Abstract
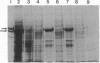
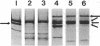
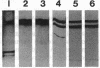
Prolyl 4-hydroxylase (EC 1.14.11.2), an alpha 2 beta 2 tetramer, catalyzes the posttranslational formation of 4-hydroxyproline in collagens. The enzyme can easily be dissociated into its subunits, but all attempts to associate a tetramer from the dissociated subunits in vitro have been unsuccessful. Molecular cloning of the catalytically important alpha subunit has identified two types of cDNA clone due to mutually exclusive alternative splicing. The beta subunit is a highly unusual multifunctional polypeptide, being identical to the enzyme protein disulfide-isomerase (EC 5.3.4.1). We report here on expression of the alpha and beta subunits of prolyl 4-hydroxylase and a fully active enzyme tetramer in Spodoptera frugiperda insect cells by baculovirus vectors. When the beta subunit was expressed alone, the polypeptide produced was found in a 0.1% Triton X-100 extract of the cell homogenate and was a fully active protein disulfide-isomerase. When either form of the alpha subunit was expressed alone, only traces of the alpha subunit could be extracted from the cell homogenate with 0.1% Triton X-100, and 1% SDS was required to obtain efficient solubilization. These alpha subunits had no prolyl 4-hydroxylase activity. When the cells were coinfected with both alpha- and beta-subunit-producing viruses, an enzyme tetramer was formed, but significant amounts of alpha and beta subunits remained unassociated. The recombinant tetramer was indistinguishable from that isolated from vertebrate tissue in terms of its specific activity and kinetic constants for cosubstrates and the peptide substrate. The two alternatively spliced forms of the alpha subunit gave enzyme tetramers with identical catalytic properties. Baculovirus expression seems to be an excellent system for mass production of the enzyme tetramer and for detailed investigation of the mechanisms involved in the association of the monomers.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andres D. A., Rhodes J. D., Meisel R. L., Dixon J. E. Characterization of the carboxyl-terminal sequences responsible for protein retention in the endoplasmic reticulum. J Biol Chem. 1991 Aug 5;266(22):14277–14282. [PubMed] [Google Scholar]
- Bassuk J. A., Kao W. W., Herzer P., Kedersha N. L., Seyer J., DeMartino J. A., Daugherty B. L., Mark G. E., 3rd, Berg R. A. Prolyl 4-hydroxylase: molecular cloning and the primary structure of the alpha subunit from chicken embryo. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7382–7386. doi: 10.1073/pnas.86.19.7382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg R. A., Prockop D. J. Affinity column purification of protocollagen proline hydroxylase from chick embryos and further characterization of the enzyme. J Biol Chem. 1973 Feb 25;248(4):1175–1182. [PubMed] [Google Scholar]
- Carmichael D. F., Morin J. E., Dixon J. E. Purification and characterization of a thiol:protein disulfide oxidoreductase from bovine liver. J Biol Chem. 1977 Oct 25;252(20):7163–7167. [PubMed] [Google Scholar]
- Cheng S. Y., Gong Q. H., Parkison C., Robinson E. A., Appella E., Merlino G. T., Pastan I. The nucleotide sequence of a human cellular thyroid hormone binding protein present in endoplasmic reticulum. J Biol Chem. 1987 Aug 15;262(23):11221–11227. [PubMed] [Google Scholar]
- Freedman R. B., Bulleid N. J., Hawkins H. C., Paver J. L. Role of protein disulphide-isomerase in the expression of native proteins. Biochem Soc Symp. 1989;55:167–192. [PubMed] [Google Scholar]
- Geetha-Habib M., Noiva R., Kaplan H. A., Lennarz W. J. Glycosylation site binding protein, a component of oligosaccharyl transferase, is highly similar to three other 57 kd luminal proteins of the ER. Cell. 1988 Sep 23;54(7):1053–1060. doi: 10.1016/0092-8674(88)90120-1. [DOI] [PubMed] [Google Scholar]
- Helaakoski T., Vuori K., Myllylä R., Kivirikko K. I., Pihlajaniemi T. Molecular cloning of the alpha-subunit of human prolyl 4-hydroxylase: the complete cDNA-derived amino acid sequence and evidence for alternative splicing of RNA transcripts. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4392–4396. doi: 10.1073/pnas.86.12.4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höyhtyä M., Myllylä R., Piuva J., Kivirikko K. I., Tryggvason K. Monoclonal antibodies to human prolyl 4-hydroxylase. Eur J Biochem. 1984 Jun 15;141(3):472–482. doi: 10.1111/j.1432-1033.1984.tb08217.x. [DOI] [PubMed] [Google Scholar]
- Kedersha N. L., Tkacz J. S., Berg R. A. Characterization of the oligosaccharides of prolyl hydroxylase, a microsomal glycoprotein. Biochemistry. 1985 Oct 8;24(21):5952–5960. doi: 10.1021/bi00342a040. [DOI] [PubMed] [Google Scholar]
- Kivirikko K. I., Helaakoski T., Tasanen K., Vuori K., Myllylä R., Parkkonen T., Pihlajaniemi T. Molecular biology of prolyl 4-hydroxylase. Ann N Y Acad Sci. 1990;580:132–142. doi: 10.1111/j.1749-6632.1990.tb17925.x. [DOI] [PubMed] [Google Scholar]
- Kivirikko K. I., Myllylä R., Pihlajaniemi T. Protein hydroxylation: prolyl 4-hydroxylase, an enzyme with four cosubstrates and a multifunctional subunit. FASEB J. 1989 Mar;3(5):1609–1617. [PubMed] [Google Scholar]
- Kivirikko K. I., Myllylä R. Posttranslational enzymes in the biosynthesis of collagen: intracellular enzymes. Methods Enzymol. 1982;82(Pt A):245–304. doi: 10.1016/0076-6879(82)82067-3. [DOI] [PubMed] [Google Scholar]
- Kivirikko K. I., Myllylä R. Recent developments in posttranslational modification: intracellular processing. Methods Enzymol. 1987;144:96–114. doi: 10.1016/0076-6879(87)44175-x. [DOI] [PubMed] [Google Scholar]
- Koivu J., Myllylä R., Helaakoski T., Pihlajaniemi T., Tasanen K., Kivirikko K. I. A single polypeptide acts both as the beta subunit of prolyl 4-hydroxylase and as a protein disulfide-isomerase. J Biol Chem. 1987 May 15;262(14):6447–6449. [PubMed] [Google Scholar]
- Koivu J., Myllylä R. Protein disulfide-isomerase retains procollagen prolyl 4-hydroxylase structure in its native conformation. Biochemistry. 1986 Oct 7;25(20):5982–5986. doi: 10.1021/bi00368a022. [DOI] [PubMed] [Google Scholar]
- Luckow V. A., Summers M. D. High level expression of nonfused foreign genes with Autographa californica nuclear polyhedrosis virus expression vectors. Virology. 1989 May;170(1):31–39. doi: 10.1016/0042-6822(89)90348-6. [DOI] [PubMed] [Google Scholar]
- Myllylä R., Tuderman L., Kivirikko K. I. Mechanism of the prolyl hydroxylase reaction. 2. Kinetic analysis of the reaction sequence. Eur J Biochem. 1977 Nov 1;80(2):349–357. doi: 10.1111/j.1432-1033.1977.tb11889.x. [DOI] [PubMed] [Google Scholar]
- Nietfeld J. J., Van der Kraan I., Kemp A. Dissociation and reassociation of prolyl 4-hydroxylase subunits after cross-linking of monomers. Biochim Biophys Acta. 1981 Sep 15;661(1):21–27. doi: 10.1016/0005-2744(81)90078-4. [DOI] [PubMed] [Google Scholar]
- Noiva R., Kimura H., Roos J., Lennarz W. J. Peptide binding by protein disulfide isomerase, a resident protein of the endoplasmic reticulum lumen. J Biol Chem. 1991 Oct 15;266(29):19645–19649. [PubMed] [Google Scholar]
- Noiva R., Lennarz W. J. Protein disulfide isomerase. A multifunctional protein resident in the lumen of the endoplasmic reticulum. J Biol Chem. 1992 Feb 25;267(6):3553–3556. [PubMed] [Google Scholar]
- Parkkonen T., Kivirikko K. I., Pihlajaniemi T. Molecular cloning of a multifunctional chicken protein acting as the prolyl 4-hydroxylase beta-subunit, protein disulphide-isomerase and a cellular thyroid-hormone-binding protein. Comparison of cDNA-deduced amino acid sequences with those in other species. Biochem J. 1988 Dec 15;256(3):1005–1011. doi: 10.1042/bj2561005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R. Control of protein exit from the endoplasmic reticulum. Annu Rev Cell Biol. 1989;5:1–23. doi: 10.1146/annurev.cb.05.110189.000245. [DOI] [PubMed] [Google Scholar]
- Pihlajaniemi T., Helaakoski T., Tasanen K., Myllylä R., Huhtala M. L., Koivu J., Kivirikko K. I. Molecular cloning of the beta-subunit of human prolyl 4-hydroxylase. This subunit and protein disulphide isomerase are products of the same gene. EMBO J. 1987 Mar;6(3):643–649. doi: 10.1002/j.1460-2075.1987.tb04803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuori K., Myllylä R., Pihlajaniemi T., Kivirikko K. I. Expression and site-directed mutagenesis of human protein disulfide isomerase in Escherichia coli. This multifunctional polypeptide has two independently acting catalytic sites for the isomerase activity. J Biol Chem. 1992 Apr 15;267(11):7211–7214. [PubMed] [Google Scholar]
- Wells W. W., Xu D. P., Yang Y. F., Rocque P. A. Mammalian thioltransferase (glutaredoxin) and protein disulfide isomerase have dehydroascorbate reductase activity. J Biol Chem. 1990 Sep 15;265(26):15361–15364. [PubMed] [Google Scholar]
- Wetterau J. R., Combs K. A., McLean L. R., Spinner S. N., Aggerbeck L. P. Protein disulfide isomerase appears necessary to maintain the catalytically active structure of the microsomal triglyceride transfer protein. Biochemistry. 1991 Oct 8;30(40):9728–9735. doi: 10.1021/bi00104a023. [DOI] [PubMed] [Google Scholar]
- Wetterau J. R., Combs K. A., Spinner S. N., Joiner B. J. Protein disulfide isomerase is a component of the microsomal triglyceride transfer protein complex. J Biol Chem. 1990 Jun 15;265(17):9800–9807. [PubMed] [Google Scholar]
- Yamauchi K., Yamamoto T., Hayashi H., Koya S., Takikawa H., Toyoshima K., Horiuchi R. Sequence of membrane-associated thyroid hormone binding protein from bovine liver: its identity with protein disulphide isomerase. Biochem Biophys Res Commun. 1987 Aug 14;146(3):1485–1492. doi: 10.1016/0006-291x(87)90817-5. [DOI] [PubMed] [Google Scholar]