeutic strategy is urgently required for improving the clinical outcome of OSCC.
Recent data have demonstrated that multiple cancers, including OSCC, contain a small subpopulation of cells that have stem cell-like properties, so-called cancer stem cells (CSCs).7–9 CSCs have the unique features of self-renewal and asymmetrical division, and show resistance to radio- and chemotherapy.10, 11 CSCs in OSCC were first isolated using cell surface marker CD44.5 The existence of CSCs may explain the high rate of recurrences after conventional therapies. Therefore, targeting of CSCs is considered to provide a new strategy to improve therapeutic effects on many cancers.
Wnt/beta-catenin signal is one of the crucial signaling pathways for self-renewal and differentiation of CSCs.12, 13 Expression levels of stem cell related genes, including CD44, are regulated by this pathway. We have previously shown that Wnt/beta-catenin signal is suppressed during the hepatic differentiation process of human mesenchymal stem cells (MSCs),14, 15 and recently developed small molecule compounds, derivatives of Wnt/beta-catenin signal inhibitors, that efficiently suppress Wnt/beta-catenin transcriptional activity in human MSCs.16, 17 In the present study, we found that these compounds also suppress the Wnt/beta-catenin signal in human OSCC cell line HSC2. Of these compounds, HC-1 effectively reduced the rate of CD44-positive cells without affecting cell viability. In addition, HC-1 sensitized HSC2 cells to 5-fluorouracil (5-FU), a DNA/RNA synthesis inhibitor,18 by virtue of enhancement of apoptotic cell death.
MATERIALS AND METHODS
Compounds
We recently developed new small molecular compounds, the derivatives of the compounds which were originally reported to be Wnt/beta-catenin signal inhibitors of colorectal cancer cells,19–21 for human MSCs.17 These compounds include HC-1, a derivative of hexachlorophene; IC-2, a derivative of ICG-001; and PN-3-13, a derivative of PNU-74654. The compounds were dissolved in dimethylsulfoxide (DMSO) whose final concentration was 1%, and used in the experiments.
Cells and culture
Human OSCC cell line, HSC2 were kindly provided by Dr. H. Kugoh (Tottori Univ., Yonago, Japan). The cells were incubated in DMEM (Life Technologies, Carlsbad, CA) supplemented with 10% FBS, 1% penicillin streptomycin, 2 mM L-glutamine, 0.2% NaHCO3 and 3.5 g/L D-glucose with or without chemical compounds.
Luciferase reporter assay
HSC2 cells were seeded onto a 24-well plate at a density of 5 × 104 cells/well. After overnight incubation, the cells were transfected with pTCF4-CMVpro-Fluc and pRL-CMV-Rluc plasmids14 using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) for 4 h, and then treated with various concentrations of compounds for 48 h. The luciferase activity was measured by Dual-LuciferaseR Reporter assay system (Promega, Madison, WI) and MiniLumat LB 9506 (Berthold Technologies, Bad Wildbad, Germany).
WST assay
HSC2 cells were seeded onto a 96-well plate at a density of 2.5 × 103 cells/well. After overnight incubation, the cells were treated with various concentrations of compounds for 48 h. Cell viability was analyzed by WST assay using Cell Counting kit-8 (Dojindo, Kumamoto, Japan) and Micro plate reader (Tecan, Männedorf, Switzerland). IC50 values are 50% cell growth inhibitory concentrations of each compound, which are obtained from the following equation: IC50 = 10^[LOG(A/B)x(50-C)/(D-C) + LOG(B)] - A, a higher concentration of two values that sandwich IC50; B, a lower concentration of two values that sandwich IC50; C, cell viability (%) at B; D, cell viability (%) at A.
Flow cytometry
HSC2 cells were seeded onto 10 cm plate at a density of 5 × 105 cells/well. After overnight incubation, the cells were treated with indicated concentrations of compounds for 48 h. Then cells were sorted by a cell sorting system (FACS-Aria, Becton Dickinson and Company, Franklin Lakes, NJ) using CD44 mouse antibody (Cell Signaling Technology, Danvers, MA) and goat-antimouse IgG Alexa 488 (Life Technologies, Carlsbad, CA).
Apoptosis assay
HSC2 cells were seeded onto a 6-well plate at a density of 1 × 105 cells/well. After overnight incubation, the cells were treated with 0.1 µM 5-FU and/or 0.1 µM HC-1 for 48 h. Then, the cells were stained by annexin-V and PI using an annexin-V-FLOUS Staining Kit (Roche Diagnostics, Indianapolis, IN), photographed with a microscope (IX-71) (Olympus, Tokyo, Japan) and analyzed using an image analysis system (inForm 2.0) (PerkinElmer, Norwalk, CT).
Western blot analysis
Cell lysis proteins (12.5 µg) were subjected to Western blot assay. The rabbit polyclonal antibody against beta-catenin (Cell Signaling Technology, Danvers, MA) and goat polyclonal antibody against Actin (Santa Cruz Biotechnology, Santa Cruz, CA) were used. Actin was served as an internal control.
Statistical analysis
All the values were expressed as mean ± SD. Multiple comparison was performed by a one-way ANOVA followed by a Tukey HSD test or Games-Howell test using predictive analytics software (SPSS, Chicago, IL). The differences between the two groups were analyzed by an unpaired two-tailed Student’s t-test. A P-value less than 0.05 was considered to be significant.
RESULTS
Small molecule compounds inhibit TCF4/beta-catenin transcriptional activity in OSCC HSC2 cells
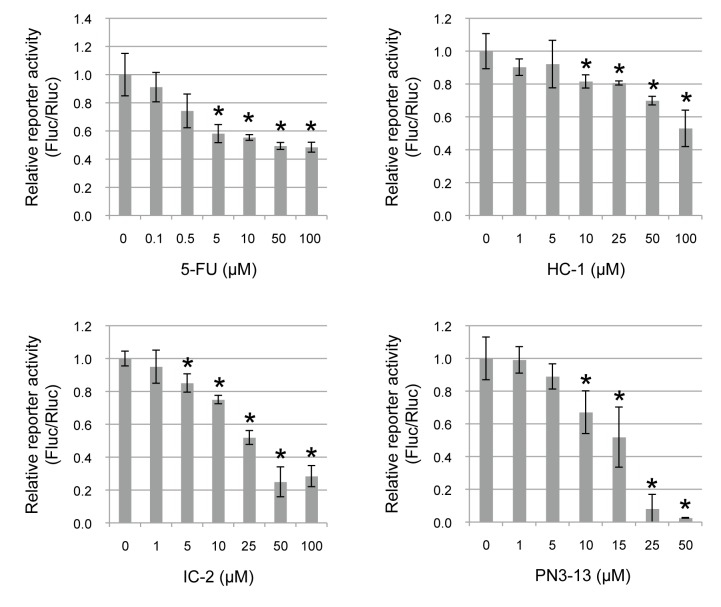
To examine the effect of derivatives of Wnt/beta-catenin signal inhibitors on TCF4/beta-catenin transcriptional activity in OSCC cells, a luciferase reporter assay was performed using HSC2 cells. The cells were treated with various concentrations of HC-1, IC-2, PN3-13 or 5-FU for 48 h (Fig. 1). All the derivatives significantly suppressed the luciferase activity in a dose-dependent manner, indicating that these compounds possess an inhibitory effect on Wnt/beta-catenin signal in HSC2 cells (Fig. 1)
Fig. 1.
Small molecule compounds inhibit TCF4/beta-catenin transcriptional activity in OSCC HSC2 cells. TCF4/beta-catenin transcriptional activity was examined by luciferase reporter assay with indicated concentrations of 5-FU (0–100 µM), HC-1 (0–100 µM), IC-2 (0–100 µM) and PN3-13 (0–50 µM). The cells were transfected with pTCF4-CMVpro-Fluc plasmid and internal control pRL-CMV-Rluc plasmid, and luciferase activity of pTCF4-CMVpro-Fluc (Fluc) was normalized by the activity of pRL-CMV-Rluc (Rluc).*P < 0.05, compared to 0 µM of each compound. The statistics were examined by unpaired Student’s t-test. Data are shown as mean ± SD of three experiments. 5-FU, 5-fluorouracil; OSCC, Oral squamous cell carcinoma.
Cell viability assay of HSC2 cells treated with chemical compounds
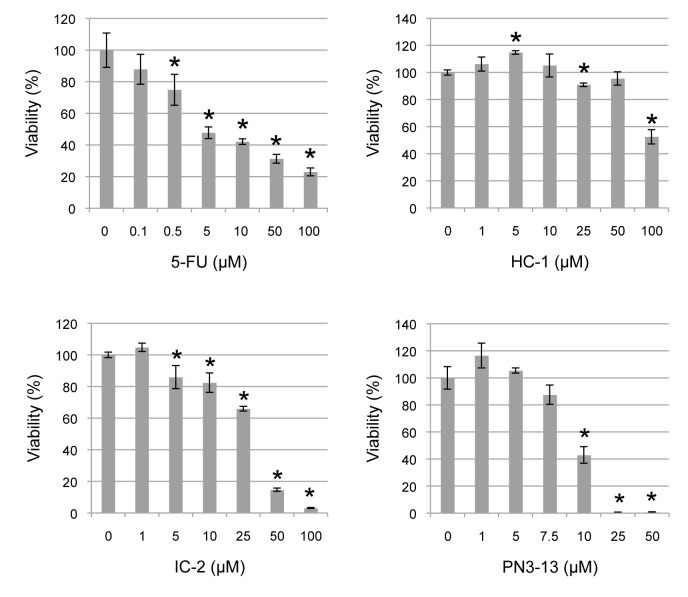
To investigate the effect of Wnt/beta-catenin signal inhibitors on proliferation of HSC2 cells, cell viability was analyzed by WST assay. The cells were treated with various concentrations of compounds for 48 h. IC-2, PN3-13 and 5-FU significantly reduced cell viability in a dose-dependent manner with IC50 at 31 µM, 9.6 µM and 4.1 µM, respectively (Fig. 2). HC-1 had little effect on cell viability up to 50 µM, whereas it reduced that to about 50% at 100 µM.
Fig. 2.
Cell viability assay of HSC2 cells treated with small molecule compounds. Cells were treated with increasing concentrations of 5-FU, HC-1, IC-2 and PN3-13 for 48 h. Cell viability was analyzed by WST assay. *P < 0.05, compared to 0 µM of each compound. The statistics were examined by unpaired Student’s t-test. Data are shown as mean ± SD of three experiments. 5-FU, 5-fluorouracil.
HC-1 reduces CD44-positive population in HSC2 cells
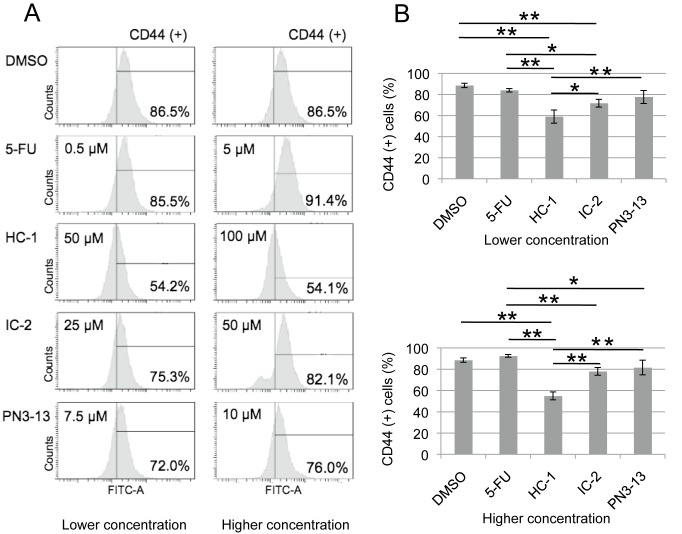
Growing evidence suggests that CSCs are highly resistant to conventional therapies and are responsible for recurrence and metastasis.10, 11 Thus, the suppression of CSCs seems to be important for cancer therapy. To examine the effect of Wnt/beta-catenin inhibitors on CSC population, we next performed FACS analysis using antibody specific to CD44, a CSC marker for OSCC (Fig. 3A).5 HSC2 cells were treated with these compounds for 48 h at two different concentrations (lower and higher) whose values are close to the IC50 of the cell viability assay (Fig. 2). Both concentrations displayed similar effects on the CD44-positive population. IC-2 showed a modest decrease of CD44-positive cells, whereas PN3-13 and 5-FU did not significantly change the rate, compared to DMSO treatment (Fig. 3B). Interestingly, HC-1 clearly and more effectively reduced the CD44-positive population, compared to other compounds (Fig. 3B), suggesting that HC-1 efficiently targets CSCs of OSCC cells.
Fig. 3.
HC-1 reduces CD44-positive population in HSC2 cells. Cells were treated by 5-FU, HC-1, IC-2 and PN3-13 for 48 h with indicated concentrations and sorted by cell sorting system using CD44 antibody.
A: Representative sorting images of the cells treated with lower or higher concentration, of which value is close to IC50 of the compounds (Fig. 2), with the ratio of CD44-positive cells. B: Quantification of CD44-positive cells treated with lower or higher concentration, of which value is close to IC50. *P < 0.05, **P < 0.01, compared between two groups. The statistics were examined by one-way ANOVA, followed by Tukey HSD test. Data are shown as mean ± SD of three experiments. 5-FU, 5-fluorouracil; DMSO, dimethylsulfoxide; IC50, inhibitory concentration 50.
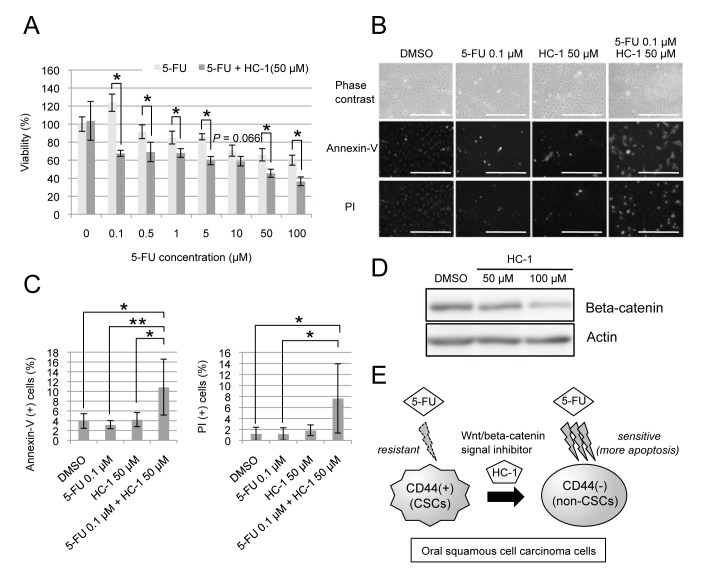
Combined treatment using 5-FU and HC-1 increases the rate of apoptotic cell death
The decrease of the CD44-positive population by HC-1 treatment raised the possibility that HC-1 increases the cytotoxicity of conventional anticancer drugs, such as 5-FU. To test the effect of the combination treatment, HSC2 cells were treated with 0 to 100 µM 5-FU alone or in combination with HC-1 (Fig. 4A). The concentration of HC-1 used was 50 µM, which showed no inhibitory effect on HSC2 proliferation (Fig. 2). Notably, HC-1 significantly enhanced the cytotoxicity of 5-FU even at 0.1 µM (Fig. 4A). To investigate how HC-1 enhanced the cytotoxicity of 5-FU, we examined whether apoptosis of the cells is induced by the treatment of 5-FU with or without HC-1 (Fig. 4B). Annexin -V and PI staining revealed that apoptosis increased when the cells were treated with 5-FU in combination with HC-1, compared to DMSO treatment (Figs. 4B and C). No induction of apoptosis was observed in the cells treated with HC-1 or 5-FU alone, which is consistent to the cell viability assay (Figs. 2 and Fig. 4). These results suggest that HC-1 increases the cytotoxic effect of 5-FU through enhancement of apoptosis in HSC2 cells.
Fig. 4.
Combined treatment using 5-FU and HC-1 increases the rate of apoptotic cell death.
A: HSC2 cells were treated with increasing concentration of 5-FU alone or in combination with 50 µM HC-1 for 48 h. Cell viability was analyzed by WST assay. *P < 0.05, compared between with and without HC-1. The statistics were examined by unpaired Student’s t-test. Data are shown as mean ± SD of three experiments. At 10 µM 5-FU, P value was 0.066. B: Cells were treated with DMSO, 0.1 µM 5-FU, 50 µM HC-1 or combination of 5-FU and HC-1 for 48 h. The representative cell images are shown [phase contrast (upper), annexin-V staining (middle) and PI staining (lower)]. Bar = 500 µm. C: Quantification of annexin-V (+) and PI (+) cells. Ten fields were randomly chosen, photographed from each group, and statistically analyzed. *P < 0.05, **P < 0.01, compared between two groups. The statistics were examined by one-way ANOVA, followed by Games-Howell test. Data are shown as mean ± SD of 10 fields. D: Western blot analysis of beta-catenin using HSC2 cells treated with DMSO, 50 µM or 100 µM HC-1 for 48 h. Actin was used as an internal control. E: Model of the chemotherapeutic effect of combined treatment with HC-1 and 5-FU to CD44-positive OSCC cells. Wnt/beta-catenin inhibitor HC-1 stimulates shift of CD44-positive cells (CSCs) to CD44-negative cells (non-CSCs), and sensitizes HSC2 cells to 5-FU through enhancement of apoptotic cell death. 5-FU, 5-fluorouracil; CSC, cancer stem cell; DMSO, dimethylsulfoxide; OSCC, Oral squamous cell carcinoma.
DISCUSSION
CSCs have self-renewal ability, higher tumor forming capacity, and show resistance to chemotherapy.10, 11 Thus, CSCs are thought to be a key factor for tumorigenesis, progression, metastasis and recurrence after treatments. To improve therapeutic effect on cancers, targeting the CSCs is considered to provide an effective strategy. CSCs use many of the same signaling pathways that have been found in normal stem cells, such as Wnt/beta-catenin, Notch and Hedgehog.22–24 Wnt/beta-catenin signal is an evolutionary conserved developmental pathway, which is crucial for self-renewal and differentiation of CSCs.12, 13 Since we have recently developed small molecule compounds, the derivatives of Wnt/beta-catenin signal inhibitors that efficiently suppress Wnt/beta-catenin transcriptional activity of human MSCs,16, 17 we tested the effects of these inhibitors on HSC2 OSCC cells. Here, we showed that HC-1, IC-2 and PN3-13 reduced Wnt/beta-catenin signal in HSC2 cells. Of these compounds, HC-1 showed efficient reduction of CD44-positive population without affecting cell viability. In addition, HC-1 sensitized HSC2 cells to 5-FU, which inhibits the synthesis of DNA/RNA in non-CSCs. These results suggest that HC-1 is an effective compound to enhance the cytotoxicity of the cells by shifting CSCs to non-CSCs (Fig. 4E).
It has been reported that one of the first successes of differentiation therapy is to use all-trans retinoic acid (ATRA), which was given to patients suffering from acute promyelocytic leukemia.25 ATRA treatment stimulates the shift of abnormal leukemic promyelocytes into mature granulocytes, and markedly improves patient survival.26 The success of this therapy has induced the concept that changes of the cell state are effectively used to treat other forms of cancers. The other agents that have similar effects to change the leukemic cell state include phorbol myristate acetate (PMA), hexamethylamine bisacetamide (HMBA), DMSO and vitamin D3.27–30 Since HC-1 efficiently shifted CSCs to non-CSCs in OSCC cells, HC-1 may also be beneficial for cancer therapy. HC-1 is a derivative of hexachlorophene. Hexachlorophene inhibits Wnt/beta-catenin signal by promoting E3 ubiquitin-protein ligase SIAH1-dependent degradation of beta-catenin.21 Consistent with this, Western blot analysis showed that HC-1 also decreased protein levels of beta-catenin (Fig. 4D), suggesting that HC-1 inhibits Wnt/beta-catenin signal through beta-catenin reduction. Therefore, beta-catenin degradation may be associated with the reduction of CSC population. Further studies are required to reveal the crucial function of HC-1 in shifting CSCs to non-CSCs, which would also facilitate understanding of CSC property and development of cancer chemotherapy.
Acknowledgments
Ackonwlegments: We thank Dr. T. Sakabe for helpful discussion on this manuscript. This work was supported by a Management Expenses Grant from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
The authors declare no conflict of interest.
REFERENCES
- 1. Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893-917. [DOI] [PubMed] [Google Scholar]
- 2. Stransky N, Egloff AM, Tward AD, Kostic AD, Cibulskis K, Sivachenko A, et al. The mutational landscape of head and neck squamous cell carcinoma. Science. 2011;333:1157-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hunter KD, Parkinson EK, Harrison PR. Profiling early head and neck cancer. Nat Rev Cancer. 2005;5:127-35. [DOI] [PubMed] [Google Scholar]
- 4. Gupta S, Kong W, Peng Y, Miao Q, Mackillop WJ. Temporal trends in the incidence and survival of cancers of the upper aerodigestive tract in Ontario and the United States. Int J Cancer. 2009;125:2159-65. [DOI] [PubMed] [Google Scholar]
- 5. Prince ME, Sivanandan R, Kaczorowski A, Wolf GT, Kaplan MJ, Dalerba P, et al. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci USA. 2007;104:973-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Leemans CR, Braakhuis BJ, Brakenhoff RH. The molecular biology of head and neck cancer. Nat Rev Cancer. 2011;11:9-22. [DOI] [PubMed] [Google Scholar]
- 7. Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105-11. [DOI] [PubMed] [Google Scholar]
- 8. Shackleton M, Quintana E, Fearon ER, Morrison SJ. Heterogeneity in cancer: cancer stem cells versus clonal evolution. Cell. 2009;138:822-9. [DOI] [PubMed] [Google Scholar]
- 9. Meacham CE, Morrison SJ. Tumour heterogeneity and cancer cell plasticity. Nature. 2013;501:328-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jordan CT, Guzman ML, Noble M. Cancer stem cells. N Engl J Med. 2006;355:1253-61. [DOI] [PubMed] [Google Scholar]
- 11. Cabrera MC, Hollingsworth RE, Hurt EM. Cancer stem cell plasticity and tumor hierarchy. World J Stem Cells. 2015;7:27-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Takahashi-Yanaga F, Kahn M. Targeting Wnt signaling: can we safely eradicate cancer stem cells?. Clin Cancer Res. 2010;16:3153-62. [DOI] [PubMed] [Google Scholar]
- 13. Takebe N, Harris PJ, Warren RQ, Ivy SP. Targeting cancer stem cells by inhibiting Wnt, Notch, and Hedgehog pathways. Nat Rev Clin Oncol. 2011;8:97-106. [DOI] [PubMed] [Google Scholar]
- 14. Yoshida Y, Shimomura T, Sakabe T, Ishii K, Gonda K, Matsuoka S, et al. A role of Wnt/beta-catenin signals in hepatic fate specification of human umbilical cord blood-derived mesenchymal stem cells. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1089-98. [DOI] [PubMed] [Google Scholar]
- 15. Shimomura T, Yoshida Y, Sakabe T, Ishii K, Gonda K, Murai R, et al. Hepatic differentiation of human bone marrow-derived UE7T-13 cells: Effects of cytokines and CCN family gene expression. Hepatol Res. 2007;37:1068-79. [DOI] [PubMed] [Google Scholar]
- 16. Itaba N, Matsumi Y, Okinaka K, Ashla AA, Kono Y, Osaki M, et al. Human mesenchymal stem cell-engineered hepatic cell sheets accelerate liver regeneration in mice. Sci Rep. 2015;5:16169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Itaba N, Sakabe T, Kanki K, Azumi J, Shimizu H, Kono Y, et al. Identification of the small molecule compound which induces hepatic differentiation of human mesenchymal stem cells. Regenerative Therapy. 2015;2:32-41. DOI: 10.1016/j.reth.2015.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Longley DB, Harkin DP, Johnston PG. 5-fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer. 2003;5:330-8. [DOI] [PubMed] [Google Scholar]
- 19. Lepourcelet M, Chen YN, France DS, Wang H, Crews P, Petersen F, et al. Small-molecule antagonists of the oncogenic Tcf/beta-catenin protein complex. Cancer cell. 2004;5:91-102. [DOI] [PubMed] [Google Scholar]
- 20. Voronkov A, Krauss S. Wnt/beta-catenin signaling and small molecule inhibitors. Curr Pharm Des. 2013;19:634-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Park S, Gwak J, Cho M, Song T, Won J, Kim DE, et al. Hexachlorophene inhibits Wnt/beta-catenin pathway by promoting Siah-mediated beta-catenin degradation. Mol Pharmacol. 2006;70:960-6. [DOI] [PubMed] [Google Scholar]
- 22. Takebe N, Miele L, Harris PJ, Jeong W, Bando H, Kahn M, et al. Targeting Notch, Hedgehog, and Wnt pathways in cancer stem cells: clinical update. Nat Rev Clin Oncol. 2015;12:445-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Espinoza I, Miele L. Notch inhibitors for cancer treatment. Pharmacol Ther. 2013;139:95-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Beachy PA, Hymowitz SG, Lazarus RA, Leahy DJ, Siebold C. Interactions between Hedgehog proteins and their binding partners come into view. Genes Dev. 2010;24:2001-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Warrell RP Jr., de Thé H, Wang ZY, Degos L. Acute promyelocytic leukemia. N Engl J Med. 1993;329:177-89. [DOI] [PubMed] [Google Scholar]
- 26. Mi JQ, Li JM, Shen ZX, Chen SJ, Chen Z. How to manage acute promyelocytic leukemia. Leukemia. 2012;26:1743-51. [DOI] [PubMed] [Google Scholar]
- 27. Carey JO, Posekany KJ, deVente JE, Pettit GR, Ways DK. Phorbol ester-stimulated phosphorylation of PU.1: association with leukemic cell growth inhibition. Blood. 1996;87:4316-24. [PubMed] [Google Scholar]
- 28. Wu H, Scher BM, Chu CL, Leonard M, Olmedo R, Scher GS, et al. Reduction in lactate accumulation correlates with differentiation-induced terminal cell division of leukemia cells. Differentiation. 1991;48:51-8. [DOI] [PubMed] [Google Scholar]
- 29. Arcangeli A, Carlà M, Del Bene MR, Becchetti A, Wanke E, Olivotto M. Polar/apolar compounds induce leukemia cell differentiation by modulating cell-surface potential. Proc Natl Acad Sci USA. 1993;90:5858-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Olsson I, Gullberg U, Ivhed I, Nilsson K. Induction of differentiation of the human histiocytic lymphoma cell line U-937 by 1 alpha, 25-dihydroxycholecalciferol. Cancer Res. 1983;43:5862-7. [PubMed] [Google Scholar]