Abstract
An unprecedented dengue outbreak involving more than 15,000 infections, including 136 dengue hemorrhagic fever (DHF) cases and 20 fatalities, occurred in Taiwan in 2014. The median age of the DHF cases was 71 years (range: 4–92 years) and most of them (N = 100, 73.5%) had comorbidities, of which the majority were hypertension (56%) and diabetes mellitus (DM; 27%). Only approximately half of the DHF cases (59/136) were classified as severe dengue, based on the 2009 WHO-revised dengue classification. The fatality rate for this DHF outbreak was 14.7%. DM (odds ratio [OR] = 3.60, 95% confidence interval [CI] = 1.22–10.63) and presentation with severe plasma leakage (OR = 6.42, 95% CI = 1.76–23.63) were independent risk factors for fatality.
Introduction
Dengue is the most rapidly spreading mosquito-borne viral disease in the world and over 2.5 billion people are living in geographic areas where dengue is endemic.1 Each year, about 50 million dengue virus (DENV) infections occur and approximately half a million people contract severe dengue (SD), causing significant morbidity and mortality worldwide.2 A noticeable shift has been observed recently, from epidemics of dengue mainly affecting children to more adult cases being reported, with a particular increase in SD in adults.1,3,4 However, research concerning adult fatalities as a result of dengue, particularly among the elderly, remains scarce.
In Taiwan, where dengue is not yet considered endemic, despite the annual occurrence of dengue outbreaks of various magnitudes,5,6 most individuals infected with dengue are adults, with dengue hemorrhagic fever (DHF) and related fatality occurring primarily in the elderly.6 This unique epidemiologic pattern has been unchanged in the past decade, with annual numbers of fatal cases ranging from 0 to 7.7 However, in 2014, an unprecedented dengue outbreak, involving more than 15,000 infections, including 136 DHF cases and 20 fatalities, occurred,8 followed by an even larger outbreak in 2015. These outbreaks suggest that dengue may have become endemic to Taiwan and that occurrence of tens of thousands of DENV infections annually might be not uncommon in future. Review of the characteristics of DHF cases and exploration of risk factors for fatality is necessary to ensure preparedness for future dengue epidemics.
Materials and Methods
Surveillance of subjects with DENV infection.
Patients with DENV infection, notified as dengue fever (DF) or DHF in Taiwan, were required to have blood samples submitted to the Taiwan Centers for Disease Control for laboratory diagnoses, and be interviewed by local public health staff to obtain information including demographic data, illness onset, prior dengue exposure, residency, and travel history 14 days preceding illness onset.9 Physicians were requested to upload the medical records of patients with DHF to the National Notifiable Disease Surveillance System.9 If patients died during hospitalization or were critically ill and discharged against medical advice, the uploaded medical records were required to include death certificates and/or discharge summaries for these patients. Medical officers of the Taiwan Centers for Disease Control scrutinized each notified case to validate the classification of DHF by reviewing the medical charts and consulting clinicians, where appropriate.
Laboratory diagnosis of DENV infection.
DENV infections were confirmed by laboratory diagnosis based on 1) positive DENV RNA, determined by reverse transcription-polymerase chain reaction (RT-PCR); 2) positive DENV nonstructural protein 1 (NS1) antigen; and 3) at least a 4-fold increase in DENV-specific IgM or IgG antibodies, where cross-reactions to Japanese encephalitis virus had been excluded.10
To detect and differentiate DENV serotypes, we performed one-step, SYBR Green I-based, real-time RT-PCR (QuantiTect SYBR Green RT-PCR kit; Qiagen, Hilden, Germany) using DENV serotype–specific primer sets in the Mx3000P quantitative PCR system (Stratagene, La Jolla, CA).11 A commercial DENV NS1 Ag strip rapid test kit (Bio-Rad Laboratories, Marnes La Coquette, France) and a SD Dengue NS1 Ag test (Standard Diagnostics, Inc., Kyonggi-do, Korea) were used to detect DENV NS1 antigen.12 Envelope/membrane-specific capture IgM and IgG enzyme-linked immunosorbent assays were used to detect DENV-specific IgM and IgG antibodies. Secondary DENV infections were defined by the positive RT-PCR and/or positive NS1, associated with IgG positivity during the acute phase (disease duration < 7 days) and an IgM/IgG ratio of < 1.2 during the convalescent phase (disease duration ≥ 7 days).13
Definition of DHF and SD.
DHF was defined according to the WHO 1997 case definition as the patient having fever, bleeding manifestations, thrombocytopenia (platelet count < 100,000/μL), and evidence of plasma leakage.14 SD was defined based on the 2009 WHO dengue classification.1 SD cases included in this study fulfilled at least one of these three criteria: 1) severe plasma leakage causing a) dengue shock syndrome (DSS; defined as systolic blood pressure < 90 mmHg) or b) fluid accumulation with respiratory distress requiring intubation, or the presence of massive pleural effusion requiring continuous drainage by pigtail or chest tubes, 2) severe bleeding requiring transfusion of red blood cell containing products, and 3) severe organ impairment, defined as injuries of a) liver (aspartate or alanine transaminase ≥ 1,000 U/L), b) central nervous system (impaired consciousness described by clinicians), c) heart (myocarditis with elevated creatine kinase-MB and troponin-I, supported by heart echography with low ejection fraction or poor heart motion), or d) kidney (≥ 2-fold increase in serum creatinine level, glomerular filtration rate decreased by ≥ 50%, or urine output < 0.5 mL/kg/hour for at least 12 hours).
Study design and population.
Because only DHF cases had medical records available on the National Notifiable Disease Surveillance System, a retrospective study to characterize DHF cases in 2014 was conducted. The age-specific incidence rate of DHF was calculated using national age-specific census mid-year estimates.15 Characteristics of each DHF subject, including demographic data, serological evidence of secondary DENV infection, comorbidities, clinical and laboratory parameters, severity category (based on the classification criteria for SD), and prognosis at 28 days after admission, were collected. To identify characteristics associated with prognosis and facilitate early fatality prediction, only risk factors that could be determined at the time of hospital presentation were selected for further analysis. This study was part of an emergency response to the dengue outbreak and was exempt from institutional review board review.
Statistical analysis.
Descriptive results for categorical variables were displayed as frequency and percentage. For continuous variables, median and interquartile range (IQR) were used. χ2 or Fisher's exact test was used for comparisons of categorical variables, whereas the Mann–Whitney U test was used for continuous variables. Data analyses were performed using Epi-info_7 software (Centers for Disease Control and Prevention, Atlanta, GA). Variables with a P value < 0.20 in univariate analysis were incorporated into a multivariate logistic regression model to calculate odds ratios (ORs) and 95% confidence intervals (CIs). A two-tailed P value < 0.05 was considered statistically significant.
Results
General characteristics of patients with DHF.
There were 140 DHF cases notified in the 2014 dengue outbreak. After exclusion of four cases not fulfilling the criteria for DHF, 136 were included in our analysis (Table 1).
Table 1.
General characteristics of 136 patients with DHF*
| Variable | No. (%) |
|---|---|
| Age (years) [median (IQR)] | 71 (60.5–76) |
| Male gender | 66 (48.5) |
| Timeliness of key indicators (days) [median (IQR)] | |
| Illness onset to admission to hospital | 3 (1–4) |
| Illness onset to notification of DHF | 6.5 (6–8) |
| Prior DENV infection | |
| Self-report of prior dengue | 7 (5.1) |
| Secondary DENV infection (N = 128) | 59 (46.1) |
| Comorbidities | |
| Any underlying disease | 100 (73.5) |
| Hypertension | 76 (55.9) |
| DM | 37 (27.2) |
| Cardiovascular disease | 19 (14.0) |
| Gastrointestinal ulcerative disease | 17 (12.5) |
| Neurological disease | 13 (9.6) |
| Liver disease | 10 (7.4) |
| Chronic kidney disease | 17 (12.5) |
| Active malignancy | 9 (6.6) |
| Chronic pulmonary disease | 8 (5.9) |
DENV = dengue virus; DHF = dengue hemorrhagic fever; DM = diabetes mellitus; IQR = interquartile range.
Values in the table refer to the number of patients (%), unless stated otherwise.
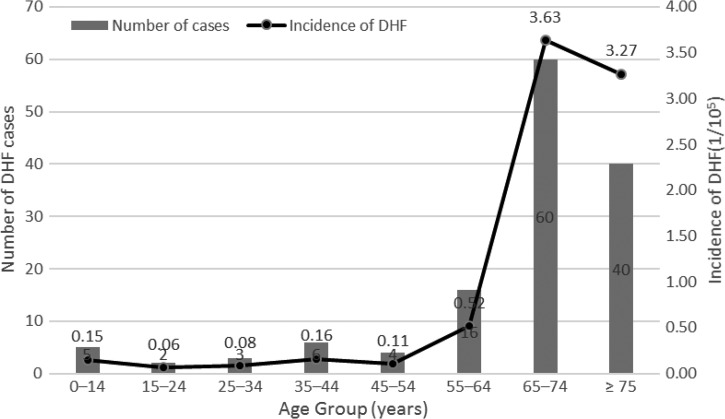
Among the 136 DHF subjects, 66 (48.5%) were male and the male:female gender ratio was 1:1.06. The majority of DHF cases were adults and only five were children or adolescents younger than 18 years. The median age was 71 years (IQR = 60.5–76) and the incidence of DHF increased sharply beyond the age of 65 (Figure 1 ).
Figure 1.
Number of cases and age-specific incidence of dengue hemorrhagic fever, Taiwan, 2014.
Of the 136 subjects, DENV infection was confirmed by NS1 antigenemia (N = 73), positive RT-PCR (N = 51, all revealed DENV-1 infection), and at least a 4-fold increase in DENV-specific IgM or IgG antibodies (N = 12). Fifty-nine (46.1%) of the 128 subjects with serological profiles available were classified as having secondary DENV infection, whereas only seven (5.1%) of 136 patients recalled experiencing prior dengue.
A total of 100 patients (73.5%) had comorbidities. The most common comorbidities were hypertension (55.9%), diabetes mellitus (DM; 27.2%), and cardiovascular disease (14.0%).
Severity categorization and prognosis of patients with DHF.
A total of 37 patients were classified as having SD at presentation. An additional 22 subjects had illness which progressed to SD after admission, resulting in a total of 59 subjects with SD among the 136 DHF patients (Table 2).
Table 2.
Severity categorization, intervention, and prognosis of 136 patients with DHF*
| Variable | No. (%) |
|---|---|
| SD categorization | 59 (43.3) |
| Severe plasma leakage | 36 (26.5) |
| Severe bleeding | 34 (0.25) |
| Severe organ impairment | 37 (27.2) |
| AST/ALT > 1,000 U/L | 7 (5.2) |
| Consciousness impairment | 26 (19.1) |
| Acute kidney injury | 21 (15.4) |
| Myocarditis | 3 (2.2) |
| Intensive care or invasive procedures | (19.9) |
| Tracheal intubation | 22 (16.2) |
| Esophagogastroduodenoscopy | 11 (8.1) |
| Hemodialysis or hemofiltration | 7 (5.2) |
| Tube thoracotomy | 1 (0.7) |
| Intra-arterial balloon pump | 1 (0.7) |
| Transfusion therapy | |
| Packed red blood cells | 36 (26.5) |
| Platelet concentrate | 63 (46.3) |
| Fresh-frozen plasma | 22 (16.2) |
| Length of hospital stay (days) (median [IQR]) | 7 (5–9) |
| ICU admission | 41 (31.2) |
| Length of ICU stay (days) (median [IQR]) | 4 (2–6) |
| Fatality | 20 (14.7) |
| Intervals between illness onset and death (days) (median [IQR]) | 7 (4–9.5) |
| Duration of hospitalization (days) (median [IQR]) | 3 (1–4) |
ALT = alanine aminotransferase; AST = aspartate aminotransferase; DHF = dengue hemorrhagic fever; ICU = intensive care unit; IQR = interquartile range; SD = severe dengue.
Values in the table refer to the number of patients (%), unless stated otherwise.
Severe plasma leakage was noted in 36 subjects, and all 36 manifested with DSS. Severe bleeding occurred in 34 subjects, among whom 32 had gastrointestinal bleeding and two had vaginal bleeding. Among 37 patients with severe organ impairment, impaired consciousness accounted for the majority (N = 26). A total of 12 presented with impaired consciousness before arrival and six had episodes of falls to the ground at home. Other infrequent manifestations of severe organ impairment included severe rhabdomyolysis with creatine phosphokinase > 1,000 IU/L (N = 3) and pancreatitis (N = 1).
A total of 20 patients died at a median age of 72.5 years, with a case fatality rate of 14.7% in this DHF case series. Only one fatal subject, a 59-year-old woman presenting with shock on arrival at the hospital, reported no comorbidities. The major cause of death was shock (15/20, 75.0%) and three patients had concomitant bacteremia (Escherichia coli plus Pseudomonas aeruginosa, Klebsiella pneumoniae, and Enterococcus faecium, respectively). A total of 40% of fatalities occurred within 3 days after admission and 13 of the 20 fatal cases were not notified as DHF/DSS until the day the patient died.
Risk factors for fatality at presentation.
In univariate analysis, neither age ≥ 65 years nor secondary DENV infection was found to be associated with fatality. Regarding comorbidities, only DM was associated with fatality. Presentation with SD on hospital arrival was associated with fatality and severe plasma leakage only remained significantly associated with death after stratification by the components of SD (Table 3). In multivariate analysis, only DM (OR = 3.60, 95% CI = 1.22–10.63) and presentation with severe plasma leakage on arrival at hospital (OR = 6.42, 95% CI = 1.76–23.63) were independently associated with death (Table 4).
Table 3.
Comparison of characteristics of patients with fatal and nonfatal DHF at the time of hospital presentation*
| Variables | Fatal (N = 20) | Nonfatal (N = 116) | P value |
|---|---|---|---|
| Age (years) (median [IQR]) | 72.5 (67.5–77) | 71 (59–76) | 0.322 |
| Age ≥ 65 | 17 (85.0) | 82 (82.8) | 0.291 |
| Male gender | 7 (35.0) | 59 (50.9) | 0.285 |
| Secondary DENV infection, n/N | 9/20 (45.0) | 51/108 (47.2) | 0.951 |
| Duration of illness preceding hospitalization (days) (median [IQR]) | 2 (0.5–3.5) | 3 (2.0–4.0) | 0.105 |
| Comorbidities | |||
| Any underlying disease | 19 (95.0) | 81 (69.8) | 0.037 |
| Hypertension | 13 (65.0) | 63 (54.3) | 0.519 |
| DM | 10 (50.0) | 27 (23.3) | 0.027 |
| Cardiovascular disease | 5 (25.0) | 14 (12.1) | 0.234 |
| Gastrointestinal ulcerative disease | 3 (15.0) | 14 (12.1) | 0.946 |
| Neurological disease | 0 (0.0) | 13 (11.2) | 0.726 |
| Liver disease | 3 (15.0) | 7 (6.0) | 0.331 |
| Chronic kidney disease | 1 (5.0) | 16 (13.8) | 0.487 |
| Malignancy | 2 (10.0) | 7 (6.0) | 0.785 |
| Chronic pulmonary disease | 3 (15.0) | 5 (4.3) | 0.188 |
| Presenting symptoms | |||
| Fever | 20 (100.0) | 113 (97.4) | 1.000 |
| Myalgia | 10 (50.0) | 48 (41.3) | 0.515 |
| Headache | 4 (20.0) | 35 (30.2) | 0.355 |
| Arthralgia | 2 (10.0) | 14 (12.1) | 1.000 |
| Bone pain | 1 (5.0) | 13 (11.2) | 0.708 |
| Lethargy | 6 (30.0) | 14 (12.1) | 0.080 |
| Retro-orbital pain | 0 (0.0) | 7 (6.0) | 1.000 |
| Rash | 3 (15.0) | 26 (22.4) | 0.677 |
| Vomiting | 8 (40.0) | 30 (25.9) | 0.302 |
| Abdominal pain or tenderness | 3 (15.0) | 26 (22.4) | 0.677 |
| Diarrhea | 3 (15.0) | 23 (19.8) | 0.878 |
| Any hemorrhage | 6 (30.0) | 46 (39.7) | 0.568 |
| Hematology findings | |||
| WBC count/μL [median (IQR)] | 5,880 (3,300–7,745) | 4,050 (2,900–5,900) | 0.130 |
| WBC count ≥ 10,000/μL | 0 (0.0) | 3 (2.6) | 0.875 |
| WBC count ≤ 4,000/μL | 6 (30.0) | 57 (49.1) | 0.179 |
| Platelet count (/μL) (median [IQR]) | 84,500 (31,500–117,500) | 60,500 (18,000–116,000) | 0.631 |
| Platelet count ≤ 20,000/μL | 4 (20.0) | 30 (25.9) | 0.780 |
| Severity categorization of dengue | 11 (55.0) | 26 (22.4) | 0.006 |
| Severe plasma leakage | 7 (35.0) | 8 (6.9) | < 0.001 |
| Severe bleeding | 2 (10.0) | 14 (12.1) | 1.000 |
| Severe organ impairment | 4 (20.0) | 10 (8.6) | 0.251 |
DENV = dengue virus; DHF = dengue hemorrhagic fever; DM = diabetes mellitus; IQR = interquartile range; WBC = white blood cell.
Values in the table refer to the number of patients (%), unless stated otherwise.
Table 4.
Multivariate logistic regression analysis of characteristics for fatality at hospital presentation in patients with DHF
| Variable | aOR | 95% CI | P value |
|---|---|---|---|
| DM | 3.60 | 1.22–10.63 | 0.020 |
| Chronic pulmonary disease | 4.17 | 0.70–24.72 | 0.115 |
| Severe plasma leakage | 6.42 | 1.76–23.63 | 0.005 |
| WBC count ≤ 4,000/μL | 0.78 | 0.25–2.48 | 0.676 |
| Lethargy | 2.25 | l0.61–8.33 | 0.223 |
aOR = adjusted odds ratio; CI = confidence interval; DM = diabetes mellitus; WBC = white blood cell.
Discussion
Compared with previous dengue epidemics, which have primarily affected children, the new challenge we are currently facing is the surging number of adult subjects with dengue of increased severity.3,16–19 Our study, which included primarily elderly adult cases of DHF with complete laboratory investigations, not only demonstrates the diverse clinical spectrum of DHF, but also highlights the devastating consequences when DHF affects the elderly. Together with global warming, the rapid expansion of DENV to new geographic areas where the elderly remain susceptible to dengue would result in significant morbidity and mortality, especially in the absence of a suitable vaccine and clinical management recommendations specific for the elderly.20,21
In sharp contrast to other countries where dengue is endemic, most DHF patients in Taiwan are elderly,6,7,16,22–25 probably as a consequence of a higher likelihood of secondary DENV infection, which is a well-known risk factor for DHF.26,27 We speculate that, despite a higher seroprevalence of dengue among the elderly (> 65 years old; P. Y. Su, unpublished data), they will not be immune to all four DENV serotypes, since Taiwan has not historically been a dengue-endemic area. Instead, the elderly would be likely to have secondary DENV infection if a dengue outbreak occurred with a DENV serotype to which they remained susceptible. In addition to secondary DENV infection, comorbidities frequent in the elderly also contributed to the development of DHF.28,29 To our knowledge, the median age of ≥ 70 years in our DHF subjects is the highest among case series published to date, and is much higher than that of a previously reported series of 93 DHF Taiwanese cases (median age, 63 years).22
We found two risk factors at presentation associated with fatality in adult DHF cases: DM and presentation of severe plasma leakage on arrival at hospital. DM is a well-recognized risk factor for fatality and severe clinical presentation of dengue.7,18,28,30 There is an immunological hypothesis that cytokine overload, attributed to diabetes, renders the endothelium more vulnerable to subsequent vascular leakage, leading to severe manifestations of dengue.28,30,31 In light of the high prevalence of diabetes in the elderly population,32,33 further studies are required to determine whether better glycemic control could avert the risk of severe manifestations or fatality of dengue.
Because DSS is a well-known risk factor for dengue fatality, ref-type="bibr">23 it was not surprising that presentation with severe plasma leakage was associated with fatality in our study. Although the exact mechanism underlying the pathophysiology of plasma leakage remains elusive, viral factors and host genetic and immunological background may be all implicated.26 It has been proposed that increased levels of particular cytokines contribute to disease severity,34 and reduction of DENV-induced cytokines may confer survival benefits in cases of vascular leakage.35 Additional efforts are required to aid development of prognostic markers and therapeutic intervention in subjects with plasma leakage.
Despite the fact that secondary DENV infection has long been considered a major risk factor for DHF/DSS and related fatality, other factors, including a sequence of two DENV infections, the interval between infections, and characteristics of human hosts, such as age and comorbidities, could also modify responses to dengue disease.36 In our study, secondary infection with DENV-1 was not found to be detrimental to prognosis, a finding consistent with previous studies which reported that secondary infection with a non-DENV-2 strain was not associated with increased fatality.6,37 However, because our study only included patients with DHF, future research also including cases with DF is needed to further clarify the interplay between secondary infections and virus strains on the outcome of DENV infection.
In our study, only approximately half of DHF subjects were classified as having SD. Moreover, 65% of fatal cases were not notified as DHF/DSS until the day the patient died. Because DHF/DSS is a reportable disease in Taiwan, which should be notified within 24 hours after diagnosis, our finding suggests limitations of the 1997 WHO DF/DHF classification when applied in clinical management of dengue. Because the 2009 WHO dengue classification is regarded as able to detect disease severity with high sensitivity and assist clinical management, potentially contributing to reduced dengue mortality,38 the Taiwan Centers for Disease Control adopted the 2009 WHO classification for dengue surveillance in May 2015.
This study had several limitations. First, our analysis of dengue fatality was limited to DHF cases and the conclusions may, therefore, not be applicable to the much larger numbers of individuals with DF. Second, the limited number of fatal cases included in the study, despite the fact that the cases occurring nationwide were included, provided insufficient statistical power to detect other significant risk factors for DHF fatality. Third, because most of our DHF subjects were in the charge of different clinicians, they may have received varied clinical management, which was not taken into consideration in our analyses. Fourth, our study was conducted through review of medical records on the National Notifiable Disease Surveillance System, which did not require medical records to be uploaded in a standardized format and may contain inadequate information. Prospective studies will be necessary to provide a more comprehensive analysis of risk factors associated with dengue fatality in the elderly.
In conclusion, an unprecedented dengue outbreak, involving 136 DHF cases associated with 20 fatalities, occurred in Taiwan in 2014. Most of these DHF cases were elderly and had comorbidities. Only approximately half (59/136) of DHF cases were classified as SD based on the 2009 WHO revised dengue classification. DM and the presentation of severe plasma leakage were associated with increased fatality of DHF.
Acknowledgments
We appreciate the comments given by Yi-Chun Lo on this manuscript. We also thank the health care personnel who provide meticulous care for subjects with DHF and the staff of local public health authorities for their unremitting efforts on the control of dengue epidemics.
Footnotes
Authors' addresses: Hsin-Yi Wei and Min-Nan Hung, Office of Preventive Medicine, Centers for Disease Control, Taipei, Taiwan, E-mails: januarylly@cdc.gov.tw and mnhung@cdc.gov.tw. Pei-Yun Shu, Center for Diagnostics and Vaccine Development, Centers for Disease Control, Taipei, Taiwan, E-mail: pyshu@cdc.gov.tw.
References
- 1.WHO Dengue: Guideline for Diagnosis, Treatment, Prevention and Control. 2009. http://whqlibdoc.who.int/publications/2009/9789241547871_eng.pdf Available at. Accessed November 4, 2015.
- 2.WHO, Media Centre Dengue and Severe Dengue. Updated April 2016. http://www.who.int/mediacentre/factsheets/fs117/en/ Available at. Accessed May 27, 2016.
- 3.Moraes GH, de Fatima Duarte E, Duarte EC. Determinants of mortality from severe dengue in Brazil: a population-based case-control study. Am J Trop Med Hyg. 2013;88:670–676. doi: 10.4269/ajtmh.11-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ooi EE, Goh KT, Gubler DJ. Dengue prevention and 35 years of vector control in Singapore. Emerg Infect Dis. 2006;12:887–893. doi: 10.3201/10.3201/eid1206.051210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin CH, Schiøler KL, Jepsen MR, Ho CK, Li SH, Konradsen F. Dengue outbreaks in high-income area, Kaohsiung City, Taiwan, 2003–2009. Emerg Infect Dis. 2012;18:1603–1611. doi: 10.3201/eid1810.111929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin CC, Huang YH, Shu PY, Wu HS, Lin YS, Yeh TM, Liu HS, Liu CC, Lei HY. Characteristic of dengue disease in Taiwan: 2002–2007. Am J Trop Med Hyg. 2010;82:731–739. doi: 10.4269/ajtmh.2010.09-0549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu YT, Fang CT, Yen JJ. Risk factors for dengue hemorrhagic fever in Taiwan, 2003–2013. Taiwan J Public Health. 2015;34:437–445. [Google Scholar]
- 8.Wang SF, Wang WH, Chang K, Chen YH, Tseng SP, Yen CH, Wu DC, Chen YM. Severe dengue fever outbreak in Taiwan. Am J Trop Med Hyg. 2016;94:193–197. doi: 10.4269/ajtmh.15-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McKerr C, Lo YC, Edeghere O, Bracebridge S. Evaluation of the National Notifiable Diseases Surveillance System for dengue fever in Taiwan, 2010–2012. PLoS Negl Trop Dis. 2015;9:e0003639. doi: 10.1371/journal.pntd.0003639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang JH, Liao TL, Chang SF, Su CL, Chien LJ, Kuo YC, Yang CF, Lin CC, Shu PY. Laboratory-based dengue surveillance in Taiwan, 2005: a molecular epidemiologic study. Am J Trop Med Hyg. 2007;77:903–909. [PubMed] [Google Scholar]
- 11.Pei-Yun Shu, Chang SF, Kuo YC, Yueh YY, Chien LJ, Sue CL, Lin TH, Huang JH. Development of group- and serotype-specific one-step SYBR green I-based real-time reverse transcription-PCR assay for dengue virus. J Clin Microbiol. 2003;41:2408–2416. doi: 10.1128/JCM.41.6.2408-2416.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shu PY, Yang CF, Kao JF, Su CL, Chang SF, Lin CC, Yang WC, Shih H, Yang SY, Wu PF, Wu HS, Huang JH. Application of the dengue virus NS1 antigen rapid test for on-site detection of imported dengue cases at airports. Clin Vaccine Immunol. 2009;16:589–591. doi: 10.1128/CVI.00475-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shu PY, Chen LK, Chang SF, Yueh YY, Chow L, Chien LJ, Chin C, Lin TH, Huang JH. Comparison of capture immunoglobulin M (IgM) and IgG enzyme-linked immunosorbent assay (ELISA) and nonstructural protein NS1 serotype-specific IgG ELISA for differentiation of primary and secondary dengue virus infections. Clin Diagn Lab Immunol. 2003;10:622–630. doi: 10.1128/CDLI.10.4.622-630.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.WHO . Dengue Hemorrhagic Fever: Diagnosis, Treatment, Prevention and Control. 2nd edition. Geneva, Switzerland: World Health Organization; 1997. http://www.who.int/csr/resources/publications/dengue/Denguepublication/en/ Available at. Accessed November 4, 2015. [Google Scholar]
- 15.Department of Household registration, Ministry of the Interior Taiwan Midyear Population by Sex and 5-Year Age Group. 2014. http://www.ris.gov.tw/web/ris3-english/history Available at. Accessed April 28, 2016.
- 16.Lee IK, Liu JW, Yang KD. Clinical characteristics, risk factors, and outcomes in adults experiencing dengue hemorrhagic fever complicated with acute renal failure. Am J Trop Med Hyg. 2009;80:651–655. [PubMed] [Google Scholar]
- 17.Leo YS, Thein TL, Fisher DA, Low JG, Oh HM, Narayanan RL, Gan VC, Lee VJ, Lye DC. Confirmed adult dengue deaths in Singapore: 5-year multi-center retrospective study. BMC Infect Dis. 2011;11:123. doi: 10.1186/1471-2334-11-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pang J, Thein TL, Leo YS, Lye DC. Early clinical and laboratory risk factors of intensive care unit requirement during 2004–2008 dengue epidemics in Singapore: a matched case-control study. BMC Infect Dis. 2014;14:649. doi: 10.1186/s12879-014-0649-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thanachartwet V, Oer-Areemitr N, Chamnanchanunt S, Sahassananda D, Jittmittraphap A, Suwannakudt P, Desakorn V, Wattanathum A. Identification of clinical factors associated with severe dengue among Thai adults: a prospective study. BMC Infect Dis. 2015;15:420. doi: 10.1186/s12879-015-1150-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garcia-Rivera EJ, Rigau-Perez JG. Dengue severity in the elderly in Puerto Rico. Rev Panam Salud Publica. 2003;13:362–368. doi: 10.1590/s1020-49892003000500004. [DOI] [PubMed] [Google Scholar]
- 21.Lloyd-Sherlock P. Population ageing in developed and developing regions: implications for health policy. Soc Sci Med. 2000;51:887–895. doi: 10.1016/s0277-9536(00)00068-x. [DOI] [PubMed] [Google Scholar]
- 22.Hsu HY, Lai SK, Kuo CH, Wu CW, Liu DP. An epidemiological analysis of dengue hemorrhagic fever cases in Taiwan from 2003 to 2011. Taiwan Epidemiology Bulletin. 2013;29:319–328. [Google Scholar]
- 23.Lee IK, Liu JW, Yang KD. Clinical and laboratory characteristics and risk factors for fatality in elderly patients with dengue hemorrhagic fever. Am J Trop Med Hyg. 2008;79:149–153. [PubMed] [Google Scholar]
- 24.Lee IK, Liu JW, Yang KD. Fatal dengue hemorrhagic fever in adults: emphasizing the evolutionary pre-fatal clinical and laboratory manifestations. PLoS Negl Trop Dis. 2012;6:e1532. doi: 10.1371/journal.pntd.0001532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee MS, Hwang KP, Chen TC, Lu PL, Chen TP. Clinical characteristics of dengue and dengue hemorrhagic fever in a medical center of southern Taiwan during the 2002 epidemic. J Microbiol Immunol Infect. 2006;39:121–129. [PubMed] [Google Scholar]
- 26.Guzman MG, Harris E. Dengue. Lancet. 2015;385:453–465. doi: 10.1016/S0140-6736(14)60572-9. [DOI] [PubMed] [Google Scholar]
- 27.Halstead SB. Neutralization and antibody-dependent enhancement of dengue viruses. Adv Virus Res. 2003;60:421–467. doi: 10.1016/s0065-3527(03)60011-4. [DOI] [PubMed] [Google Scholar]
- 28.Pang J, Salim A, Lee VJ, Hibberd ML, Chia KS, Leo YS, Lye DC. Diabetes with hypertension as risk factors for adult dengue hemorrhagic fever in a predominantly dengue serotype 2 epidemic: a case control study. PLoS Negl Trop Dis. 2012;6:e1641. doi: 10.1371/journal.pntd.0001641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rowe EK, Leo YS, Wong JG, Thein TL, Gan VC, Lee LK, Lye DC. Challenges in dengue fever in the elderly: atypical presentation and risk of severe dengue and hospital-acquired infection. PLoS Negl Trop Dis. 2014;8:e2777. doi: 10.1371/journal.pntd.0002777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chaturvedi UC, Agarwal R, Elbishbishi EA, Mustafa AS. Cytokine cascade in dengue hemorrhagic fever: implications for pathogenesis. FEMS Immunol Med Microbiol. 2000;28:183–188. doi: 10.1111/j.1574-695X.2000.tb01474.x. [DOI] [PubMed] [Google Scholar]
- 31.Sierra B, Perez AB, Vogt K, Garcia G, Schmolke K, Aguirre E, Alvarez M, Kern F, Kourí G, Volk HD, Guzman MG. Secondary heterologous dengue infection risk: disequilibrium between immune regulation and inflammation? Cell Immunol. 2010;262:134–140. doi: 10.1016/j.cellimm.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 32.Jiang YD, Chang CH, Tai TY, Chen JF, Chuang LM. Incidence and prevalence rates of diabetes mellitus in Taiwan: analysis of the 2000–2009 Nationwide Health Insurance database. J Formos Med Assoc. 2012;111:599–604. doi: 10.1016/j.jfma.2012.09.014. [DOI] [PubMed] [Google Scholar]
- 33.Kirkman MS, Briscoe VJ, Clark N, Florez H, Haas LB, Halter JB, Huang ES, Korytkowski MT, Munshi MN, Odegard PS, Pratley RE, Swift CS. Diabetes in older adults. Diabetes Care. 2012;35:2650–2664. doi: 10.2337/dc12-1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rothman AL. Immunity to dengue virus: a tale of original antigenic sin and tropical cytokine storms. Nat Rev Immunol. 2011;11:532–543. doi: 10.1038/nri3014. [DOI] [PubMed] [Google Scholar]
- 35.Shresta S, Sharar KL, Prigozhin DM, Beatty PR, Harris E. Murine model for dengue virus-induced lethal disease with increased vascular permeability. J Virol. 2006;80:10208–10217. doi: 10.1128/JVI.00062-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guzman MG, Alvarez M, Halstead SB. Secondary infection as a risk factor for dengue hemorrhagic fever/dengue shock syndrome: an historical perspective and role of antibody-dependent enhancement of infection. Arch Virol. 2013;158:1445–1459. doi: 10.1007/s00705-013-1645-3. [DOI] [PubMed] [Google Scholar]
- 37.Thomas L, Verlaeten O, Cabié A, Kaidomar S, Moravie V, Martial J, Najioullah F, Plumelle Y, Fonteau C, Dussart P, Césaire R. Influence of the dengue serotype, previous dengue infection, and plasma viral load on clinical presentation and outcome during a dengue-2 and dengue-4 co-epidemic. Am J Trop Med Hyg. 2008;78:990–998. [PubMed] [Google Scholar]
- 38.Horstick O, Jaenisch T, Martinez E, Kroeger A, See LL, Farrar J, Ranzinger SR. Comparing the usefulness of the 1997 and 2009 WHO dengue case classification: a systematic literature review. Am J Trop Med Hyg. 2014;91:621–634. doi: 10.4269/ajtmh.13-0676. [DOI] [PMC free article] [PubMed] [Google Scholar]