Abstract
Background:
Intrahepatic cholangiocarcinoma (ICC) is a rapidly progressing malignancy; only a minority of the tumours can be resected and the palliative regimens have shown limited success. The aim of this study was to assess overall survival (OS), tumour response and the safety of radioembolization with yttrium-90 (90Y-TARE) in patients with unresectable/recurrent ICC.
Methods:
Survival was calculated from the date of the 90Y-TARE procedure. Target and overall Response Evaluation Criteria in Solid Tumors (RECIST) and modified RECIST (mRECIST) and European Association for the Study of the Liver (EASL)—measuring delayed-phase contrast enhancement—treatment responses were assessed at 3 months.
Results:
The overall median survival was 17.9 months (95% CI: 14.3–21.4 months). Significantly longer survival was obtained in naive patients as compared with patients in whom TARE was preceded by other treatments, including surgery (52 vs 16 months, P=0.009). Significantly prolonged OS was recorded for patients with a response based on mRECIST and the EASL criteria while RECIST responses were not found to be associated with survival. Treatment was well-tolerated, and no mortality was reported within 30 days.
Conclusions:
In unresectable ICC, 90Y-TARE is safe and offers a survival benefit in naive patients, as well as in responders.
Keywords: intrahepatic cholangiocarcinoma, yttrium-90 radioembolization, 90Y-TARE, survival, radiological tumour response, adverse events
Intrahepatic cholangiocarcinoma (ICC) is the second most common primary liver cancer after hepatocellular carcinoma (HCC) (Bridgewater et al, 2014). Although relatively rare, its incidence has been reported to be increasing worldwide, accounting for up to 15% of primary liver cancers with an age-adjusted rate of about 2.1 per 1 00 000 people per year in western countries (Yang et al, 2012; Bridgewater et al, 2014).
Surgical treatment is the only potential curative treatment for ICC. However, only 30–40% of ICCs are diagnosed at a stage which meets the criteria for curative resection (Bridgewater et al, 2014). Unresectable ICCs have a median survival of <8 months if untreated (Chou et al, 1997; Roayaie et al, 1998), which can be increased to ∼12 months with systemic chemotherapy (gemcitabine and cisplatin) (Valle et al, 2010; Bridgewater et al, 2014). The results of curative-intent surgery, which increases median overall survival (OS) with a range from 27 to 36 months, are mainly limited by the high recurrence rate of this cancer.
For these reasons, locoregional therapies, such as radiofrequency ablation (RFA) or transarterial chemoembolization (TACE), are part of the therapeutic armamentarium for ICC. RFA has been proven to achieve good results in patients with small, single ICCs, leading to a median survival ranging from 33 to 38.5 months and 3-year survival rates ranging from 43.3 to 83.3% (Kamphues et al, 2010; Kim et al, 2011a, b, c). However, the effectiveness of RFA is poor for tumours >5 cm and for those located close to large vessels or in the subcapsular region (Razumilava and Gores, 2014). In a recent meta-analysis, TACE has also been demonstrated to be superior to supportive care in patients with unresectable ICC, allowing cumulative median OS rate of 12.4 months from the date of treatment (Boehm et al, 2015). Nevertheless, these data must be interpreted with caution due to the heterogeneity of the studies and to the absence of standard-of-care systemic therapy to be used as a benchmark (Razumilava and Gores, 2014).
Considering the relative radiosensitivity of ICC (Grove et al, 1991; Nathan et al, 2007), Yttrium-90 radioembolization (90Y-TARE) represents a promising alternative treatment for this neoplasm. The advantage of 90Y-TARE is the ability to deliver a high dose of radiation directly to the tumour, thus powering the tumoricidal effect with minimal collateral damage to the normal liver parenchyma or surrounding tissues. To date, seminal data regarding the safety and efficacy of 90Y-TARE in ICC patients have been reported by several small studies in which the median survival ranged from 9.3 to 22 months (Bridgewater et al, 2014; Al-Adra et al, 2015).
The present study, based on one of the largest case series of those already published, provides additional information regarding survival, tumour response and the safety of 90Y-TARE in patients with unresectable ICC treated at a tertiary referral centre.
Materials and Methods
Patients
In this single-centre study, the data of 23 consecutive patients with ICC undergoing 90Y-TARE between July 2010 and September 2015 were retrospectively analysed; all treated ICC were mass-forming type. This therapeutic choice was undertaken by the multidisciplinary tumour board of our Institution after a careful and comprehensive evaluation of the clinical characteristics and a radiological imaging work-up unequivocally showing the presence of an unresectable primary or recurrent ICC.
Patients were eligible for this treatment if they met the following criteria: (1) histologically proven ICC; (2) unresectable naive tumour or disease relapse/persistence after various treatments, including liver resection; (3) Eastern Cooperation Oncology Group (ECOG) performance status of 0–2; (4) adequate liver function with bilirubin <2.0 mg dl−1; (5) granulocyte count ⩾1.5 × 109 l−1; (6) platelet count ⩾50 × 109 l−1 and (7) amenability to visceral angiography. The exclusion criteria included: (1) flow to the gastrointestinal tract not correctable by coil embolization or (2) estimated lung exposition to a radiation dose >30 Gy in a single administration or 50 Gy cumulatively. Patients with liver cirrhosis in Child–Pugh class C were also excluded.
The study conforms to the ethics guidelines of the Declaration of Helsinki and was approved by our Institutional Review Board. All patients provided informed, written consent to the procedure.
Treatment protocol
All patients evaluated for 90Y-TARE underwent pre-treatment angiography to detect liver arterial variants and intratumoral artero-venous shunting; thereafter, technetium-99 m macroaggregated albumin scanning was performed. Radioembolization therapy was performed according to the technique previously described in detail (Golfieri et al, 2015; Gramenzi et al, 2015). The device used was 90Y resin microspheres (SIR-Spheres; Sirtex Medical, Lane Cove, NSW, Australia), which is an approved treatment for unresectable liver tumours in the European Union and other countries. Treatment was always unilobar and, in patients with bilobar distribution of ICC, a second 90Y-TARE, if deemed useful and feasible, was repeated 30–60 days after the first one.
Clinical follow-up and response to treatment
Before treatment, all patients underwent clinical evaluation, which included history, physical examination, a specific laboratory profile (liver function tests, complete blood count, coagulation profiles, albumin and total bilirubin) and detailed radiological assessment using computed tomography (CT) scan or magnetic resonance (MR) imaging.
After 90Y-TARE, the patients were regularly evaluated at 1 and 3 months, and thereafter at 3-month intervals. At each visit, the clinical and toxicity data were recorded, and CT or MRI was performed. In the case of incomplete tumour targeting or progressive intrahepatic disease, the patients were retreated. Biochemical toxicities occurring at any time after treatment were reported. The Common Terminology Criteria for Adverse Events of the National Cancer Institute were used to categorise toxicities (Cirillo et al, 2009). All clinical, laboratory and imaging data were prospectively acquired.
Tumour response was assessed by both tumour size criteria (Response Evaluation Criteria In Solid Tumors, RECIST 1.1) (Eisenhauer et al, 2009) and enhancement criteria, such as modified RECIST (Lencioni and Llovet, 2010) and the European Association for the Study of the Liver (EASL)) criteria (Bruix et al, 2001). These criteria were adapted and applied on delayed acquisitions, as previously reported by Camacho et al (2014), given that ICCs usually show peripheral/centripetal enhancement on the equilibrium/delayed phase after contrast medium administration on either CT or MRI (Lazaridis and Gores, 2005).
For RECIST and mRECIST, target and overall assessments were obtained while only target assessment was carried out for the EASL criteria since it was proposed as a locoregional assessment tool. The objective response (OR) was calculated as the sum of the complete response (CR) and the partial response (PR), while disease control (DC) included CR, PR and stable disease (SD). The radiological response was evaluated by two fellowship-trained abdominal radiologists.
Statistical analysis
The primary endpoint of this study was OS. The secondary endpoints were radiological tumour response and safety.
The quantitative variables were expressed as mean±s.d. or median and range, as appropriate. The categorical variables were presented as numbers and percentages. OS was calculated from the time of the first 90Y-TARE to death, with the data terminating on 30 September 2015 (end of the study) or at the last patient evaluation. The Kaplan–Meier method was used to calculate median survival and the pertinent 95% confidence interval. Median survivals of different categories of variables (performance status, portal vein thrombosis (PVT), prior radical procedure or systemic chemotherapy, tumour distribution, extrahepatic metastases, the presence of cirrhosis and tumour response) were compared using the log-rank test. A two-tailed P-value <−0.05 was considered statistically significant. The statistical analysis was carried out using SPSS 16.0 software (SPSS, Chicago, IL, USA).
Results
The baseline demographical and clinical characteristics of the 23 ICC patients are summarised in Table 1. The majority of the patients were male (61%), ECOG 0 (78%) and without cirrhosis (65%). The majority of them presented with bilobar and multifocal disease. Extrahepatic metastases were found in two cases (8%): one patient had two pulmonary lesions (<1 cm) and one had regional lymph node metastases (maximum diameter: 3 cm).
Table 1. Baseline characteristics of patients.
| Characteristics | |
|---|---|
| Age (years), range | 65±10, 42–82 |
| Sex | |
| Male | 14 (61%) |
| Female | 9 (39%) |
| ECOG | |
| 0 | 18 (78%) |
| 1 | 5 (22%) |
| No prior treatment for ICC | 4 (17%) |
| Previous surgical procedures | |
| None | 7 (30%) |
| Resection | 10 (44%) |
| Lobectomy | 6 (26%) |
| Previous vascular procedures | |
| None | 15 (65%) |
| TACE | 4 (17%) |
| TAE | 4 (17%) |
| Systemic chemotherapy | |
| GEMOX | 9 (39%) |
| Gemcitabine | 3 (13%) |
| Percutaneous procedure | 2 (9%) |
| Portal vein occlusion | |
| Patent | 19 (83%) |
| Branch complete | 4 (17%) |
| Main complete | 0 |
| Bilobar disease | 16 (70%) |
| Number of nodules | |
| Monofocal | 2 (9%) |
| Paucifocal (2–5) | 10 (43%) |
| Multifocal (>5) | 11 (48%) |
| Metastases | |
| None | 21 (91%) |
| Lymphonodal | 1 (4%) |
| Pulmonary | 1 (4%) |
| Increased CA-19.9 levela | 11 (48%) |
| Cirrhosis | 8 (35) |
| Ascites | 2 (9%) |
Abbreviations: CA=carbohydrate antigen; ECOG=Eastern Cooperative Oncology Group; GEMOX=gemcitabine combined with oxaliplatin; ICC=intrahepatic cholangiocarcinoma; TACE=transarterial chemoembolization; TAE=transarterial embolization.
>37 U ml−1.
Only four patients (17%) had a naive unresectable ICC, i.e., they had not received any type of prior treatment. Sixteen tumours (70%) had recurred after curative-intent surgical procedures (resection or lobectomy), and 13 of them had already undergone systemic or locoregional treatment. Overall, 12 patients (52%) had previously been treated with systemic chemotherapy.
90Y-TARE treatment
The injected activity, tumour volume, target volume, percentage of targeted to total liver volume, lung shunt and average lung dose are reported in Table 2. The characteristics of the TARE treatment are also reported in Table 2. Three (13%) patients repeated the procedure.
Table 2. Characteristics of 90Y-TARE treatment.
| BSA | 1.9±0.3 (range 1.4–2.3) |
|---|---|
| Lung shunt study (%) | Median: 3 (1.4–6) |
| Treatment target | |
| Whole liver | 0 |
| Right lobe | 5 (22%) |
| Left lobe | 3 (13%) |
| Segmental | 5 (22%) |
| Multisegmental | 10 (43%) |
| Targeted liver volume (ml) | 1056±484 (range: 297–1820) |
| Targeted tumour volume (ml) | 180±163 (range: 7–572) |
| Delivered activity (GBq) | 1.5±0.4 (range: 0.8–2.24) |
| Length of hospitalisation (hours) | |
| <24 | 0 |
| 24–72 | 18 (86%) |
| >72 | 3 (14%) |
Abbreviation: BSA=body surface area.
Radiological response
Regular radiological assessment was completed in 20 patients (87%), since 2 patients died before the first assessment and 1 was treated in such close proximity to the end of the study that he did not reach the time of the first evaluation. Table 3 shows the radiological response at 3 months. Target lesion OR rates (CR+PR) were 20% for RECIST 1.1, 70% for mRECIST and 60% for the EASL criteria. Overall OR rates were 15% for RECIST 1.1 and 45% for mRECIST.
Table 3. Imaging responses based on overall and target RECIST 1.1, mRECIST and the EASL criteria (20 points).
|
Target |
Overall |
||||
|---|---|---|---|---|---|
| Response, n (%) | RECIST 1.1 | mRECIST | EASL | RECIST 1.1 | mRECIST |
| CR | 0 | 1 (5%) | 1 (5%) | 0 | 1 (5%) |
| PR | 4 (20%) | 13 (65%) | 11 (55%) | 3 (15%) | 8 (40%) |
| SD | 11 (55%) | 3 (15%) | 5 (25%) | 6 (30%) | 3 (15%) |
| PD | 5 (25%) | 3 (15%) | 3 (15%) | 11 (55%) | 8 (40%) |
Abbreviations: CR=complete response; EASL=European Association for the Study of the Liver; mRECIST=modified RECIST; PD=progressive disease; PR=partial response; RECIST=response evaluation criteria in solid tumors; SD=stable disease.
Survival
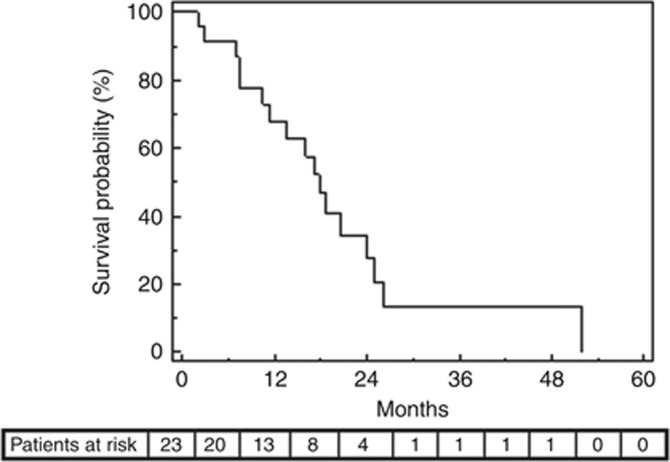
During a median follow-up of 16 months (range: 2–52 months), 17 patients (74%) died. The median survival was 17.9 months (95% CI: 14.3–21.4 months). The cumulative survival rate was 67.9% at 1 year and 20.6% at 2 years (Figure 1).
Figure 1.
Overall survival after 90Y-TARE (Kaplan–Meier curve).
Table 4 summarises the median survivals on the basis of baseline characteristics.
Table 4. Factors affecting overall survival: univariate analysis.
| Median survival (month) | 95% CI | P | |
|---|---|---|---|
|
Agea | |||
| <68 years (n=11) | 24 | 9.5–39 | 0.876 |
| ⩾68 years (n=12) | 16 | 10.5–21.5 | |
|
Sex | |||
| Male (n=14) | 16 | 10–22 | 0.821 |
| Female (n=9) | 20.5 | 17.5–23.5 | |
|
Prior treatments | |||
| Naive (n=4) | 52 | NA | 0.009 |
| Any prior treatment (n=19) | 16 | 9–22.5 | |
|
Prior surgical treatments | |||
| None (n=7) | 52 | NA | 0.223 |
| Surgical treatments (n=16) | 18 | 15–21 | |
|
Portal vein occlusion | |||
| None (n=7) | 18 | NA | 0.924 |
| Present (n=16) | 17 | 14–21 | |
|
Bilobar disease | |||
| No (n=7) | 18 | 16–20 | 0.735 |
| Yes (n=16) | 19 | 7.5–30 | |
|
ECOG | |||
| 0 (n=18) | 18 | 15–21 | 0.663 |
| 1 (n=5) | 24 | 7–42 | |
|
Metastases | |||
| No (n=21) | 17 | 11.5–23 | 0.543 |
| Yes (n=2) | 20.5 | NA | |
|
Cirrhosis | |||
| No (n=15) | 14 | 5–22 | 0.056 |
| Yes (n=8) | 20.5 | 16–25 | |
|
Target RECIST disease controlb | |||
| Yes (n=15) | 20.5 | 15–26 | 0.571 |
| No (n=5) | 16 | 0–35 | |
|
Overall RECIST disease controlb | |||
| Yes (n=9) | 24 | 17–32 | 0.075 |
| No (n=11) | 14 | 9–19.5 | |
|
Target RECIST objective responseb | |||
| Yes (n=4) | 20.5 | NA | 0.207 |
| No (n=16) | 16 | 9–23 | |
|
Overall RECIST objective responseb | |||
| Yes (n=3) | NA | NA | 0.132 |
| No (n=17) | 17 | 11–23 | |
|
Target mRECIST disease controlb | |||
| Yes (n=17) | 20.5 | 15–26 | 0.014 |
| No (n=3) | 7 | 0–15 | |
|
Overall mRECIST disease controlb | |||
| Yes (n=12) | 24 | 17–31 | 0.013 |
| No (n=8) | 11 | 7–16 | |
|
Target mRECIST objective responseb | |||
| Yes (n=14) | 19 | 14–23 | 0.169 |
| No (n=6) | 10.5 | 0–23.5 | |
|
Overall mRECIST objective responseb | |||
| Yes (n=9) | 20.5 | 17–24 | 0.114 |
| No (n=11) | 14 | 8–19.5 | |
|
EASL disease control | |||
| Yes (n=17) | 20.5 | 15–26 | 0.014 |
| No (n=3) | 7 | 0–15 | |
|
EASL objective response | |||
| Yes (n=12) | 17 | 13–21 | 0.878 |
| No (n=8) | 18 | 0–37 | |
Abbreviations: CI=confidence interval; EASL=European Association for the Study of the Liver; ECOG=Eastern Cooperative Oncology Group; mRECIST=modified response evaluation criteria in solid tumors; NA=not available as a result of small sample size; RECIST=response evaluation criteria in solid tumors.
Median survival was calculated from the date of 90Y-radioembolization.
Dichotomised according to the median value.
20 patients.
At univariate analysis, no statistically significant differences in OS were observed according to age, sex, ECOG, PVT, bilobar disease, and number of nodules or metastases. The four treatment-naive patients showed significantly better median survival as compared with previously treated patients (52 vs 16 months, P=0.009). The median survival of the 16 patients previously treated with curative-intent surgery was shorter than that of the other patients, but the difference did not reach the statistical significance (18 vs 52 months, P=0.223).
No statistically significant differences in survival were noted when the radiological response was assessed by RECIST, while they manifested when using both mRECIST and the EASL criteria. In particular, according to mRECIST, patients with DC had significantly prolonged survival as compared with patients with progression disease (PD) regardless of whether target lesions (median survival: 21 vs 7 months, P=0.014) or overall response (median survival: 24 vs 11 months, P=0.013) were considered. Similarly, according to the EASL criteria, patients with DC had significantly prolonged survival when compared with PD patients (median survival: 21 vs 7 months, P=0.014).
Four patients (17%) received systemic chemotherapy (gemcitabine or gemcitabine plus oxaliplatin) after TARE due to disease progression as judged by the treating physician.
Toxicities
Periprocedural adverse events occurred in 10 patients and consisted of grade 1 abdominal pain in 5 cases, grade 1 fever in 3 and grade 1 fatigue in 2. Routine medications allowed obtaining a complete remission of the pain and fever. Fatigue resolved in <3 months.
Late complications included a grade 3 bilirubin increase, which spontaneously resolved 3 months after 90Y-TARE and one case of cholecystitis requiring medical management. Mild ascites occurred in five patients (22%) starting from 15 to 90 days after the procedure. All of them were cirrhotic patients and the ascites resolved with diuretic therapy in all cases.
Discussion
Transarterial radioembolization with 90Y microspheres has been indicated as a treatment option alternative to other locoregional or systemic therapies for patients with unresectable ICC. However, to date, the literature addressing this issue is scanty, and the few studies evaluating the outcome of such a treatment have reported heterogeneous and difficult-to-scrutinise results, showing median survival which ranges from 9.3 (Saxena et al, 2010) to 22 months (Hoffmann et al, 2012). This difference can be principally attributed to the extreme heterogeneity of the patient population enroled, which is an unavoidable feature of clinical series dealing with (relatively) rare diseases. In fact, even our case series included patients with either naive unresectable ICC or tumours which relapsed after various treatments (including liver resection), who can be considered as having reached different time points in the natural history of their disease. Therefore, since carrying out robust sub-analyses within single-case series is highly improbable, only meta-analysis and meta-regression of published studies can obtain compelling, evidence-based and patient-tailored information.
It should be noted that 90Y-TARE was performed successfully with no mortality and a very low morbidity rate (Ibrahim et al, 2008; Rafi et al, 2013).
The median OS of 17.9 months obtained in our study is encouraging, and competes favourably with the pooled mean figure of 15.5 months recently reported by a systematic review of 12 studies by Al-Adra et al (2015). However, it is worth noting that the already mentioned heterogeneity of ICC-treated population limits the reliability of these comparisons. Such a heterogeneity can be ascribed not only to tumour extent, the presence or absence of portal involvement and extrahepatic disease, but also to the treatment regimens used before TARE (surgery, systemic chemotherapy, intra-arterial therapies). In any case, in our study, as well as in previous case series, the majority of patients was treated with TARE at a late stage of the disease and had a poor prognosis. Therefore, the OS observed probably underestimated the effects of radioembolization in patients at an earlier time point in the natural history of unresectable ICC (Al-Adra et al, 2015). This hypothesis is supported by the notably longer survival obtained in patients who did not receive any prior treatment, as compared with patients in whom TARE was preceded by other treatments including surgery. It can therefore be inferred that TARE could be used with good results as a first-line therapy in patients with unresectable ICC. It is pertinent to note that recently published data show median survival figures of 11.7 months for unresectable ICCs undergoing systemic cisplatin–gemcitabine chemotherapy (Valle et al, 2010) and of 12.4 months for those treated with TACE (Boehm et al, 2015). Our data represent a good background for a randomised controlled trial comparing TARE with other therapies, the only method of definitely asserting the superiority of 90Y-TARE as a standard-of-care for unresectable ICC.
Unlike other studies (Saxena et al, 2010; Hoffmann et al, 2012; Mouli et al, 2013), our study did not observe any association between ECOG performance status and survival. It is likely that this result simply reflects the better performance status of our study population as compared with other series. In fact, the majority of our patients had a performance status of ECOG 0 and none had a performance status of ECOG 2.
Similarly, OS did not vary significantly between patient subgroups with extrahepatic metastasis, multifocal tumours or portal invasion. Even this result can be ascribed to an insufficient number of cases in each subgroup.
As suggested by Camacho et al (2014), our team assessed the radiological response to 90Y-TARE using three different criteria. This allowed us to obtain some useful clinical information. First, as previously reported for HCCs treated with sorafenib (Gillmore et al, 2011), our study found that the response measured with the RECIST criteria does not predict survival. Second, the response rates at 3 months measured with both mRECIST and the EASL criteria applied to the delayed phases were higher as compared with those obtained with RECIST. Third, significantly improved survival was observed in patients demonstrating a radiological response, according to mRECIST and the EASL criteria.
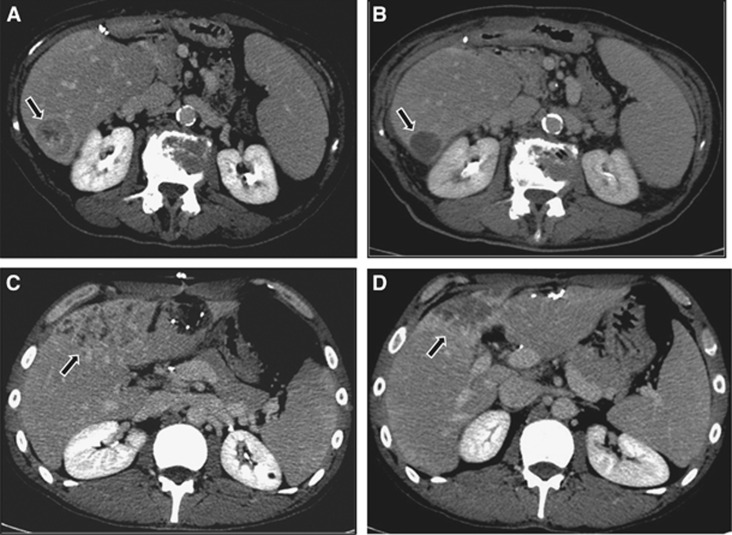
Although the choice of evaluation criteria of the response is still a matter of debate, our results endorsed the experience of Camacho et al (2014), confirming the superiority of ‘enhancement' criteria over ‘dimensional' criteria in evaluating the ICC response to 90Y-TARE. The accurate imaging evaluation of the response after locoregional therapies is essential for cancer patient management. The RECIST criteria have been widely utilised in the majority of studies regarding ICC treated with TARE, but a clear correlation with survival has never been reported. This corroborated the demonstrated belief that tumour shrinkage measured as a single diameter in the RECIST criteria is inaccurate, due to the potential underestimation of the response, particularly following locoregional therapies. It could be correlated to the concept that in ICC, as well as in HCC evaluation, angiogenesis partially reflects carcinogenesis (Bruix et al, 2001; Lencioni and Lovet, 2010). Hence, the enhancement of a viable tumour as measured by mRECIST and the EASL criteria can evaluate tumour response not only in HCC, in the arterial phase, but also in ICC by assessing the decrease of peripheral enhancement in the delayed phases of contrast-enhanced CT or MRI (Figure 2).
Figure 2.
CT imaging of ICC pre and post TARE treatment.Two cases of intrahepatic ICC comparing pre-treatment delayed-phase CT imaging (arrows) with post-treatment contrast-enhanced examination (arrows) according to modified mRECIST and the EASL criteria; A and B show a CR, while C and D demonstrate a PR at 3 months after 90Y-TARE.
A number of limitations of our study should be considered. The first limitation is certainly the small sample size, which did not allow the identification of prognostic factors. Second, the absence of a control group. However, the rare occurrence of ICC makes it difficult to conduct large prospective and randomised control trials in this context by a single centre alone. An additional limitation is related to the heterogeneity of the study population. Indeed, the majority of the patients enroled had already undergone prior surgical and/or medical or intravascular therapies before 90Y-TARE. The heterogeneity of ICC, especially in advanced stages, is a confounding factor for the majority of published experiences regarding TARE, and represents a significant challenge in drawing firm conclusions regarding treatment efficacy. This heterogeneity includes both patient factors, tumour features and treatment factors (prior interventions and concomitant chemotherapy). Nevertheless, it is representative of the real-world clinical setting.
In conclusion, this case series, conducted in a real-world clinical setting of a tertiary referral centre, confirms that, in patients with unresectable primary or recurrent ICC, 90Y-TARE is safe and shows a survival benefit especially in responders at 3 months as defined by mRECIST or the EASL criteria by measuring delayed-phase contrast enhancement. Since ICC still represents a complex and heterogeneous scenario in which no evidence-based algorithms of care exist, future multicentric randomised controlled trials are required to establish the exact role and timing of 90Y-TARE in the management of this cancer.
The authors declare no conflict of interest.
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License.
References
- Al-Adra DP, Gill RS, Axford SJ, Shi X, Kneteman N, Liau SS (2015) Treatment of unresectable intrahepatic cholangiocarcinoma with yttrium-90 radioembolization: a systematic review and pooled analysis. Eur J Surg Oncol 41: 120–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm LM, Jayakrishnan TT, Miura JT, Zacharias AJ, Johnston FM, Turaga KK, Gamblin TC (2015) Comparative effectiveness of hepatic artery based therapies for unresectable intrahepatic cholangiocarcinoma. J Surg Oncol 111: 213–220. [DOI] [PubMed] [Google Scholar]
- Bridgewater J, Galle PR, Khan SA, Llovet JM, Park JW, Patel T, Pawlik TM, Gores GJ (2014) Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol 60: 1268–1289. [DOI] [PubMed] [Google Scholar]
- Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, Christensen E, Pagliaro L, Colombo M, Rodés J EASL Panel of Experts on HCC (2001) Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol 35: 421–430. [DOI] [PubMed] [Google Scholar]
- Camacho JC, Kokabi N, Xing M, Prajapati HJ, El-Rayes B, Kim HS (2014) Modified Response Evaluation Criteria in Solid Tumors and European Association for the Study of the Liver criteria using delayed-phase imaging at an early time point predict survival in patients with unresectable intrahepatic cholangiocarcinoma following yttrium-90 radioembolization. J Vasc Interv Radiol 25: 256–265. [DOI] [PubMed] [Google Scholar]
- Chou FF, Sheen-Chen SM, Chen YS, Chen MC, Chen CL (1997) Surgical treatment of cholangiocarcinoma. Hepatogastroenterology 44: 760–765. [PubMed] [Google Scholar]
- Cirillo M, Venturini M, Ciccarelli L, Coati F, Bortolami O, Verlato G (2009) Clinician versus nurse symptom reporting using the National Cancer Institute-Common Terminology Criteria for Adverse Events during chemotherapy: results of a comparison based on patient's self-reported questionnaire. Ann Oncol 20: 1929–1935. [DOI] [PubMed] [Google Scholar]
- Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J (2009) New response evaluation criteria in solid tumors: revised RECIST guideline (version1.1). Eur J Cancer 45: 228–247. [DOI] [PubMed] [Google Scholar]
- Gillmore R, Stuart S, Kirkwood A, Hameeduddin A, Woodward N, Burroughs AK, Meyer T (2011) EASL and mRECIST responses are independent prognostic factors for survival in hepatocellular cancer patients treated with transarterial embolization. J Hepatol 55: 1309–1316. [DOI] [PubMed] [Google Scholar]
- Golfieri R, Mosconi C, Cappelli A, Giampalma E, Galaverni MC, Pettinato C, Renzulli M, Monari F, Angelelli B, Pini P, Terzi E, Ascanio S, Garzillo G, Piscaglia F, Bolondi L, Trevisani F (2015) Efficacy of radioembolization according to tumor morphology and portal vein thrombosis in intermediate-advanced hepatocellular carcinoma. Future Oncol 11: 3133–3142. [DOI] [PubMed] [Google Scholar]
- Gramenzi A, Golfieri R, Mosconi C, Cappelli A, Granito A, Cucchetti A, Marinelli S, Pettinato C, Erroi V, Fiumana S, Bolondi L, Bernardi M, Trevisani F BLOG (Bologna Liver Oncology Group) (2015) Yttrium-90 radioembolization vs sorafenib for intermediate-locally advanced hepatocellular carcinoma: a cohort study with propensity score analysis. Liver Int 35: 1036–1047. [DOI] [PubMed] [Google Scholar]
- Grove MK, Hermann RE, Vogt DP, Broughan TA (1991) Role of radiation after operative palliation in cancer of the proximal bile ducts. Am J Surg 161: 454–458. [DOI] [PubMed] [Google Scholar]
- Hoffmann RT, Paprottka PM, Schön A, Bamberg F, Haug A, Dürr EM, Rauch B, Trumm CT, Jakobs TF, Helmberger TK, Reiser MF, Kolligs FT (2012) Transarterial hepatic yttrium-90 radioembolization in patients with unresectable intrahepatic cholangiocarcinoma: factors associated with prolonged survival. Cardiovasc Intervent Radiol 35: 105–116. [DOI] [PubMed] [Google Scholar]
- Ibrahim SM, Mulcahy MF, Lewandowski RJ, Sato KT, Ryu RK, Masterson EJ, Newman SB, Benson A 3rd, Omary RA, Salem R (2008) Treatment of unresectable cholangiocarcinoma using yttrium-90 microspheres: results from a pilot study. Cancer 113: 2119–2128. [DOI] [PubMed] [Google Scholar]
- Kamphues C, Seehofer D, Eisele RM, Denecke T, Pratschke J, Neumann UP, Neuhaus P (2010) Recurrent intrahepatic cholangiocarcinoma: single-center experience using repeated hepatectomy and radiofrequency ablation. J Hepatobiliary Pancreat Sci 17: 509–515. [DOI] [PubMed] [Google Scholar]
- Kim JH, Won HJ, Shin YM, Kim KA, Kim PN (2011. a) Radiofrequency ablation for the treatment of primary intrahepatic cholangiocarcinoma. AJR Am J Roentgenol 196: W205–W209. [DOI] [PubMed] [Google Scholar]
- Kim JH, Won HJ, Shin YM, Kim PN, Lee SG, Hwang S (2011. b) Radiofrequency ablation for recurrent intrahepatic cholangiocarcinoma after curative resection. Eur J Radiol 80: e221–e225. [DOI] [PubMed] [Google Scholar]
- Kim SA, Lee JM, Lee KB, Kim SH, Yoon SH, Han JK, Choi BI (2011. c) Intrahepatic mass-forming cholangiocarcinomas: enhancement patterns at multiphasic CT, with special emphasis on arterial enhancement pattern–correlation with clinicopathologic findings. Radiology 260: 148–157. [DOI] [PubMed] [Google Scholar]
- Lencioni R, Llovet JM (2010) Modified RECIST(mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis 30: 52–60. [DOI] [PubMed] [Google Scholar]
- Lazaridis KN, Gores GJ (2005) Cholangiocarcinoma. Gastroenterology 128: 1655–1667. [DOI] [PubMed] [Google Scholar]
- Mouli S, Memon K, Baker T, Benson AB 3rd, Mulcahy MF, Gupta R, Ryu RK, Salem R, Lewandowski RJ (2013) Yttrium-90 radioembolization for intrahepatic cholangiocarcinoma: safety, response, and survival analysis. J Vasc Interv Radiol 24: 1227–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan H, Pawlik TM, Wolfgang CL, Choti MA, Cameron JL, Schulick RD (2007) Trends in survival after surgery for cholangiocarcinoma: a 30-year population-based SEER database analysis. J Gastrointest Surg 11: 1488–1496. [DOI] [PubMed] [Google Scholar]
- Rafi S, Piduru SM, El-Rayes B, Kauh JS, Kooby DA, Sarmiento JM, Kim HS (2013) Yttrium-90 radioembolization for unresectable standard-chemorefractory intrahepatic cholangiocarcinoma: survival, efficacy, and safety study. Cardiovasc Intervent Radiol 36: 440–448. [DOI] [PubMed] [Google Scholar]
- Razumilava N, Gores GJ (2014) Cholangiocarcinoma. Lancet 383: 2168–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roayaie S, Guarrera JV, Ye MQ, Thung SN, Emre S, Fishbein TM, Guy SR, Sheiner PA, Miller CM, Schwartz ME (1998) Aggressive surgical treatment of intrahepatic cholangiocarcinoma: predictors of outcomes. J Am Coll Surg 187: 365–372. [DOI] [PubMed] [Google Scholar]
- Saxena A, Bester L, Chua TC, Chu FC, Morris DL (2010) Yttrium-90 radiotherapy for unresectable intrahepatic cholangiocarcinoma: a preliminary assessment of this novel treatment option. Ann Surg Oncol 17: 484–491. [DOI] [PubMed] [Google Scholar]
- Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, Madhusudan S, Iveson T, Hughes S, Pereira SP, Roughton M, Bridgewater J ABC-02 Trial Investigators (2010) Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 362: 1273–1281. [DOI] [PubMed] [Google Scholar]
- Yang JD, Kim B, Sanderson SO, Sauver JS, Yawn BP, Larson JJ, Therneau TM, Roberts LR, Gores GJ, Kim WR (2012) Biliary tract cancers in Olmsted County, Minnesota, 1976–2008. Am J Gastroenterol 107: 1256–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]