Abstract
Oropouche virus (OROV), genus Orthobunyavirus, family Bunyaviridae, is an important cause of human illness in tropical South America. Herein, we report the isolation, complete genome sequence, genetic characterization, and phylogenetic analysis of an OROV species reassortant, Madre de Dios virus (MDDV), obtained from a sick monkey (Cebus olivaceus Schomburgk) collected in a forest near Atapirire, a small rural village located in Anzoategui State, Venezuela. MDDV is one of a growing number of naturally occurring OROV species reassortants isolated in South America and was known previously only from southern Peru.
Introduction
Viruses in the family Bunyaviridae are currently classified into five genera: Orthobunyavirus, Hantavirus, Nairovirus, Phlebovirus, and Tospovirus.1 The Orthobunyavirus genus includes a number of zoonotic pathogens transmitted by arthropod vectors like culicoides midges, mosquitoes, phlebotomine sandflies, and ticks; it is the largest of the five genera within the family.2 Orthobunyaviruses are enveloped viruses with negative sense RNA genomes consisting of three segments: small (S), medium (M), and large (L). The S segment encodes the nucleocapsid and a small nonstructural protein, NSs. The M segment encodes the glycoproteins Gn and Gc, whereas the L segment encodes the viral polymerase.3
Oropouche virus (OROV) is a species within the Simbu serogroup of the Orthobunyavirus genus, and it causes a febrile illness in humans characterized by headache, dizziness, weakness, myalgia, and arthralgia.4–6 OROV was first isolated from a pool of Coquillettidia venezuelensis mosquitoes collected in Trinidad,7 and later from Aedes (Ochlerotatus) serratus and Culex quinquefasciatus mosquitoes,8 and frequently from the midge Culicoides paraensis in Brazil.7,9,10
The virus has also been isolated from several wild mammals including a three-toed sloth, Bradypus tridactylus11 and the marmoset, Callithrix penicillata (Nunes and others.12 Because of the clinical similarity of OROV infection to other endemic arboviral illness diseases such as dengue, Mayaro, chikungunya, and Zika fevers, and the paucity of cases confirmed by laboratory tests, the true public health burden of OROV infection in South American remains unclear.
Genetic reassortment among orthobunyaviruses of the same serogroup occurs frequently in nature and has led to the emergence of new viruses, occasionally with increased pathogenicity.13 On the basis of genetic and antigenic analyses, several reassortant viruses involving OROV and other unknown Simbu serogroup viruses have been described. These include Iquitos virus (IQTV), Madre de Dios virus (MDDV), and Perdões virus (PDEV), which contain the S and L segments of OROV, and the M segments of other still unrecognized Simbu serogroup viruses.14–16
Herein, we report the complete genome sequence of an isolate of an OROV reassortant obtained from a moribund monkey (Cebus olivaceus Schomburgk) collected in Venezuela during an epizootic of monkey deaths in 2010. This is the first report of the isolation, complete genome sequence, and evidence of an OROV reassortment event in Venezuela. We also discuss the importance of this evolutionary mechanism in terms of the epidemiology of OROV.
Materials and Methods
Study site and epizootic background.
Atapirire village is located in the southeastern part of Venezuela, within Anzoategui State, Miranda municipality. Atapirire lies 51 km south of El Tigre city and 96 km north of the largest city in the region, Ciudad Bolivar, Capitol of Bolivar State, which borders Brazil (Figure 1 ). Atapirire is a small rural village with a population of ∼800, surrounded by gallery and secondary forests in close proximity to the Orinoco river. The region is part of the biogeographic Central Llanos (lowland plains or savannas) of Venezuela. The llanos are part of the Orinoco river basin; the river separates this biogeographic region from the Guiana Shield and Amazonian regions in southern Venezuela, adjacent to the borders with Colombia and Brazil (Figure 1).
Figure 1.
Geographic location of the epizootic and source of INHRR 17a-10 isolate. (A) Venezuela location in the geographical context of South America. (B) Political map of Venezuela showing location of the outbreak, Atapirire village, Municipality of Miranda, Anzoategui State, C. MODIS image of central plains (Llanos) showing forest patches los Llanos surrounding the urban-rural village, the main cities near Atapirire and the Orinoco river (MODIS VCF. 2010, percent tree cover, Townsend and others, 2011). Bright green color = tree cover > 50%.
In August of 2010, an epizootic of illness among sylvatic monkeys near Atapirire was reported to the Central Office of Environmental Health (COEH), and a field commission was sent to investigate. Given the difficult access to the forests around Atapirire and the delay in outbreak notification, carcasses of many dead monkeys were observed upon arrival of the field team. Two moribund monkeys were found: a white-faced monkey (C. olivaceus) and red howler monkey (Alouatta seniculus Linnaeus). COEH personnel are authorized by the Venezuelan Ministry of Health to collect samples and euthanize sick monkeys in situations like this, as part of the national yellow fever surveillance program. According to protocol, the monkeys were euthanized to obtain tissue samples, which were sent immediately to the National Institute of Hygiene Rafael Rangel (INHRR) in Caracas.
Virus isolation and identification.
At the INHRR, tissue samples from both sick monkeys were first tested by reverse transcription polymerase chain reaction for yellow fever virus (YFV) and yielded a negative result. Tissue homogenates were also inoculated into flask cultures of Vero cells. A 20% tissue homogenate (w/v) of lung and liver samples of each animal was prepared in minimum essential medium supplemented with 2% fetal bovine serum and antibiotics (200 mg of streptomycin and 200 U/mL of penicillin). A total of 100 μL of the filtered homogenate was inoculated into flasks of confluent African green monkey kidney (Vero E6) cells, as previously described.15,17 Vero cell cultures were maintained at 37°C, and were examined daily for 7 days for evidence of viral cytopathic effects (CPEs). CPE was observed in a culture of a lung homogenate from the C. olivaceus, identified as INHRR 17a-10. Spot slides of the infected Vero cells were subsequently prepared, and an indirect immunofluorescence assay was performed using polyclonal broadly reacting antibodies against alphaviruses, flaviviruses, and bunyaviruses provided by the Centers for Disease Control and Prevention, Fort Collins, CO (Supplemental Table 1). In 2010, there also were concurrent outbreaks of YFV infection in monkeys, and Mayaro virus (MAYV) infection in humans in other regions of Venezuela.18 After confirming that the etiologic agent from monkey INHRR 17a-10 was in fact a bunyavirus and not YFV or MAYV, OROV-specific polyclonal mouse immune ascitic fluids were used to identify the agent.
RNA extraction, genome sequencing, and assembling.
Total RNA was isolated from the supernatant of infected Vero-E6 cells using the Qiamp Viral RNA minikit (Qiagen, Valencia, CA), according to the manufacturer's instructions. The RNA was used for genome sequencing utilizing the ion semiconduction method implemented in the Ion Torrent PGM device,19 as previously described.20 Genome assembly was carried out using a de novo assembler strategy implemented in MIRA software v.4.9.2 (Bastien Chevreux, San Francisco, CA).21 Final contigs (assembled with at least five reads) were inspected for quality and used to reconstruct the RNA segments. Terminal noncoding sequences (3′ and 5′ noncoding region [NCR]) for the virus isolate INHRR 17a-10 were obtained using both the 3′ and 5′ rapid amplification of cDNA ends method, as previously described,22 using a specific set of primers (Supplemental Table 2). Complete sequences for the S, M, and LRNAs of INHRR 17a-10 were deposited in the GenBank database (from KJ866389-R1 to KJ866391-R1).
Genome characterization.
The genome size, open reading frame (ORF) descriptions, 5′ and 3′ terminal NCRs, as well as conserved motifs, glycosylation sites, cysteine residues, and cleavage sites were determined using the Geneious v R7,23 InterProScan (http://www.ebi.ac.uk/Tools/pfa/iprscan5/), and NetNGlyc v.1.0 Server (http://www.cbs.dtu.dk/services/NetNGlyc/). RNA secondary structure (termini cyclization) was predicted, using the RNA fold structure application available on Geneious v.R7 software.23
Genetic variability and phylogenetic analyses.
Genetic variability was determined using the complete ORF sequences for the S, M, and LRNA segments of virus isolate INHRR 17a-10 and other viruses belonging to the Simbu group (Supplemental Table 3) using the multiple sequencing alignment approach implemented in Geneiuos vR7 software,23 which estimates the evolutionary divergence between sequences (number of amino acid or nucleotide substitutions per site between sequences). Analyses were conducted using the JTT matrix-based model. The rate of variation among sites was modeled with a gamma distribution (shape parameter = 1). The genetic relationships were also assessed by estimating the genetic divergences and plotting the results as a box-plot graphic using the R core team software (http://www.R-project.org). Cutoff values were established according to Williamson.24
Phylogenetic reconstructions were made, using the complete coding sequences for the N, NSs, Gn, Gc, and polymerase genes. Initially, the appropriate DNA substitution model was determined by the RAxML v.8 software (Alexandros Stamatakis, Lausanne, Switzerland), and was used to construct the phylogenetic relationship with complete ORF sequences available for other selected orthobunyaviruses belonging to the Simbu serogroup. Bunyamwera virus (BUNV) was used as the outgroup25 (Supplemental Table 3). Both maximum likelihood (ML) and Bayesian methods were performed. For ML, trees were constructed using the RAxML v.8 software, whereas Bayesian analyses were implemented in BEAUTi and the BEAST programs (Alexei J. Drummond, Marc A. Suchard, Dong Xie, and Andrew Rambaut, Oxford University, Oxford, United Kingdom).26,27 Phylogenetic trees were selected according to the highest probabilistic values and visualized using Figtree (Andrew Rambaut, University of Edinburgh, Edinburgh, United Kingdom).28
Analysis of genetic reassortment.
Evidence of genome reassortment was evaluated using the complete coding sequences for the N, M polyprotein, and polymerase gene of the INHRR 17a-10 isolate together with selected members of the Simbu serogroup. Bootscan analysis was performed with Simplot software.29 Concatenated coding regions (S, M, and LRNAs) of INHRR 17a-10 virus were compared with concatenated homologous sequences of closely related viruses within the Simbu serogroup. The analysis was conducted in a screenshot window of 200 nucleotides (nt) and along the genomes (X-axis); values for phylogenetic permutation trees or PPTV (percentage of phylogenetic similarity in a given genomic position) was expressed in percentages along the Y-axis and evaluated as high (PPTV > 90%), medium (50% > PPTV > 90%), and low (PPTV < 50) values. Genome reassortment was considered when PPTV was equal or higher than 90% across the entire genome segment (genome coverage above 95%).
Results
Nucleotide sequence and protein prediction.
Complete genome sequences were obtained for all three RNA segments of the INHRR 17a-10 isolate. The SRNA was 949 nt (220 × coverage), the MRNA was 4,395 nt (105 × coverage), and LRNA was 6,850 nt in length (95 × coverage). After the ORF predictions, six coding regions were observed: N and NSs (S RNA segment), M polyprotein (MRNA), and L polyprotein (LRNA). The evaluation of the gene products depicted six proteins: three structural (N, Gn, and Gc) and three nonstructural (NSs, NSm, and L). Table 1 shows the genomic organization and predicted ORFs for INHRR 17a-10 in comparison to other Simbu viruses.
Table 1.
Genome organization of INHRR 17a-10 isolate in comparison to other Simbu viruses according to strain, 5′ and 3′ NCR, RNA segments, genes, and protein sizes
| RNA segment | Virus | Strain | Genome regions (nt/aa) | Total lenght | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 5′–NNNNNNNN | N | NSs | M polyprotein | L polyprotein | 3′-NNNNNNN | ||||||
| SRNA | INHRR 17a-10 | TVP 19255 | 44 | AGTAGTGTACT-CCAC… | 696 (231aa) | 279 (92aa) | NA | NA | 209 | …TGGGAGCACACTACT | 949 |
| MIS 0397 | TVP 19261 | 44 | AGTAGTGTACT-CCAC… | 696 (231aa) | 279 (92aa) | NA | NA | 203 | …TGGGAGCACACTACT | 943 | |
| PDEV | BeAn790177 | 44 | AGTAGTGTACT-CCAC… | 696 (231aa) | 279 (92aa) | NA | NA | 207 | …TGGGAGCACACTACT | 947 | |
| OROV | BEAN 19991 | 44 | AGTAGTGTACT-CCAC… | 696 (231aa) | 279 (92aa) | NA | NA | 218 | …TGGGAGCACACTACT | 958 | |
| JTBV | BeAn 423380 | 44 | AGTAGTGTACT-CCAC… | 696 (231aa) | 279 (92aa) | NA | NA | 200 | …TGGGAGCACACTACT | 940 | |
| SIMV | SA Ar 53 | 33 | AGTAGTGTACT-CCAC… | 702 (233aa) | 276 (91aa) | NA | NA | 134 | …TGGGAGCACACTACT | 860 | |
| AKAV | OBE-1 | 33 | AGTAGTGTACT-CCAC… | 702 (233 aa) | 276 (91aa) | NA | NA | 123 | …TGGGAGCACACTACT | 858 | |
| Mean | 922 | ||||||||||
| MRNA | INHRR 17a-10 | TVP 19255 | 30 | AGTAGTGTACTACCA… | NA | NA | 4.272 (1.423aa) | NA | 108 | …TGGTAGCACACTACT | 4.395 |
| MIS 0397 | TVP 19261 | 30 | AGTAGTGTACTACCA… | NA | NA | 4.257 (1.418aa) | NA | 92 | …TGGTAGCACACTACT | 4.379 | |
| PDEV | BeAn790177 | 23 | AGTAGTGTACTACCA… | NA | NA | 4257 (1.418aa) | NA | 138 | …TGGTAGCACACTACT | 4.418 | |
| OROV | BEAN 19991 | 31 | AGTAGTGTACTACCA… | NA | NA | 4.263 (1.420aa) | NA | 91 | …TGGTAGCACACTACT | 4.385 | |
| JTBV | BeAn 423380 | 23 | AGTAGTGTACTCCCA… | NA | NA | 4.266 (1.421aa) | NA | 114 | …TGGTAGCACACTACT | 4.403 | |
| SIMV | SA Ar 53 | 23 | AGTAGTGAACTACCA… | NA | NA | 4.230 (1.409aa) | NA | 154 | …TGGTAGCACACTACT | 4.407 | |
| AKAV | OBE-1 | 22 | AGTAGTGAACTACCA… | NA | NA | 4.206 (1.401aa) | NA | 81 | …TGGTAGAACACTACT | 4.309 | |
| Mean | 4385 | ||||||||||
| LRNA | INHRR 17a-10 | TVP 19255 | 44 | AGTAGTGTACTCCTA… | NA | NA | NA | 6.756 (2.251 aa) | 50 | …TAGGAGCACACTACT | 6.850 |
| MIS 0397 | TVP 19261 | 43 | AGTAGTGTACTCCTA… | NA | NA | NA | 6.759 (2.252 aa) | 50 | …TAGGAGCACACTACT | 6.852 | |
| PDEV | BeAn790177 | 43 | AGTAGTGTACTCCTA… | NA | NA | NA | 6.759 (2.252 aa) | 50 | …TAGGAGCACACTACT | 6.852 | |
| OROV | BEAN 19991 | 43 | AGTAGTGTACTCCTA… | NA | NA | NA | 6.759 (2.252 aa) | 50 | …TAGGAGCACACTACT | 6.852 | |
| JTBV | BeAn 423380 | 42 | AGTAGTGTACTCCTA… | NA | NA | NA | 6.759 (2.252 aa) | 47 | …TAGGAGCACACTACT | 6.848 | |
| SIMV | SA Ar 53 | 28 | AGTAGTGTACCCCTA… | NA | NA | NA | 6.762 (2.253 aa) | 71 | …TAGGGGCACACTACT | 6.861 | |
| AKAV | OBE-1 | 42 | AGTAGTGTACTCCTA… | NA | NA | NA | 6.759 (2.252 aa) | 47 | …TAGGAGCACACTACT | 6.848 | |
| Mean | 6.852 | ||||||||||
aa = amino acid; AKAV = Akabane virus; JTBV = Jatobal virus; NA = not applied; NCR = noncoding region; nt = nucleotide; OROV = Oropouche virus; PDEV = Perdões virus; SIMV = Simbu virus.
SRNA, MRNA, and LRNA are small, medium, and large segments of RNA, respectively.
Cysteine residues, glycosylation, and M polyprotein cleavage sites.
A total of 106 cysteine residues was found for N (N = 1), NSs (N = 0), Gn (N = 17), NSm (N = 8), Gc (N = 40), and L (N = 36) proteins. Furthermore, three N-glycosylation sites (Gn = 2; NSm = 0; Gc = 1) were observed, as well as M polyprotein cleavage sites were assessed for the INHRR 17a-10 isolate.
5′–3′ Terminal sequences and predicted RNA folding structures.
Terminal sequences of S, M, and LRNAs for the isolate INHRR 17a-10 showed highly conserved nucleotide sequences as shown in Table 1. RNA folding structures revealed evidence of sequence complementarity (Supplemental Figure 1).
Conserved motifs.
As observed with other orthobunyavirus polymerases, conserved motifs designated as I, II, III, and IV were observed.30 Furthermore, the PreA (KGQKTAKDREIFLGEFEAKMCLYLVERIAK), A (GLKIEINADMSKWSAQDV), B (TVEIKRNWLQGNLNYTSSYLHSC), C (EALVNSMVHSDDNQT), D (GNQANMKKTYLT), and E (IKEFVSLFNIHGEPFSIYG) domains were also assessed for the virus INHRR 17a-10. Our analyses involving the M segment polyprotein indicated the presence of a Zinc finger motif within the Gn protein. Within the NSm protein, the domains I to VI were observed. There was high conservation noted in domain I, in comparison to BUNV in domain I, and only moderate conservation for domains II, IV, and V. Low conservation was observed in domain III for INHRR 17a-10 virus as TNKCGTCICGC. At the Gc protein level, the highly conserved transmembrane region (amino acid positions 1,060 and 1,086) was described, as well as the fusion peptide motif WGCEExGCLAxxxGCV(F/Y)GSCQD. For the N gene, amino acid residues involved in the bunyavirus ribonucleoprotein packaging process (P113, G138, Y86 e I231), RNA synthesis (F18, F145, L161, Y186, L82, K80, Y186, W194, M195, F226), and virus RNA ligation to viral proteins (R41, R95 e K51) were observed.
Analysis of evolutionary divergence.
A matrix of nucleotide and amino acid similarities, based on the complete ORFs for INHRR 17a-10 and selected members of the Simbu serogroup, revealed different degrees of genetic relatedness according to the RNA segment studied. For the S and LRNA segments, low means of evolutionary divergences were determined: ranging from 0.07 to 0.1 nucleotide substitutions/site, and from 0 to 0.03 amino acid substitutions/site, respectively. In the case of MRNA, the mean evolutionary divergence was estimated as 0.7 nucleotide substitutions/site and 0.65 amino acid substitutions/site. Minimal evolutionary divergences were observed with OROV isolates, and with MDDV isolate FMD1303 when comparing the MRNAs (Table 2).
Table 2.
Matrix of nucleotide and amino acidic evolutionary distances among INHRR 17a-10 isolate and other Simbu viruses (within the black frame box)
| Genome segment | Viruses | Pairwise distance (Nucleotide) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pairwise distance (amino acid) | ||||||||||||||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | |||
| SRNA | 1 | KP026181_Oropouche_TRVL9760 | – | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.069 | 0.385 | 0.496 | 0.498 | 0.490 | 0.421 | 0.506 | 0.497 | 0.489 | 0.494 | 0.494 | 0.960 | 1.670 |
| 2 | KP052852_Oropouche_BeAn19991 | 0.015 | – | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.069 | 0.385 | 0.496 | 0.498 | 0.490 | 0.421 | 0.506 | 0.497 | 0.489 | 0.494 | 0.494 | 0.960 | 1.670 | |
| 3 | MIS-0397 | 0.063 | 0.049 | – | 0.000 | 0.000 | 0.000 | 0.000 | 0.069 | 0.385 | 0.496 | 0.498 | 0.490 | 0.421 | 0.506 | 0.497 | 0.489 | 0.494 | 0.494 | 0.960 | 1.670 | |
| 4 | KF697144_Iquitos_IQT9924 | 0.063 | 0.049 | 0.000 | – | 0.000 | 0.000 | 0.000 | 0.069 | 0.385 | 0.496 | 0.498 | 0.490 | 0.421 | 0.506 | 0.497 | 0.489 | 0.494 | 0.494 | 0.960 | 1.670 | |
| 5 | KP691626_Perdoes_BeAn_789726 | 0.049 | 0.039 | 0.037 | 0.037 | – | 0.000 | 0.000 | 0.069 | 0.385 | 0.496 | 0.498 | 0.490 | 0.421 | 0.506 | 0.497 | 0.489 | 0.494 | 0.494 | 0.960 | 1.670 | |
| 6 | TVP_19255_INHRR 17a-10 | 0.094 | 0.090 | 0.095 | 0.095 | 0.088 | – | 0.000 | 0.069 | 0.385 | 0.496 | 0.498 | 0.490 | 0.421 | 0.506 | 0.497 | 0.489 | 0.494 | 0.494 | 0.960 | 1.670 | |
| 7 | KF697146_Madre_de_Dios_FMD1303 | 0.094 | 0.090 | 0.095 | 0.095 | 0.088 | 0.000 | – | 0.069 | 0.385 | 0.496 | 0.498 | 0.490 | 0.421 | 0.506 | 0.497 | 0.489 | 0.494 | 0.494 | 0.960 | 1.670 | |
| 8 | JQ675601_Jatobal_strain_BeAN_423380 | 0.202 | 0.197 | 0.212 | 0.212 | 0.212 | 0.198 | 0.198 | – | 0.408 | 0.501 | 0.504 | 0.497 | 0.422 | 0.516 | 0.494 | 0.486 | 0.490 | 0.490 | 1.014 | 1.616 | |
| 9 | HE795089_Aino | 0.561 | 0.541 | 0.544 | 0.544 | 0.563 | 0.524 | 0.524 | 0.535 | – | 0.592 | 0.596 | 0.588 | 0.616 | 0.648 | 0.694 | 0.700 | 0.700 | 0.700 | 1.031 | 1.860 | |
| 10 | HE800143_Shuni | 0.542 | 0.542 | 0.536 | 0.536 | 0.569 | 0.496 | 0.496 | 0.526 | 0.066 | – | 0.004 | 0.009 | 0.203 | 0.219 | 0.251 | 0.250 | 0.255 | 0.255 | 0.918 | 1.602 | |
| 11 | HE795095_Peaton | 0.561 | 0.560 | 0.586 | 0.586 | 0.601 | 0.555 | 0.555 | 0.526 | 0.102 | 0.095 | – | 0.004 | 0.209 | 0.225 | 0.245 | 0.244 | 0.249 | 0.249 | 0.913 | 1.605 | |
| 12 | HE795092_Douglas | 0.499 | 0.481 | 0.507 | 0.507 | 0.520 | 0.486 | 0.486 | 0.463 | 0.299 | 0.298 | 0.311 | – | 0.203 | 0.219 | 0.239 | 0.238 | 0.244 | 0.244 | 0.904 | 1.624 | |
| 13 | HE795104_Sathuperi | 0.556 | 0.551 | 0.548 | 0.548 | 0.573 | 0.526 | 0.526 | 0.486 | 0.302 | 0.314 | 0.314 | 0.081 | – | 0.225 | 0.233 | 0.239 | 0.238 | 0.238 | 1.040 | 1.716 | |
| 14 | HE795107_Shamonda | 0.517 | 0.511 | 0.524 | 0.524 | 0.528 | 0.502 | 0.502 | 0.478 | 0.336 | 0.316 | 0.316 | 0.080 | 0.081 | – | 0.248 | 0.252 | 0.257 | 0.257 | 0.963 | 1.786 | |
| 15 | JX853181_Schmallenberg | 0.507 | 0.502 | 0.514 | 0.514 | 0.518 | 0.502 | 0.502 | 0.469 | 0.330 | 0.316 | 0.322 | 0.090 | 0.079 | 0.024 | – | 0.004 | 0.009 | 0.009 | 0.996 | 1.702 | |
| 16 | NC_018477_Simbu | 0.513 | 0.522 | 0.510 | 0.510 | 0.518 | 0.507 | 0.507 | 0.401 | 0.306 | 0.315 | 0.322 | 0.259 | 0.285 | 0.297 | 0.297 | – | 0.004 | 0.004 | 0.994 | 1.698 | |
| 17 | NC_009896_Akabane | 0.528 | 0.509 | 0.488 | 0.488 | 0.514 | 0.509 | 0.509 | 0.491 | 0.326 | 0.337 | 0.338 | 0.301 | 0.293 | 0.320 | 0.324 | 0.296 | – | 0.000 | 0.999 | 1.696 | |
| 18 | JX983192_Oya | 0.447 | 0.451 | 0.477 | 0.477 | 0.470 | 0.473 | 0.473 | 0.457 | 0.602 | 0.545 | 0.568 | 0.632 | 0.688 | 0.671 | 0.689 | 0.563 | 0.602 | – | 0.999 | 1.696 | |
| 19 | HM627177_Leanyer | 0.939 | 0.938 | 0.978 | 0.978 | 0.918 | 0.949 | 0.949 | 0.875 | 0.878 | 0.819 | 0.819 | 0.837 | 0.836 | 0.851 | 0.822 | 0.905 | 0.904 | 1.054 | – | 1.632 | |
| 20 | NC_001927_Bunyamwera | 1.576 | 1.524 | 1.555 | 1.555 | 1.631 | 1.668 | 1.668 | 1.620 | 1.410 | 1.538 | 1.611 | 1.627 | 1.590 | 1.790 | 1.788 | 1.577 | 1.754 | 1.778 | 1.580 | – | |
| Pairwise distance (Nucleotide) | ||||||||||||||||||||||
| Pairwise distance (amino acid) | ||||||||||||||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | |||
| MRNA | 1 | KP026181_Oropouche_TRVL9760 | – | 0.010 | 0.658 | 0.658 | 1.177 | 0.666 | 0.666 | 1.183 | 2.049 | 2.077 | 2.344 | 2.193 | 2.150 | 2.352 | 2.174 | 1.896 | 2.541 | 1.036 | 2.060 | 2.519 |
| 2 | KP052852_Oropouche_BeAn19991 | 0.022 | – | 0.657 | 0.657 | 1.180 | 0.668 | 0.668 | 1.180 | 2.053 | 2.090 | 2.347 | 2.204 | 2.161 | 2.362 | 2.185 | 1.902 | 2.540 | 1.041 | 2.064 | 2.505 | |
| 3 | MIS-0397 | 0.764 | 0.763 | – | 0.000 | 1.142 | 0.171 | 0.171 | 1.129 | 1.994 | 1.983 | 2.337 | 2.196 | 2.149 | 2.377 | 2.177 | 1.944 | 2.415 | 1.033 | 2.102 | 2.372 | |
| 4 | KF697144_Iquitos_IQT9924 | 0.764 | 0.763 | 0.000 | – | 1.142 | 0.171 | 0.171 | 1.129 | 1.994 | 1.983 | 0.764 | 0.763 | 0.000 | – | 1.142 | 0.171 | 0.171 | 1.129 | 1.994 | 1.983 | |
| 5 | KP691626_Perdoes_BeAn_789726 | 1.077 | 1.075 | 1.120 | 1.120 | – | 1.188 | 1.188 | 0.304 | 2.148 | 2.103 | 2.279 | 2.189 | 2.178 | 2.334 | 2.160 | 1.958 | 2.389 | 1.065 | 2.073 | 2.480 | |
| 6 | TVP_19255_INHRR 17a-10 | 0.791 | 0.791 | 0.336 | 0.336 | 1.059 | – | 0.000 | 1.165 | 1.974 | 1.996 | 2.385 | 2.190 | 2.145 | 2.398 | 2.160 | 1.938 | 2.412 | 1.033 | 2.152 | 2.367 | |
| 7 | KF697146_Madre_de_Dios_FMD1303 | 0.791 | 0.791 | 0.336 | 0.336 | 1.059 | 0.003 | – | 1.165 | 1.974 | 1.996 | 2.385 | 2.190 | 2.145 | 2.398 | 2.160 | 1.938 | 2.412 | 1.033 | 2.152 | 2.367 | |
| 8 | JQ675601_Jatobal_strain_BeAN_423380 | 1.047 | 1.055 | 1.100 | 1.100 | 0.474 | 1.056 | 1.056 | – | 2.077 | 2.007 | 2.346 | 2.208 | 2.191 | 2.439 | 2.152 | 1.934 | 2.398 | 1.084 | 2.054 | 2.396 | |
| 9 | HE795089_Aino | 1.685 | 1.644 | 1.659 | 1.659 | 1.731 | 1.646 | 1.646 | 1.459 | – | 0.315 | 2.062 | 0.823 | 0.822 | 2.110 | 0.830 | 1.038 | 2.090 | 2.161 | 2.559 | 2.750 | |
| 10 | HE800143_Shuni | 1.607 | 1.628 | 1.494 | 1.494 | 1.572 | 1.651 | 1.651 | 1.415 | 0.477 | – | 2.151 | 0.854 | 0.846 | 2.077 | 0.847 | 1.062 | 2.014 | 2.178 | 2.551 | 2.672 | |
| 11 | HE795095_Peaton | 1.727 | 1.737 | 1.803 | 1.803 | 1.597 | 1.794 | 1.794 | 1.684 | 1.767 | 1.675 | – | 2.002 | 2.019 | 1.275 | 2.048 | 2.030 | 1.122 | 2.328 | 2.702 | 2.912 | |
| 12 | HE795092_Douglas | 1.817 | 1.814 | 1.729 | 1.729 | 1.710 | 1.794 | 1.794 | 1.701 | 0.859 | 0.854 | 1.611 | – | 0.086 | 2.049 | 0.122 | 1.068 | 2.053 | 2.128 | 2.578 | 2.757 | |
| 13 | HE795104_Sathuperi | 1.760 | 1.756 | 1.758 | 1.758 | 1.723 | 1.741 | 1.741 | 1.659 | 0.879 | 0.879 | 1.681 | 0.187 | – | 2.040 | 0.103 | 1.066 | 2.083 | 2.083 | 2.533 | 2.716 | |
| 14 | HE795107_Shamonda | 1.790 | 1.786 | 1.885 | 1.885 | 2.007 | 1.963 | 1.963 | 1.818 | 1.838 | 1.702 | 1.220 | 1.668 | 1.675 | – | 2.050 | 1.996 | 1.211 | 2.469 | 2.690 | 2.847 | |
| 15 | JX853181_Schmallenberg | 1.704 | 1.720 | 1.715 | 1.715 | 1.728 | 1.694 | 1.694 | 1.644 | 0.884 | 0.886 | 1.722 | 0.265 | 0.257 | 1.701 | – | 1.044 | 2.092 | 2.079 | 2.589 | 2.745 | |
| 16 | NC_018477_Simbu | 1.546 | 1.530 | 1.715 | 1.715 | 1.430 | 1.606 | 1.606 | 1.453 | 0.962 | 0.967 | 1.714 | 1.049 | 1.036 | 1.610 | 1.051 | – | 2.062 | 1.940 | 2.450 | 2.630 | |
| 17 | NC_009896_Akabane | 1.863 | 1.853 | 1.917 | 1.917 | 1.908 | 1.920 | 1.920 | 1.826 | 1.896 | 1.722 | 1.148 | 1.738 | 1.632 | 1.220 | 1.753 | 1.895 | – | 2.449 | 2.643 | 2.926 | |
| 18 | JX983192_Oya | 1.061 | 1.060 | 1.000 | 1.000 | 1.066 | 1.047 | 1.047 | 0.997 | 1.704 | 1.597 | 1.830 | 1.663 | 1.673 | 1.932 | 1.641 | 1.557 | 1.885 | – | 1.976 | 2.440 | |
| 19 | HM627177_Leanyer | 1.729 | 1.735 | 1.785 | 1.785 | 1.639 | 1.937 | 1.937 | 1.630 | 2.035 | 1.972 | 1.948 | 1.923 | 1.948 | 2.205 | 1.977 | 1.881 | 2.137 | 1.687 | – | 2.669 | |
| 20 | NC_001927_Bunyamwera | 1.848 | 1.815 | 1.815 | 1.815 | 1.935 | 1.910 | 1.910 | 1.812 | 2.057 | 1.847 | 2.079 | 2.179 | 2.012 | 2.121 | 2.093 | 1.808 | 2.060 | 1.826 | 2.052 | – | |
| LRNA | 1 | KP026181_Oropouche_TRVL9760 | – | 0.0084 | 0.0151 | 0.0151 | 0.0164 | 0.0544 | 0.0532 | 0.1519 | 0.7766 | 0.7647 | 0.7766 | 0.8072 | 0.7998 | 0.8071 | 0.8052 | 0.7775 | 0.7731 | 0.5276 | 0.7644 | 1.1467 |
| 2 | KP052852_Oropouche_BeAn19991 | 0.0228 | – | 0.0156 | 0.0156 | 0.0178 | 0.0535 | 0.0523 | 0.1488 | 0.7737 | 0.7618 | 0.7742 | 0.8000 | 0.7932 | 0.8024 | 0.8005 | 0.7723 | 0.7704 | 0.5241 | 0.7599 | 1.1398 | |
| 3 | MIS-0397 | 0.1158 | 0.1168 | – | 0.0000 | 0.0075 | 0.0511 | 0.0489 | 0.1488 | 0.7693 | 0.7586 | 0.7709 | 0.8061 | 0.7933 | 0.8018 | 0.8019 | 0.7704 | 0.7680 | 0.5201 | 0.7549 | 1.1412 | |
| 4 | KF697144_Iquitos_IQT9924 | 0.1158 | 0.1168 | 0.0000 | – | 0.0075 | 0.0511 | 0.0489 | 0.1488 | 0.7693 | 0.7586 | 0.7709 | 0.8061 | 0.7933 | 0.8018 | 0.8019 | 0.7704 | 0.7680 | 0.5201 | 0.7549 | 1.1412 | |
| 5 | KP691626_Perdoes_BeAn_789726 | 0.1212 | 0.1252 | 0.0548 | 0.0548 | – | 0.0530 | 0.0518 | 0.1486 | 0.7724 | 0.7629 | 0.7747 | 0.8045 | 0.7949 | 0.8001 | 0.7995 | 0.7704 | 0.7695 | 0.5209 | 0.7583 | 1.1419 | |
| 6 | TVP_19255_INHRR 17a-10 | 0.2274 | 0.2245 | 0.2327 | 0.2327 | 0.2443 | – | 0.0018 | 0.1490 | 0.7887 | 0.7793 | 0.7836 | 0.7994 | 0.7976 | 0.8057 | 0.8028 | 0.7686 | 0.7779 | 0.5308 | 0.7649 | 1.1559 | |
| 7 | KF697146_Madre_de_Dios_FMD1303 | 0.2274 | 0.2245 | 0.2327 | 0.2327 | 0.2443 | 0.0000 | – | 0.1472 | 0.7837 | 0.7743 | 0.7786 | 0.7943 | 0.7925 | 0.8006 | 0.7978 | 0.7637 | 0.7730 | 0.5271 | 0.7610 | 1.1483 | |
| 8 | JQ675601_Jatobal_strain_BeAN_423380 | 0.3744 | 0.3650 | 0.3703 | 0.3703 | 0.3816 | 0.3743 | 0.3743 | – | 0.7809 | 0.7751 | 0.7619 | 0.7947 | 0.7904 | 0.7962 | 0.7883 | 0.7664 | 0.7960 | 0.5279 | 0.7761 | 1.1493 | |
| 9 | HE795089_Aino | 0.8489 | 0.8407 | 0.8263 | 0.8263 | 0.8399 | 0.8289 | 0.8289 | 0.8197 | – | 0.0459 | 0.1232 | 0.4880 | 0.4885 | 0.4864 | 0.4814 | 0.4339 | 0.4942 | 0.8162 | 0.9124 | 1.2299 | |
| 10 | HE800143_Shuni | 0.8239 | 0.8265 | 0.8402 | 0.8402 | 0.8417 | 0.8459 | 0.8459 | 0.8244 | 0.2030 | – | 0.1204 | 0.4881 | 0.4873 | 0.4909 | 0.4851 | 0.4350 | 0.4869 | 0.8063 | 0.9171 | 1.2387 | |
| 11 | HE795095_Peaton | 0.8374 | 0.8347 | 0.8207 | 0.8207 | 0.8331 | 0.8450 | 0.8450 | 0.8331 | 0.3232 | 0.3243 | – | 0.4900 | 0.4909 | 0.4902 | 0.4823 | 0.4366 | 0.4865 | 0.8280 | 0.9451 | 1.2474 | |
| 12 | HE795092_Douglas | 0.8657 | 0.8552 | 0.8710 | 0.8710 | 0.8819 | 0.8596 | 0.8596 | 0.8780 | 0.6483 | 0.6244 | 0.6433 | – | 0.0373 | 0.0558 | 0.0512 | 0.4517 | 0.4453 | 0.8512 | 0.9236 | 1.2442 | |
| 13 | HE795104_Sathuperi | 0.8918 | 0.8801 | 0.8838 | 0.8838 | 0.8914 | 0.8745 | 0.8745 | 0.8761 | 0.6413 | 0.6377 | 0.6317 | 0.1692 | – | 0.0420 | 0.0412 | 0.4466 | 0.4337 | 0.8433 | 0.9101 | 1.2280 | |
| 14 | HE795107_Shamonda | 0.8914 | 0.8875 | 0.8646 | 0.8646 | 0.8797 | 0.8928 | 0.8928 | 0.8898 | 0.6562 | 0.6491 | 0.6471 | 0.2144 | 0.2086 | – | 0.0157 | 0.4541 | 0.4363 | 0.8440 | 0.9179 | 1.2257 | |
| 15 | JX853181_Schmallenberg | 0.8719 | 0.8635 | 0.8756 | 0.8756 | 0.8778 | 0.8726 | 0.8726 | 0.8746 | 0.6258 | 0.6412 | 0.6098 | 0.2127 | 0.2168 | 0.0824 | – | 0.4519 | 0.4359 | 0.8429 | 0.9173 | 1.2174 | |
| 16 | NC_018477_Simbu | 0.8691 | 0.8618 | 0.8486 | 0.8486 | 0.8572 | 0.8603 | 0.8603 | 0.8674 | 0.5780 | 0.5948 | 0.6002 | 0.5961 | 0.5971 | 0.6059 | 0.5936 | – | 0.4393 | 0.7958 | 0.9018 | 1.2326 | |
| 17 | NC_009896_Akabane | 0.8654 | 0.8536 | 0.8658 | 0.8658 | 0.8577 | 0.8511 | 0.8511 | 0.8945 | 0.6309 | 0.6577 | 0.6436 | 0.6157 | 0.6013 | 0.6082 | 0.5970 | 0.6001 | – | 0.8092 | 0.9311 | 1.1822 | |
| 18 | JX983192_Oya | 0.6833 | 0.6797 | 0.6823 | 0.6823 | 0.6914 | 0.6631 | 0.6631 | 0.6359 | 0.8666 | 0.8466 | 0.9053 | 0.8699 | 0.8896 | 0.8863 | 0.9045 | 0.8495 | 0.8790 | – | 0.8133 | 1.2055 | |
| 19 | HM627177_Leanyer | 0.8031 | 0.8070 | 0.7946 | 0.7946 | 0.7914 | 0.8110 | 0.8110 | 0.8217 | 0.9361 | 0.9522 | 0.9711 | 0.9170 | 0.8973 | 0.9223 | 0.9114 | 0.9088 | 0.9266 | 0.8683 | – | 1.2306 | |
| 20 | NC_001927_Bunyamwera | 1.1130 | 1.1012 | 1.1171 | 1.1171 | 1.1044 | 1.1339 | 1.1339 | 1.1277 | 1.1025 | 1.1396 | 1.1202 | 1.1231 | 1.1627 | 1.1639 | 1.1314 | 1.1626 | 1.1103 | 1.1441 | 1.1174 | – | |
SRNA, MRNA, and LRNA are small, medium, and large segments of RNA, respectively.
Nucleotide and amino acid genetic distances are highlighted in light blue color. Grey boxes represent no genetic distance between the same virus isolate.
Calculations were carried out based on the nucleotide and amino acid distances for the three RNA segments of the isolate INHRR 17a-10, and other selected members of the genus Orthobunyavirus, using pairwise distances; Simbu virus intragroup and intergroup distances are depicted in Supplemental Figure 2.
Phylogenetic analysis.
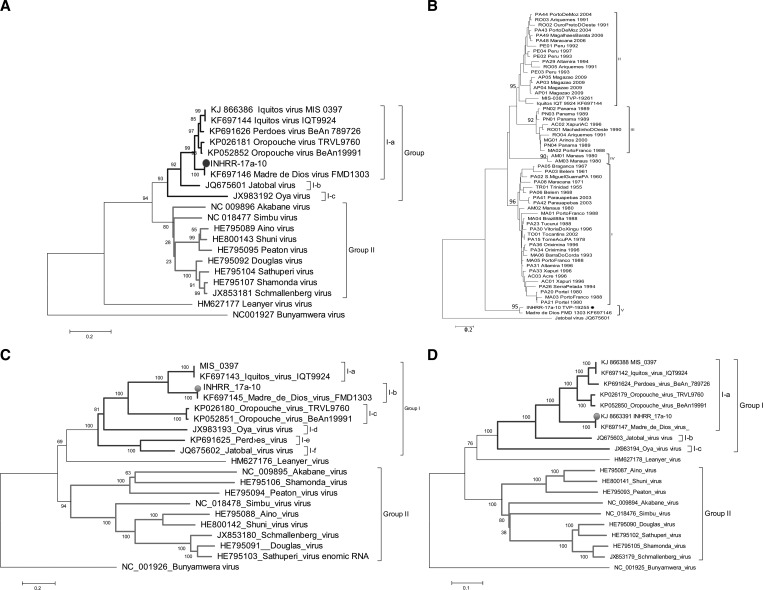
Regardless of the RNA segment analyzed, our phylogenetic reconstructions grouped the INHRR 17a-10 virus together with other members of the Simbu group, and depicted two major groups (I and II) corresponding to the main genetic clades of the Simbu virus group, genus Orthobunyavirus. For the SRNA (N gene), the INHRR 17a-10 isolate fell into the group I with Bayesian posterior probability (BPP) equal to 93, and more specifically within the subclade I-a (BPP = 92) together with MDDV (BPP = 100), and closely related to different OROV, PDEV, and IQTV SRNAs (BPP = 99) (Figure 2A ). A more specific analysis using 56 OROV, PPDV, and IQTV N genes available in the GenBank database, revealed that IHNRR 17a-10 is closely related to MDDV (Figure 2B). Analyses using complete M polyprotein also included the INHRR 17a-10 virus in group I (BBP = 100), clustering together with MDDV (BBP = 100) within the subgroup I-b, and separately from subgroups I-a (IQTV and MIS 0397) and I-c (OROV isolates) (Figure 2C). In case of L polyprotein, as observed in N gene analysis, the INHRR 17a-10 isolate showed a strong relationship to OROV with BPP equal to 100 (Figure 2D).
Figure 2.
Phylogenetic analysis using the entire (A, B) N, M polyprotein (C) and polymerase gene (D) coding regions of the INHRR 17a-10 virus and selected orthobunyaviruses using the Bayesian inference. Values over each tree node represent the Bayesian posterior probabilities expressed in percentage. Main phylogenetic groups (I and II) are highlighted within collared (blue and red) brackets. Subgroups are indicated as I-a to I-f. The INHRR 17a-10 isolate is indicated with a blue circle.
Genetic reassortment.
Analyses of reassortments (segment exchange), using the entire ORFs of INHRR 17a-10 and selected Simbu members demonstrated high phylogenetic permutation tree values (PPTV) for the SRNA (PPTV > 96% with OROV SRNA), and MDDV MRNA (PPTV > 95%), and moderate values (PPTV > 80%) for the LRNA (with OROV LRNA), suggesting that the INHH-17a-10 isolate is a reassortant virus involving OROV and MDDV (Supplemental Figure 3).
Discussion
The Brazilian Amazon is one of the world's richest ecosystems in terms of biodiversity; approximately 5,000 species of vertebrates, 50,000 species of insects, and 10 to 15 million plants and innumerable microorganisms (viruses, bacteria, and fungi) coexist.4,31–34 OROV is one of the most important arboviruses affecting humans in the Amazon region. More than 1,500,000 people have been infected with OROV in the Amazon region of Brazil, since it was first described in Brazil in 1961.6,11,35–38 Since the original detection and description of OROV in Trinidad in 1955,7 more than 30 outbreaks of Oropouche fever have been reported from Brazil, Peru, and Panama.6,35–38
Molecular studies, using sequence information for the genomic segments of OROV and other Simbu viruses, have provided a better understanding of the molecular epidemiology and evolution of viruses included in the OROV species and the Simbu virus serogroup.15,16,39,40
Our recovery of MDDV from a sick monkey is the first evidence that this virus occurs in Venezuela. Sick and dying monkeys in South America are usually associated with YFV infections.41 However, in the present case, the monkey was infected with an OROV reassortant. It is unknown whether INHRR 17a-10 virus was the cause of the severe illness in the animal, or if it was responsible for the other monkey deaths observed in the vicinity.
The INHRR 17a-10 virus has similar genomic organization and genetic characteristics to other orthobunyaviruses, including three genome segments with compatible size, functional ORFs (N, NSs, M polyprotein, and L polyprotein), structural (N, Gn, and Gc) and nonstructural proteins (NSs, NSm, and polymerase), conserved motifs, protein cleavage sites, cysteine residues, and glycosylation sites (Table 1).
RNA fold analysis has been used to determine the RNA curvature, structural stabilization, and prediction of complementary 5′–3′ terminal genomic regions over a given energy level for other RNA viruses.42–44 In the case of INHRR 17a-10, the RNA structures generated for the three segments (S, M, L) showed similar folding structures with a high complementarity level at the 3′–5′ ends. Together with RACE 5′ and 3′ termini recovering strategies, it indicates that the genome segments were complete (Supplemental Figure 1A). Regarding size heterogeneity, differences in 3′ termini were noted. We analyzed the 3′ ends of distinct OROV strains in comparison to INHRR 17a-10 virus (Supplemental Figure 1B), and observed that size heterogeneity is present among OROV SRNAs; however, these differences appear not to be related to geographic location, as observed previously among YFV strains.45 Further studies are necessary to evaluate if the 3′ NCR heterogeneity could be related to virus establishment, selection, and adaptive mechanisms to a given host.
Previous work involving the genetic characterization and phylogenetic analysis of Simbu serogroup viruses have provided a better understanding of molecular and evolutionary aspects of this group of viruses.30,46,47 Reassortment is a common mechanism described for orthobunyaviruses of the Bunyamwera, Wyeomyia, and Simbu serogroups.15,47–49 In the case of INHRR 17a-10 virus, we used extensive and robust analyses for testing the possibility of genetic reassortment of the isolate, including matrix of evolutionary distance, phylogenetic reconstructions, and Simplot method. Phylogenetic analyses indicated distinct origins for the S, M, and LRNA segments; the S and LRNAs were related to OROV, whereas the MRNA was related to MDDV (Figure 1). A more in-depth analysis using 95 sequences for the N gene of distinct OROV strains isolated in Brazil, Trinidad, Panamá, and Peru, indicated that INHRR 17-10a, although related to OROV, has a distinct SRNA segment from other available OROV sequences, clustering separately and together with MDDV (Figure 1B).
OROV is an important human pathogen, and it appears to be involved in different processes of genetic reassortment as a parental virus, contributing to the emergence of new human pathogens such as IQTV in Peru, PDEV in Brazil, and MDDV in Peru and Venezuela.14–16 Interestingly, PDEV was isolated from a dead marmoset (C. penicillata) collected in Minas Gerais State, in western Brazil in 2013.14 This is the same geographic area and host from which OROV was previously isolated in 2005.37
Matrices of evolutionary distances (Table 2), as well as box plots (Supplemental Figure 2), and Simplot (Supplemental Figure 3) analyses in our study indicate that INHHR 17a-10 represents a strain of MDDV, a previously characterized reassortant virus16 involving OROV and a still unknown Simbu member as parental viruses.
Systematic virus surveillance programs are essential for the evaluation of the true prevalence of a specific viral agent in a given geographic area. Surveillance programs have contributed substantially to virus discovery and detection of emerging pathogens in the Amazon region of Brazil and in other South American countries.4,6,37,38 Evidence of OROV circulation is periodically reported within endemic areas, especially in Brazil39 and sporadically in areas considered to be endemic or enzootic.37,50 The isolation of an OROV reassortant in Venezuela, in a biogeographic area completely distinct from the usual Amazonian endemic transmission region, indicates that the virus is present in other ecologic zones and this finding is of epidemiological importance.
The data presented here constitute the first isolation, molecular description, complete genome sequencing, genetic characterization, and evolutionary analyses of MDDV in Venezuela. MDDV has been associated with human illness in Peru. Further surveillance and molecular epidemiologic studies of OROV and OROV reassortants in tropical America are needed to allow us to better understand the bunyavirus biodiversity and its impact on human and animal health in the region.
Supplementary Material
Acknowledgments
We thank Cinda Martinez from Direccion General de Salud Ambiental, Ministry of Health, Venezuela. We also thank Barbara Johnson, from the CDC, Fort Collins, CO, for the donation of monoclonal antibodies used to diagnose OROV.
Footnotes
Financial support: This work was supported by a grant from FONACIT-Mision Ciencia-Venezuela (2008000911-4) to Juan-Carlos Navarro; the Western Regional Center of Excellence for Biodefense and Emerging Infectious Disease Research, National Institutes of Health (NIH) grant U54 AIO57156; NIH contract HHSN272202000040I/HHSN200004/D04; the Robert E. Shope International Fellowship in Infectious Diseases from the American Society of Tropical Medicine and Hygiene; CNPq (Brazilian National Council for Research and Development) grant no. 302032/2011-8. Albert J. Auguste was supported by the James W. McLaughlin endowment fund.
Authors' addresses: Juan-Carlos Navarro, Lab Biología de Vectores, Instituto de Zoología y Ecología Tropical, Universidad Central de Venezuela, Caracas, Venezuela, and Universidad Internacional SEK, Quito, Ecuador, E-mail: jcnavac@gmail.com. Dileyvic Giambalvo, Lab Biología de Vectores, Instituto de Zoología y Ecología Tropical, Universidad Central de Venezuela, Caracas, Venezuela, E-mail: dileyvic@gmail.com. Rosa Hernandez, Instituto Nacional de Higiene “Rafael Rangel” (INHRR), Caracas, Venezuela, E-mail: rosahernandez08@gmail.com. Albert J. Auguste and Robert B. Tesh, Department of Microbiology and Immunology, Institute for Human Infections and Immunity, University of Texas Medical Branch, Galveston, TX, and Department of Pathology, University of Texas Medical Branch, Galveston, TX, E-mails: aj1augus@utmb.edu and rtesh@utmb.edu. Scott C. Weaver, Department of Pathology, University of Texas Medical Branch, Galveston, TX, E-mail: sweaver@utmb.edu. Humberto Montañez, Dirección General de Salud Ambiental, Ministerio del Poder Popular Para la Salud, Caracas, Venezuela, E-mail: virus.arbo@yahoo.com. Jonathan Liria, Departamento de Biología, Facultad Experimental de Ciencias y Tecnología (FACYT), Universidad de Carabobo, Valencia, Venezuela, E-mail: jonathan.liria@gmail.com. Anderson Lima, Jorge Fernando Soares Travassos da Rosa, Sandro P. da Silva, Janaina M. Vasconcelos, Rodrigo Oliveira, João L. S. G. Vianez Jr., and Marcio R. T. Nunes, Centro de Inovações Tecnológicas, Instituto Evandro Chagas, Para, Ananindeua, Brazil, E-mails: andersonfcl@hotmail.com, jorgetravassos@iec.pa.gov.br, spatroca@gmail.com, janaina.mvasconcelos@yahoo.com.br, rodrigodeoliveira01@gmail.com, joao.vianezjr@gmail.com, and marcionunes@iec.pa.gov.br.
References
- 1.Dietzgen RG, Calisher CH, Kurath G, Kuzman IV, Rodriguez LL, Stone DM, Tesh RB, Tordo N, Walker PJ, Wetzel T, Whitfield AE. Virus taxonomy. In: King AMQ, Adams MJ, Carstens EB, Lefkowitz EJ, editors. Ninth Report of the International Committee on Taxonomy of Viruses. San Diego, CA: Elsevier; 2012. pp. 654–681. [Google Scholar]
- 2.Schmaljohn CS, Nichol ST. In: Fields Virology. 5th edition. Knipe DM, Howley PM, editors. Vol. 2. Philadelphia, PA: Wolters Kluwer/Lippincott Williams and Wilkins; 2007. pp. 1741–1789. (Bunyaviridae). [Google Scholar]
- 3.Elliott RM, Schaljohn CS. In: Fields Virology. 6th edition. Knipe DM, Howley PM, editors. Vol. 1. Philadelphia, PA: Wolter Kluwer/Lippincott Williams and Wilkins; 2013. pp. 1244–1282. (Bunyaviridae). [Google Scholar]
- 4.Travassos da Rosa JFS, Travassos da Rosa APA, Vasconcelos PFC, Pinheiro FP, Rodrigues SG, Travassos da Rosa ES, Dias L, Cruz A. Arboviruses isolated in the Evandro Chagas Institute, including some described for the first time in the Brazilian Amazon region, their known host, and their pathology for man. In: Travassos da Rosa APA, Vasconcelos PFC, Travassos da Rosa JFS, editors. An Overview of Arbovirology in Brazil and Neighbouring Countries. Belem, Brazil: Instituto Evandro Chagas; 1988. pp. 19–31. [Google Scholar]
- 5.Vasconcelos PFC, Travassos da Rosa APA, Degallier N, Travassos da Rosa JFS, Pinheiro FP. Clinical and ecoepidemiological situation of human arboviruses in Brazilian Amazonia. Cienc e Cult (Sao Paulo) 1992;44:117–124. [Google Scholar]
- 6.LeDuc JW, Pinheiro FP. Oropouch fever. In: Monath TP, editor. The Arboviruses: Epidemiology and Ecology. Vol. 4. Boca Raton, FL: CRC Press Inc.; 1988. pp. 1–14. [Google Scholar]
- 7.Anderson CR, Spence L, Downs WG, Aitken THG. Oropouche virus: a new human disease agent from Trinidad, West Indies. Am J Trop Med Hyg. 1961;10:574–578. doi: 10.4269/ajtmh.1961.10.574. [DOI] [PubMed] [Google Scholar]
- 8.Pinheiro FP, Travassos da Rosa APA, Ishak R, Freitas RB, Gomes MLC, LeDuc JW, Oliva OFP. Oropouche virus. 1. A review of clinical, epidemiological, and ecological findings. Am J Trop Med Hyg. 1981;30:149–160. [PubMed] [Google Scholar]
- 9.Pinheiro FP, Hoch AL, Gomes MLC, Roberts DR. Oropouche virus. IV. Laboratory transmission by Culicoides paraensis. Am J Trop Med Hyg. 1981;30:172–176. [PubMed] [Google Scholar]
- 10.Roberts DR, Hock AL, Dixon KE, Llewellyn CH. Oropouche virus. III. Entomological observations from three epidemics in Para, Brazil, 1975. Am Trop Med Hyg. 1981;30:165–171. [PubMed] [Google Scholar]
- 11.Pinheiro FP, Travassos da Rosa AP, Travassos da Rosa JF, Bensabath G. An outbreak of Oropouche virus disease in the vicinity of Santarem, Para, Brazil. Tropenmed Parasitol. 1976;27:213–223. [PubMed] [Google Scholar]
- 12.Nunes MR, Caricio Martins L, Guerreiro Rodrigues S, Chiang JO, Da Silva Azevedo RDS, Travassos da Rosa APA, da Costa Vasconcelos PF. Oropouche virus isolation, southeast Brazil. Emerg Infect Dis. 2005;11:1610–1613. doi: 10.3201/eid1110.050464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bowen MD, Trappier SG, Sanchez AJ, Meyer RF, Goldsmith CS, Zaki SR, Dunster LM, Peters CJ, Ksiazek TG, Nichol ST. A reassortant bunyavirus isolated from acute hemorrhagic fever cases in Kenya and Somalia. Virology. 2001;29:185–190. doi: 10.1006/viro.2001.1201. [DOI] [PubMed] [Google Scholar]
- 14.Tilston-Lunel N, Hughes J, Acrani G, da Silva D, Azevedo R, Rodrigues S, Vasconcelos P, Nunes M, Elliott R. A genetic analysis of the Oropouche virus species and identification of a novel M segment sequence. J Gen Virol. 2015;96:1636–1650. doi: 10.1099/vir.0.000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aguilar PV, Barrett AD, Saeed MF, Watts DM, Russell K, Guevara C, Ampuero JS, Suarez L, Cespedes M, Montgomery JM, Halsey ES, Kochel TJ. Iquitos virus: a novel reassortant Orthobunyavirus associated with human illness in Peru. PLoS Negl Trop Dis. 2011;5:e1315. doi: 10.1371/journal.pntd.0001315. doi:10.1371/journal.pntd.0001315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ladner J, Savji N, Lofts L, Travassos da Rosa A, Wiley M, Gestole M, Rosen G, Guzman H, Vasconcelos P, Nunes M, Kochel T, Lipkin W, Tesh R, Palacios G. Genomic and phylogenetic characterization of viruses included in the Manzanilla and Oropouche species complexes of the genus Orthobunyavirus, family Bunyaviridae. J Gen Virol. 2014;95:1055–1066. doi: 10.1099/vir.0.061309-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tan R, Ksiazek TG, Olson JG. Comparative sensitivity of mosquito inoculation and mammalian cell culture for isolation of some arboviruses in Indonesia. Southeast Asian J Trop Med Public Health. 1981;12:544–548. [PubMed] [Google Scholar]
- 18.Auguste AJ, Liria J, Forrester NL, Giambalvo D, Long KC, Morón D, De Manzione N, Tesh RB, Halsey S, Kochel TJ, Hernandez R, Navarro J-C, Weaver SC. Evolutionary and ecological characterization of Mayaro virus strains isolated during an outbreak, Venezuela. Emerg Infect Dis. 2015;21:1742–1750. doi: 10.3201/eid2110.141660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rothberg JM, Hinz W, Rearick TM, Schultz J, Mileski W, Davey M, Leamon JH, Johnson K, Milgrew MJ, Edwards M, Hoon J, Simons JF, Marran D, Myers JW, Davidson JF, Branting A, Nobile JR, Puc BP, Light D, Clark TA, Huber M, Branciforte JT, Stoner IB, Cawley SE, Lyons M, Fu Y, Homer N, Sedova M, Miao X, Reed B, Sabina J, Feierstein E, Schorn M, Alanjary M, Dimalanta E, Dressman D, Kasinskas R, Sokolsky T, Fidanza JA, Namsaraev E, McKernan KJ, Williams A, Roth GT, Bustillo J. An integrated semiconductor device enabling non-optical genome sequencing. Nature. 2011;475:348–352. doi: 10.1038/nature10242. [DOI] [PubMed] [Google Scholar]
- 20.Wanzeller ALM, Martins LC, Diniz Júnior JAP, de Almeida Medeiros DB, Cardoso JF, da Silva DEA, de Oliveira LF, de Vasconcelos JM, Nunes MRT, Vianez JL, Jr, Vasconcelos PF. Xiburema virus, a hitherto undescribed virus within the family Rhabdoviridae isolated in the Brazilian Amazon region. Genome Announc. 2014;2:e00454–e14. doi: 10.1128/genomeA.00454-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chevreux B, Pfisterer T, Drescher B, Driesel AJ, Müller WEG, Wetter T, Suhai S. Using the miraEST assembler for reliable and automated mRNA transcript assembly and SNP detection in sequenced ESTs. Genome Res. 2004;14:1147–1159. doi: 10.1101/gr.1917404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roche 5/3 RACE Kit, 2nd Generation. Manual. 2011;Vol 1–31 doi:10.1016/j.eurpsy.2007.01.525. [Google Scholar]
- 23.Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, Thierer T, Ashton B, Meintjes P, Drummond A. Geneious basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012;28:1647–1649. doi: 10.1093/bioinformatics/bts199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Williamson DF, Parker RA, Kendrick JS. The box plot: a simple visual method to interpret data. Ann Intern Med. 1989;110:916–921. doi: 10.7326/0003-4819-110-11-916. [DOI] [PubMed] [Google Scholar]
- 25.Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol. 2010;59:307–321. doi: 10.1093/sysbio/syq010. doi:10.1093/sysbio/syq010. [DOI] [PubMed] [Google Scholar]
- 27.Drummond AJ, Suchard MA, Xie D, Rambaut A. Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol Biol Evol. 2012;29:1969–1973. doi: 10.1093/molbev/mss075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rambaut A. FigTree, a Graphical Viewer of Phylogenetic Trees. Inst Evol Biol Univ Edinburgh. 2009. http://tree.bio.ed.ac.uk/software/figtree/ Available at.
- 29.Escoto-Delgadillo M, Flores Romero L, Gomez Flores-Ramos L, Vazquez Torres BM, Torres Mendoza BM, Vazquez Valls E. Comparing RIP, REGA and SIMPLOT Software to Define the HIV-1 Recombination in Mexican Population. XVII International AIDS Conference; August 3–8, 2008; Mexico City, Mexico. 2008. [Google Scholar]
- 30.Savji N, Palacios G, Travassos Da Rosa A, Hutchison S, Celone C, Hui J, Briese T, Calisher CH, Tesh RB, Lipkin WI. Genomic and phylogenetic characterization of Leanyer virus, a novel Orthobunyavirus isolated in northern Australia. J Gen Virol. 2011;92:1676–1687. doi: 10.1099/vir.0.028308-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mares MA. Neotropical mammals and the myth of Amazonian biodiversity. Science. 1992;255:976–979. doi: 10.1126/science.255.5047.976. [DOI] [PubMed] [Google Scholar]
- 32.Regalado A. Biodiversity. Brazil says rate of deforestation in Amazon continues to plunge. Science. 2010;329:1270–1271. doi: 10.1126/science.329.5997.1270-b. [DOI] [PubMed] [Google Scholar]
- 33.Jones-Walters L, Čivić K. Wilderness and biodiversity. J Nat Conserv. 2010;18:338–339. [Google Scholar]
- 34.Moura NG, Lees AC, Andretti CB, Davis BJW, Solar RRC, Aleixo A, Barlow J, Ferreira J, Gardner TA. Avian biodiversity in multiple-use landscapes of the Brazilian Amazon. Biol Conserv. 2013;167:339–348. [Google Scholar]
- 35.Dixon KE, Travassos da Rosa APA, Travassos da Rosa JF, Llewellyn CH. Oropouche virus. II. Epidemiological observations during an epidemic in Santarem, Para, Brazil in 1975. Am J Trop Med Hyg. 1981;30:161–164. [PubMed] [Google Scholar]
- 36.Vasconcelos HB, Azevedo RSS, Casseb SM, Nunes-Neto JP, Chiang JO, Cantuária PC, Segura MNO, Martins LC, Monteiro HAO, Rodrigues SG, Nunes MRT, Vasconcelos PFC. Oropouche fever epidemic in northern Brazil: epidemiology and molecular characterization of isolates. J Clin Virol. 2009;44:129–133. doi: 10.1016/j.jcv.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 37.Nunes MRT, Martins LC, Rodrigues SG, Chiang JO, Azevedo RDSDS, Da Rosa APAT, Vasconcelos PFDC. Oropouche virus isolation, southeast Brazil. Emerg Infect Dis. 2005;11:1610–1613. doi: 10.3201/eid1110.050464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Azevedo RDSDS, Nunes MRT, Chiang JO, Bensabath G, Vasconcelos HB, Pinto AYDN, Martins LC, Monteiro HADO, Rodrigues SG, Vasconcelos PFDC. Reemergence of Oropouche fever, northern Brazil. Emerg Infect Dis. 2007;13:912–915. doi: 10.3201/eid1306.061114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vasconcelos HB, Nunes MRT, Casseb LMN, Carvalho VL, da Silva EVP, Silva M, Casseb SMM, Vasconcelos PFC. Molecular epidemiology of Oropouche virus, Brazil. Emerg Infect Dis. 2011;17:800–806. doi: 10.3201/eid1705.101333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saeed MF, Wang H, Nunes M, Vasconcelos PFC, Weaver SC, Shope RE, Watts DM, Tesh RB, Barrett ADT. Nucleotide sequences and phylogeny of the nucleocapsid gene of Oropouche virus. J Gen Virol. 2000;81:743–748. doi: 10.1099/0022-1317-81-3-743. [DOI] [PubMed] [Google Scholar]
- 41.Monath TP, Vasconcelos PF. Yellow fever. J Clin Virol. 2015;64:160–173. doi: 10.1016/j.jcv.2014.08.030. [DOI] [PubMed] [Google Scholar]
- 42.Nunes MRT, Palacios G, Cardoso JF, Martins LC, Sousa EC, de Lima CPS, Medeiros DBA, Savji N, Desai A, Rodrigues SG, Carvalho VL, Lipkin WI, Vasconcelos PFC. Genomic and phylogenetic characterization of Brazilian yellow fever virus strains. J Virol. 2012;86:13263–13271. doi: 10.1128/JVI.00565-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Villordo SM, Gamarnik AV. Genome cyclization as strategy for flavivirus RNA replication. Virus Res. 2009;139:230–239. doi: 10.1016/j.virusres.2008.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guu TSY, Zheng W, Tao YJ. Bunyavirus: structure and replication. Adv Exp Med Biol. 2012;726:245–266. doi: 10.1007/978-1-4614-0980-9_11. [DOI] [PubMed] [Google Scholar]
- 45.Bryant JE, Vasconcelos PFC, Rijnbrand RCA, Mutebi JP, Higgs S, Barrett ADT. Size heterogeneity in the 3′ noncoding region of South American isolates of yellow fever virus. J Virol. 2005;79:3807–3821. doi: 10.1128/JVI.79.6.3807-3821.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yanase T, Yoshida K, Ohashi S, Kato T, Tsuda T. Sequence analysis of the medium RNA segment of three Simbu serogroup viruses, Akabane, Aino, and Peaton viruses. Virus Res. 2003;93:63–69. doi: 10.1016/s0168-1702(03)00066-2. [DOI] [PubMed] [Google Scholar]
- 47.Saeed MF, Li L, Wang H, Weaver SC, Barrett ADT. Phylogeny of the Simbu serogroup of the genus Bunyavirus. J Gen Virol. 2001;82:2173–2181. doi: 10.1099/0022-1317-82-9-2173. [DOI] [PubMed] [Google Scholar]
- 48.Briese T, Bird B, Kapoor V, Nichol ST, Lipkin WI. Batai and Ngari viruses: M segment reassortment and association with severe febrile disease outbreaks in east Africa. J Virol. 2006;80:5627–5630. doi: 10.1128/JVI.02448-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chowdhary R, Street C, Travassos Da Rosa A, Nunes M, Tee KK, Hutchison SK, Vasconcelos PFC, Tesh R, Lipkin IW, Briese T. Genetic characterization of the Wyeomyia group of orthobunyaviruses and their phylogenetic relationships. J Gen Virol. 2012;93:1023–1034. doi: 10.1099/vir.0.039479-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Terzian ACB, De Bronzoni RVM, Drumond BP, Da Silva-Nunes M, Da Silva NS, Ferreira MU, Sperança MA, Nogueira ML. Sporadic Oropouche virus infection, Acre, Brazil. Emerg Infect Dis. 2009;15:348–350. doi: 10.3201/eid1502.080401. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.