Abstract
Whole parasite immunization strategies employing genetically attenuated parasites (GAP), which arrest during liver-stage development, have been applied successfully for induction of sterile malaria protection in rodents. Recently, we generated a Plasmodium berghei GAP-lacking expression of multidrug resistance-associated protein (MRP2) (PbΔmrp2) that was capable of partial schizogony in hepatocytes but showed complete growth arrest. Here, we investigated the protective efficacy after intravenous (IV) immunization of BALB/c and C57BL/6J mice with PbΔmrp2 sporozoites. Low-dose immunization using 400 PbΔmrp2 sporozoites induced 100% sterile protection in BALB/c mice after IV challenge with 10,000 wild-type sporozoites. In addition, almost full protection (90%) was obtained after three immunizations with 10,000 sporozoites in C57BL/6J mice. Parasite liver loads in nonprotected PbΔmrp2-challenged C57BL/6J mice were reduced by 86% ± 5% on average compared with naive control mice. The mid-to-late arresting PbΔmrp2 GAP was equipotent in induction of protective immunity to the early arresting PbΔb9Δslarp GAP. The combined data support a clear basis for further exploration of Plasmodium falciparum parasites lacking mrp2 as a suitable GAP vaccine candidate.
Introduction
Plasmodium berghei genetically attenuated parasite (GAP) immunization strategies using whole, live parasites have been applied successfully for induction of sterile malaria protection in mice.1–3 Compared with immunizations using radiation-attenuated parasites4 or sporozoites under chloroquine cover,5 GAPs have the advantages of consisting of homogenous populations of attenuated parasites, and circumvent the necessity of prophylactic drug use. This potentiates their use as a vaccine strategy to prevent malaria, which is a severe disease causing more than 400,000 deaths of (mostly) children under 5 years of age, annually.6 Multiple rodent GAPs have been generated that abort development in the liver at different time -points after invasion of hepatocytes resulting in different levels of protection.7,8 By comparing two Plasmodium yoelii GAPs, it was found that the late arresting P. yoelii PyΔfabb/f GAP9 resulted in enhanced protective immunity as compared with the early arresting PyΔsap110,11 highlighting the potential benefit that late-arresting GAPs provide. Next to inducing high-level protection, a critical requirement for a vaccine consisting of GAP is complete attenuation of the sporozoites and, hence, the absence of breakthrough blood-stage infections after immunization.12 In contrast to the late-arresting PyΔfabb/f GAP, immunization of mice with the orthologous P. berghei Δfabb/f GAP resulted in breakthrough blood infection in a number of mice, emphasizing the need to generate fully attenuated GAPs. Recently, early arresting P. berghei GAPs have been generated by removing genes encoding sporozoite and liver asparagine-rich protein (SLARP)13 (ortholog of P. yoelii SAP1) or B9.14 Immunization of mice with mutant sporozoites lacking B9 mutant resulted in low-grade blood-stage breakthrough infections.14 However, using a double gene deletion mutant lacking both the b9 and the slarp gene (Δb9Δslarp), a complete arrest was obtained at early liver stage with absence of both nuclear division of parasites and exported protein 1 expression, a marker for the parasitophorous vacuole membrane (PVM).15 High protective efficacy was obtained after immunization of C57BL/6J mice with an immunization scheme of 3 × 10,000 PbΔb9Δslarp sporozoites.15 More recently, we generated P. berghei mutants lacking expression of the adenosine triphosphate–binding cassette transport protein MRP2 (Δmrp2) that showed a severely compromised phenotype during liver-stage development resulting in complete arrest at mid-to-late liver stage.16 Although PbΔmrp2 liver-stage parasites show nuclear division and are still present at 52 hours postinfection, breakthrough blood infections were never observed, even after administration of very high doses of sporozoites.16 Here, our main objective was to address the protective efficacy induced by immunization with PbΔmrp2 sporozoites in two different mouse strains, BALB/c and C57BL/6J. Furthermore, we compared the induced protection to early arresting PbΔb9Δslarp GAP immunizations performed in parallel.
Methods
Experimental mice and P. berghei lines.
Animal experiments were performed in female C57BL/6J mice (from 8 weeks; Janvier, Le Genest-Saint-Isle, France) and approved by the Radboud University Experimental Animal Ethical Committee (RUDEC 2014-170). In animal experiments performed at the Leiden University Medical Center, female BALB/c (4–6 weeks old) from Charles River were used, as approved by the Animal Experiments Committee of the Leiden University Medical Center (DEC 12111). The Dutch Experiments on Animal Act is established under European guidelines on protection of animals used for scientific purposes (EU directive no. 2010/63/EU). Two previously generated P. berghei ANKA mutant parasite lines were used to immunize mice: PbΔb9Δslarp (1844 cl1)15 and PbΔmrp2 (1025 cl2).16 For challenge of the immunized mice with wild-type (WT) parasites, we used the reference P. berghei ANKA line 676m1cl1 (PbGFP-Luccon)17 that expresses the reporter fusion protein green fluorescent protein (GFP)-luciferase.
Immunizations and challenge.
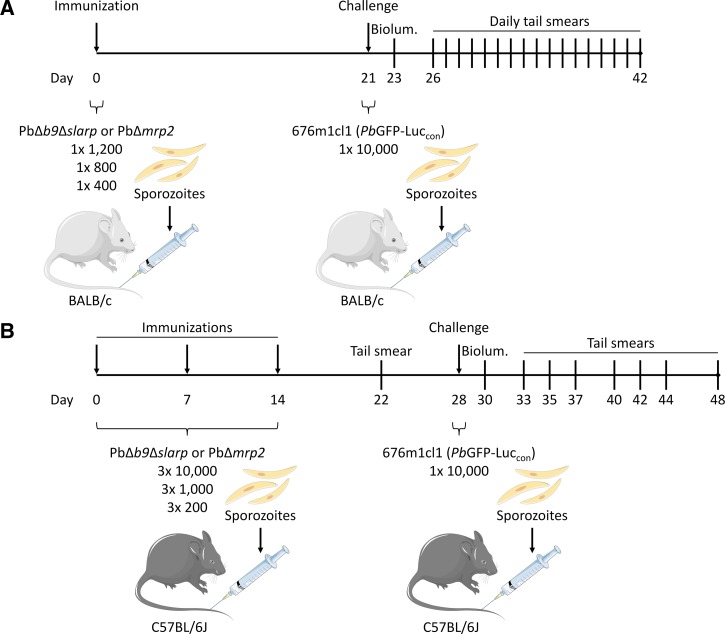
Sporozoites of the three above mentioned P. berghei lines were produced by feeding infected mice to Anopheles stephensi mosquitoes. After 3–4 weeks of parasite development, mosquitoes were hand dissected, and salivary glands were homogenized in Dulbecco's Modified Eagle Medium (+4.5 g/L d-glucose, +NEAA, -l-glutamine, -pyruvate; Life Technologies, Bleiswijk, The Netherlands) supplemented with 1% human albumin (Albuman; Sanquin, Nijmegen, The Netherlands) using a homemade glass grinder. Sporozoites were then quantified using a Bürker-Türk counting chamber using phase contrast microscopy to prepare immunization or challenge solutions. After anesthetizing using isoflurane, female BALB/c and C57BL/6J mice were immunized and challenged by intravenous (IV) injection of 200 μL sporozoite solution in the tail vein according to the schedule presented in Figure 1A and B . Thirty-one BALB/c mice were divided into groups of 16 and 15, receiving either PbΔb9Δslarp or PbΔmrp2 immunizations. These groups were subdivided in three groups of five (six in the high PbΔb9Δslarp dose group) mice receiving immunization doses of 1 × 400, 1 × 800, or 1 × 1,200 sporozoites. Immunized mice and five age-matched naive mice were challenged after 21 days by 10,000 wild type PbGFP-Luccon sporozoites, which express the GFP-luciferase reporter protein. For the C57BL/6J strain, 60 mice were divided into two equal groups, receiving either PbΔb9Δslarp or PbΔmrp2 immunizations. These groups were subdivided in three groups of 10 mice receiving immunization doses of 3 × 10,000, 3 × 1,000, or 3 × 200 sporozoites with a 1-week interval between each immunization. Immunized mice and three age-matched naive mice were challenged after 14 days by 10,000 wild type PbGFP-Luccon sporozoites. Subsequently, liver loads were determined by measuring luciferase activity by bioluminescence imaging (see section In vivo liver-stage bioluminescence imaging) 48 hours after injection of sporozoites, and tail blood was analyzed by Giemsa-stained films for blood-stage positivity from 5 to 21 days post challenge.
Figure 1.
Schematic setup of mouse immunizations, challenge, and follow-up. (A) Thirty-one BALB/c mice were separated in groups of five (and one group of six), and (B) 60 C57BL/6J mice were separated in groups of 10, receiving either PbΔb9Δslarp or PbΔmrp2 genetically attenuated parasites immunization doses (BALB/c high: 1 × 1,200, medium: 1 × 800, low: 1 × 400 sporozoites; C57BL/6J high: 3 × 10,000, medium: 3 × 1,000, low: 3 × 200 sporozoites) on the indicated days. All immunized mice and five (BALB/c) or three (C57BL/6J) naive controls were challenged with 10,000 WT sporozoites 2 weeks succeeding the last immunization. Liver-stage parasite development was monitored by bioluminescence (Biolum.) 2 days after challenge. Finally, tail smears were prepared to screen for malaria blood-stage positivity from daum 5 to 20 (BALB/c) or 21 (C57BL/6J) (with varying intervals) after challenge.
In vivo liver-stage bioluminescence imaging.
Forty-eight hours after challenge with PbGFP-Luccon parasites, the parasite liver load was determined in abdomen-shaved mice by measuring luciferase activity by real-time in vivo imaging using the IVIS Lumina II system (Caliper Life Sciences, Hopkinton, MA) as described previously18,19 with minor adaptations. In brief, BALB/c mice were injected subcutaneously in the neck with 60 μL (C57BL/6J: 200 μL) d-luciferin (120 mg/kg [C57BL/6J: 150 mg/kg]; PerkinElmer, Groningen, The Netherlands) dissolved in PBS (Life Technologies, Gibco) 3 minutes (C57BL/6J: 4 minutes) before imaging. After anesthetization by isoflurane, mice were imaged using a field of view of 12.5 cm, medium binning factor and an exposure time of 25–120 seconds (C57BL/6J: 300 sec.). Luminescence intensity was quantified and visualized using the Living Image 4.5 software (Caliper Life Sciences), shown as rainbow plots with automatic scale bars per measurement and represented as radiance units (photons/second/cm2/steradian). The region of interest (ROI) was set to include the liver, quantified the total flux of photons (photons/second) per mouse and per dose group and is represented as the mean ± standard error of the mean (SEM).
Statistical analysis.
The different immunization groups were scored on a binominal distribution (protected, blood smear negative 20 or 21 days post challenge, or nonprotected, blood smear positive) and significant differences between these groups were determined using the Fisher's exact test. The ROIs of these immunization groups and naive controls, calculated by the Living Image 4.5 software, were statistically compared with a one-way analysis of variance using the Tukey's posttest, and the overall percent reduction in C57BL/6J liver load was depicted as mean (%) ± SEM. All tests were performed using GraphPad Prism version 5.03 (GraphPad Software, San Diego, CA).
Results and Discussion
To study the protective efficacy induced by immunization of mice with sporozoites of the mid-to-late arresting PbΔmrp2 GAP,16 we followed an immunization scheme employing single doses of 1,200 (high), 800 (medium), or 400 (low) sporozoites in BALB/c mice followed by a challenge after 3 weeks (Figure 1A). Immunized mice were challenged with 10,000 GFP-luciferase expressing WT sporozoites showing sterile protection in all mice immunized with the low (5/5) and high dose (5/5), whereas three of five mice were protected with the medium dose (Supplemental Figure 1). Immunization of BALB/c mice with sporozoites of the early arrester PbΔb9Δslarp induced a similar level of protection (Supplemental Figure 1), as all mice immunized with the low (5/5) and high (6/6) doses were protected against a subsequent challenge, whereas four of five mice were protected with the medium dose. As expected, challenging naive BALB/c mice with 10,000 WT sporozoites resulted in all five mice becoming liver- and blood-stage positive (Supplemental Figure 1). As summarized in Table 1, there was no significant difference in protective efficacy induced by immunization of BALB/c mice with PbΔb9Δslarp or PbΔmrp2 sporozoites.
Table 1.
Protection in BALB/c or C57BL/6J mice immunized with PbΔmrp2 or PbΔb9Δslarp sporozoites
| Mouse strain | Parasite line | Immunization dose(s) (sporozoites) | No. protected/no. challenged |
|---|---|---|---|
| BALB/c | PbΔmrp2 | 1 × 1,200 | 5/5* |
| 1 × 800 | 3/5† | ||
| 1 × 400 | 5/5 | ||
| PbΔb9Δslarp | 1 × 1,200 | 6/6‡ | |
| 1 × 800 | 4/5 | ||
| 1 × 400 | 5/5 | ||
| Naive control | – | 0/5 | |
| C57BL/6 | PbΔmrp2 | 3 × 10,000 | 9/10§ |
| 3 × 1,000 | 0/10‖ | ||
| 3 × 200 | 0/10 | ||
| PbΔb9Δslarp | 3 × 10,000 | 10/10¶** | |
| 3 × 1,000 | 3/10 | ||
| 3 × 200 | 0/10 | ||
| Naive control | – | 0/3 |
P = 0.44 (Fisher's exact test) for PbΔmrp2 1 × 1,200 vs. 1 × 800.
P = 1 (Fisher's exact test) for PbΔmrp2 1 × 800 vs. PbΔb9Δslarp 1 × 800.
P = 0.45 (Fisher's exact test) for PbΔb9Δslarp 1 × 1,200 vs. 1 × 800.
P = 0.0001 (Fisher's exact test) for PbΔmrp2 3 × 10,000 vs. 3 × 1,000 or 3 × 200.
P = 0.21 (Fisher's exact test) for PbΔmrp2 3 × 1,000 vs. PbΔb9Δslarp 3 × 1,000.
P = 0.0031 (Fisher's exact test) for PbΔb9Δslarp 3 × 10,000 vs. 3 × 1,000.
P < 0.0001 (Fisher's exact test) for PbΔb9Δslarp 3 × 10,000 vs. 3 × 200.
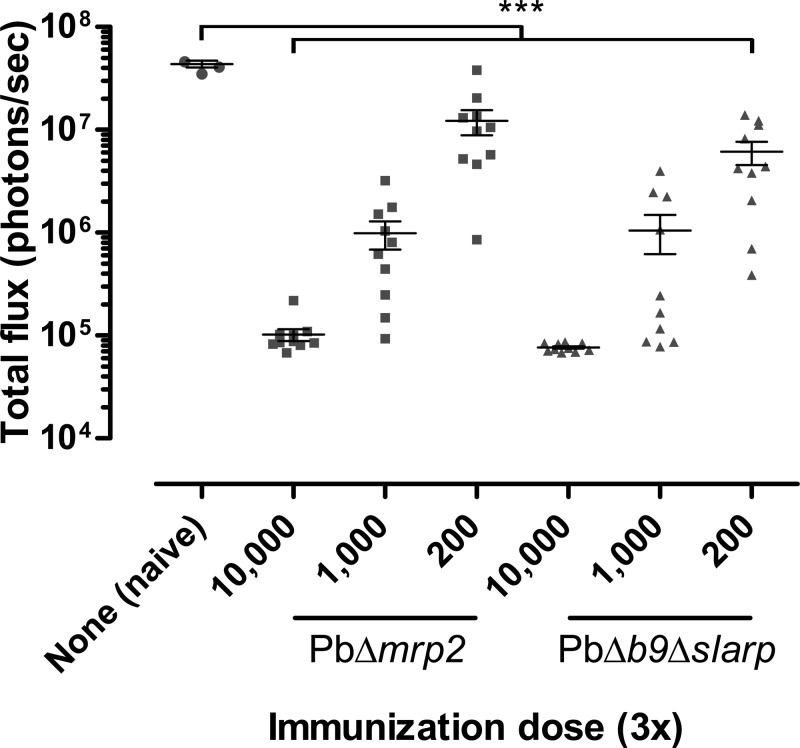
Next, we immunized C57BL/6J mice, known as a more stringent model for inducing protective immunity against P. berghei,20 according to the scheme shown in Figure 1B. Here, three weekly immunizations of 10,000 (high), 1,000 (medium), or 200 (low) were followed by challenge with 10,000 GFP-luciferase expressing WT sporozoites 2 weeks after the last immunization. Eight days after the last immunization, before challenge, we analyzed tail blood for the presence of possible breakthrough blood infections. Neither immunization with PbΔmrp2 nor PbΔb9Δslarp GAP parasites caused breakthrough infections, confirming previously reported complete attenuation of these parasites.15,16 Nine of 10 mice immunized with the high dose of PbΔmrp2 were completely protected against a WT challenge (Supplemental Figure 1), which was clearly superior to the medium- and low-dose immunization groups where none of the mice were protected (P < 0.0001) (Table 1). Previously, it had been shown that after three weekly immunizations of 10,000 sporozoites of the early arresting PbΔb9Δslarp, sterile protection was obtained against a WT challenge.15 Here, three PbΔb9Δslarp GAP immunizations (Supplemental Figure 1) also resulted in sterile protection when using the high dose. Protection declined dose-dependently, resulting in 3/10 protected mice in the medium dose group (versus 6/1015) and 0/10 protected mice in the low-dose group. As summarized in Table 1, the high dose provided significant better protection than the medium- (P = 0.0031) and low-dose groups (P < 0.0001). As expected, all three naive mice became liver- and blood-stage positive after challenge with 10,000 WT sporozoites (Supplemental Figure 1). Although most C57BL/6J mice in the medium- and low-dose groups became blood smear positive after immunization using both GAPs, parasite liver loads in all immunized groups were strongly reduced (P < 0.0001) compared with control mice (Figure 2 ). The nonprotected mice had an average reduction in liver load of 86% ± 5% and 90% ± 2% upon immunization with PbΔmrp2 and PbΔb9Δslarp, respectively, highlighting the immune potency of both GAPs.
Figure 2.
Parasite liver-stage development after challenge. The parasite liver load of entire C57BL/6J livers was quantified by measuring the total flux of photons within each region of interest (red circles in Supplemental Figure 1) and is depicted as mean (photons/second) ± standard error of the mean. ***P < 0.0001 as determined with a one-way analysis of variance using the Tukey's posttest.
It has been suggested that GAPs arresting late during liver stage provide superior protection presumably due to expression of additional parasite proteins during late liver-stage development and formation of daughter merozoites by schizogony.7,8,11 PbΔmrp2 parasites enter schizogony as shown by nuclear division, and develop to an average size of ∼50% compared with fully developed liver-stage schizonts of WT parasites.16 In contrast, PbΔb9Δslarp abort development soon after invasion of the hepatocytes. These parasites remain very small, have a compromised PVM, and show no features of schizogony.14 Apparently, this difference in degree of development between the two GAPs is not sufficient to translate into a difference in induction of protective immune responses.
PbΔmrp2 parasites do produce a PVM in contrast to PbΔb9Δslarp parasites. As the PVM is considered to function as decoy for host defense mechanisms including autophagy,21 it is possible that PbΔb9Δslarp parasites might be more prone to intracellular degradation, resulting in relatively effective antigen presentation. This might explain why PbΔb9Δslarp parasites, despite expressing fewer antigens, induce comparable protective immune responses as PbΔmrp2 parasites. While the late arresting P. yoelii Δfabb/f GAP9 forms a PVM and lacks expression of MSP1, a similar phenotype as compared with the mid-to-late arresting P. berghei Δmrp2 GAP,16 the PyΔfabb/f GAP induces stronger protective immune responses compared with the early arresting PyΔsap1 GAP.11 Whether this is caused by parasite species (P. yoelii versus P. berghei) or difference in antigen presentation between the PyΔfabb/f GAP and the PbΔmrp2 GAP remains to be investigated in comparable immunization/challenge protocols.
While potency is important, it is absolutely essential that GAP immunizations will be safe and do not result in breakthrough blood infections. Several P. berghei GAPs do generate protective immunity, but most produce breakthrough blood infections at high doses.12 The only safe P. berghei GAPs based on single gene deletions are slarp10,13,15 or mrp2 gene knock-out parasites.16 As we have reported here, both studied GAPs are capable of generating highly potent protective immunity in different strains of mice. To further reduce the risks for breakthrough infections, it has been recommended to delete multiple genes targeting vital but independent functions during liver-stage development.22 Currently, two multiple gene deletion GAPs have been generated in P. falciparum, and in both lines the slarp gene has been removed.15,23 These early arresting P. falciparum GAPs have undergone preclinical evaluation for safety and are now being advanced into Phase 1 clinical trials.15,23,24 Combining PfΔslarp with PfΔmrp2, active in unrelated pathways, would remove two genes both of which independently result in complete liver-stage arrest (increases the safety profile of the GAP). Moreover, as PbΔmrp2 is the first mid-to-late arresting GAP that shows both protective efficacy and no evidence for breakthrough blood infections, we believe that P. falciparum GAPs lacking the mrp2 gene merit investigation to further develop a GAP vaccine that is suitable and potent for human use.
Supplementary Material
ACKNOWLEDGMENTS
We thank Stefanie Schönfeld, Mike Peters, Kitty Lemmens-Hermans, Bianca Lemmers-van de Weem, and Iris Lamer-Elemans for their excellent mouse handling. We are grateful for the excellent mosquito handling of Jolanda Klaassen, Astrid Pouwelsen, Laura Pelser-Posthumus, and Jacqueline Kuhnen. Images from the PowerPoint image bank of Servier Medical Art (http://servier.com/) were used for construction of the immunization scheme.
Footnotes
Authors' addresses: Maarten van der Velden, Frans G. M. Russel, and Jan B. Koenderink, Department of Pharmacology and Toxicology, Radboud University Medical Center, Nijmegen, The Netherlands, E-mails: maarten.vandervelden@radboudumc.nl, frans.russel@radboudumc.nl, and jan.koenderink@radboudumc.nl. Sanna R. Rijpma, Geert-Jan van Gemert, Marga van de Vegte-Bolmer, Robert W. Sauerwein, Department of Medical Microbiology, Radboud University Medical Center, Nijmegen, The Netherlands, E-mails: sanna.rijpma@radboudumc.nl, geert-jan.vangemert@radboudumc.nl, marga.vandevegte-bolmer@radboudumc.nl, and robert.sauerwein@radboudumc.nl. Vivienne Verweij, Donders Institute for Brain, Cognition, Radboud University Medical Center, Nijmegen, The Netherlands, and Behaviour, and Department of Anatomy, Radboud University Medical Center, Nijmegen, The Netherlands, E-mail: vivienne.verweij@radboudumc.nl. Séverine Chevalley-Maurel, Blandine M. Franke-Fayard, and Chris J. Janse, Leiden Malaria Research Group, Department of Parasitology, Center of Infectious Diseases, Leiden University Medical Center, Leiden, The Netherlands, E-mails: s.c.chevalley@lumc.nl, b.franke-fayard@lumc.nl, and c.j.janse@lumc.nl.
References
- 1.Mueller AK, Camargo N, Kaiser K, Andorfer C, Frevert U, Matuschewski K, Kappe SH. Plasmodium liver stage developmental arrest by depletion of a protein at the parasite-host interface. Proc Natl Acad Sci USA. 2005;102:3022–3027. doi: 10.1073/pnas.0408442102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mueller AK, Labaied M, Kappe SH, Matuschewski K. Genetically modified Plasmodium parasites as a protective experimental malaria vaccine. Nature. 2005;433:164–167. doi: 10.1038/nature03188. [DOI] [PubMed] [Google Scholar]
- 3.van Dijk MR, Douradinha B, Franke-Fayard B, Heussler V, van Dooren MW, van Schaijk B, van Gemert GJ, Sauerwein RW, Mota MM, Waters AP, Janse CJ. Genetically attenuated, P36p-deficient malarial sporozoites induce protective immunity and apoptosis of infected liver cells. Proc Natl Acad Sci USA. 2005;102:12194–12199. doi: 10.1073/pnas.0500925102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nussenzweig RS, Vanderberg J, Most H, Orton C. Protective immunity produced by the injection of x-irradiated sporozoites of Plasmodium berghei. Nature. 1967;216:160–162. doi: 10.1038/216160a0. [DOI] [PubMed] [Google Scholar]
- 5.Belnoue E, Costa FT, Frankenberg T, Vigario AM, Voza T, Leroy N, Rodrigues MM, Landau I, Snounou G, Renia L. Protective T cell immunity against malaria liver stage after vaccination with live sporozoites under chloroquine treatment. J Immunol. 2004;172:2487–2495. doi: 10.4049/jimmunol.172.4.2487. [DOI] [PubMed] [Google Scholar]
- 6.WHO . Guidelines for Treating Malaria. 3rd edition. Geneva, Switzerland: World Health Organization; 2015. [Google Scholar]
- 7.Nganou-Makamdop K, Sauerwein RW. Liver or blood-stage arrest during malaria sporozoite immunization: the later the better? Trends Parasitol. 2013;29:304–310. doi: 10.1016/j.pt.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 8.Butler NS, Vaughan AM, Harty JT, Kappe SH. Whole parasite vaccination approaches for prevention of malaria infection. Trends Immunol. 2012;33:247–254. doi: 10.1016/j.it.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 9.Vaughan AM, O'Neill MT, Tarun AS, Camargo N, Phuong TM, Aly AS, Cowman AF, Kappe SH. Type II fatty acid synthesis is essential only for malaria parasite late liver stage development. Cell Microbiol. 2009;11:506–520. doi: 10.1111/j.1462-5822.2008.01270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aly AS, Mikolajczak SA, Rivera HS, Camargo N, Jacobs-Lorena V, Labaied M, Coppens I, Kappe SH. Targeted deletion of SAP1 abolishes the expression of infectivity factors necessary for successful malaria parasite liver infection. Mol Microbiol. 2008;69:152–163. doi: 10.1111/j.1365-2958.2008.06271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Butler NS, Schmidt NW, Vaughan AM, Aly AS, Kappe SH, Harty JT. Superior antimalarial immunity after vaccination with late liver stage-arresting genetically attenuated parasites. Cell Host Microbe. 2011;9:451–462. doi: 10.1016/j.chom.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khan SM, Janse CJ, Kappe SH, Mikolajczak SA. Genetic engineering of attenuated malaria parasites for vaccination. Curr Opin Biotechnol. 2012;23:908–916. doi: 10.1016/j.copbio.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 13.Silvie O, Goetz K, Matuschewski K. A sporozoite asparagine-rich protein controls initiation of Plasmodium liver stage development. PLoS Pathog. 2008;4:e1000086. doi: 10.1371/journal.ppat.1000086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Annoura T, van Schaijk BC, Ploemen IH, Sajid M, Lin JW, Vos MW, Dinmohamed AG, Inaoka DK, Rijpma SR, van Gemert GJ, Chevalley-Maurel S, Kielbasa SM, Scheltinga F, Franke-Fayard B, Klop O, Hermsen CC, Kita K, Gego A, Franetich JF, Mazier D, Hoffman SL, Janse CJ, Sauerwein RW, Khan SM. Two Plasmodium 6-Cys family-related proteins have distinct and critical roles in liver-stage development. FASEB J. 2014;28:2158–2170. doi: 10.1096/fj.13-241570. [DOI] [PubMed] [Google Scholar]
- 15.van Schaijk BC, Ploemen IH, Annoura T, Vos MW, Foquet L, van Gemert GJ, Chevalley-Maurel S, van de Vegte-Bolmer M, Sajid M, Franetich JF, Lorthiois A, Leroux-Roels G, Meuleman P, Hermsen CC, Mazier D, Hoffman SL, Janse CJ, Khan SM, Sauerwein RW. A genetically attenuated malaria vaccine candidate based on P. falciparum b9/slarp gene-deficient sporozoites. Elife. 2014;3:e03582. doi: 10.7554/eLife.03582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rijpma SR, van der Velden M, Gonzalez-Pons M, Annoura T, van Schaijk BC, van Gemert GJ, van den Heuvel JJ, Ramesar J, Chevalley-Maurel S, Ploemen IH, Khan SM, Franetich JF, Mazier D, de Wilt JH, Serrano AE, Russel FG, Janse CJ, Sauerwein RW, Koenderink JB, Franke-Fayard BM. Multidrug ABC transporters are essential for hepatic development of Plasmodium sporozoites. Cell Microbiol. 2016;18:369–383. doi: 10.1111/cmi.12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Janse CJ, Franke-Fayard B, Mair GR, Ramesar J, Thiel C, Engelmann S, Matuschewski K, van Gemert GJ, Sauerwein RW, Waters AP. High efficiency transfection of Plasmodium berghei facilitates novel selection procedures. Mol Biochem Parasitol. 2006;145:60–70. doi: 10.1016/j.molbiopara.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 18.Ploemen I, Behet M, Nganou-Makamdop K, van Gemert GJ, Bijker E, Hermsen C, Sauerwein R. Evaluation of immunity against malaria using luciferase-expressing Plasmodium berghei parasites. Malar J. 2011;10:350. doi: 10.1186/1475-2875-10-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Franke-Fayard B, Waters AP, Janse CJ. Real-time in vivo imaging of transgenic bioluminescent blood stages of rodent malaria parasites in mice. Nat Protoc. 2006;1:476–485. doi: 10.1038/nprot.2006.69. [DOI] [PubMed] [Google Scholar]
- 20.Jaffe RI, Lowell GH, Gordon DM. Differences in susceptibility among mouse strains to infection with Plasmodium berghei (ANKA clone) sporozoites and its relationship to protection by gamma-irradiated sporozoites. Am J Trop Med Hyg. 1990;42:309–313. doi: 10.4269/ajtmh.1990.42.309. [DOI] [PubMed] [Google Scholar]
- 21.Prado M, Eickel N, De Niz M, Heitmann A, Agop-Nersesian C, Wacker R, Schmuckli-Maurer J, Caldelari R, Janse CJ, Khan SM, May J, Meyer CG, Heussler VT. Long-term live imaging reveals cytosolic immune responses of host hepatocytes against Plasmodium infection and parasite escape mechanisms. Autophagy. 2015;11:1561–1579. doi: 10.1080/15548627.2015.1067361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Annoura T, Ploemen IH, van Schaijk BC, Sajid M, Vos MW, van Gemert GJ, Chevalley-Maurel S, Franke-Fayard BM, Hermsen CC, Gego A, Franetich JF, Mazier D, Hoffman SL, Janse CJ, Sauerwein RW, Khan SM. Assessing the adequacy of attenuation of genetically modified malaria parasite vaccine candidates. Vaccine. 2012;30:2662–2670. doi: 10.1016/j.vaccine.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 23.Mikolajczak SA, Lakshmanan V, Fishbaugher M, Camargo N, Harupa A, Kaushansky A, Douglass AN, Baldwin M, Healer J, O'Neill M, Phuong T, Cowman A, Kappe SH. A next-generation genetically attenuated Plasmodium falciparum parasite created by triple gene deletion. Mol Ther. 2014;22:1707–1715. doi: 10.1038/mt.2014.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bijker EM, Borrmann S, Kappe SH, Mordmuller B, Sack BK, Khan SM. Novel approaches to whole sporozoite vaccination against malaria. Vaccine. 2015;33:7462–7468. doi: 10.1016/j.vaccine.2015.09.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.