Abstract
Estimates of malaria transmission intensity (MTI) typically rely upon microscopy or rapid diagnostic testing (RDT). However, these methods are less sensitive than nucleic acid amplification techniques and may underestimate parasite prevalence. We compared microscopy, RDT, and polymerase chain reaction (PCR) for the diagnosis of Plasmodium falciparum parasitemia as part of an MTI study of 800 children and adults conducted in Lilongwe, Malawi. PCR detected more cases of parasitemia than microscopy or RDT. Age less than 5 years predicted parasitemia detected by PCR alone (adjusted odds ratio = 1.61, 95% confidence interval = 1.09–2.38, Wald P = 0.02). In addition, we identified one P. falciparum parasite with a false-negative RDT result due to a suspected deletion of the histidine-rich protein 2 (hrp2) gene and used a novel, ultrasensitive PCR assay to detect low-level parasitemia missed by traditional PCR. Molecular methods should be considered for use in future transmission studies as a supplement to RDT or microscopy.
Plasmodium falciparum infects hundreds of millions of individuals and kills 600,000 persons every year.1 A recently completed phase three trial of the RTS,S/ASO1 malaria vaccine demonstrated moderate and varying efficacy in different transmission settings.2 Study sites conducted annual malaria transmission intensity (MTI) studies during the trial using microscopy and rapid diagnostic test (RDT) parasite prevalence as a surrogate for transmission intensity.
Both microscopy and RDT are known to produce negative results in Africa when parasitemia levels are beneath their limits of detection, 50 and 200 parasites/μL blood, respectively.3–5 Infections not detected by microscopy or RDT, often referred to as subpatent infections, can often be detected by nucleic acid detection methods.6 Though infections detected by microscopy and RDT are likely responsible for the majority of transmission, further studies are needed to assess the impact of subpatent infections on the transmission reservoir.7,8 In the context of vaccine trials, these low-level, subpatent parasitemias may confound estimates of transmission intensity used to study vaccine efficacy.
To evaluate the impact of subpatent parasitemias on parasite prevalence estimates, we collected dried blood spots from all participants during the final year of the 3-year MTI study in Lilongwe, Malawi, to allow for nucleic acid detection of parasitemia. Using clinical data and a spatial database to evaluate ecological factors that may influence transmission, we attempted to identify risk factors for subpatent parasitemia. We also investigated the impact of other factors on discordances between polymerase chain reaction (PCR) and other assays (RDT and microscopy) including 1) non-falciparum parasitemia, 2) the occurrence of histidine-rich protein 2 (hrp2) gene deletions on false-negative RDTs, and 3) low-level parasitemia detectable by ultrasensitive PCR targeting high copy number genes.9 This work adds to the growing literature concerning the relationship between testing characteristics for malaria detection and provides new information about hrp2 deletions in Malawi, and the use of ultrasensitive, falciparum-specific, high-copy telomere-associated repetitive element 2 (TARE-2) PCR to address diagnostic discordances.
The MTI study was conducted in Lilongwe from 2011 to 2013 within the catchment area of the phase three RTS,S/ASO1 trial.2 A total of 800 participants were included each year from randomly selected households in the area surrounding the Malawi Ministry of Health Area 18 Health Center in Lilongwe. Each annual cohort of enrollees in the MTI study included 400 subjects ≥ 6 months and < 5 years of age, 200 subjects ≥ 5 years of age and < 20 years of age, and 200 subjects ≥ 20 years of age. Participants in the phase three RTS,S/ASO1 trial were excluded from the MTI study. This study includes only the 2013 cohort, all of whom had a finger prick with blood collected for RDT and microscopy and dried on Whatman 3 filter paper (GE Healthcare, Piscataway, NJ). Microscopy slides were read by two expert microscopists who received training every 4 months, with discordances referred to a third microscopist. RDT testing was performed using SD Bioline HRP-2 Kits (Gyeonggi-do, Republic of Korea). Filter papers were labeled with an identifier and stored individually with dessicant at −20°C. This study was approved by the Malawian National Health Sciences Research Committee and the University of North Carolina (UNC) Institutional Review Board. All participants provided written informed consent.
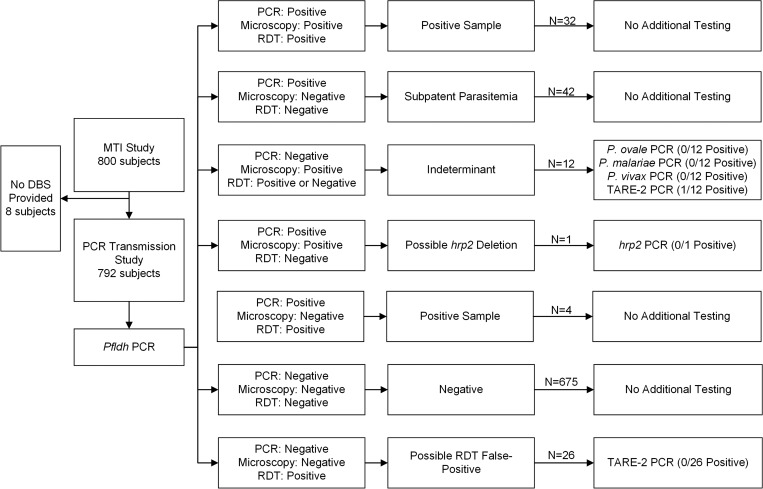
DNA was extracted from filter paper using the PureLink Pro 96 Genomic DNA Purification Kit (Life Technologies, Grand Island, NY) at UNC. We used a predetermined screening algorithm for testing samples (Figure 1 and Supplemental Table 1). Initial testing for parasitemia involved a real-time PCR assay to detect the falciparum-specific lactate dehydrogenase (pfldh) gene.10 All samples were tested with 1 μL of template DNA per reaction in duplicate, followed by 4 μL of template DNA in duplicate for discordant replicates and for microscopy-positive, PCR-negative samples.
Figure 1.
Diagnostic testing algorithm and results.
The clinical characteristics of subjects were compared using Fisher's exact test for categorical variables, the Student's t test for normally distributed continuous variables, and the Wilcoxon rank-sum test for non-normally distributed continuous variables. Risk factors for subpatent malaria were investigated by constructing a binomial regression model. We used directed acyclic graphs to assess all possible relationships between risk factors of interest and subpatent malaria and to identify potential confounders. We calculated crude and adjusted odds ratios for each risk factor. Kappa statistics were calculated to quantify the agreement between diagnostic testing methods in subjects who underwent testing with all three methods (PCR, microscopy, and RDT), in addition to sensitivity and specificity using PCR as the gold standard. Statistical analyses were performed using SAS 9.4 (SAS Institute, Cary, NC).
The majority of malaria infections identified in 2013 were subpatent (Table 1 and Figure 2 ). This finding was most prominent among children younger than 5 years, in whom RDT and microscopy failed to detect two-thirds of all PCR-positive parasitemias. These children were more likely to have subpatent malaria than patent malaria, defined as PCR positive and either microscopy or RDT positive (P = 0.04). Subjects with subpatent and patent malaria had similar demographic and clinical characteristics (Table 1). Modeling confirmed that age less than 5 years predicted subpatent malaria in our cohort (adjusted odds ratio = 1.61, 95% confidence interval [CI] = 1.09–2.38, Wald P = 0.02; Supplemental Table 2). Neither bed net use nor anemia predicted subpatent malaria in the crude or adjusted models.
Table 1.
Patient characteristics
| Entire cohort | Patent malaria | Subpatent malaria | P value | |
|---|---|---|---|---|
| N (%) | 800 (100) | 38 (4.8) | 42 (5.3) | – |
| Age (years), n (%) | ||||
| < 5 | 400 (50.0) | 11 (29.0) | 22 (52.4) | 0.04 |
| ≥ 5 to < 20 | 200 (25.0) | 16 (42.1) | 11 (26.2) | 0.16 |
| ≥ 20 | 200 (25.0) | 11 (29.0) | 9 (21.4) | 0.45 |
| Females, n (%) | 494 (62.5) | 24 (63.2) | 25 (59.5) | 0.82 |
| Household members, mean (SD) | 5.0 (1.6) | 4.6 (1.4) | 5.2 (1.7) | 0.10 |
| Electricity, n (%) | 164 (20.6) | 4 (10.8) | 9 (21.4) | 0.23 |
| Number of bed nets per household, mean (SD) | 1.7 (1.3) | 1.5 (1.2) | 1.7 (1.1) | 0.34 |
| Slept under a bed net prior night, n (%)* | 629 (78.6) | 26 (68.4) | 32 (76.2) | 0.46 |
| Using a treated net | 514 (81.7) | 23 (88.5) | 28 (87.5) | 1.00 |
| Using a torn net | 239 (38.0) | 9 (34.6) | 11 (34.4) | 1.00 |
| Reported fever within prior 24 hours, n (%) | 108 (13.5) | 11 (29.0) | 6 (14.3) | 0.17 |
| Malaria treatment during prior 2 weeks, n (%) | 50 (6.3) | 2 (5.3) | 1 (2.4) | 0.60 |
| Hospitalized during preceding 3 months for malaria, n (%) | 17 (2.1) | 0 (0) | 0 (0) | – |
| Hemoglobin (g/dL), mean (SD) | 12.0 (1.7) | 11.5 (1.8) | 12.1 (1.9) | 0.12 |
| Age group (years) | ||||
| < 5 | 11.2 (1.3) | 10.8 (2.2) | 11.5 (1.6) | 0.37 |
| ≥ 5 to < 20 | 12.4 (1.3) | 11.6 (1.3) | 12.5 (1.0) | 0.05 |
| ≥ 20 | 13.1 (1.8) | 12.1 (1.9) | 13.4 (2.6) | 0.22 |
| Parasites/mL blood, median (IQR) | – | 896 (198–15,798) | – | – |
| Age group (years) | ||||
| < 5 | – | 834 (595–20,673) | – | – |
| ≥ 5 to < 20 | – | 5,794 (303–21,710) | – | – |
| ≥ 20 | – | 675 (23–1,905) | – | – |
IQR = interquartile range; SD = standard deviation.
Proportion calculated using all subjects. All other proportions exclude those with missing data.
Figure 2.
Proportional Venn diagram comparing Plasmodium falciparum prevalence by diagnostic method. Number of results and percent overall prevalence by testing method. Diagram was generated using eulerAPE, version 3.0.0.14
PCR was the most sensitive diagnostic test for parasitemia, producing a prevalence 76% higher than microscopy and 16% higher than RDT in 2013. Malaria was detected by pfldh PCR in 79 (10.0%) subjects, by microscopy in 45 (5.7%), and by RDT in 68 (8.6%). There was moderate agreement between all the three tests (Figure 2). RDT and microscopy had the highest level of agreement (kappa = 0.64, 95% CI = 0.54–0.74), PCR and microscopy had less agreement (kappa = 0.50, 95% CI = 0.38–0.61), and PCR and RDT had the lowest level of agreement (kappa = 0.44, 95% CI = 0.33–0.54). Using PCR as the gold standard, both microscopy and RDT lacked sensitivity but were specific. For microscopy, the sensitivity and specificity were 41.8% (95% CI = 30.9−52.7) and 98.3% (95% CI = 97.4–99.3), whereas RDT had a sensitivity and specificity of 45.6% (95% CI = 34.6–56.6) and 95.5% (95% CI = 94.0–97.0). Among 12 subjects with indeterminant microscopy results (microscopy positive but RDT and pfldh PCR negative), there were no cases of non-falciparum malaria, but one subject with parasitemia below the pfldh PCR assay's limit of detection was detected using ultrasensitive TARE-2 PCR (Figure 1). All 26 subjects evaluated for possible RDT false-positive results (RDT positive but microscopy and pfldh PCR negative) had negative TARE-2 PCR results. Notably, one parasite detected by both PCR and microscopy but not detected by RDT likely harbored a deletion of the hrp2 gene, which is responsible for production of the antigen detected by most RDTs. The geographical distribution of positive test results by PCR, microscopy, and RDT was similar.
These results confirm that studies that rely upon RDT and/or microscopy underestimate malaria prevalence. The striking difference in parasitemia prevalence estimates among children younger than 5 years is especially concerning, as these children are at highest risk of malaria-related morbidity and mortality.1 Even if most subpatent infections in these young children are asymptomatic, they may represent a transmission reservoir that requires further study and may need to be addressed by ongoing malaria control efforts.6
Age less than 5 years was a risk factor for subpatent malaria in our cohort, a finding that differs from other published reports. Specifically, submicroscopic malaria has been associated with older age, presumably due to partial immunity.6,15,16 Although high levels of bed net usage during the study period suggested that bed nets may be partly responsible for this finding, bed net usage was not a risk factor for subpatent infection in our models, a finding consistent with prior reports.16 Submicroscopic infections are also thought to be more common in settings of low transmission intensity.11,17 Because PCR testing was only performed in the final year of the study, we could not assess changes in subpatent malaria over time.
Using individual-level data, we found a lower level of concordance between microscopy and RDT results than a recent analysis of prevalence studies, which depended upon cluster-level data.6,10 Although malaria prevalence as determined by PCR and RDT differed by only 16%, there was only moderate agreement between tests (kappa = 0.44), likely due in part to the large number of “false-positive” RDT results (Figures 1 and 2). “False-positive” RDT results have been described in the setting of persistent circulating HRP2 antigen after clearance of P. falciparum parasitemia, indicating not a failure of the assay itself but recently resolved malaria.5,18,19 We confirmed the absence of low-level parasitemia in samples with false-positive RDT results using TARE-2 PCR, a novel ultrasensitive PCR assay that can detect P. falciparum parasitemias of 0.03–0.15 parasites/μL blood. We also used this ultrasensitive assay to evaluate microscopy-positive but RDT-/PCR-negative results, and successfully identified low-level parasitemia in one of 12 subjects.
The hrp2-deleted P. falciparum parasite identified in this study represents the first suspected case reported in Malawi. There are increasing reports of these stealth parasites, which evade detection by RDTs that depend upon identification of circulating P. falciparum HRP2 antigen, but their public health significance remains unknown.9,12
In conclusion, we used an epidemiological and molecular approach to evaluate the diagnostic methods used during a large MTI study conducted as part of the RTS,S/ASO1 vaccine trials. Our findings confirm that RDT and microscopy underestimate the prevalence of P. falciparum parasitemia. RDTs may currently lack the sensitivity and specificity for accurate assessment of transmission intensity.12,19 Molecular methods should be considered for use in future transmission studies as a supplement to RDT or microscopy.
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank the staff of UNC Project-Malawi, the MTI study team, and the subjects who participated in the MTI study. We would also like to thank Corinna Keeler for assistance with an earlier version of this manuscript.
Footnotes
Financial support: This work was supported by the National Institutes of Allergy and Infectious Diseases (5T32AI007151 to Jonathan B. Parr, 5 T32AI07001–36 to Veronica Escamillia, and R01AI089819 to Jonathan J. Juliano), the UNC Summer Undergraduate Research Gold Fellowship for Connor Belson, a GlaxoSmithKline post-graduate fellowship to Veronica Escamillia, the Population Research Infrastructure Program through funding awarded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development to the Carolina Population Center [R24 HD050924 to Veronica Escamillia], and the National Science Foundation [BCS-1339949 to Michael Emch]. The MTI study was sponsored by GlaxoSmithKline and funded by GlaxoSmithKline and PATH MVI.
Authors' addresses: Jonathan B. Parr and Irving F. Hoffman, Division of Infectious Diseases, University of North Carolina, Chapel Hill, NC, E-mails: jonathan_parr@med.unc.edu and irving_hoffman@med.unc.edu. Connor Belson, Department of Biology, University of North Carolina, Chapel Hill, NC, E-mail: connorbelson@gmail.com. Jaymin C. Patel and Steven R. Meshnick, Department of Epidemiology, Gillings School of Global Public Health, Chapel Hill, NC, E-mails: jaymin86@email.unc.edu and meshnick@email.unc.edu. Portia Kamthunzi, Francis Martinson, Gerald Tegha, and Isaac Thengolose, University of North Carolina Project-Malawi, Lilongwe, Malawi, E-mails: portia@globemw.net, fmartinson@unclilongwe.org, gtegha@unclilongwe.org, and ithengolose@unclilongwe.org. Chris Drakeley, Faculty of Infectious and Tropical Diseases, London School of Hygiene and Tropical Medicine, London, United Kingdom, E-mail: chris.drakeley@lshtm.ac.uk. Veronica Escamilla, Center for Infectious Diseases, University of North Carolina, Chapel Hill, NC, and Carolina Population Center, University of North Carolina, Chapel Hill, NC, E-mail: escamill@email.unc.edu. Michael Emch, Department of Geography, University of North Carolina, Chapel Hill, NC, E-mail: emch@med.unc.edu. Jonathan J. Juliano, Division of Infectious Diseases, Department of Medicine, University of North Carolina, Chapel Hill, NC, E-mail: jonathan_juliano@med.unc.edu.
References
- 1.World Health Organization . World Malaria Report 2014. Geneva, Switzerland: World Health Organization; 2014. [Google Scholar]
- 2.RTS,S Clinical Trials Partnership Efficacy and safety of RTS,S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: final results of a phase 3, individually randomised, controlled trial. Lancet. 2015;386:31–45. doi: 10.1016/S0140-6736(15)60721-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mwingira F, Genton B, Kabanywanyi AN, Felger I. Comparison of detection methods to estimate asexual Plasmodium falciparum parasite prevalence and gametocyte carriage in a community survey in Tanzania. Malar J. 2014;13:433. doi: 10.1186/1475-2875-13-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kilian AH, Metzger WG, Mutschelknauss EJ, Kabagambe G, Langi P, Korte R, von Sonnenburg F. Reliability of malaria microscopy in epidemiological studies: results of quality control. Trop Med Int Health. 2000;5:3–8. doi: 10.1046/j.1365-3156.2000.00509.x. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization . Malaria Rapid Diagnostic Test Performance. Results of WHO Product Testing of Malaria RDTs: Round 6 (2015) Geneva, Switzerland: World Health Organization; 2015. [Google Scholar]
- 6.Wu L, van den Hoogen LL, Slater H, Walker PG, Ghani AC, Drakeley CJ, Okell LC. Comparison of diagnostics for the detection of asymptomatic Plasmodium falciparum infections to inform control and elimination strategies. Nature. 2015;528:S86–S93. doi: 10.1038/nature16039. [DOI] [PubMed] [Google Scholar]
- 7.Ouedraogo AL, Goncalves BP, Gneme A, Wenger EA, Guelbeogo MW, Ouedraogo A, Gerardin J, Bever CA, Lyons H, Pitroipa X, Verhave JP, Eckhoff PA, Drakeley C, Sauerwein R, Luty AJ, Kouyate B, Bousema T. Dynamics of the human infectious reservoir for malaria determined by mosquito feeding assays and ultrasensitive malaria diagnosis in Burkina Faso. J Infect Dis. 2016;213:90–99. doi: 10.1093/infdis/jiv370. [DOI] [PubMed] [Google Scholar]
- 8.Lin JT, Ubalee R, Lon C, Balasubramanian S, Kuntawunginn W, Rahman R, Saingam P, Heng TK, Vy D, San S, Nuom S, Burkly H, Chanarat N, Ponsa C, Levitz L, Parobek C, Chuor CM, Somethy S, Spring M, Lanteri C, Gosi P, Meshnick SR, Saunders DL. Microscopic Plasmodium falciparum gametocytemia and infectivity to mosquitoes in Cambodia. J Infect Dis. 2016;213:1491–1494. doi: 10.1093/infdis/jiv599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akinyi S, Hayden T, Gamboa D, Torres K, Bendezu J, Abdallah JF, Griffing SM, Quezada WM, Arrospide N, De Oliveira AM, Lucas C, Magill AJ, Bacon DJ, Barnwell JW, Udhayakumar V. Multiple genetic origins of histidine-rich protein 2 gene deletion in Plasmodium falciparum parasites from Peru. Sci Rep. 2013;3:2797. doi: 10.1038/srep02797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pickard AL, Wongsrichanalai C, Purfield A, Kamwendo D, Emery K, Zalewski C, Kawamoto F, Miller RS, Meshnick SR. Resistance to antimalarials in southeast Asia and genetic polymorphisms in pfmdr1. Antimicrob Agents Chemother. 2003;47:2418–2423. doi: 10.1128/AAC.47.8.2418-2423.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lefterova MI, Budvytiene I, Sandlund J, Farnert A, Banaei N. Simple real-time PCR and amplicon sequencing method for identification of Plasmodium species in human whole blood. J Clin Microbiol. 2015;53:2251–2257. doi: 10.1128/JCM.00542-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koita OA, Doumbo OK, Ouattara A, Tall LK, Konare A, Diakite M, Diallo M, Sagara I, Masinde GL, Doumbo SN, Dolo A, Tounkara A, Traore I, Krogstad DJ. False-negative rapid diagnostic tests for malaria and deletion of the histidine-rich repeat region of the hrp2 gene. Am J Trop Med Hyg. 2012;86:194–198. doi: 10.4269/ajtmh.2012.10-0665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hofmann N, Mwingira F, Shekalaghe S, Robinson LJ, Mueller I, Felger I. Ultra-sensitive detection of Plasmodium falciparum by amplification of multi-copy subtelomeric targets. PLoS Med. 2015;12:e1001788. doi: 10.1371/journal.pmed.1001788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Micallef L, Rodgers P. eulerAPE: drawing area-proportional 3-venn diagrams using ellipses. PLoS One. 2014;9:e101717. doi: 10.1371/journal.pone.0101717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okell LC, Bousema T, Griffin JT, Ouedraogo AL, Ghani AC, Drakeley CJ. Factors determining the occurrence of submicroscopic malaria infections and their relevance for control. Nat Commun. 2012;3:1237. doi: 10.1038/ncomms2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walldorf JA, Cohee LM, Coalson JE, Bauleni A, Nkanaunena K, Kapito-Tembo A, Seydel KB, Ali D, Mathanga D, Taylor TE, Valim C, Laufer MK. School-age children are a reservoir of malaria infection in Malawi. PLoS One. 2015;10:e0134061. doi: 10.1371/journal.pone.0134061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harris I, Sharrock WW, Bain LM, Gray KA, Bobogare A, Boaz L, Lilley K, Krause D, Vallely A, Johnson ML, Gatton ML, Shanks GD, Cheng Q. A large proportion of asymptomatic Plasmodium infections with low and sub-microscopic parasite densities in the low transmission setting of Temotu Province, Solomon Islands: challenges for malaria diagnostics in an elimination setting. Malar J. 2010;9:254. doi: 10.1186/1475-2875-9-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abba K, Deeks JJ, Olliaro P, Naing CM, Jackson SM, Takwoingi Y, Donegan S, Garner P. Rapid diagnostic tests for diagnosing uncomplicated P. falciparum malaria in endemic countries. Cochrane Database Syst Rev. 2011:CD008122. doi: 10.1002/14651858.CD008122.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iqbal J, Siddique A, Jameel M, Hira PR. Persistent histidine-rich protein 2, parasite lactate dehydrogenase, and panmalarial antigen reactivity after clearance of Plasmodium falciparum monoinfection. J Clin Microbiol. 2004;42:4237–4241. doi: 10.1128/JCM.42.9.4237-4241.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.