Abstract
Background
Genetic diagnoses provide beneficial information to patients and families. However, traditional genetic diagnoses are often difficult even for experienced clinicians and require recognition of characteristic patterns of signs or symptoms to guide targeted genetic testing for the confirmation of diagnoses. Next-generation sequencing (NGS) is a powerful genetic diagnostic tool. However, whole-genome and whole-exome sequencing (WES) are expensive, and the interpretation of results is difficult. Hence, target gene capture sequencing of gene panels has recently been applied to genetic diagnoses. Herein, we demonstrate that targeted sequencing approaches using gene panel testing are highly efficient for the diagnosis of Mendelian disorders.
Methods
NGS using TruSight one gene panel was performed in 17 families and 20 patients, and we developed a bioinformatic pipeline at our institution for detecting mutations.
Results
We detected causative mutations in 6 of 17 (35%) families. In particular, 11 (65%) families had syndromic diagnosis and 6 (35%) had no syndromic diagnosis before NGS testing. The number of positive diagnoses was 5 of 11 (45%) in the syndromic group and were 1 of 6 (17%) among patients of the no syndromic diagnosis group.
Conclusion
Diagnostic yields in the present study were higher than in previous reports of genetic and chromosomal tests and WES. The present comprehensive gene-targeted panel test is a powerful diagnostic tool for Mendelian disorders.
Keywords: candidate gene analyses, diagnosis high-throughput DNA sequencing
Traditional genetic diagnosis requires the recognition of characteristic patterns of signs or symptoms to guide targeted genetic testing for the confirmation of diagnoses. Because genetic diagnoses are often difficult, even for experienced clinicians, the traditional approach for diagnosing patients with suspected genetic disorders is on the threshold of a paradigm shift due to next-generation sequencing (NGS). Whole-exome sequencing (WES) was first used in 2010 to identify causative genes of a Mendelian disease1 and is rapidly becoming an attractive tool for diagnostic testing in general medical genetics.
Typical exomes contain more than 30,000 variants compared with human reference sequences and approximately 10,000 of these represent nonsynonymous amino acid substitutions, alterations of conserved splice site residues, or small insertions or deletions.2 Thus, subsequent steps are required to determine the significance of mutations by identifying benign variants, and genetic diagnoses using NGS remain challenging.
NGS is performed by sequencing small DNA fragments, and the sequenced “reads” can be 25–100 bps from one or both ends. The massive capacity of NGS allows sequencing of multiple randomly overlapping DNA fragments. Therefore, all nucleotides in targeted regions may be included in many reads, allowing repeated analysis and depth of coverage. Increased depth of coverage usually improves sequencing accuracy and has a major influence on the performance of targeted capture for NGS.3
Although NGS is becoming an important tool for genetic diagnosis, whole-genome sequencing (WGS) and WES remain expensive, and the volume of information obtained hampers interpretation of the results. Recently, target gene capture sequencing using gene panels has been applied to genetic diagnoses.2, 4 In particular, restricted target gene sequencing has high sequencing accuracy and deep achievable coverage of targeted genes, giving rise to significant cost saving and feasible data sets for bioinformatic analyses that are functionally interpretable.3 The present study demonstrates that a targeted sequencing approach using gene panel testing is highly efficient for the diagnosis of Mendelian disorders.
SUBJECTS AND METHODS
Subjects
Patients were recruited for targeted gene testing from October 2014 through September 2015 at the Tottori University Hospital. Patients were divided into two groups of syndromic diagnoses and no syndromic diagnoses. The syndromic diagnosis group comprised patients who received specific diagnoses such as Sotos syndrome, Joubert syndrome, and Neuro-fibromatosis type 1. The no syndromic diagnosis group included patients with no specific diagnoses of epilepsy, polydactyly, or intellectual disability (ID), among others (Tables 1 and 2).
Table 1.
Clinical Characteristics of 17 families, 20 patients
| Cohort (n = 20) | ||
| Gender | ||
| Male | 12 | |
| Female | 8 | |
| Age (years old) | ||
| 0–10 | 13 | |
| 10–20 | 3 | |
| > 20 | 4 | |
| Group (n = 17) | ||
| Syndromic diagnosis (family: n = 17) | 11 | |
| No syndromic diagnosis | 6 | |
| Previous exploration | ||
| Array CGH | 1 | |
| Karyotype | 12 | |
CGH, comparative genomic hybridization.
Table 2.
List of all patients in our study
| Sex | Age | Phenotype | Group | Genetic diagnosis | Family history | Genetic test | |
| F1 | M | 1 year 11 months | Joubert syndrome | S | AHI1 | none | G-band |
| F2 | F | 2 years | Epilepsy, Macrocephaly, ID | N | FGFR3 | none | NSD1 gene, G-band |
| F3 | F | 4 years | Sotos syndrome | S | NSD1 | none | FISH |
| F4 | M | 6 years | Sotos syndrome | S | NSD1 | none | FISH |
| F5 | F | 31 years | Nonsyndromic hearing loss | S | WFS1 | none | none |
| F6 | F | 47 years | Neurofibromatosis type 1 | S | NF1 | none | none |
| F7 | F | 10 months | Hydranencephaly | S | ND | Sister (hydranencephaly) | G-band |
| F8 | M | 2 years | ID | N | ND | none | G-band |
| F9-1 | F | 3 years | Polydactyly, Congenital heart disease, Craniosynostosis, ID | N | ND | Brother (F9-2) | G-band |
| F9-2 | M | 5 years | Hydrocephaly, polydactyly | N | ND | Sister (F9-1) | G-band, Array CGH |
| F10 | M | 5 years | Leigh syndrome | S | ND | None | mt-DNA |
| F11-1 | M | 6 years | Joint hyperextension, ID | N | ND | Brother (F11-2, 3) | none |
| F11-2 | M | 9 years | Joint hyperextension, ID | N | ND | Brother (F11-1, 3) | none |
| F11-3 | M | 12 years | Joint hyperextension, ID | N | ND | Brother (F11-1, 2) | none |
| F12 | M | 7 years | Anhidrotic ectodermal dysplasia | S | ND | None | Nemo gene |
| F13 | M | 7 years | ID | N | ND | None | G-band |
| F14 | M | 10 years | Congenital disorders of glycosylation | S | ND | None | G-band |
| F15 | F | 13 years | Cyclic vomiting, peripheral neuropathy, ID | N | ND | None | G-band, mt-DNA |
| F16 | M | 42 years | Arrhythmogenic right ventricular cardiomyopathy | S | ND | Father and Sister | none |
| F17 | F | 63 years | Progressive Familial Intrahepatic Cholestasis | S | ND | None | none |
AHI1, abelson helper integration site 1; CGH, comparative genomic hybridization; F, female; FGFR3, fibroblast growth factor receptor 3; FISH, fluorescence in situ hybridization; ID, intellectual disability; M, male; N, non clinical diagnosis group; ND, not detective; NF1, neurofibromatosis type 1; NSD1, nuclear receptor binding SET domain protein 1; S, syndromic diagnosis group; WFS1, wolframin ER transmembrane glycoprotein.
Tests were performed after patients were informed of the risks and benefits and provided written informed consent. Informed written consent was obtained from adult subjects and parents of children. Each patient was informed about incidental findings, which were defined as conditions unrelated to the present symptoms. Additionally, we explained that only genes related to the patient’s present symptoms would be investigated in this clinical study. Peripheral-blood samples were provided in all cases, and clinical data were collected from medical records. The study was approved by the ethics committee at the Tottori University (dated September 22, 2014, approval number G152).
Library preparation and Sequencing
Genomic DNA was extracted from peripheral-blood samples. Targeted resequencing was performed using TruSight One sequencing panels (Illumina, San Diego, CA), which included 4,813 genes that are associated with known clinical phenotypes. TruSight one sequencing panels contain all the reagents required for amplification, amplicon enrichment, and indexing of samples, and procedures were performed according the manufacturer’s instructions. After constructing the sequence library, a MiSeq next-generation sequencer (Illumina) was used to sequence 152-bp paired-end reads.
Bioinformatic pipeline and variant ranking
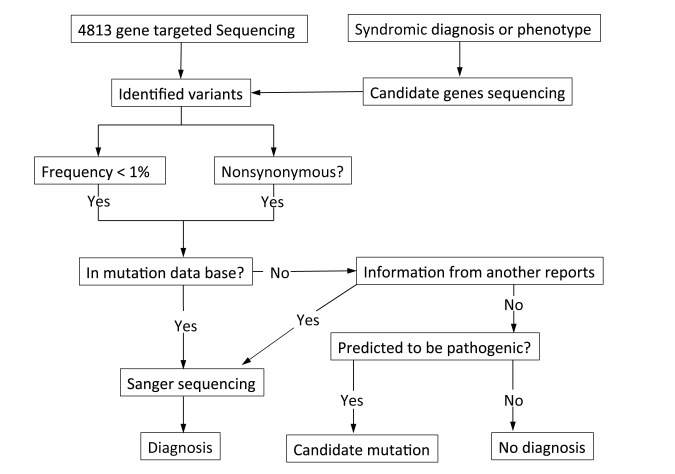
A bioinformatic pipeline was constructed in our institution to detect mutations (Fig. 1). Extracted data were mapped to a reference genome (GRCh37/hg19) using a Burrows-Wheeler Aligner (BWA). BWA is a software package for mapping low-divergent sequences against large reference genomes, such as the human genome. Variant calling and assembly of variant files were performed using standard procedures with software such as SAMtools, genome analysis toolkit, and picard, which produce binary formats (BAM) from raw data. Variant filtering was performed using SnpEff and SnpSift software, which collects variant-specific information according to predicted pathogenicity (putative effects on protein, conservation scores, splice site predictions, and allelic frequencies in all patients, and in control cohorts such as the variant databases, Human Genetic Variation Database (HGVD), GWAS, and dbSNP. The HGVD is a Japanese mutation database. Additionally, to narrow candidate variants, we prepared a list of candidate genes for each symptom and clinical diagnosis (Table 3).
Fig. 1.
Flowchart for bioinformatic analyses.
Targeted resequencing was performed using a TruSight One sequencing panel (Illumina, San Diego, CA), which includes 4,813 genes. Data were mapped to a reference genome (GRCh37/hg 19), and variant calling using the bioinformatic pipeline was constructed in our facility. Variant filtering was performed using predicted pathogenicities and frequencies according to several mutation databases. Finally, we used the list of candidate genes to narrow candidate variants. In cases of the variant that were predicted to be pathogenic, we detected the variant as a candidate mutation, and all candidate mutations were validated using Sanger sequencing.
Table 3.
Overview of gene list of our method
| Brain malformation | ARFGEF2 ARX DCX EOMES FKRP FKTN FLNA GPR56 LAMC3 LARGE NDE1 OCLN PAFAH1B1 POMGNT1 POMT1 POMT2 PQBP1 RELN SRPX2 TUBA1A TUBA8 TUBB2B TUBB3 VLDLR |
| Hereditary hearing loss | ACTG1 BSND CCDC50 CDH23 CHRN CLDN14 COCH COL11A2 DFNA5 DFNB59 DIAPH1 DSPP ESPN ESRRB EYA4 FAM189A2 GJB2 GJB3 GJB6 GPSM2 GRHL2 GRXCR1 HGF KCNQ4 LHFPL5 LOXHD1 LRTOMT MARVELD2 MIR96 MYH14 MYH9 MYO15A MYO1A MYO3A MYO6 MYO7A OTOA OTOF P2RX2 PCDH15 POU3F4 POU4F3 PRPS1 PTPRQ RDX SIX1 SLC17A8 SLC26A4 SLC26A5 SMPX STRC SYNE4 TECTA TJP2 TMC1 TMIE TMPRSS3 TPRN TRIOBP USH1C WFS1 |
| Epilepsy | ALDH7A1 ARFGEF2 ARHGEF9 ARX ATP1A2 ATP2A2 ATP6AP2 ATP6V0A2 ATRX CACNA1A CASK CASR CCDC88C CDKL5 CHRNA2 CHRNA4 CHRNB2 CLCNKA CLCNKB CLN3 CLN5 CLN6 CLN8 CNTNAP2 VPS13B COL18A1 COL4A1 CPT2 CSTB CTSD CUL4B OFD1 DCX DEPDC5 DNAJC5 EFHC1 EMX2 EPM2A FGD1 FGFR3 FKRP FKTN FLNA FOXG1 GABRA1 GABRB3 GABRD GABRG2 GPC3 GPR56 GRIA3 GRIN2A HSD17B10 KDM5C KCNA1 KCNJ1 KCNJ10 KCNMA1 KCNQ2 KCNQ3 KCNT1 KCTD7 KIAA1279 LAMA2 LARGE LBR LGI1 MBD5 ME2 MECP2 MEF2C MFSD8 MLL2 NHLRC1 NIPBL NOTCH3 NRXN1 OPHN1 PAFAH1B1 PAK3 PANK2 PAX6 PCDH19 PEX7 PHF6 SERPINI1 PIGV PLA2G6 PLP1 PNKP POLG POMGNT1 POMT1 POMT2 PPT1 PQBP1 PRICKLE1 PRICKLE2 PRRT2 RAB39B RAB3GAP1 RAI1 RELN RNASEH2A RNASEH2B RNASEH2C SAMHD1 SCARB2 SCN1A SCN1B SCN2A SCN8A SCN9A SETBP1 SLC25A22 SLC2A1 SLC4A10 SLC9A6 SMC1A SMC3 SMS SNAP29 SPTAN1 SRPX2 STXBP1 SYNGAP1 SYP TBC1D24 TBX1 TCF4 TPP1 TREX1 TSC1 TSC2 TUBA1A TUBA8 TUBB2B UBE3A VPS13A WDR62 ZEB2 |
| Hereditary sensory and autonomic neuropathy | ATL1 CCT5 DNMT1 FAM134B HSN2 IKBKAP KIF1A NGFB NTRK1 RAB7 SCN9A SPTLC1 SPTLC2 WNK1 |
| Intellectual disability | ABCC9 ABCD1 ABCD4 ABHD5 ACAD9 ACO2 ACOX1 ACSF3 ACSL4 ACTB ACTG1 ACVR1 ADAR ADCK3 ADSL AFF2 AGA AGPAT2 AGTR2 AHCY AHI1 AIFM1 AIMP1 AK1 AKT3 ALDH18A1 ALDH3A2 ALG1 ALG12 ALG13 ALG2 ALG3 ALG6 ALG9 AMT ANKH ANKRD11 ANO10 AP1S2 AP3B1 AP4B1 AP4E1 AP4S1 APTX ARFGEF2 ARHGEF6 ARHGEF9 ARID1A ARID1B ARL13B ARL6 ARX ASB11 ASL ASPA ASPM ASXL1 ATP1A2 ATP2A2 ATP6AP2 ATP6V0A2 ATR ATRX AUH B3GALTL B4GALT1 B4GALT7 BBS1 BBS10 BBS12 BBS2 BBS4 BBS5 BBS7 BBS9 BCKDHA BCKDHB BCOR BCS1L BIVMERCC5 BLM BRAF BRWD3 BSCL2 BUB1B C5orf42 CA2 CACNA1C CACNG2 CASK CC2D1A CC2D2A CCBE1 CCDC78 CDH15 CDK5RAP2 CDKL5 CDON CENPJ CEP135 CEP152 CEP290 CEP41 CHD7 CHKBCPT1B CNTNAP2 COG1 COG7 COG8 COL4A1 COL4A2 COLEC11 COQ2 COX15 CRBN CREBBP CTDP1 CTNNB1 CUL4 CYB5R3 D2HGDH DBT DCX DDHD2 DDX26B DHCR24 DHCR7 DHFR DHTKD1 DIP2B DKC1 DLD DLG3 DMD DMPK DNAJC19 DNMT3B DOCK8 DPAGT1 DPM1 DPYD DYM DYNC1H1 DYRK1A EFTUD2 EHMT1 EIF2AK3 ELOVL4 EMX2 ENOX2 EP300 EPB41L1 ERCC2 ERCC3 ERCC6 ERLIN2 ESCO2 ETHE1 FANCB4 FANCD2 FAM120C FBN1 FGD1 FGFR2 FGFR3 FH FKRP FKTN FLNA FMR1 FOXG1 FOXP1 FRAS1 FTO FTSJ1 FUCA1 GAD1 GALE GALT GAMT GATAD2B GATM GCH1 GCSH GDI1 GFAP GJC2 GK GLDC GLI3 GNAS GNPAT GNS GPC3 GPHN GPM6B GPR56 GRIA3 GRIK2 GRIN1 GRIN2A GRIN2B GSS GTF2H5 GUCY2F GUSB HAX1 HCCS HCFC1 HDAC4 HDAC8 HESX1 HLCS HOXA1 HPD HPRT1 HRAS HSD17B10 IDS IDUA IER3IP1 IGF1 IKBKG INPP5E IQSEC2 ISPD JAM3 KANSL1 KAT6B KCNJ11 KCNK9 KCNQ2 KCNT1 KCTD7 KDM5C KDM6A KIAA1279 KIF11 KIF7 KIRREL3 KLHL13 KMT2D KRAS KRBOX4 L1CAM L2HGDH LAMA2 LAMC3 LAMP2 LARGE LARP7 LIG4 LRP2 LRPPRC MAGT1 MAN1B1 MANBA MAOA MAP2K1 MAP2K2 MAT1A MBD5 MCCC1 MCCC2 MCOLN1 MCPH1 MECP2 MED12 MED17 MED23 MEF2C MGAT2 MID1 MKKS MLYCD MMAA MMACHC MMADHC MOCS1 MOCS2 MPDU1 MPLKIP MRPS22 MTR MTRR MUT MVK MYO5A NAA10 NAGA NAGLU NBN NDE1 NDP NDUFA1 NDUFA11 NDUFA12 NDUFS1 NDUFS2 NDUFS3 NDUFS4 NDUFS7 NDUFS8 NDUFV1 NEU1 NF1 NHS NIPBL NKX2-1 NLGN3 NLGN4 NLGN4X NLRP3 NPHP1 NRXN1 NSD1 NSDHL NSUN2 NTRK1 OCRL OFD1 OPHN1 ORC1 PACS1 PAFAH1B1 PAK3 PANK2 PAX6 PC PCDH19 PCNT PDHA1 PDSS1 PDSS2 PDZB11 PEPD PEX1 PEX10 PEX11B PEX13 PEX26 PEX5 PEX7 PGK1 PHF6 PHF8 PHGDH PIGN PIGO PIGV PIK3R2 PLCB1 PMM2 PNKP PNP POC1A POLR3A POLR3B POMGNT1 POMT1 POMT2 PORCN PPOX PQBP1 PRODH PRPS1 PRSS12 PTCH1 PTCHD1 PTEN PTPN11 PUS1 PVRL1 PYCR1 RAB18 RAB27A RAB39B RAB3GAP1 RAB3GAP2 RAB40AL RAD21 RAF1 RAI1 RARS2 RELN REPS2 RFT1 RMND1 RNASEH2A RNASEH2B RNASEH2C ROGDI RPGRIP1L RPS6KA3 SALL1 SATB2 SC5D SCN1A SCN2A SCN8A SCO2 SDHA SERAC1 SETBP1 SHH SHOC2 SHROOM4 SIL1 SIX3 SKI SLC12A6 SLC16A2 SLC17A5 SLC25A15 SLC25A22 SLC2A1 SLC35C1 SLC4A4 SLC6A8 SLC9A6 SMAD4 SMARCA2 SMARCA4 SMARCB1 SMC1A SMC3 SMCX SMOC1 SMPD1 SMS SNAP29 SOBP SOS1 SOX10 SOX2-OT SOX3 SPRED1 SRCAP SRD5A3 SRPX2 ST3GAL3 STIL STRA6 STXBP1 SUOX SURF1 SYNGAP1 SYP SYN1 SYT14 TAT TBC1D24 TBCE TBX22 TCF4 TECR TGFBR1 TGFBR2 THRB TIMM8A TMCO1 TMEM165 TMEM231 TMEM237 TMEM67 TRAPPC9 TREX1 TRPC5 TSC1 TSC2 TSPAN7 TTC8 TUBA1A TUBB2B TUSC3 UBE2A UBE3A UBR1 UPB1 UPF3B VPS13B WDR62 XPA ZDHHC9 ZEB2 ZIC2 ZNF41 ZNF592 ZNF674 ZNF711 ZNF81 |
| Leigh syndrome | BCS1L C12orf65 COX10 COX15 EARS2 ECHS1 FARS2 FOXRED1 GFM1 HIBCH LRPPRC MTFMT MT-ATP6 MT-ND1 MT-ND2 MT-ND3 MT-ND4 MT-ND5 MT-ND6 MT-TK MT-TV MT-TW NDUFA1 NDUFA9 NDUFA10 NDUFA11 NDUFA2 NDUFA4 NDUFAF2 NDUFAF5 NDUFAF6 NDUFS1 NDUFS2 NDUFS3 NDUFS4 NDUFS7 NDUFS8 NDUFV1 PET100 PDHA1 PDHB PDSS2 POLG SCO2 SDHA SDHAF1 SERAC1 SUCLA2 SUCLG1 SURF1 TACO1 TTC19 UQCRQ |
| Progressive familial intrahepatic cholestasis | ATP8B1 ABCB11 ABCB4 TJP2 |
| Macrocephaly | ASPA BRAF CA2 CDKN1C CLCN7 D2HGD FGFR3 FMR1 GCDH GFAP GPC3 HEXA HRAS IDS IDUA KRAS L1CAM LRP5 MEK1 MEK2 MLC1 NF1 NSD1 OSTM1 PLEKHM1 PTEN PTPN11 RAF1 SOS1 TCIRG1 TNFSF11 VG5Q |
| Skeletal dysplasia | ACAN ACVR1 ADAMTS10 ADAMTSL2 AGPS ALPL ALX4 ANKH ARSE BMPR1B CA2 CASR CHST3 CLCN7 COL10A1 COL11A1 COL11A2 COL1A1 COL1A2CRTAP COL2A1 COL9A1 COL9A2 COL9A3 COMP CRTAP CTSK CUL7 CXORF5 DDR2 DHCR24 DHPAT DLL3 DLX3 DTDST DYM DYNC2H1 EBP EFNB EIF2AK3 ESCO2 EVC1 EVC2 EXT1 EXT2 FBN1 FGF10 FGF23 FGF9 FGFR1 FGFR2 FGFR3 FLNA FLNB GDF5 GDF5COMP GJA1 GLI3 GNAS1 HOXA11 HOXD13 HSPG2 IFT80 IHH IKBKG LBR LEMD3 LEPRE LFNG LIFR LMBR1 LMNA LMX1B LRP5 MATN3 MESP2 MGP MMP13 MMP2 MSX2 NEMO NOG NPR2 OSTM1 P63 PAPSS2 PCTN2 PEX7 PHEX PLOD2 POR PPIB PTHR1 RANKL RECQL4 RMRP ROR2 RUNX2 SALL1 SALL4 SBDS SCYL1BP1 SEDL SHH SHOX SLC34A3 SMARCAL1 SOST SOX9 TBCE TBX15 TBX3 TBX4 TBX5 TBXAS1 TCIRG1 TGFB1 TMEM16E TNFRSF11A TNFRSF11B TNFSF11 TP63 TRIP11 TRPS1 TRPV4 TWIST1 WISP3 WNT3 WNT7A ZMPSTE24 |
| Congenital Disorders of Glycosylation | ALDH18A1 ALDOB ALG1 ALG11 ALG12 ALG13 ALG2 ALG3 ALG6 ALG8 ALG9 ATP6V0A2 B3GALTL B3GNT1 B4GALT1 BSCL2 CACNA1C CANT1 CDH3 CEP290 CHRNE CHST14 CHSY1 CNTN4 COG1 COG4 COG5 COG6 COG7 COG8 COL1A1 COL1A2 CSF2RA DAG1 DDOST DOLK DPAGT1 DPM1 DPM2 DPM3 EIF2AK3 F13A1 FBN1 FBN2 FGA FGF10 FGFR1 FGG FKRP FKTN G3GALNT2 G6PC3 GARS GDNF GFPT1 GH1 GMPPB GPR143 GPR98 IFNGR1 IFNGR2 IL12B IL12RB1 ISPD ITGB2 JAM3 LARGE LHCGR LMNA LRP2 LRP5 MBTPS2 MGAT2 MOGS MPDU1 MPI MYOC NEU1 NGLY1 NRXN1 NTRK2 P2RX1 PDE4D PGM1 PIGN PKD1 PKHD1 PMM2 PMP22 POMGNT1 POMGNT2 POMT1 POMT2 PRKAG2 PSAP PYCR1 RELN RET RFT1 RHO RNASET2 RPGR SEC23B SERPINA7 SERPINC1 SGK196 SHH SI SIL1 SLC10A2 SLC26A4 SLC35A1 SLC35A2 SLC35C1 SLC4A1 SLC4A11 SLC5A7 SPINK5 SPINT2 SRD5A1 SRD5A3 STAT1 STT3A STT3B SYNE1 TGFBR1 TMEM15 TMEM165 TMEM5 TSHR TYR VPS45A VWF WFS1 |
| Joubert syndrome | AHI1 ARL13B C5orf42 CC2D2A CEP290 (NPHP6) CEP41 INPP5E KIF7 NPHP1 OFD1 RPGRIP1L TCTN1 TCTN2 TCTN3 TMEM138 TMEM216 TMEM231 TMEM237 TMEM67 (MKS3) |
| Hypohydrotic Ectodermal dysplasia | EDA EDAR EDARADD |
| Arrhythmogenic Right Ventricular Dysplasia | DSC2 DSG2 DSP JUP PKP2 RYR2 TGFB3 TMEM43 |
Candidate variants were selected when harboring a frequency that was compatible with the incidence of the disease and expectedly accounted for less than 1%. Variants with suboptimal quality scores were removed from consideration. Remaining variants were compared computationally with the list of reported mutations from the Human Gene Mutation Database (HGMD). Variants in this database with minor allele frequencies of less than 1% according to either HGVD were retained. Variants that are not described in the HGMD, including synonymous variants, intronic variants that were more than 5 bp from exon boundaries (unlikely to affect messenger RNA splicing), and common variants (minor allele frequency, > 1%) were also discarded.
Mutation validation
All candidate mutations were validated by Sanger sequencing, and co-segregation analyses were performed when possible.
RESULTS
Patient characteristics
Detailed clinical phenotypes of 17 families and 20 patients are provided in the clinical descriptions of patient section in Table 2. Subjects that were submitted to the clinical laboratory for targeted gene testing were primarily pediatric patients. Clinical characteristics are presented in Table 1 and include 13 children younger than 10 years (59%), 3 children and adolescents from 10 to 20 years of age (18%), and 4 adults older than 20 years (24%; Table 1). Office settings of the ordering physicians were as follows: pediatrics (13 families), cardiology (1 family), and gastroenterology (1 family). A total of 11 (65%) families had syndromic diagnoses and 6 (35%) families had no syndromic diagnoses. Samples were available from both parents for five families. The majority of patients had phenotypes that were related to intellectual disabilities (40%), and 13 of 20 patients had received chromosomal or other genetic tests, such as array comparative genomic hybridization (CGH) and fluorescence in situ hybridization (FISH) analysis.
Sequencing data and false-positive calls for SNVs
The median sequence coverage was 27.5, and an average of 84% of all targeted exons was covered by at least 10 sequence reads (Table 4). Approximately, 15,000–37,000 single-nucleotide variants and small insertion and deletion changes were identified in each patient’s personal genome by comparison with the current reference haploid human genome sequence (GRCh37/hg 19). After removing low-frequency variants and synonymous variants, an average of 742 exon variants per sample was analyzed for the 20 samples. No cases of detected causative genes showed false positives when confirmed by Sanger sequencing.
Table 4.
Overview of sequencing data of our method
| Family | Raw reads | Unmapped reads | Read mapped (%) | Duplicate reads (%) | Median coverage | Mean coverage | > 1x (%) | > 10x (%) | > 20x (%) | > 40x (%) | Variant | EXON variant | Variant (freq < 0.05) | EXON Variant (freq < 0.05) | Variant (freq < 0.01) | EXON variant (freq < 0.01) |
| 1 | 13324086 | 2602 | 99.98 | 3.23 | 40 | 43.69 | 98.73 | 89.62 | 76.53 | 48.74 | 26423 | 7080 | 4057 | 943 | 3297 | 691 |
| 2 | 9625914 | 1978 | 99.98 | 5.74 | 27 | 30.86 | 97.47 | 83.55 | 64.3 | 26.75 | 24607 | 7045 | 3751 | 927 | 3048 | 672 |
| 3 | 12116082 | 1956 | 99.98 | 14.05 | 36 | 43.65 | 97.5 | 86.14 | 72.03 | 44.71 | 23864 | 6910 | 4132 | 1020 | 3509 | 775 |
| 4 | 12164432 | 3055 | 99.97 | 3.17 | 37 | 41.06 | 98.9 | 89.67 | 75.27 | 44.97 | 27983 | 7300 | 3999 | 950 | 3339 | 729 |
| 5 | 9980608 | 2009 | 99.98 | 5.55 | 28 | 31.86 | 97.68 | 84.48 | 65.79 | 28.36 | 25371 | 6975 | 3750 | 902 | 3134 | 680 |
| 6 | 14588198 | 4130 | 99.97 | 3.46 | 42 | 46.15 | 98.41 | 90.33 | 78.48 | 52.48 | 28846 | 7241 | 4553 | 1053 | 3826 | 799 |
| 7 | 8989404 | 2419 | 99.97 | 4.92 | 27 | 33.16 | 97.23 | 80.34 | 61.54 | 31.57 | 21784 | 6970 | 3387 | 984 | 2834 | 756 |
| 8 | 9580736 | 2624 | 99.97 | 2.68 | 26 | 29.31 | 97.68 | 82.22 | 62.12 | 25.36 | 23113 | 6816 | 3305 | 876 | 2809 | 660 |
| 9-1 | 7061216 | 2349 | 99.97 | 3.75 | 20 | 25.23 | 95.8 | 73.27 | 49.97 | 18.9 | 20582 | 6622 | 2982 | 849 | 2493 | 646 |
| 9-2 | 8989404 | 2419 | 99.97 | 4.92 | 27 | 33.16 | 97.23 | 80.34 | 61.54 | 31.57 | 21784 | 6970 | 3387 | 984 | 2834 | 756 |
| 10 | 9625914 | 1978 | 99.98 | 5.74 | 27 | 30.86 | 97.47 | 83.55 | 64.3 | 26.75 | 24607 | 7045 | 3751 | 927 | 3048 | 672 |
| 11-1 | 9866094 | 5432 | 99.94 | 3.6 | 26 | 30.43 | 99.12 | 85.95 | 62.24 | 24.92 | 31105 | 7182 | 5410 | 1014 | 4731 | 801 |
| 11-2 | 6644172 | 5035 | 99.92 | 3.09 | 16 | 19.74 | 98.27 | 71.2 | 37.77 | 8.06 | 26623 | 6794 | 4552 | 977 | 3937 | 753 |
| 11-3 | 5527400 | 3323 | 99.94 | 3.24 | 15 | 18.76 | 96.47 | 64.86 | 35.17 | 8.64 | 20299 | 6721 | 3209 | 897 | 2689 | 656 |
| 12 | 13309866 | 2767 | 99.98 | 7.37 | 39 | 43.62 | 98.74 | 89.81 | 77.04 | 47.91 | 27920 | 7114 | 4538 | 1017 | 3762 | 769 |
| 13 | 10407584 | 2717 | 99.97 | 4.99 | 33 | 36.08 | 97.91 | 85.79 | 70.47 | 37.68 | 23220 | 7073 | 3516 | 948 | 2965 | 731 |
| 14 | 30800624 | 12121 | 99.96 | 10.43 | 80 | 100.32 | 99.68 | 97.1 | 91.82 | 77.41 | 37720 | 7433 | 6461 | 1120 | 5541 | 873 |
| 15 | 6600806 | 1192 | 99.98 | 11.67 | 20 | 25.74 | 94.28 | 71.3 | 49.32 | 20.23 | 15756 | 6542 | 2652 | 960 | 2250 | 752 |
| 16 | 16279852 | 7220 | 99.95 | 8.66 | 43 | 51.38 | 99.35 | 93.22 | 81.1 | 53.57 | 31755 | 7341 | 6033 | 1077 | 5193 | 839 |
| 17 | 17014786 | 4078 | 99.98 | 6.6 | 53 | 57.64 | 98.52 | 92.19 | 83.53 | 64.11 | 28550 | 7459 | 4749 | 1094 | 3962 | 839 |
CSII, continuous subcutaneous insulin infusion.
Diagnosis based on target resequencing
Positive diagnoses were observed in 5 of 11 (45%) patients of the syndromic diagnosis group and in 1 of 6 (17%) of those in the no syndromic diagnosis group. We detected certainly causative mutations in 6 of 17 (35%) families, including five families with autosomal dominant diseases and one with autosomal recessive disease. All five mutations of autosomal dominant diseases were described in HGMD (Table 5). Due to the absence of consent, parental studies were not performed in all five families, and one autosomal recessive family demonstrated compound heterozygosity of two distinct mutations. Nonetheless, parental studies indicated that patients inherited mutant alleles from each carrier parent, and both mutations were not present in HGMD. The c.841 G > T mutation causes a stop codon. At the c. 2015 C > T mutation, the prediction of function using Polyphen2 shows probably damaging. Additionally, this mutation was detected in two cases of Joubert syndrome at our facility, and both mutations were confirmed by Sanger sequencing.
Table 5.
Summary of disease-causing mutations in 6 clinical cases
| Age, y | Gene | Mutation | References | Inheritance | Phenotype | |
| F1 | 1 | AHI1 | E281X c.841 G > T | In house data, prediction of function | AR | Joubert syndrome |
| T702M c.2105 C > T | ||||||
| F2 | 2 | FGFR3 | N540K c.1620 C > A | 13 | AD | Hypochondroplasia |
| F3 | 4 | NSD1 | R2117X c.6349 C > T | 14 | AD | Sotos syndrome |
| F4 | 6 | NSD1 | T2055I c.6164 C > T | 15 | AD | Sotos syndrome |
| F5 | 31 | WFS1 | D797N c.2389 G > A | 16 | AD | Nonsyndromic hearing loss |
| F6 | 47 | NF1 | W2208X c.6623 G > A | 17 | AD | Neurofibromatosis-1 |
AD, autosomal dominant; AHI1, abelson helper integration site 1; AR, autosomal recessive; FGFR3, fibroblast growth factor receptor 3; NF1; neurofibromatosis type 1; NSD1, nuclear receptor binding SET domain protein 1; WFS1, wolframin ER transmembrane glycoprotein; y, year(s).
DISCUSSION
Patient characteristics
Certainly causative mutations were detected in 6 of 17 (35%) families. Among families who received genetic diagnoses, five in six were syndromically diagnosed before targeted gene testing. In the cases F2, F3 and F6 (Tables 2 and 5), the size of NSD1 and NF1 genes was large, and no family history was found. To confirm the absence of other gene mutations, we used TruSight one gene panel sequencing instead of Sanger sequencing. In the case of F1 (Tables 2 and 5), Joubert syndrome has genetically heterogeneous. Accordingly, 19 genes were associated with Joubert syndrome in the OMIM database. Recently, reports of causative genes have increased in number, and genetic heterogeneity of Mendelian disorders is more widely understood. Hence, genetic diagnosis using NGS is more important as a diagnostic alternate to Sanger sequencing.
In the present study, most cases were children, and only four cases were adults. ID cases were identified in 6 of 17 families. IDs are common neurodevelopmental disorders and are reported in 1.5%–2% of children and adolescents.5 Syndromic diagnoses of ID patients are made according to clinical symptoms but remain difficult even for experienced professional clinicians. However, although most ID disorders lack therapeutic drugs, understanding of genetic causes can benefit patients and families by providing prognostic information and precluding further unnecessary invasive testing. Moreover, diagnoses often facilitate access to appropriate medical and supportive care, and family members may benefit from knowledge of the risks of recurrence counseling and possible prenatal diagnoses.6 In the present study, physicians performed extensive clinical diagnostic workups on some subjects before ordering gene panel testing, and some of these may have exceeded the time and cost of gene panel testing. For example, one patient (F2 in Table 2) had gene panel testing at 2 years of age following evaluation using karyotype analysis, chromosomal CGH array analysis, and NSD1 single-gene sequencing tests. This patient carried a mutation in the FGFR3 gene, which is associated with hypochondroplasia. Bone dysplasia of this patient was mild and was unnoticed, and clinical diagnosis had been difficult. Thus, information from genetic diagnosis provides benefits for patient and families with genetic disorders and ID.
Target gene sequencing
Upon applying gene panel testing to genetic diagnoses of 17 families, we observed a molecular diagnostic yield of 35%, which was higher than the positive rates of other genetic tests, such as karyotype analysis (5%–15%) and chromosomal microarray analysis (30%).7 A recent study of diagnostic WES in 250 unselected, consecutive cases achieved a diagnostic yield of 25%,7 and another larger study of subjects with ID reached a diagnostic yield of 16%.6 Although comparisons of diagnostic yields between studies are statistically difficult, the results of the present study show higher rates of definitive genetic diagnosis than other genetic and chromosomal tests and WES reports, potentially reflecting testing of more specific subjects. Accordingly, among 2,000 WES samples, the molecular diagnosis rate was 36.1% in children with specific neurological findings (36.1%).7 In agreement, the present syndromic diagnostic rate was 45%, whereas that among subjects of the no syndromic diagnosis group was only 17%.
Genetic diagnosis of patients with certain clinical diagnoses and suspected causative genes are limited, warranting use of disease-restricted targeted gene panel sequencing, such as cancer panels, hearing loss panels, and muscular dystrophy panels. Hence, depending on specialization of facilities, disease-restricted targeted gene panel sequencing may be sufficient. Conversely, in facilities with patients carrying various diseases, multiple-restricted gene panels are required. However, numerous causative genes have been identified for heterogeneous diseases such as ID. In these cases, a large number of gene-targeted panels, such as TruSight one gene panel, may find utility.
Various clinical studies report diagnoses using TruSight one gene panels.9, 10 Accordingly, TruSight one gene panels target 4813 genes that are potentially associated with known clinical phenotypes. Because genes are numerous, suspected variants are also numerous, and interpretations of variants remain poorly established. Genetic diagnosis in approximately 35% of our six families with positive cases was based on disease-related gene mutations that were reported in mutation databases and in in-house data. However, most gene mutations that underlie Mendelian disorders remain to be discovered. We expect that diagnostic rates of gene panel testing will increase with the identification of additional patients who have mutations in novel candidate genes. Additionally, in undiagnosed cases, etiological mutations may be located in noncoding regions, such as regulatory or deep intronic regions, and copy number variations are currently not detected by the present gene panel tests.
NGS is used to sequence many randomly overlapping DNA fragments. Moreover, increased depth of coverage usually improves sequencing accuracy. In this study, the depth of coverage at 20× was 66% and was lower than in another report using the Trusight one gene panel.11 Hence, low depth of coverage may influence sequencing accuracy. Although the necessity for confirmation of Sanger sequencing after NGS has not reached consensus, NGS variants with lower coverage of depth are likely sufficient for confirmation of Sanger sequencing-based diagnoses.12 In this study, we confirmed all mutations by Sanger sequencing and considered that this approach can compensate for the low depth of coverage.
In conclusion, the use of the present gene panel test to analyze 17 clinical cases yielded diagnoses in 35% of cases. Hence, large numbers of gene-targeted panel tests are warranted as diagnostic tests for patients carrying nonspecific or unusual disease presentations with possible genetic causes and for patients with clinical diagnoses of heterogeneous genetic conditions.
The authors declare no conflict of interest.
REFERENCES
- 1. Ng SB, Buckingham KJ, Lee C, Bigham AW, Tabor HK, Dent KM, et al. Exome sequencing identifies the cause of a mendelian disorder. Nat Genet. 2010;42:30-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zemojtel T, Köhler S, Mackenroth L, Jäger M, Hecht J, Krawitz P, et al. Effective diagnosis of genetic disease by computational phenotype analysis of the disease-associated genome. Sci Transl Med. 2014;6:252ra123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lin X, Tang W, Ahmad S, Lu J, Colby CC, Zhu J, et al. Applications of targeted gene capture and next-generation sequencing technologies in studies of human deafness and other genetic disabilities. Hear Res. 2012;288:67-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vrijenhoek T, Kraaijeveld K, Elferink M, de Ligt J, Kranendonk E, Santen G, et al. Next-generation sequencing-based genome diagnostics across clinical genetics centers: implementation choices and their effects. Eur J Hum Genet. 2015;23:1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Redin C, Gérard B, Lauer J, Herenger Y, Muller J, Quartier A, et al. Efficient strategy for the molecular diagnosis of intellectual disability using targeted high-throughput sequencing. J Med Genet. 2014;51:724-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. de Ligt J, Willemsen MH, van Bon BW, Kleefstra T, Yntema HG, Kroes T, et al. Diagnostic exome sequencing in persons with severe intellectual disability. N Engl J Med. 2012;367:1921-9. [DOI] [PubMed] [Google Scholar]
- 7. Yang Y, Muzny DM, Reid JG, Bainbridge MN, Willis A, Ward PA, et al. Clinical whole-exome sequencing for the diagnosis of Mendelian disorders. N Engl J Med. 2013;369:1502-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yang Y, Muzny DM, Xia F, Niu Z, Person R, Ding Y, et al. Molecular findings among patients referred for clinical whole-exome sequencing. JAMA. 2014;312:1870-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dello Russo C, Di Giacomo G, Mesoraca A, D’Emidio L, Iaconianni P, Minutolo E, et al. Next generation sequencing in the identification of a rare genetic disease from preconceptional couple screening to preimplantation genetic diagnosis. J Prenat Med. 2014;8:17-24. [PMC free article] [PubMed] [Google Scholar]
- 10. Kato T, Morisada N, Nagase H, Nishiyama M, Toyoshima D, Nakagawa T, et al. Somatic mosaicism of a CDKL5 mutation identified by next-generation sequencing. Brain Dev. 2015;37:911-5. [DOI] [PubMed] [Google Scholar]
- 11. Yamamoto T, Igarashi N, Shimojima K, Sangu N, Sakamoto Y, Shimoji K, et al. Use of targeted next-generation sequencing for molecular diagnosis of craniosynostosis: identification of a novel de novo mutation of EFNB1. Congenit Anom (Kyoto). 2015. [DOI] [PubMed] [Google Scholar]
- 12. Baudhuin LM, Lagerstedt SA, Klee EW, Fadra N, Oglesbee D, Ferber MJ. Confirming Variants in Next-Generation Sequencing Panel Testing by Sanger Sequencing. J Mol Diagn. 2015;17:456-61. [DOI] [PubMed] [Google Scholar]
- 13. Bellus GA, McIntosh I, Smith EA, Aylsworth AS, Kaitila I, Horton WA, et al. A recurrent mutation in the tyrosine kinase domain of fibroblast growth factor receptor 3 causes hypochondroplasia. Nat Genet. 1995;10:357-9. [DOI] [PubMed] [Google Scholar]
- 14. Tong TM, Hau EW, Lo IF, Chan DH, Lam ST. Spectrum of NSD1 gene mutations in southern Chinese patients with Sotos syndrome. Chin Med J (Engl). 2005;118:1499-506. [PubMed] [Google Scholar]
- 15. Duno M, Skovby F, Schwartz M. Leukocyte cDNA analysis of NSD1 derived from confirmed Sotos syndrome patients. Ann Hum Genet. 2007;71:713-8. [DOI] [PubMed] [Google Scholar]
- 16. Bai X, Lv H, Zhang F, Liu J, Fan Z, Xu L, et al. Identification of a novel missense mutation in the WFS1 gene as a cause of autosomal dominant nonsyndromic sensorineural hearing loss in all-frequencies. Am J Med Genet A. 2014;164A:3052-60. [DOI] [PubMed] [Google Scholar]
- 17. van Minkelen R, van Bever Y, Kromosoeto JN, Withagen-Hermans CJ, Nieuwlaat A, Halley DJ, et al. A clinical and genetic overview of 18 years neurofibromatosis type 1 molecular diagnostics in the Netherlands. Clin Genet. 2014;85:318-27. [DOI] [PubMed] [Google Scholar]