Abstract
Background:
Cancer-related venous thromboembolism (VTE) heralds a poor prognosis, especially in pancreatic adenocarcinoma (PAC). Tissue factor (TF) is implicated as one of the main culprits in PAC-associated VTE and disease progression.
Methods:
In a prospective cohort study of 79 PAC patients, we measured plasma CA19–9 and microparticle-associated TF activity (MP-TF activity). In addition, we enumerated TF+MPs and MUC1+MPs in plasma (n=55), and studied the expression of TF, MUC1, CD31 and CD68 in tumour tissue (n=44).
Results:
Plasma MP-TF activity was markedly elevated in PAC patients with VTE compared with those without (median: 1925 vs 113 fM Xa min−1; P<0.001) and correlated with the extent of thromboembolic events, metastatic disease and short survival. Similar results were found for CA19–9. Patients with massively progressing thrombosis and cerebral embolisms despite anticoagulant therapy (n=3) had the highest MP-TF activities (12 118–40 188 fM Xa min−1) and CA19–9 (40 730–197 000 kU l−1). All tumours expressed MUC1 and TF. MP-TF activity did not correlate with intensity of TF expression in adenocarcinoma cells, but corresponded with numbers of TF+ macrophages in the surrounding stroma.
Conclusions:
Circulating TF+MPs and mucins may concertedly aggravate coagulopathy in PAC. Understanding of underlying mechanisms may result in new treatment strategies for VTE prevention and improvement of survival.
Keywords: macrophage, microparticles, mucins, pancreatic cancer, survival, thrombosis, tissue factor
Pancreatic adenocarcinoma (PAC) is the most lethal among adenocarcinomas with a mortality rate that equals the incidence (Torre et al, 2015). Less than 20% of patients are candidates for curative surgery and more than 75% of patients die within 1 year (Bilimoria et al, 2007). After ‘curative' resection, PAC invariably recurs and rapidly disseminates (Michl and Gress, 2013). Up to 5 years ago, median survival time was 7 months for patients with locally advanced PAC and 4.5 months for patients with metastatic disease (Bilimoria et al, 2007). Since then, treatment of advanced stage PAC patients with FOLFIRINOX (i.e., 5-FU/LV, irinotecan and oxaliplatin) has resulted in a median survival of 11 months for only those fit enough to undergo this toxic regimen (Conroy et al, 2011).
Cancer patients have an increased risk of venous thromboembolism (VTE) and the risk is especially high in PAC (58-fold increased) (Blom et al, 2006). Thus, in addition to a short lifespan, quality of life is often hampered by thromboembolic complications such as deep vein thrombosis (DVT), pulmonary embolism (PE) and occasionally marantic endocarditis or phlegmasia cerulea dolens, that is, massive venous thrombosis resulting in ischaemia (Howard, 1960; Smeglin et al, 2008).
Tissue Factor (TF) is expressed in exocrine pancreatic cells upon malignant transformation and may be one of the key proteins involved in cancer-related thrombosis and rapid metastatic spread in PAC (Kakkar et al, 1995). Complex formation with coagulation factor VII (FVII) not only initiates the coagulation cascade, but also activates intracellular protease-activated receptor 2 signalling after β1-integrin ligation, thus contributing to angiogenesis, tumour cell proliferation and migration (Ruf et al, 2010). In a cohort of 113 PAC patients with a median follow-up of 16 months, overall survival was significantly shorter in case of high tumour-TF expression (Nitori et al, 2005). A study of 122 PAC patients with a similar follow-up period did not confirm these findings, but showed a higher prevalence of VTE in patients with high tumour-TF expression (26.3%) compared with patients with low tumour-TF expression (4.5%) in a subgroup analysis of 41 patients (Khorana et al, 2007).
As procoagulant TF on tumour cells does not explain why thrombosis is typically found at sites distant from the primary tumour and its metastases, it is hypothesised that circulating TF attached to submicron cellular membrane vesicles – so-called microparticles (MPs) – may be one of the factors contributing to the coagulopathy (Falati et al, 2003; Tesselaar et al, 2007; Zwicker et al, 2009; Bharthuar et al, 2013). Whether procoagulant TF+MPs cause clinically manifest thrombosis or represent an epiphenomenon reflecting aggressive tumour biology remains under debate (Soff, 2014). In addition, TF+MPs are assumed to originate from tumour cells, but may very well be released by macrophages or endothelial cells upon activation (Ahamed et al, 2007; Østerud, 2010).
In order to gain more insight into the role of TF in PAC with respect to coagulopathy and survival, we measured numbers of TF+MPs, plasma MP-TF activity and tumour-TF expression within a cohort of 79 patients. In addition, we assessed whether mucins in the tumour and in the circulation could have contributed to the observed thromboembolic events.
Patients and Methods
Study design
From December 2000 to June 2009, pancreatic cancer patients who were referred to the Departments of Surgery and Clinical Oncology of the Leiden University Medical Centre were screened for eligibility for study participation. Inclusion criteria were age ⩾18 years and any stage of PAC, including ampullary cancer. Exclusion criteria were revised histological classification other than adenocarcinoma, duodenal carcinoma or ‘adenocarcinoma of unknown primary'. Patients were also excluded when blood sample processing within 1 h after venepuncture was not possible. All patients gave their informed consent.
Seventy-nine patients were included in the study. We collected blood from all patients at inclusion. Two patients were unintentionally included twice and thus a second plasma sample was obtained during follow-up. In 65 of 79 patients, PAC was confirmed by surgical biopsy (n=32), core needle biopsy (n=23) or fine needle aspiration (n=10). Fourteen patients (failed biopsy: n=2) were diagnosed on typical radiological findings and elevated CA19–9 levels. In 44 patients, tumour tissue specimens were suitable for immunohistochemical analysis. In a representative subset of 37 patients with similar sex and age distributions as the original cohort of 79 patients, tissue samples were obtained within a median interval of 0 days after study entry (IQR 0–3; range −70 to 30 days), enabling assessment of the relation between plasma MP-TF activity, CA19–9 levels and antigen expression in the tumour.
Procedures and definitions
Demographic and clinical data were obtained by reviewing the medical records and subsequently cross-referenced with the Leiden Cancer Registry. Tumour stage (i.e., local, locally advanced and metastatic disease) was established based on findings at surgery and thoraco-abdominal computed tomography. Radiologic evidence of VTE was recorded and patients were followed until death or 1 October 2014, whichever came first. The extent of thrombosis was semi-quantitatively scored as: no thrombosis, small VT, DVT and/or PE, massive VTE. ‘Small VT' was defined as any coincidental radiological diagnosis of VT visible over a very short distance in the absence of clinical symptoms (e.g., splenic vein thrombosis), whereas ‘massive VTE' was defined as extensive VT involving a long trajectory of veins. ‘Cancer-related thrombosis' was defined as unprovoked thrombosis in cancer patients in the absence of chemotherapeutic treatment, venous catheters or recent surgery. To assess the relation between plasma MP-TF activity, CA19–9 and the severity of VTE, the maximum interval between blood sampling and the occurrence of VTE was arbitrarily set at ±16 days.
Isolation of microparticles, measurement of plasma MP-TF activity and CA19–9
Upon inclusion into the study, venous blood samples were collected in citrated BD Vacutainer tubes (Beckton Dickinson, Franklin Lakes, NJ, USA) using minimal venostasis and discarding the first tube. Within 1 h, platelet poor plasma was prepared (20 min, 1550 g, room temperature), snap frozen in liquid nitrogen and stored in aliquots at −80 °C until analysis. After thawing of deep-frozen platelet poor plasma samples, blood MPs were pelleted and washed twice with filtered 0.32% citrate/PBS buffer, pH 7.45 (30 min at 17 570 g with minimum brake, 20 °C) (VanWijk et al, 2002). Isolated MPs were immediately tested for MP-associated TF (MP-TF) activity, as previously described (Tesselaar et al, 2007; Woei-A-Jin et al, 2014). Results are reported as FVII/TF-dependent FXa formation (fM Xa min−1) in plasma. Mean and median plasma MP-TF activity in 66 healthy volunteers without medical history or current illness were 70 and 69 fM Xa min−1, respectively (range 3–238). Plasma MP-TF activities higher than the mean plus 2 s.d. (i.e., >155 fM Xa min−1) were considered to be elevated. All samples were analysed batchwise within 1 year after blood collection. No difference in MP-TF activity was found following prolonged frozen storage (>15 months; n=16).
CA19–9, the sialylated lacto-N-fucopentaose II (Lewisa blood group) antigen, was measured in plasma using an immunoenzymatic assay on an IMX Automated Immunoassay Analyzer (Abbott Diagnostics, Lake Forest, IL, USA). CA19–9 levels >37 kU l−1 were considered to be elevated (Galli et al, 2013).
Flowcytometric analysis of microparticles
From 55 of 79 patients, frozen plasma samples were available for further analysis. Microparticles were isolated from plasma as described above, resuspended in 2.5 mM CaCl2/PBS buffer, pH 7.4 and incubated in the dark for 15 min at room temperature with Annexin V (AnnV) labelled with Allophycocyanin (APC) (Caltag Laboratories, Burlingame, CA, USA) and one of the following monoclonal antibodies labelled with fluorescein isothiocyanate (FITC): anti-epithelial membrane antigen (EMA/MUC1)-IgG1-FITC (clone B24.1; Biomeda, Foster City, CA, USA), anti-TF-IgG1-FITC (No. 4508CJ, American Diagnostica, Stamford, CT, USA) and mouse IgG1-FITC (clone X40; BD Biosciences, San Jose, CA, USA). The MP suspension was washed with excess 2.5 mM CaCl2/PBS buffer, pH 7.4, thus diluting the antibody concentration 52 ×. Following centrifugation (30 min at 17 570 g with minimum brake, 20 °C), removal of the supernatant and resuspension in 2.5 mM CaCl2/PBS buffer, pH 7.4, samples were analysed on a FACSCalibur using the Cell Quest software (BD Biosciences). Forward and sideward scatter of light were set at logarithmic gain. Microparticles were identified on the basis of their size, density and capacity to bind Ann V (Nieuwland et al, 1997). Fluorescent colour profiles of the selected population were subsequently recorded for 1 min. The number of MP per litre plasma was calculated as previously described (VanWijk et al, 2002). In seven patients, measurement of AnnV+TF+MPs was not possible due to insufficient plasma.
For our experiments, we used one single batch of FITC-labelled anti-TF-IgG1 antibody (clone 4508CJ from American Diagnostica, currently Sekisui Diagnostics). This is an inhibitory antibody directed against the first 25 amino acids of TF. Previously, the same batch of antibody was used to demonstrate the presence of CD14+TF+ MPs in plasma from a patient with fulminant meningococcal sepsis and disseminated intravascular coagulopathy (Nieuwland et al, 2000), and to detect a TF expressing subset of MPs derived from activated HUVEC cells (Abid Hussein et al, 2003). We confirmed the sensitivity and specificity of the anti-TF antibody using MPs generated from TF-expressing breast cancer cell lines MDA-231 (high TF) and MCF-7 (low TF) after stimulation with Ca2+ ionophore A23187. In addition, we used MPs derived from cells that do not express TF. Simultaneous staining of MPs with AnnV-APC, anti-TF-IgG1-FITC and either anti-CD235a-IgG1-PE (clone JC159; Dako, Glostrup, Denmark) or anti-CD66e-IgG1-PE (clone CLB-gran/10, IH4Fc; Sanquin, Amsterdam, Netherlands) showed that neither erythrocyte nor granulocyte-derived MPs expressed TF.
Immunohistochemistry
Formalin-fixed 4 μm sections were deparaffinised with xylene and rehydrated through graded ethanol and demineralised water. Antigens were retrieved by boiling in 10 mM citrate buffer, pH 6.0 in a pressure cooker. Non-specific binding was blocked using Antibody Diluent S2022 (Dako). Consecutive tissue sections were incubated with the following primary monoclonal mouse anti-human antibodies diluted in Antibody Diluent S2022 (Dako): anti-TF (No. 4509, American Diagnostica), anti-Epithelial Membrane Antigen (MUC1) (clone E29), anti-CD68 (clone KP1) and anti-CD31 (Clone JC70A) from Dako. After blocking endogenous peroxidases, staining was visualised using a peroxidase-labelled polymer conjugated to goat anti-mouse immunoglobulins and 3,3′-diaminobenzidine (K4007, Mouse EnVision+ kit, Dako). Sections were decolourised, counterstained with haematoxylin (1 : 1 dilution with demineralised water; Merck, Darmstadt, Germany), decolourised again, dehydrated and finally permanently mounted.
Mouse IgG1 (BD Biosciences) and omission of the primary antibody served as specificity controls. Normal pancreatic islet cells, tonsils and ductal breast cancer cells served as positive control for TF, CD68 and MUC1 staining, respectively. No separate control was prepared for CD31 staining.
Tumour grading and scoring of antigen expression
TF, MUC1, CD31 and CD68 antigen expression was assessed at × 100 magnification. The intensity of TF staining on tumour cells was qualitatively scored as weak, intermediate or strong. All tissue sections were reviewed independently by two investigators blinded for clinical data. Discrepancies were resolved by consensus. To prevent observer bias, tumour grade was determined by one pathologist not involved in the study. The presence of TF+CD68+ macrophages and vascular TF expression was established by examining serial sections at × 200 magnification. Numbers of macrophages expressing TF were semi-quantitatively scored as absent (no TF+CD68+ macrophages), sporadic (solitary dispersed TF+CD68+ macrophages) or in clusters (increased number of TF+CD68+ macrophages in groups).
Statistical analysis
All data were recorded and analysed using SPSS software (version 20.0; SPSS, Inc., Chicago, IL, USA). Survival time was defined as the interval from enrolment to death and compared between groups using the log-rank test. The Mann–Whitney U-test was applied for univariate analysis and the differences between independent categories were determined with the Kruskal–Wallis test (reported as H(df), P-value). Correlations were assessed using the Spearman's rho (rs) test. Finally, the association between TF expression on tumour cells and differentiation grade was investigated using the χ2-test. P-values <0.05 were considered significant.
Results
Patient cohort
Characteristics of the study population of 79 patients are shown in Table 1. The median age was 63 years (range 39–81) and there was a fairly even distribution among sexes (50.6% male) and tumour stage. The majority of patients (92.4%) were chemo-naive; six patients already received chemotherapy prior to inclusion. The overall survival of the 79 patients was poor (median 6.3 months) and 72% of patients died within 1 year. Only six patients were alive at the end of the observation period. In total, twenty pancreatic cancer patients had developed a thromboembolic event during their disease course. None of the VTE were catheter- or chemotherapy-related.
Table 1. Patient characteristics according to tumour stage.
| Local | Locally advanced | Metastasis | |
|---|---|---|---|
| Pancreatic cancer patients (n=79) | (n=26) | (n=19) | (n=34) |
| Male, n (%) | 12 (46.2) | 8 (42.1) | 20 (58.8) |
| Age (years), median (IQR) | 65 (59–70) | 58 (52–64) | 64 (57–68) |
| Localisation of primary tumour, n (%) | |||
| Caput | 20 (76.9) | 15 (78.9) | 18 (52.9) |
| Corpus | 0 (0) | 2 (10.5) | 4 (11.8) |
| Cauda | 1 (3.8) | 2 (10.5) | 10 (29.4) |
| Vater's papilla | 5 (19.2) | 0 (0) | 2 (5.9) |
| MP-TF activity (fM Xa min−1), median (IQR) | 46 (24–149) | 123 (41–181) | 328 (111–1184) |
| CA19–9 (kU l−1), median (IQR)a | 39 (9–374) | 1820 (72–3000) | 2139 (268–39 241) |
| Venous thromboembolism at inclusion, n (%) | 1 (3.8) | 0 (0) | 13 (38.2) |
| Overall survival (months), median (IQR) | 21.5 (11.9–41.6) | 6.9 (5.2–9.6) | 2.1 (1.1–4.7) |
| Death during follow-up, n (%) | 20 (76.9) | 19 (100) | 34 (100) |
| Flowcytometric subset analysis (n=55) | (n=11) | (n=16) | (n=28) |
| Total AnnV+MP (× 106 l−1), median (IQR) | 3204 (1677–5873) | 4114 (2600–6106) | 4249 (3118–6864) |
| AnnV+TF+MP (× 106 l−1), median (IQR) | 0 (0–40) | 0 (0–0) | 83 (0–307) |
| AnnV+MUC1+MP (× 106 l−1), median (IQR) | 0 (0–0) | 0 (0–282) | 152 (0–314) |
Abbreviations: IQR=interquartile range; MP=microparticle; TF=tissue factor; AnnV=Annexin V.
CA19–9, 6 missing values.
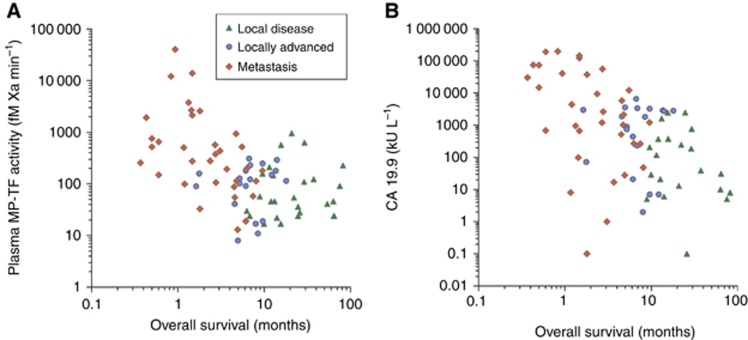
The median MP-TF activity in pancreatic cancer patients (128 fM Xa min−1 (IQR 46–376)) was higher than in healthy controls (69 fM Xa min−1 (IQR 41–92); P<0.001) and increased significantly with tumour stage (Kruskal–Wallis test: H(2)=18.13, P<0.001). Intra-individual analysis of one patient who progressed from local to metastatic disease revealed an increase in MP-TF activity from 211 to 780 fM Xa min−1. Overall survival was shorter in pancreatic cancer patients with an elevated MP-TF activity (>155 fM Xa min−1) than in patients with MP-TF activities within the normal range (2.9 vs 8.5 months, log-rank: P=0.002) and was related to tumour stage (Figure 1A). Similarly, patients with elevated CA19–9 had a shorter median survival than patients with normal CA19–9 (5.0 vs 9.9 months, log-rank: P=0.004) (Figure 1B). Multiple regression analysis (Cox proportional hazards model, stepwise exclusion of gender, age, stage, elevated MP-TF activity and elevated CA19–9 levels) identified tumour stage as the only variable independently affecting survival.
Figure 1.
Plasma MP-TF activity (A) and CA19–9 levels (B) show a moderate negative correlation with overall survival (rs −0.471 and −0.499, respectively; P<0.001) and are related to tumour stage.
AnnV+TF+MPs and AnnV+MUC1+MPs were almost exclusively observed in metastatic patients. Not all TF+MPs were MUC1-positive. Finally, numbers of AnnV+TF+MPs correlated weakly but significantly with MP-TF activity (rs 0.61; P<0.0001).
Thromboembolic events and refractory fulminant thrombosis
Within the set interval of 16 days before and after blood sampling (i.e., inclusion), 11 patients were diagnosed with VTE. Three other patients already had thrombosis 41–61 days before inclusion (plasma MP-TF activity 200, 937 and 2662 fM Xa min−1) and six patients developed thrombosis during follow-up (median 155; range 35–328 days). The 11 patients with VTE at inclusion had a higher median plasma MP-TF activity than the 59 patients who never developed VTE (1925 fM Xa min−1 (IQR 99–12 120) vs 113 fM Xa min−1 (IQR 33–211); P<0.001). Median MP-TF activity did not differ significantly between the 59 patients without thrombosis and the 6 patients who developed VTE during follow-up (301 fM Xa min−1 (IQR 37–470); P=0.185).
Flowcytometric analysis in a representative subset of patients indicated that median numbers of AnnV+TF+MPs were higher in patients with VTE (345 × 106 l−1 (IQR 229–585); n=6) than in patients without VTE (0 × 106 l−1 (IQR 0–27); n=42; P<0.001). Similar results were found for numbers of AnnV+MUC1+MPs in patients with VTE (194 × 106 l−1 (IQR 27–392); n=9) and without VTE (0 × 106 l−1 (IQR 0–221); n=46; P=0.046).
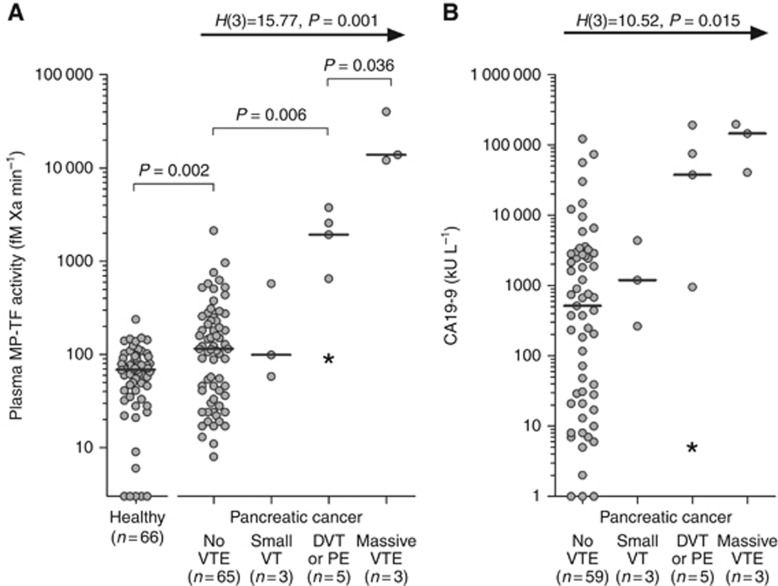
Categorisation of patients according to the magnitude of thrombosis at inclusion (i.e., no thrombosis, small thrombosis, DVT and/or PE, massive VTE) demonstrated an increase in plasma MP-TF activity with increasing extent of thrombosis (Figure 2A) (Kruskal–Wallis test: H(3)=15.77, P=0.001). All patients with VTE had metastatic disease and elevated plasma MP-TF activities, except for one patient with local disease who developed PE following surgery (plasma MP-TF activity 91 fM Xa min−1). Median plasma CA19–9 was higher in patients with VTE than in patients without VTE and increased with the extent of thrombosis (Figure 2B). Similarly, serial measurements in one metastatic patient with a plasma MP-TF activity of 151 fM Xa min−1 demonstrated an increase to 2567 fM Xa min−1 at the time DVT was diagnosed, whereas CA19–9 levels increased from 20 to 37 751 kU l−1.
Figure 2.
Magnitude of VTE and (A) plasma MP-TF activity (n=76) and (B) plasma CA19–9 levels (n=70). Differences between groups were analysed with the Kruskal–Wallis test (H(df), P-value). Three patients had been diagnosed with VTE before inclusion and were excluded from this analysis. All patients with VTE had metastatic disease, except for one patient (*) with local disease who developed PE following surgery.
The highest MP-TF activities (12 118–40 188 fM Xa min−1) were detected in three metastatic cancer patients with striking fulminant venous thrombosis and even cerebral arterial thromboembolic events in two of the three cases. One patient, who presented with intraocular lesions, was diagnosed with extensive DVT reaching from the popliteal into the common femoral vein. Despite low-molecular-weight heparin (LMWH) treatment, surgical closure of a patent foramen ovale and insertion of a caval vein filter, he persistently suffered multiple bilateral ischaemic cerebral vascular attacks and died 22 days later. This patient had the highest MP-TF activity (40 188 fM Xa min−1; CA19–9 of 40 730 kU l−1; no enumeration of MPs).
Another patient presented with a popliteal vein thrombosis for which vitamin K antagonists and low-dose LMWH were started. Nevertheless, the thrombosis progressed into the iliac vein within 2 weeks, resulting in phlegmasia cerulia dolens and venous gangrene. He died within 44 days. Both MP-TF activity and CA19–9 were very high (13 887 fM Xa min−1 and 145 110 kU l−1, respectively). Numbers of AnnV+TF+MPs and AnnV+MUC1+MPs were 353 (>75th percentile) and 194 × 106 l−1 (>50th percentile), respectively.
The third patient presented with massive thrombosis ranging from the popliteal to iliac vein, bilateral PEs and a cerebral infarction despite anticoagulant treatment with a vitamin K antagonist and INR>6. The VTE-related symptoms improved after switching to LMWH, but he succumbed 25 days later. This patient had the highest CA19–9 levels of 197 000 kU l−1 and the highest numbers of circulating AnnV+MUC1+MPs (1700 × 106 l−1). Plasma MP-TF activity was 12 118 fM Xa min−1 and the number of AnnV+TF+MPs was 252 × 106 l−1.
Expression of TF in pancreatic adenocarcinoma and circulating microparticles
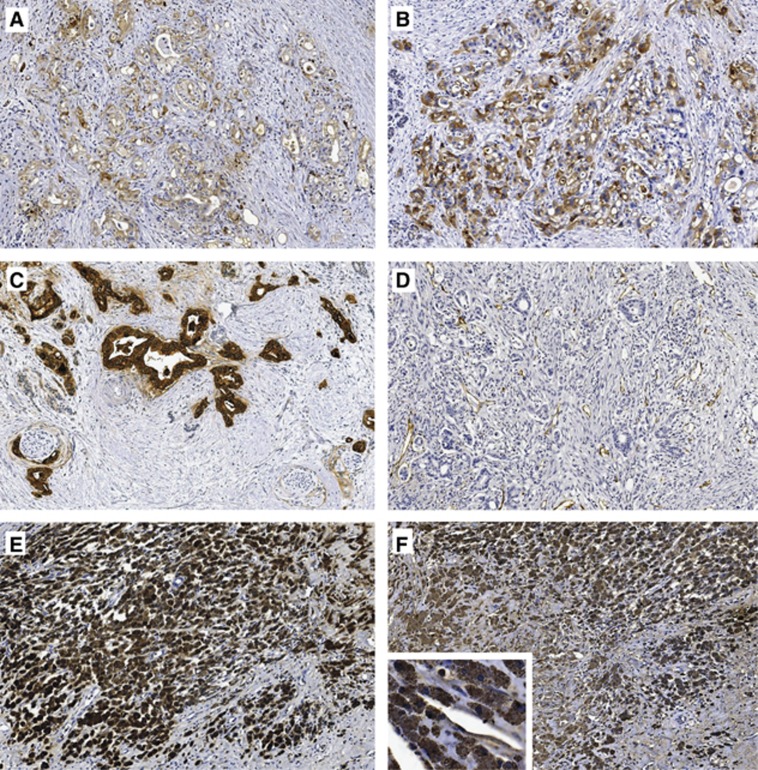
In all 44 tumour specimens, adenocarcinoma cells expressed TF and MUC1 (representative specimens shown in Figure 3A–C). The intensity of tumour-TF expression (low: n=21, intermediate: n=18, strong: n=5) increased with histological tumour grade (χ2=32.62, df=4, P<0.0001). No significant correlation between tumour-TF expression and tumour stage was found. Nevertheless, local disease patients with intermediate/strong tumour-TF expression (n=16) had a shorter overall survival than patients with low tumour-TF expression (n=12) (median 12.7 vs 25.8 months, log-rank: P=0.008). The difference in survival was not significant for patients with (locally) advanced disease. During follow-up, 6 of 44 patients developed DVT or PE, two of which were within the immediate post-surgery period of 6 weeks. The unprovoked VTE rate was similar in patients with intermediate/high tumour-TF expression (2 of 21; 10%) and patients with low tumour-TF expression (2 of 23; 9%).
Figure 3.
Representative pancreatic tumour tissue samples at × 10 magnification. (A) Intermediate and (B) strong TF staining in moderately and poorly differentiated pancreatic adenocarcinoma, respectively, (C) MUC1 staining, (D) CD31 staining of endothelium, (E) CD68 staining of large clusters of macrophages and (F) corresponding very strongly TF+ macrophages.
Endothelial cells stained positive for CD31 (Figure 3D), but were predominantly TF-negative. CD68+ macrophages were unevenly distributed throughout the stroma and adipose tissue. In 16 of 43 tumours (one biopsy specimen too small for reliable assessment of macrophages), TF+ macrophages were detected. In eight of these, moderate to large clusters of TF+ macrophages were found in the tumour environment (Figure 3E–F). All TF+ macrophages showed very strong TF expression compared with tumour cells.
In the 37 patients in whom tumour tissue was collected in a pre-specified time frame of −70 to 30 days following blood sampling, we found no correlation between the intensity of TF expression in adenocarcinoma cells and plasma MP-TF activity. Subgroup analysis of 19 patients also showed no correlation between the intensity of TF expression in adenocarcinoma cells and numbers of circulating AnnV+TF+MPs. In contrast, in patients with large clusters of TF+ macrophages infiltrating the tumour-surrounding stroma, median plasma MP-TF activity was higher (743 fM Xa min−1 (IQR 267–5468); n=6) than in patients with sporadic infiltration of TF+ macrophages (57 fM Xa min−1 (IQR 33–123); n=7) or without TF+ macrophages (44 fM Xa min−1 (IQR 24–124); n=24; P<0.001).
Discussion
This prospective cohort study in 79 patients reflects the natural disease course in PAC as it was conducted in the era before introduction of the FOLFIRINOX chemotherapeutic regimen (Conroy et al, 2011). Survival rates were similar as those reported in the literature (Bilimoria et al, 2007). We confirmed that high plasma MP-TF activity correlates with short survival (Tesselaar et al, 2007; Thaler et al, 2012, 2013; Bharthuar et al, 2013), but additionally demonstrated that this is mostly related to its association with tumour stage. PAC cells expressed MUC1 and TF. In accordance with the published data, tumour-TF expression increased with histological grade (Kakkar et al, 1995; Nitori et al, 2005; Khorana et al, 2007). We confirmed in a homogeneous group of local disease patients (n=28) that high tumour-TF expression corresponds with poor survival (Nitori et al, 2005).
Patients with cancer-related thrombosis had higher MP-TF activities than patients without thrombosis, which is in agreement with previous observations (Tesselaar et al, 2007; Bharthuar et al, 2013). Although all cancer-related VTE patients had metastatic disease, we demonstrated that the magnitude of VTE increased significantly with plasma MP-TF activity, indicating that the release of TF+MPs is not merely an epiphenomenon, but may contribute to the development of thrombosis.
The intensity of TF expression by adenocarcinoma cells did not correlate with plasma MP-TF activity. In some patients with AnnV+TF+MPs, no AnnV+MUC1+MPs were detected. These findings are consistent with a study of three pancreatic cancer patients, which showed that 50% of TF+MPs were MUC1-negative (Zwicker et al, 2009) and may be related to an additional source of TF+MPs other than tumour cells. Interestingly, tissue specimens of patients with high plasma MP-TF activity demonstrated large clusters of very strongly stained TF+ macrophages invading the vast tumour-surrounding stroma. Thus, in addition to PAC cells, macrophages may also form a significant source of procoagulant MP-TF activity. We hypothesise that the pro-inflammatory state in advanced stage pancreatic cancer induces the activation of monocytes and macrophages expressing large amounts of TF and that both TF+ tumour cells and activated TF+ macrophages in the tumour environment are the source of TF+MPs in the circulation.
Alternative pathways may contribute to the thrombotic phenomena, early dissemination and strikingly poor prognosis of pancreas adenocarcinoma patients. Adenocarcinomas and in particular PAC express aberrantly glycosylated structures on their cell surface and shed large quantities of mucins into the circulation. These glycan structures may mediate crosstalk between the tumour and its microenvironment. Their presence seems to correlate with cancer progression and may affect tumour cell migration and dissemination (Tei et al, 2002). In the absence of a method to quantify mucins in blood, CA19–9 may serve as a surrogate marker as it binds to apomucins, including MUC1, MUC5AC and MUC16 (Yue et al, 2011). In our study cohort, plasma CA19–9 levels correlated with stage, short survival and – albeit to a lesser extent than MP-TF activity – with the severity of VTE.
Experimental studies in mice provided evidence that intravenous injection of carcinoma mucins carrying selectin ligands resulted in the formation of platelet-rich microthrombi, whereas thrombosis was markedly diminished in P-selectin or L-selectin-deficient mice (Wahrenbrock et al, 2003).
Three patients with fulminant massive thromboembolisms despite anticoagulant therapy had extremely high MP-TF activity and CA19–9 levels. Devastating warfarin-refractory arterial and venous thromboembolisms have been described previously and heparins may be more effective (Bell et al, 1985; Walsh-McMonagle and Green, 1997). In contrast to vitamin K antagonists, which decrease thrombin production, heparin additionally blocks binding of mucins to selectins thus hampering platelet aggregation. In our study, the patient with the highest levels of AnnV+MUC1+MPs and extremely high CA19–9 (which may reflect levels of circulating mucins) developed overwhelming progressive thrombosis while receiving vitamin K antagonist treatment. Strikingly, this patient improved clinically with heparin treatment in accordance with the hypothesis that circulating tumour-derived mucins also have a role in cancer-related thrombosis.
In conclusion, our current findings support the notion that circulating procoagulant TF+ MPs are mechanistically related to the severe coagulopathy observed in PAC patients. Until now, studies focussed on the role of TF in pancreatic cancer-associated VTE, but TF may not be the only player. Circulating MUC1 attached to MPs and other soluble mucins may also initiate and aggravate clotting. We hypothesise that PAC cells, as well as stromal macrophages, release procoagulant TF+MPs. These TF+MPs may, in a concerted action with soluble- or MP-bound mucins, potentiate coagulopathy and the aggressive behaviour of PAC. Improved insight into these mechanisms are relevant to develop optimal treatment strategies targeting TF/FVII on tumour cells and macrophages, as well as blocking circulating CA19–9 and mucins released by tumour cells.
Acknowledgments
This work was supported by the Dutch Cancer Society (KWF UL 2006–3618).
The authors declare no conflict of interest.
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License.
References
- Abid Hussein MN, Meesters EW, Osmanovic N, Romijn FP, Nieuwland R, Sturk A (2003) Antigenic characterization of endothelial cell-derived microparticles and their detection ex vivo. J Thromb Haemost 1(11): 2434–2443. [DOI] [PubMed] [Google Scholar]
- Ahamed J, Niessen F, Kurokawa T, Lee YK, Bhattacharjee G, Morrissey JH, Ruf W (2007) Regulation of macrophage procoagulant responses by the tissue factor cytoplasmic domain in endotoxemia. Blood 109(12): 5251–5259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell WR, Starksen NF, Tong S, Porterfield JK (1985) Trousseau's syndrome. Devastating coagulopathy in the absence of heparin. Am J Med 79(4): 423–430. [DOI] [PubMed] [Google Scholar]
- Bharthuar A, Khorana AA, Hutson A, Wang JG, Key NS, Mackman N, Iyer RV (2013) Circulating microparticle tissue factor, thromboembolism and survival in pancreaticobiliary cancers. Thromb Res 132(2): 180–184. [DOI] [PubMed] [Google Scholar]
- Bilimoria KY, Bentrem DJ, Ko CY, Ritchey J, Stewart AK, Winchester DP, Talamonti MS (2007) Validation of the 6th edition AJCC Pancreatic Cancer Staging System: report from the National Cancer Database. Cancer 110(4): 738–744. [DOI] [PubMed] [Google Scholar]
- Blom JW, Osanto S, Rosendaal FR (2006) High risk of venous thrombosis in patients with pancreatic cancer: a cohort study of 202 patients. Eur J Cancer 42(3): 410–414. [DOI] [PubMed] [Google Scholar]
- Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardiere C, Bennouna J, Bachet JB, Khemissa-Akouz F, Pere-Verge D, Delbaldo C, Assenat E, Chauffert B, Michel P, Montoto-Grillot C, Ducreux M (2011) FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 364(19): 1817–1825. [DOI] [PubMed] [Google Scholar]
- Falati S, Liu Q, Gross P, Merrill-Skoloff G, Chou J, Vandendries E, Celi A, Croce K, Furie BC, Furie B (2003) Accumulation of tissue factor into developing thrombi in vivo is dependent upon microparticle P-selectin glycoprotein ligand 1 and platelet P-selectin. J Exp Med 197(11): 1585–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli C, Basso D, Plebani M (2013) CA 19-9: handle with care. Clin Chem Lab Med 51(7): 1369–1383. [DOI] [PubMed] [Google Scholar]
- Howard EJ (1960) Phlegmasia cerulea dolens secondary to carcinoma of the pancreas. Angiology 11: 319–322. [DOI] [PubMed] [Google Scholar]
- Kakkar AK, Lemoine NR, Scully MF, Tebbutt S, Williamson RC (1995) Tissue factor expression correlates with histological grade in human pancreatic cancer. Br J Surg 82(8): 1101–1104. [DOI] [PubMed] [Google Scholar]
- Khorana AA, Ahrendt SA, Ryan CK, Francis CW, Hruban RH, Hu YC, Hostetter G, Harvey J, Taubman MB (2007) Tissue factor expression, angiogenesis, and thrombosis in pancreatic cancer. Clin Cancer Res 13(10): 2870–2875. [DOI] [PubMed] [Google Scholar]
- Michl P, Gress TM (2013) Current concepts and novel targets in advanced pancreatic cancer. Gut 62(2): 317–326. [DOI] [PubMed] [Google Scholar]
- Nieuwland R, Berckmans RJ, Rotteveel-Eijkman RC, Maquelin KN, Roozendaal KJ, Jansen PG, ten Have K, Eijsman L, Hack CE, Sturk A (1997) Cell-derived microparticles generated in patients during cardiopulmonary bypass are highly procoagulant. Circulation 96(10): 3534–3541. [DOI] [PubMed] [Google Scholar]
- Nieuwland R, Berckmans RJ, McGregor S, Böing AN, Romijn FP, Westendorp R, Hack CE, Sturk A (2000) Cellular origin and procoagulant properties of microparticles in meningocollal sepsis. Blood 95(3): 930–935. [PubMed] [Google Scholar]
- Nitori N, Ino Y, Nakanishi Y, Yamada T, Honda K, Yanagihara K, Kosuge T, Kanai Y, Kitajima M, Hirohashi S (2005) Prognostic significance of tissue factor in pancreatic ductal adenocarcinoma. Clin Cancer Res 11(7): 2531–2539. [DOI] [PubMed] [Google Scholar]
- Østerud B (2010) Tissue factor expression in blood cells. Thromb Res 125(Suppl 1): S31–S34. [DOI] [PubMed] [Google Scholar]
- Ruf W, Yokota N, Schaffner F (2010) Tissue factor in cancer progression and angiogenesis. Thromb Res 125(Suppl 2): S36–S38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeglin A, Ansari M, Skali H, Oo TH, Maysky M (2008) Marantic endocarditis and disseminated intravascular coagulation with systemic emboli in presentation of pancreatic cancer. J Clin Oncol 26(8): 1383–1385. [DOI] [PubMed] [Google Scholar]
- Soff GA (2014) Commentary on ‘microparticle-associated tissue factor activity in patients with metastatic pancreatic cancer and its effect on fibrin clot formation'. Transl Res 163(2): 136–140. [DOI] [PubMed] [Google Scholar]
- Tei K, Kawakami-Kimura N, Taguchi O, Kumamoto K, Higashiyama S, Taniguchi N, Toda K, Kawata R, Hisa Y, Kannagi R (2002) Roles of cell adhesion molecules in tumor angiogenesis induced by cotransplantation of cancer and endothelial cells to nude rats. Cancer Res 62(21): 6289–6296. [PubMed] [Google Scholar]
- Tesselaar ME, Romijn FP, van der Linden IK, Prins FA, Bertina RM, Osanto S (2007) Microparticle-associated tissue factor activity: a link between cancer and thrombosis? J Thromb Haemost 5(3): 520–527. [DOI] [PubMed] [Google Scholar]
- Thaler J, Ay C, Mackman N, Bertina RM, Kaider A, Marosi C, Key NS, Barcel DA, Scheithauer W, Kornek G, Zielinski C, Pabinger I (2012) Microparticle-associated tissue factor activity, venous thromboembolism and mortality in pancreatic, gastric, colorectal and brain cancer patients. J Thromb Haemost 10(7): 1363–1370. [DOI] [PubMed] [Google Scholar]
- Thaler J, Ay C, Mackman N, Metz-Schimmerl S, Stift J, Kaider A, Mullauer L, Gnant M, Scheithauer W, Pabinger I (2013) Microparticle-associated tissue factor activity in patients with pancreatic cancer: correlation with clinicopathological features. Eur J Clin Invest 43(3): 277–285. [DOI] [PubMed] [Google Scholar]
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A (2015) Global cancer statistics, 2012. CA Cancer J Clin 65(2): 87–108. [DOI] [PubMed] [Google Scholar]
- VanWijk MJ, Nieuwland R, Boer K, van der Post JA, VanBavel E, Sturk A (2002) Microparticle subpopulations are increased in preeclampsia: possible involvement in vascular dysfunction? Am J Obstet Gynecol 187(2): 450–456. [DOI] [PubMed] [Google Scholar]
- Wahrenbrock M, Borsig L, Le D, Varki N, Varki A (2003) Selectin-mucin interactions as a probable molecular explanation for the association of Trousseau syndrome with mucinous adenocarcinomas. J Clin Invest 112(6): 853–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh-McMonagle D, Green D (1997) Low-molecular-weight heparin in the management of Trousseau's syndrome. Cancer 80(4): 649–655. [PubMed] [Google Scholar]
- Woei-A-Jin FJ, van der Starre WE, Tesselaar ME, Garcia Rodriguez P, van Nieuwkoop C, Bertina RM, van Dissel JT, Osanto S (2014) Procoagulant tissue factor activity on microparticles is associated with disease severity and bacteremia in febrile urinary tract infections. Thromb Res 133(5): 799–803. [DOI] [PubMed] [Google Scholar]
- Yue T, Maupin KA, Fallon B, Li L, Partyka K, Anderson MA, Brenner DE, Kaul K, Zeh H, Moser AJ, Simeone DM, Feng Z, Brand RE, Haab BB (2011) Enhanced discrimination of malignant from benign pancreatic disease by measuring the CA 19-9 antigen on specific protein carriers. PLoS One 6(12): e29180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwicker JI, Liebman HA, Neuberg D, Lacroix R, Bauer KA, Furie BC, Furie B (2009) Tumor-derived tissue factor-bearing microparticles are associated with venous thromboembolic events in malignancy. Clin Cancer Res 15(22): 6830–6840. [DOI] [PMC free article] [PubMed] [Google Scholar]