Abstract
Background
Daisaikoto (DSKT), a classical traditional Chinese herbal formula, has been used for treating digestive diseases for 1800 years in China. Therefore, in this study, we are going to investigate the effect of DSKT on diabetic fatty liver rats induced by a high-fat diet and streptozotocin (STZ), and the effects of DSKT on silent mating type information regulation 2 homolog 1 (SIRT1) and nuclear factor kappa B (NF-kappaB).
Methods
Diabetic fatty liver rat model was selected to establish a high-fat diet and STZ. Sixty Wistar rats were divided into six groups (n = 10): control group, high-fat diet + STZ group, simvastatin treatment group, DSKT low dose, medial dose and high dose treatment groups. After 8 weeks of drug intervention, body and liver weights, blood chemistry, blood glucose and insulin were examined. The expressions of sirtuin 1 and NF-kappaB in the liver were observed by RT-PCR and immunohistochemistry, respectively.
Results
A high-fat diet increased body, liver weights, and serum cholesterol concentrations. Intraperitoneal injection of STZ increased blood glucose and decreased body weights. DSKT improved them. Homeostasis model assessment-estimated insulin resistance (HOMA-IR) indices were increased in the high-fat diet groups. DSKT improved them too. In histological examinations of the liver, we observed a significant improvement after treatment. Immunostaining expression of NF-kappaB in the liver was improved by DSKT and simvastatin. The mRNA expressions of SIRT1 in the liver were increased by DSKT and simvastatin.
Conclusion
We have demonstrated that DSKT is capable of reversing dyslipidemia and insulin resistance induced by a high-fat diet and STZ. High dose DSKT reveals a stronger effect than simvastatin on the expressions of SIRT1 and NF-kappaB. Furthermore, DSKT has shown a strong dose-depended protective effect on diabetic fatty liver.
Keywords: daisaikoto, diabetes, fatty liver, nuclear factor kappa B, silent mating type information regulation 2 homolog 1
Nonalcoholic fatty liver disease (NAFLD), the most common cause of chronic liver disease, shows an increasing prevalence among adults.1 Recently, it has been proven that NAFLD is commonly associated with many symptoms of metabolic syndrome such as obesity, type 2 diabetes mellitus (T2DM), dyslipidemia and insulin resistance.2 The incidence of NAFLD is reported approximately in the 20–30% range of the general population in various countries. However, it reaches nearly 70–75% in individuals with T2DM, and is almost certainly increasing.3, 4 Accordingly, NAFLD seems to be linked to metabolic syndrome, and may precede T2DM development.5 Compared with other patients, those with T2DM appear to have an increased risk of developing NAFLD and certainly have a higher risk of developing fibrosis and cirrhosis.3 Inhibition of excessive lipid synthesis and uptake is recognized to be an effective intervention for NAFLD, such as statins and fibrates.6–8 However, they cannot meet the demands for treatment due to differences in individuals, drug dependence and some potential side effects.9, 10
Many cytokines are associated with the progression of NAFLD and T2DM, such as sirtuin 1 (SIRT1; silent mating type information regulation 2 homolog 1) and nuclear factor kappa B (NF-kappaB). SIRT1, an NAD+-dependent deacetylase, is implicated in diverse cellular processes, including metabolism, inflammation and apoptosis.11–13 Increasing evidence shows that it may be involved in the development of NAFLD.14 Deng, XQ et al proved that SIRT1 protein levels are reduced in NAFLD induced by a high-fat diet in rats.15 It has the ability to activate or repress many non-histone proteins such as NF-kappaB.16 NF-kappaB, an oxidative stress sensitive transcription factor, plays an important role in regulation of the expression of many genes involved in inflammatory responses, and NF-kappaB-induced cytokines have appeared widely in inflammatory diseases, including NAFLD and T2DM.17–19 However, research on SIRT1 and NF-kappaB on daisaikoto (DSKT) treatment has not yet been included.
DSKT (“Da-chai-hu-tang” in Chinese), a classical traditional Chinese herbal formula, has been used for treating digestive diseases for 1800 years in China. It was created by the well-known Chinese clinician Zhang Zhongjing in Eastern Han. Nowadays, it is used in Asian countries in patients with hypertension and associated symptoms,20 hyperlipidemia and diabetes mellitus.21 Yamamoto et al. and Iizuka et al. have reported that DSKT has the effect of lowering hepatic triglyceride biosynthesis in HepG2 human hepatocyte cells or experimental hypercholesterolemic models.22, 23 However, the effect of DSKT on diabetic fatty liver has not been proven and its effects on SIRT1 and NF-kappaB remain unclear.
Therefore, in this study, we are going to investigate the effect of DSKT on diabetic fatty liver in rats induced by a high-fat diet and STZ, and the effects of DSKT on homeostasis of SIRT1 and NF-kappaB.
MATERIALS AND METHODS
Animals
Eighty-five adult male Wistar rats (8 weeks, 200 ± 20 g, No. SCXK. Lu 20130003) were purchased from Shandong University of Traditional Chinese Medicine Laboratory Animal Center (Jinan, China). Rats were housed in individual cages under controlled environmental conditions (22 ± 2 °C relative humidity 40–60%, 12 h dark/light cycles, food and water ad libitum). Animals were used in the present study in accordance with the guidelines of the Shandong University of Traditional Chinese Medicine Institutional Animal Care and Use Committee. All studies were performed with approval from the committee (2013005-KY).
Reagents
Simvastatin was purchased from Qilu Pharmaceutical (Jinan, China). Streptozotocin (STZ) was purchased from Sigma, St. Louis, MO. Anti-NF-kappaB and goat anti-rabbit antibody were obtained from Beijing Boosen Biological Technology (Beijing, China). Insulin ELISA Kit was purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
Composition and preparation of DSKT
DSKT consists of eight traditional Chinese herbs (Table 1). Each of the herbs were purchased from Zhong-sheng Company of Traditional Crude Drugs (Qingdao, China), and carefully authenticated by Dr. Jun-Ying Shi, Shandong University of Traditional Chinese Medicine (Jinan, China). Voucher specimens (numbers were listed in Table 1) were deposited at the Herbarium of Shandong University of Traditional Chinese Medicine (SDU.TCM, Jinan, China). After drying, herbs were mixed in proportion, and 84 g of this material was combined with 300 mL of distilled water and boiled for 30 min at 100 °C. The extract was filtered, and the residual medicine was boiled in water following the same procedure once more. Finally, the pool of the extracts from two boilings and filterings was mixed and stored at 4 °C.
Table 1.
Constituents of daisaikoto (DSKT) formulation
| Components | Part used | Voucher specimens number | Amount used (g) |
| Bupleuri Radix (Bupleurum falcatum L.) | Root | SDU.TCM 0062 | 15 |
| Scutellariae Radix (Scutellaria baicalensis Georgi) | Root | SDU.TCM 0098 | 9 |
| Rhei Rhizoma (Rheum palmatumL.) | Rhizome | SDU.TCM 0076 | 6 |
| Aurantii Fructus Immaturus (Citrus aurantium L. var. daidai Makino) | Fruit | SDU.TCM 0203 | 9 |
| Paeoniae Radix (Paeonia lactiflora Pallas) | Root | SDU.TCM 0046 | 9 |
| Pinnelliae Tuber (Pinellia ternata Breit) | Tuber | SDU.TCM 0057 | 9 |
| Zingiberis Rhizoma (Zingiber officinale Roscoe) | Rhizome | SDU.TCM 0132 | 15 |
| Zizyphi Fructus (Zizyphus jujuba Mill. var. inermis Rehder) | Fruit | SDU.TCM 0080 | 12 |
Animal experiments
After 3 days adaptation, rats were randomly divided into six groups: control group (C, n = 10), high-fat diet + STZ group (H, n = 15), high-fat diet + STZ with simvastatin treated group (HS, n = 15), high-fat diet + STZ with low dose of DSKT treated group (HDL, n = 15), high-fat diet + STZ with medial dose of DSKT treated group (HDM, n = 15), high-fat diet + STZ with high dose of DSKT treated group (HDH, n = 15). Group C was fed a standard rat diet. A high-fat diet was supplied for the other groups; it was made by adding 10% lard oil, 20% sucrose, 10% egg yolk powder and 0.5% cholic acid to the standard diet (purchased from Keao Xieli Feed, Beijing, China). After 6 weeks, STZ (30 mg/kg, 2% citrate buffer solution, pH 4.2) was supplied by intraperitioneal injection for rats in Groups H, HS, HDL, HDM, HDH. After 72 h of injection, rats with a level of fasting blood glucose over 11.1 mmol/L were regarded as successful models. Ten successful model rats in each group were selected for the following experiment. Rats in Groups C (n = 10) and H (n = 10) were treated with 0.9% saline by intragastric administration (10 mL/kg body weight per day); rats in Group HS (n = 10) were treated with simvastatin by intragastric administration (0.9 mg/10 mL/kg body weight per day); rats in Groups HDL (n = 10), HDM (n = 10) and HDH (n = 10) were treated with DSKT by intragastric administration (3.75, 7.5, 15 g/10 mL/kg body weight per day, respectively). Dosages of drugs were calculated according to body surface area. The drug dosage for rats was equally proportionate to adult humans who take simvastatin (10 mg) and DSKT (84 g) per day, respectively. The body surface area conversion coefficient from humans to rats is 0.018. Dosage of drug for adult humans × 0.018 = dosage of drug for rats weighing 200 g. So the dosage of simvastatin for rats can be calculated according to the above formula: 10 mg × 0.018 = 0.18 mg for rats weighing 200 g. This results in the dosage for rats weighing 1 kg as 0.9 mg. The dosage of DSKT for rats can also be calculated similarly according to the above formula: 84 g × 0.018 = 1.5 g for rats weighing 200 g where the dosage for rats weighing 1 kg is 7.5 g, a medial dose; 3.75 g is defined as a low dose; 15 g is defined as a high dose. The drug intervention period is continued for 8 weeks. The amount of feed for each rat was regulated to 25 g/day and water was supplied ad libitum. Body weights were measured at 0, 6, 10 and 14 weeks. Blood glucose was measured at 0 week, 6 weeks, 6 weeks + 3 days, 8 weeks, 10 weeks, 12 weeks and 14 weeks.
Sample collection
At the end of 14 weeks, rats in each group were sacrificed by collecting blood from the heart under pentobarbital anesthesia after fasting for 12 h. Liver tissues were removed, and then portions of the samples were stored in 10% formalin solution for hematoxylin and eosin (HE) staining. The remaining samples were immediately transferred into EP tubes containing 500 μL of RNA later, quickly frozen in liquid nitrogen, and stored at –80°C. Serum levels of TC (total cholesterol), HDL-C (high-density lipoprotein cholesterol), LDL-C (low-density lipoprotein cholesterol), ALT (total bilirubin, alanine aminotransferase), ALP (alkaline phosphatase) and TG (triglyceride) levels were analyzed for rats using an auto analyzer at an accredited clinical laboratory (Affiliated Hospital of Shandong University of Traditional Chinese Medicine, Jinan, China).
Insulin linked immunosorbent assay (ELISA)
Serum samples were applied for an ELISA of Insulin (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer’s instructions. Insulin resistance was evaluated according to the homeostasis model assessment-estimated insulin resistance (HOMA-IR) index. The HOMA-IR index was calculated according to the following equation reported by Matthews et al.24, 25 HOMA-IR index = fasting plasma insulin (mIU/L) × fasting plasma glucose (mmol/L)/22.5.
RT-PCR of SIRT1
The tissues were quickly immersed in RNA later and stored at –80 °C. The total RNA was sequentially extracted using TRIZOL Reagent (Sigma-Aldrich, St. Louis, MO) according to the manufacturer’s instructions. The RNA was treated with DNase (DNAfree, Ambion, Austin, TX) in order to remove contaminating genomic DNA, followed by phenol, chloroform extraction and ethanol precipitation. Total RNA was assessed for purity using the NanoDrop system. Total RNA was performed in a two-step procedure as described by Power SYBR Green PCR Master Mix kits (Applied Biosystems, Waltham, MA). Briefly, in the first step, cDNA was prepared from 500 ng RNA by reverse transcription in a final volume of 20 μL in a thermal cycler (Tgradient 96, Whatman Biometra, Niedersachsen, Germany). The samples were incubated at 37 °C for 60 min and 95 °C for 5 min. The cDNA were stored at –20 °C. The rat-specific primers for the genes of PPT, NEP and beta-actin were designed using Primer Premier 5.0 (Premier Biosoft, Palo Alto, CA). The primer sequences are shown in Table 2. Primers were synthesized by BioAsia (Shanghai, China). In the second step, quantitative real-time PCR was performed on a LightCycler apparatus (Roche Diag Diagnostics, Mannheim, Germany) using Power SYBR Green. PCR Master Mix kits (Applied Biosystems). The reaction was conducted with an initial denaturing at 95 °C for 10s, and then involved 40 cycles of 55 °C 10 s, 72 °C 15 s, and terminated by a cooling step 30 s at 40 °C. A melting-curve analysis was performed to confirm the absence of primer dimers in specific PCR products. The efficiency of PCR was assessed with serial dilutions of a sample of cDNA from the normal control group. Each experiment was performed in duplicate and the data were analyzed using Light Cycler Software 4.0 (Roche Diagnostics, Basel, Switzerland). Beta-actin mRNA was used as the housekeeping gene, and all data are represented using the 2−ΔΔCT method .
Table 2.
The sequence of each PCR primer
| Gene | Forward | Reverse |
| Beta-actin | 5’-GAGGGAAATCGTGCGTGAC-3’ | 5’- GGACTCATCGTACTCCTGCTTG-3’ |
| SIRT1 | 5’-CCAGAACAGTTTCATAGAGCC-3’ | 5’TCTTACTTTCAGAGAAGACCCAATA-3’ |
PCR, polymerase chain reaction; SIRT1, sirtuin 1; silent mating type information regulation 2 homolog 1.
Immunohistochemistry of NF-kappaB
The samples were fixed in 10% buffered formalin at 4 °C for 24–48 h. The samples were sectioned (4 μm thick) using a freezing microtome (ASpZr 35) and standard immunohistochemical procedures were used to visualize NF-kappaB protein. Sections were washed 4 × 10 min in PBS (pH 7.4) at room temperature and were blocked with 10% normal goat serum. Sections were then incubated with anti- NF-kappaB (Beijing Bioss Biological Technology, Beijing, China) overnight on a shaker at room temperature. Following washing (4 × 10 min) with PBS, sections were incubated for 2 h with goat anti-rabbit antibody. Finally, sections were washed (3 × 10 min) in PBS, transferred onto gelatin-coated slides. Tissue processed without the primary NF-kappaB antibody served as a negative control. Adjacent sections were mounted onto plus-coated slides and stained using Neutral Red (0.5%), washed through a series of increasing alcohol concentrations, cleared with xylene and coverslipped with Neutral Blasam.
Five fields from every section were randomly selected for examination using an Olympus CKX41-32PH microscope equipped with an imaging system (Olympus Optical, Tokyo, Japan). The distribution and staining of positive expression of liver tissue were observed under a 400 x light microscope. The optical densities were measured by Image-Pro Plus v 6.0 software (Media Cybernetics, Rockville, MD).
Statistical analysis
All statistical analyses were performed using SPSS 17.0 software (SPSS, Chicago, IL). Data were expressed as mean ± S.E. Analysis of variance (ANOVA) was performed when more than two groups were compared. Values of P < 0.05 were considered statistically significant.
RESULTS
Body and liver weights
As Table 3 shows, there were no significant differences in the baseline of body weights at the beginning of the experiment. High-fat diet increased body weights in Groups H, HS, HDL, HDM and HDH compared with Group C after 6 weeks (P < 0.05). Intraperitoneal injection of STZ decreased body weights in Group H compared with Group C after 10 and 14 weeks (P < 0.05). DSKT ameliorated body weights in a dose dependent manner (P < 0.05). Liver weights were significantly higher in Group H than Group C after 14 weeks (P < 0.05). Groups HS, HDL, HDM and HDH had decreased liver weights after 14 weeks (P < 0.05). Groups HS and HDH had a better effect than HDL (P < 0.05).
Table 3.
Body and liver weights
| Group C | Group H | Group HS | Group HDL | Group HDM | Group HDH | |
| Body weights (g) | ||||||
| 0W | 208.30 ± 11.95 | 206.10 ± 12.58 | 205.40 ± 11.69 | 208.80 ± 7.84 | 207.90 ± 11.29 | 207.70 ± 10.31 |
| 6W | 355.60 ± 27.37 | 427.40 ± 27.59* | 425.20 ± 26.60 | 422.70 ± 23.86 | 428.80 ± 17.25 | 430.40 ± 15.64 |
| 10W | 398.60 ± 20.92 | 432.90 ± 31.07* | 428.40 ± 22.10 | 446.30 ± 25.32 | 453.00 ± 25.28 | 469.50 ± 25.01† |
| 14W | 452.70 ± 15.30 | 388.90 ± 34.55* | 390.10 ± 24.78 | 423.40 ± 21.43†‡ | 428.90 ± 26.77†‡ | 450.60 ± 27.92†‡§ |
| Liver weights (g) | ||||||
| 14W | 14.79 ± 1.29 | 21.39 ± 2.52* | 15.14 ± 1.69† | 16.83 ± 1.29†‡ | 16.41 ± 1.35† | 15.02 ± 2.12†§ |
C: normal control group, standard rat diet for 14 weeks and treated with 0.9% saline 10 mL/kg every day (n = 10); H: high-fat diet + STZ group, high-fat diet for 14 weeks and treated with 0.9% saline 10 mL/kg every day (n = 15 at 0 week and n = 10 at 6, 10 and 14 weeks); HS: high-fat diet + STZ with simvastatin treated group, high-fat diet for 14 weeks and treated with simvastatin 0.9 mg/10 mL/kg every day (n = 15 at 0 week and n = 10 at 6, 10 and 14 weeks); HDL: high-fat diet + STZ with low dose of DSKT treated group, high-fat diet for 14 weeks and treated with DSKT 3.75 g/10 mL/kg every day (n = 15 at 0 week and n = 10 at 6, 10 and 14 weeks); HDM: high-fat diet + STZ with medial dose of DSKT treated group, high-fat diet for 14 weeks and treated with DSKT 7.5 g/10 mL/kg every day (n = 15 at 0 week and n = 10 at 6, 10 and 14 weeks); HDH: high-fat diet + STZ with high dose of DSKT treated group, high-fat diet for 14 weeks and treated with DSKT 15 g/10 mL/kg every day (n = 15 at 0 week and n = 10 at 6, 10 and 14 weeks). *P < 0.05 vs. Group C, †P < 0.05 vs. Group H, ‡P < 0.05 vs. Group HS, §P < 0.05 vs. Group HDL, ||P < 0.05 vs. Group HDM. Data are expressed as the means ± standard deviation. 0W, 0 week; 6W, 6 weeks; 10W, 10 weeks; 14W, 14 weeks; DSKT, daisaikoto; STZ, streptozotocin.
Histological examination of liver
From hematoxylin-eosin staining we could find that the fatty degeneration (steatosis) of the liver was observed in the high-fat-diet-fed groups, but not in Group C. The tiny and large vacuoles as well as pleomorphic nuclei were more conspicuous in the treatment groups than in Group H (Fig. 1). Groups HS and HDH seem to show a better effect (× 400).
Fig. 1.
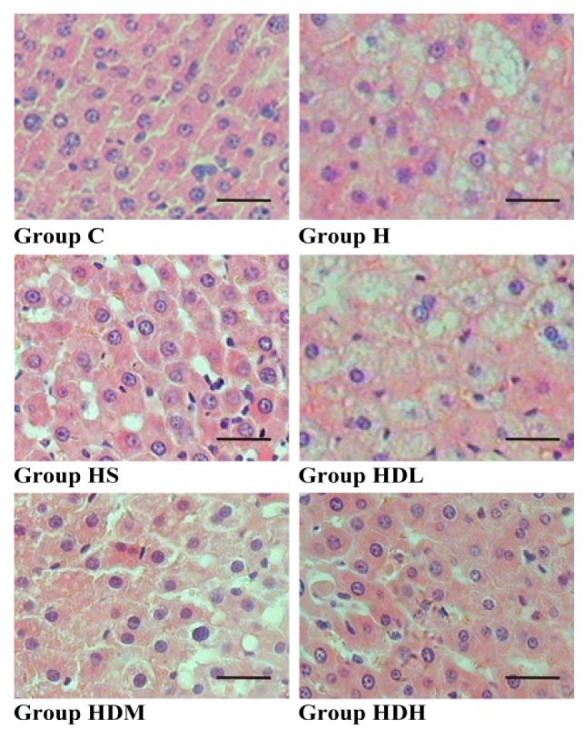
Histological examination of the liver.
Fatty degeneration (steatosis) of the liver is observed in high-fat diet-fed groups (H, HS, HDL, HDM and HDH), but not in Group C in hematoxylin-eosin stained tissues. C: normal control group, standard rat diet for 14 weeks and treated with 0.9% saline 10 mL/kg every day (n = 10); H: high-fat diet + STZ group, high-fat diet for 14 weeks and treated with 0.9% saline 10 mL/kg every day (n = 10); HS: high-fat diet + STZ with simvastatin treated group, high-fat diet for 14 weeks and treated with simvastatin 0.9 mg/10 mL/kg every day (n = 10); HDL: high-fat diet + STZ with low dose of DSKT treated group, high-fat diet for 14 weeks and treated with DSKT 3.75 g/10 mL/kg every day (n = 10); HDM: high-fat diet + STZ with medial dose of DSKT treated group, high-fat diet for 14 weeks and treated with DSKT 7.5 g/10 mL/kg every day (n = 10); HDH: high-fat diet + STZ with high dose of DSKT treated group, high-fat diet for 14 weeks and treated with DSKT 15 g/10 mL/kg every day (n = 10). Bars express 25 μm. DSKT, daisaikoto; STZ, streptozotocin.
Blood chemistry and cholesterol concentrations
As Table 4 shows, the high-fat diet increased the concentrations of LDL-C, TC, TG, ALT, ALP and reduced the concentrations of HDL-C in Group H compared with Group C after 14 weeks in serum (P < 0.05). Compared with Group H, the concentrations of LDL-C, TC, TG, ALT and ALP decreased in Groups HS, HDL, HDM and HDH (P < 0.05), while the concentration of HDL-C had an opposite trend (P < 0.05). Groups HS and HDH had a better effect (P < 0.05). DSKT regulated blood chemistry and cholesterol concentrations in a dose dependent manner (P < 0.05).
Table 4.
Blood chemistry and cholesterol concentrations
| Group C | Group H | Group HS | Group HDL | Group HDM | Group HDH | |
| LDL-C (mmol/L) | 0.65 ± 0.08 | 2.09 ± 0.18* | 0.96 ± 0.08† | 1.88 ± 0.16†‡ | 1.35 ± 0.14†‡§ | 1.00 ± 0.10†§|| |
| HDL-C (mmol/L) | 1.09 ± 0.22 | 0.54 ± 0.13* | 0.91 ± 0.14† | 0.67 ± 0.08†‡ | 0.84 ± 0.09†§ | 0.89 ± 0.14†§ |
| TC (mmol/L) | 1.18 ± 0.26 | 2.49 ± 0.51* | 1.32 ± 0.4† | 1.98 ± 0.4†‡ | 1.70 ± 0.44†‡ | 1.38 ± 0.44†§ |
| TG(mmol/L) | 0.8 ± 0.27 | 2.29 ± 0.42* | 1.79 ± 0.34† | 1.93 ± 0.49† | 1.70 ± 0.32† | 1.36 ± 0.43†‡§ |
| ALT (U/L) | 39.11 ± 2.55 | 61.75 ± 5.18* | 63.4 ± 6.96 | 55.14 ± 3.79†‡ | 49.96 ± 3.48†‡§ | 45.14 ± 4.00†‡§|| |
| ALP (U/L) | 100.45 ± 9.95 | 154.69 ± 17.54* | 168.8 ± 7.05† | 127.38 ± 9.59†‡ | 120.37 ± 10.33†‡ | 112.47 ± 6.57†‡§ |
C: normal control group, standard rat diet for 14 weeks and treated with 0.9% saline 10 mL/kg every day (n = 10); H: high-fat diet + STZ group, high-fat diet for 14 weeks and treated with 0.9% saline 10 mL/kg every day (n = 10); HS: high-fat diet + STZ with simvastatin treated group, high-fat diet for 14 weeks and treated with simvastatin 0.9 mg/10 mL/kg every day (n = 10); HDL: high-fat diet + STZ with low dose of DSKT treated group, high-fat diet for 14 weeks and treated with DSKT 3.75 g/10 mL/kg every day (n = 10); HDM: high-fat diet + STZ with medial dose of DSKT treated group, high-fat diet for 14 weeks and treated with DSKT 7.5 g/10 mL/kg every day (n = 10); HDH: high-fat diet + STZ with high dose of DSKT treated group, high-fat diet for 14 weeks and treated with DSKT 15 g/10 mL/kg every day (n = 10). *P < 0.05 vs. Group C, †P < 0.05 vs. Group H, ‡P < 0.05 vs. Group HS, §P < 0.05 vs. Group HDL, ||P < 0.05 vs. Group HDM. Data are expressed as the means ± standard deviation. ALP, alkaline phosphatase; ALT, alanine aminotransferase; DSKT, daisaikoto; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; STZ, streptozotocin; TC, total cholesterol; TG, triglyceride.
Blood glucose level
As shown in Fig. 2, there were no significant differences in the baseline of blood glucose level at the beginning of the experiment. The high fat diet increased blood glucose level slightly in Groups H, HS, HDL, HDM and HDH than in Group C after 6 weeks, but there were no statistical differences. Blood glucose increased greatly 3 days after intraperitoneal injection of STZ in Groups H, HS, HDL, HDM and HDH. After treatment, blood glucose obviously decreased in Groups HDL, HDM and HDH compared with Group H, but not in Group HS after 8, 10, 12 and 14 weeks.
Fig. 2.
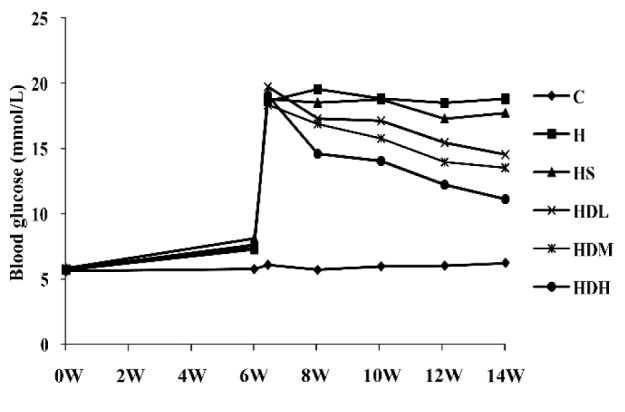
Blood glucose level
Blood glucose increased greatly 3 days after intraperitioneal injection of STZ and decreased after treatment in Groups HDL, HDM and HDH. C: normal control group, standard rat diet for 14 weeks and treated with 0.9% saline 10 mL/kg every day (n = 10); H: high-fat diet + STZ group, high-fat diet for 14 weeks and treated with 0.9% saline 10 mL/kg every day (n = 15 at 0 and 6 weeks and n = 10 at 6 weeks + 3 days, 8 weeks, 10 weeks, 12weeks and 14 weeks; HS: high-fat diet + STZ with simvastatin treated group, high-fat diet for 14 weeks and treated with simvastatin 0.9 mg/10 mL/kg every day (n = 15 at 0 and 6 weeks and n = 10 at 6 weeks + 3 days, 8 weeks, 10 weeks, 12weeks and 14 weeks); HDL: high-fat diet + STZ with low dose of DSKT treated group, high-fat diet for 14 weeks and treated with DSKT 3.75 g/10 mL/kg every day (n = 15 at 0 and 6 weeks and n = 10 at 6 weeks + 3 days, 8 weeks, 10 weeks, 12weeks and 14 weeks); HDM: high-fat diet + STZ with medial dose of DSKT treated group, high-fat diet for 14 weeks and treated with DSKT 7.5 g/10 mL/kg every day (n = 15 at 0 and 6 weeks and n = 10 at 6 weeks + 3 days, 8 weeks, 10 weeks, 12weeks and 14 weeks); HDH: high-fat diet + STZ with high dose of DSKT treated group, high-fat diet for 14 weeks and treated with DSKT 15 g/10 mL/kg every day (n = 15 at 0 and 6 weeks and n = 10 at 6 weeks + 3 days, 8 weeks, 10 weeks, 12weeks and 14 weeks). 0W, 0 week; 2W, 2 weeks; 4W, 4weeks; 6W, 6weeks; 8W, 8 weeks; 10W, 10 weeks; 12W, 12 weeks; 14W, 14 weeks. 6 weeks + 3 days means 3 days after intraperitioneal injection of STZ. DSKT, daisaikoto; STZ, streptozotocin.
Insulin in serum and HOMA-IR Index
Insulin concentrations were increased in Group H compared with Groups C and HDH after 14 weeks (Fig. 3, P < 0.05). HOMA-IR obviously increased in Groups H, HS, HDL, HDM and HDH compared with Group C (P < 0.05). After treatment, HOMA-IR decreased in Groups HS, HDL, HDM and HDH (P < 0.05). DSKT decreased HOMA-IR in a dose dependent manner (P < 0.05).
Fig. 3.
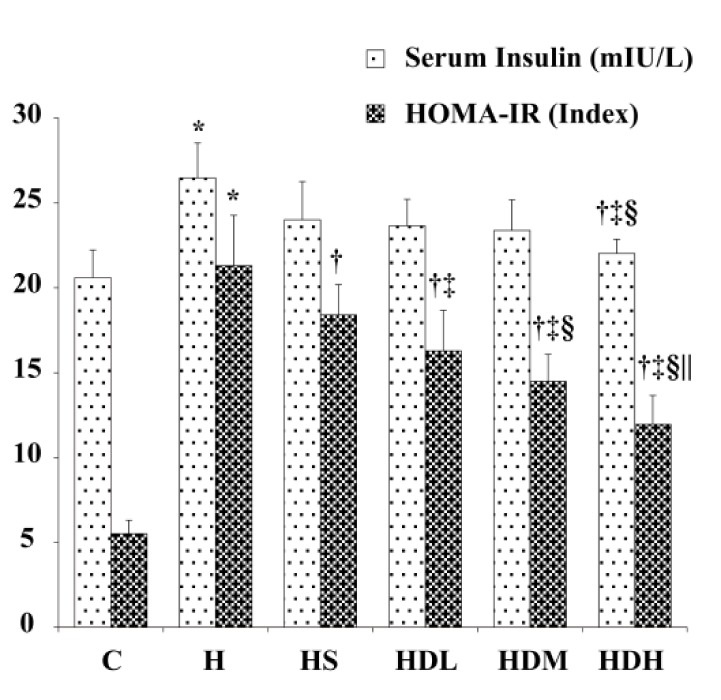
Insulin in serum and HOMA-IR Index.
C: normal control group, standard rat diet for 14 weeks and treated with 0.9% saline 10 mL/kg every day (n = 10); H: high-fat diet + STZ group, high-fat diet for 14 weeks and treated with 0.9% saline 10 mL/kg every day (n = 10); HS: high-fat diet + STZ with simvastatin treated group, high-fat diet for 14 weeks and treated with simvastatin 0.9 mg/10 mL/kg every day (n = 10); HDL: high-fat diet + STZ with low dose of DSKT treated group, high-fat diet for 14 weeks and treated with DSKT 3.75 g/10 mL/kg every day (n = 10); HDM: high-fat diet + STZ with medial dose of DSKT treated group, high-fat diet for 14 weeks and treated with DSKT 7.5 g/10 mL/kg every day (n = 10); HDH: high-fat diet + STZ with high dose of DSKT treated group, high-fat diet for 14 weeks and treated with DSKT 15 g/10 mL/kg every day (n = 10). HOMA-IR index = fasting plasma insulin (mIU/L) × fasting plasma glucose (mmol/L)/22.5. *P < 0.05 vs. Group C, †P < 0.05 vs. Group H, ‡P < 0.05 vs. Group HS, §P < 0.05 vs. Group HDL, ||P < 0.05 vs. Group HDM. DSKT, daisaikoto; HOMA-IR, Homeostasis model assessment-estimated insulin resistance; STZ, streptozotocin.
SIRT1 mRNA expression in the liver
As Fig. 4 shows, SIRT1 mRNA expression in the liver in Group H was significantly decreased compared to that in other groups (P < 0.05). Groups HS, HDL, HDM and HDH increased SIRT1 mRNA expression in the liver to a various degree (P < 0.05). Group HDH had a better effect. DSKT increased SIRT1 mRNA expression in the liver in a dose dependent manner (P < 0.05).
Fig. 4.
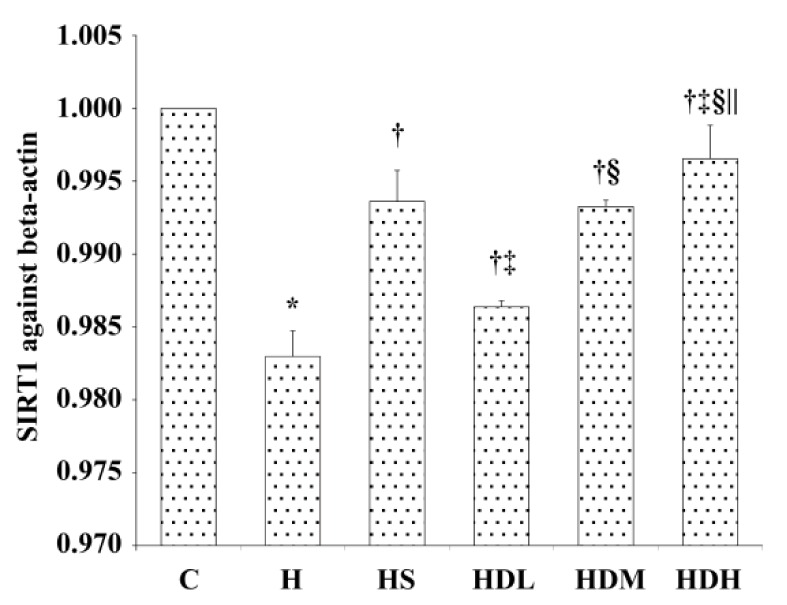
Changes of mRNA expression of SIRT1 in liver after 14 weeks.
Levels of SIRT1 against beta-actin mRNA expression are shown in the above histogram. C: normal control group, standard rat diet for 14 weeks and treated with 0.9% saline 10 mL/kg every day (n = 10); H: high-fat diet + STZ group, high-fat diet for 14 weeks and treated with 0.9% saline 10 mL/kg every day (n = 10); HS: high-fat diet + STZ with simvastatin treated group, high-fat diet for 14 weeks and treated with simvastatin 0.9 mg/10 mL/kg every day (n = 10); HDL: high-fat diet + STZ with low dose of DSKT treated group, high-fat diet for 14 weeks and treated with DSKT 3.75 g/10 mL/kg every day (n = 10); HDM: high-fat diet + STZ with medial dose of DSKT treated group, high-fat diet for 14 weeks and treated with DSKT 7.5 g/10 mL/kg every day (n = 10); HDH: high-fat diet + STZ with high dose of DSKT treated group, high-fat diet for 14 weeks and treated with DSKT 15 g/10 mL/kg every day (n = 10). *P < 0.05 vs. Group C, †P < 0.05 vs. Group H, ‡P < 0.05 vs. Group HS, §P < 0.05 vs. Group HDL, ||P < 0.05 vs. Group HDM. DSKT, daisaikoto; SIRT1, sirtuin 1, silent mating type information regulation 2 homolog 1; STZ, streptozotocin.
NF-kappaB immunostaining expression in liver
As shown in Fig. 5A, after 14 weeks, NF-kappaB immunostaining (Yellow-brown deposits indicate positive staining) was seldom found in hepatic cells in Group C. Significantly positive NF-kappaB immunostaining was observed in Group H and mainly localized in cell cytoplasm and the nucleus. Positive NF-kappaB immunostaining was also observed in Groups HS, HDL, HDM and HDH, but less than in Group H (× 400). Mean optical density values of NF-kappaB are shown in Fig. 5B. The photographs generated quantitatively analyzed the optical density of NF-kappaB by Image - Pro Plus. After treatment, the expressions in Groups HS, HDL, HDM and HDH decreased more than in Group H. Also, Group HDH was significantly lower compared with Groups HS, HDL and HDM (P < 0.05).
Fig. 5.
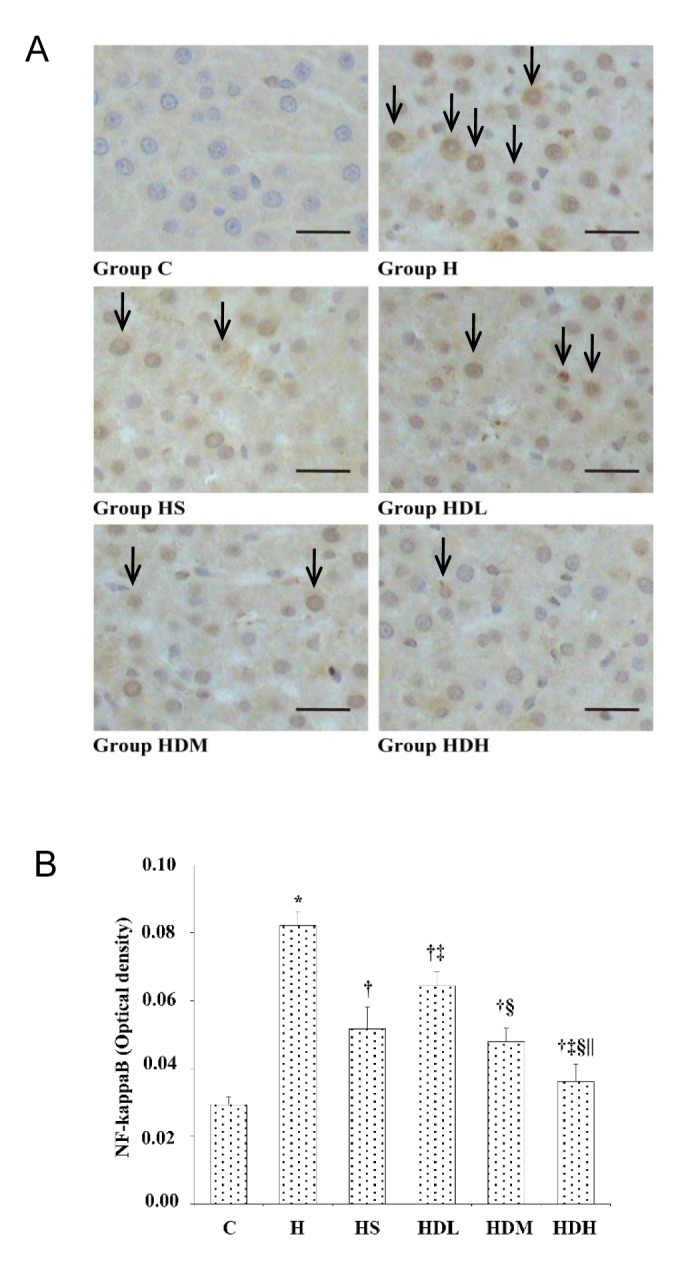
NF-kappaB immunostaining expression in liver after 14 weeks.
A: Immunohistochemistry expression of NF-kappaB in liver. Arrows show the positive NF-kappaB immunostaining. B: Mean optical density values of NF-kappaB. The photographs generated were quantitatively analyzed the optical density of NF-kappaB with Image - Pro Plus. C: normal control group, standard rat diet for 14 weeks and treated with 0.9% saline 10 mL/kg every day (n = 10); H: high-fat diet + STZ group, high-fat diet for 14 weeks and treated with 0.9% saline 10 mL/kg every day (n = 10); HS: high-fat diet + STZ with simvastatin treated group, high-fat diet for 14 weeks and treated with simvastatin 0.9 mg/10 mL/kg every day (n = 10); HDL: high-fat diet + STZ with low dose of DSKT treated group, high-fat diet for 14 weeks and treated with DSKT 3.75 g/10 mL/kg every day (n = 10); HDM: high-fat diet + STZ with medial dose of DSKT treated group, high-fat diet for 14 weeks and treated with DSKT 7.5 g/10 mL/kg every day (n = 10); HDH: high-fat diet + STZ with high dose of DSKT treated group, high-fat diet for 14 weeks and treated with DSKT 15 g/10 mL/kg every day (n = 10). *P < 0.05 vs. Group C, †P < 0.05 vs. Group H, ‡P < 0.05 vs. Group HS, §P < 0.05 vs. Group HDL, ||P < 0.05 vs. Group HDM. Bars express 25 μm. DSKT, daisaikoto; NF-kappaB, nuclear factor kappa B; STZ, streptozotocin.
DISCUSSION
NAFLD is commonly associated with many symptoms of metabolic syndrome such as obesity and T2DM.2 Patients with T2DM appear to have an increased risk of developing NAFLD and certainly have a higher risk of developing fibrosis and cirrhosis.3 Individuals always present simultaneously with characteristics of high blood glucose, insulin resistance, hyperlipidemia and fatty liver. It has been reported that the abnormal serum levels of HDL-C and LDL-C are associated with NAFLD.26 And NAFLD with T2DM patients always show up-regulated TC and LDL-C and down-regulated HDL-C levels.27, 28 Statins, one kind of lipid-lowering drug, can inhibit cholesterol synthesis. They can definitely decrease LDL-C and increase HDL-C. However, there is a risk that they sometimes are instrumental in causing rhabdomyolysis and hepatitis. Traditional Chinese medicine is always considered a safe way to harmonize the abnormal serum levels of HDL-C and LDL-C, to improve high blood glucose as well as minimize side effects. In a previous study of ours, we found fatty liver, up-regulated TC and LDL-C as well as down-regulated HDL-C in high-cholesterol diet rats.29, 30 In this experiment, fatty steatosis and high blood glucose were observed after administration of a high-fat diet and STZ in Group H. Body weights decreasing after intraperitoneal injection of STZ may be associated with the elevated blood glucose; it could be ameliorated by DSKT. DSKT and simvastatin could decrease the concentrations of LDL-C and TC and increase HDL-C. However, ALT and ALP concentrations in Group HS were significantly elevated than in the DSKT groups. The reason may be that DSKT has fewer side effects than simvastatin. On the other hand, DSKT can reduce the high blood glucose levels, while simvastatin does not have very stable effects and cannot improve high blood glucose effectively. Insulin secretion increasing in Group H compared with Group C may be a compensatory response against the increased blood glucose and impaired insulin function. HOMA-IR increased obviously in Groups H, HS, HDL, HDM and HDH compared with Group C. This shows that the body is not sensitive to insulin, and leads to difficulty in decreased blood glucose. DSKT also obviously inhibits insulin resistance. Furthermore, DSKT has shown a strong dose-dependent effect on diabetic fatty liver.
SIRT1, an NAD+-dependent deacetylase, is implicated in diverse cellular processes, including metabolism, inflammation and apoptosis.11–13 Increasing evidence has shown that it may be involved in the development of NAFLD.14 Deng, XQ et al have proven that SIRT1 protein levels are reduced in NAFLD induced by high-fat diet in rats.15 SIRT1 target multiple cellular proteins take part in metabolic homeostasis, oxidative stress as well as in inflammation, such as NF-kappaB.31 It is reported that hepatic SIRT1 is a key regulator of lipid homeostasis, in particular fatty acid oxidation. Loss of SIRT1 in hepatocytes can lead to a decrease in fatty acid oxidation, resulting in the development of hepatic steatosis and inflammation on high-fat diet mice.32 In this study, hepatic mitochondrial SIRT1 expression was significantly reduced in Group H, as previously shown by others. DSKT tended to increase hepatic SIRT1 expression, explaining in part its beneficial effect with respect to high-fat diet induced disorders.
An additional mechanism related to DSKT could elicit beneficial effects through NF-kappaB. As is well known, long-time intake of a high-fat diet could result in low-grade inflammation. This inflammation state is characterized by the activation of the NF-kappaB pathway.33 NF-kappaB, an oxidative stress sensitive transcription factor, plays an important role in regulation of the expression of many genes involved in inflammatory responses, and NF-kappaB-induced cytokines appear widely in inflammatory diseases, including NAFLD and T2DM.17–19 Andrade JM et al proved that NF-kappaB was increased in high-fat diet mice.34 Group H in our study displayed higher hepatic NF-kappaB activity as compared to Group C. According to Morgan,35 this activation could have been triggered, at least partially, by reactive oxygen species (ROS), which occurs in excess in the liver. However, Group HDH showed the strongest effect to prevent liver NF-kappaB activation and this reveals a dose-depend trend. The antioxidant effects of a high dose of DSKT likely contributed to the inhibition of NF-kappaB activation.
In conclusion, we have demonstrated that DSKT is capable of reversing blood lipid and blood glucose disorders induced by high-fat diet and STZ. DSKT improves insulin resistance as well as liver oxidative and inflammatory status, which are closely linked to the development of NAFLD with T2DM. Similarly, the antioxidant properties of DSKT contribute, at least partially, to improving the inflammatory status of the liver. Molecular targets involved in these beneficial effects include NF-kappaB inhibition and the involvement of SIRT1. These results may be a guide for clinical use in the treatment of T2DM with fatty liver.
Acknowledgments
Ackonwlegments: This work was supported by the Natural Science Foundation of Shandong Province, China (ZR2012HM093) and the Science and Technology Research Project of Traditional Chinese Medicine in Shandong Province, China (2011-038).
The authors declare no conflict of interest.
REFERENCES
- 1. Clark JM, Brancati FL, Diehl AM. Nonalcoholic fatty liver disease. Gastroenterology. 2002;122:1649-57. [DOI] [PubMed] [Google Scholar]
- 2. Adams LA, Angulo P. Recent concepts in non-alcoholic fatty liver diseas. Diabet Med. 2005;22:1129-33. [DOI] [PubMed] [Google Scholar]
- 3. Targher G, Bertolini L, Padovani R, Rodella S, Tessari R, Zenari L, et al. Prevalence of nonalcoholic fatty liver disease and its association with cardiovascular disease among type 2 diabetic patients. Diabetes care. 2007;30:1212-8. [DOI] [PubMed] [Google Scholar]
- 4. Jia G, Di F, Wang Q, Shao J, Gao L, Wang L, et al. Non-Alcoholic Fatty Liver Disease Is a Risk Factor for the Development of Diabetic Nephropathy in Patients with Type 2 Diabetes Mellitus. PloS one. 2015;10:e0142808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Younossi ZM, Gramlich T, Matteoni CA, Boparai N, McCullough AJ. Nonalcoholic fatty liver disease in patients with type 2 diabetes. Clin Gastroenterol Hepatol. 2004;2:262-5. [DOI] [PubMed] [Google Scholar]
- 6. Miele L, Forgione A, Gasbarrini G, Grieco A. Noninvasive assessment of fibrosis in non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH). Transl Res. 2007;149:114-25. [DOI] [PubMed] [Google Scholar]
- 7. Tseng PH, Liu CJ, Kao JH, Shun CT, Chen PJ, Chen DS. Disease progression in a patient with nonalcoholic steatohepatitis. J Formos Med Assoc. 2008;107:816-21. [DOI] [PubMed] [Google Scholar]
- 8. Musso G, Gambino R, Cassader M. Recent insights into hepatic lipid metabolism in non-alcoholic fatty liver disease (NAFLD). Prog Lipid Res. 2009;48:1-26. [DOI] [PubMed] [Google Scholar]
- 9. Alsheikh-Ali AA, Kuvin JT, Karas RH. Risk of adverse events with fibrates. Am J Cardiol. 2004;94:935-8. [DOI] [PubMed] [Google Scholar]
- 10. Zaitone S, Hassan N, El-Orabi N, El-Awady el S. Pentoxifylline and melatonin in combination with pioglitazone ameliorate experimental non-alcoholic fatty liver disease. Eur J Pharmacol. 2011;662:70-7. [DOI] [PubMed] [Google Scholar]
- 11. Morris KC, Lin HW, Thompson JW, Perez-Pinzon MA. Pathways for ischemic cytoprotection: role of sirtuins in caloric restriction, resveratrol, and ischemic preconditioning. J Cereb Blood Flow Metab. 2011;31:1003-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kim HJ, Joe Y, Yu JK, Chen Y, Jeong SO, Mani N, et al. Carbon monoxide protects against hepatic ischemia/reperfusion injury by modulating the miR-34a/SIRT1 pathway. Biochim Biophys Acta. 2015;1852:1550-9. [DOI] [PubMed] [Google Scholar]
- 13. Takata T, Munemura C, Fukui T, Fukuda S, Murawaki Y. Influence of Olmesartan on Sirtuin 1 mRNA Expression in 5/6 Nephrectomized Spontaneously Hypertensive Rats. Yonago Acta Med. 2015;58:63-8. [PMC free article] [PubMed] [Google Scholar]
- 14. Romain C, Bresciani L, Gaillet S, Feillet-Coudray C, Calani L, Bonafos B, et al. Moderate chronic administration of Vineatrol-enriched red wines improves metabolic, oxidative, and inflammatory markers in hamsters fed a high-fat diet. Mol Nutr Food Res. 2014;58:1212-25. [DOI] [PubMed] [Google Scholar]
- 15. Deng XQ, Chen LL, Li NX. The expression of SIRT1 in nonalcoholic fatty liver disease induced by high-fat diet in rats. Liver Int. 2007;27:708-15. [DOI] [PubMed] [Google Scholar]
- 16. Rahman I, Kinnula VL, Gorbunova V, Yao H. SIRT1 as a therapeutic target in inflammaging of the pulmonary disease. Prev Med. 2012;54 Suppl:S20-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pamukcu B, Lip GY, Shantsila E. The nuclear factor--kappaB pathway in atherosclerosis: a potential therapeutic target for atherothrombotic vascular disease. Thromb Res. 2011;128:117-23. [DOI] [PubMed] [Google Scholar]
- 18. Sharma M, Mitnala S, Vishnubhotla RK, Mukherjee R, Reddy DN, Rao PN. The Riddle of Nonalcoholic Fatty Liver Disease: Progression From Nonalcoholic Fatty Liver to Nonalcoholic Steatohepatitis. J Clin Exp Hepatol. 2015;5:147-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu F, Fu Y, Wei C, Chen Y, Ma S, Xu W. The expression of GPR109A, NF-kB and IL-1beta in peripheral blood leukocytes from patients with type 2 diabetes. Ann Clin Lab Sci. 2014;44:443-8. [PubMed] [Google Scholar]
- 20. Kuohsiung L, Wang HK, Itokawa H, Morris-Natschke SL. Current perspectives on Chinese medicines and dietary supplements in China, Japan and the United States. J Food Drug Anal. 2000;8:219-28. [Google Scholar]
- 21. Goto M, Inoue H, Seyama Y, Yamashita S, Inoue O, Yumioka E, et al. [Comparative effects of traditional Chinese medicines (dai-saiko-to, hatimi-zio-gan and byakko-ka-ninzin-to) on experimental diabetes and hyperlipidemia]. Nihon Yakurigaku Zasshi. 1989;93:179-86. Japanese. [DOI] [PubMed] [Google Scholar]
- 22. Yamamoto K, Ogawa Y, Yanagita T, Morito F, Fukushima N, Ozaki I, et al. Pharmacological effects of dai-saiko-to on lipid biosynthesis in cultured human hepatocyte HepG2 cells. J Ethnopharmacol. 1995;46:49-54. [DOI] [PubMed] [Google Scholar]
- 23. Iizuka A, Iijima OT, Yoshie F, Makino B, Amagaya S, Komatsu Y, et al. Inhibitory effects of Dai-saiko-to (Da-Chai-Hu-Tang) on the progression of atherosclerotic lesions in Kurosawa and Kusanagi-hypercholesterolemic rabbits. J Ethnopharmacol. 1998;63:209-18. [DOI] [PubMed] [Google Scholar]
- 24. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412-9. [DOI] [PubMed] [Google Scholar]
- 25. Huang DW, Chang WC, Wu JS, Shih RW, Shen SC. Vescalagin from Pink Wax Apple [Syzygium samarangense (Blume) Merrill and Perry] Alleviates Hepatic Insulin Resistance and Ameliorates Glycemic Metabolism Abnormality in Rats Fed a High-Fructose Diet. J Agric Food Chem. 2016;64:1122-9. [DOI] [PubMed] [Google Scholar]
- 26. Rader DJ. High-density lipoproteins and atherosclerosis. Am J Cardiol. 2002;90:62i-70i. [DOI] [PubMed] [Google Scholar]
- 27. Sanyal D, Mukherjee P, Raychaudhuri M, Ghosh S, Mukherjee S, Chowdhury S. Profile of liver enzymes in non-alcoholic fatty liver disease in patients with impaired glucose tolerance and newly detected untreated type 2 diabetes. Indian J Endocrinol Metab. 2015;19:597-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jia G, Li X, Wang L, Li Q, Yang L, Li N, et al. [Relationship of socioeconomic status and non-alcoholic fatty liver disease in patients with type 2 diabetes mellitus]. Zhonghua Gan Zang Bing Za Zhi. 2015;23:760-4. Chinese. [DOI] [PubMed] [Google Scholar]
- 29. Qian W, Hasegawa J, Tsuno S, Endo Y, Matsuda A, Miura N. Effects of kampo formulas on the progression of hypercholesterolemia and Fatty liver induced by high-cholesterol diet in rats. Yonago Acta Med. 2014;57:147-58. [PMC free article] [PubMed] [Google Scholar]
- 30. Qian W, Hasegawa J, Cai X, Yang J, Ishihara Y, Ping B, Tsuno S, Endo Y, Matsuda A, Miura N. Components of Boiogito Suppress the Progression of Hypercholesterolemia and Fatty Liver Induced by High-Cholesterol Diet in Rats. Yonago Acta Med. 2016;59:67-80. [PMC free article] [PubMed] [Google Scholar]
- 31. Nogueiras R, Habegger KM, Chaudhary N, Finan B, Banks AS, Dietrich MO, et al. Sirtuin 1 and sirtuin 3: physiological modulators of metabolism. Physiol Rev. 2012;92:1479-514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Purushotham A, Schug TT, Xu Q, Surapureddi S, Guo X, Li X, et al. Hepatocyte-specific deletion of SIRT1 alters fatty acid metabolism and results in hepatic steatosis and inflammation. Cell Metab. 2009;9:327-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ying HZ, Liu YH, Yu B, Wang ZY, Zang JN, Yu CH. Dietary quercetin ameliorates nonalcoholic steatohepatitis induced by a high-fat diet in gerbils. Food Chem Toxicol. 2013;52:53-60. [DOI] [PubMed] [Google Scholar]
- 34. Andrade JM, Paraiso AF, de Oliveira MV, Martins AM, Neto JF, Guimaraes AL, et al. Resveratrol attenuates hepatic steatosis in high-fat fed mice by decreasing lipogenesis and inflammation. Nutrition. 2014;30:915-9. [DOI] [PubMed] [Google Scholar]
- 35. Morgan MJ, Liu ZG. Crosstalk of reactive oxygen species and NF-kappaB signaling. Cell Res. 2011;21:103-15. [DOI] [PMC free article] [PubMed] [Google Scholar]


