Abstract
Strongyloides hyperinfection syndrome and disseminated strongyloidiasis frequently occur in immunocompromised persons and can lead to high complication and mortality rates. Thus, detection of Strongyloides stercolaris in those patients is crucial. The present study aimed to determine the prevalence of strongyloidiasis and compare the detection rates of different strongyloidiasis detection methods. We conducted a cross-sectional study of 135 adults with various immunocompromising conditions (corticosteroid usage, chemotherapy, hematologic malignancies, organ transplants, use of immunosuppressive agents, and symptomatic human immunodeficiency virus infection) in Phramongkutklao Hospital, Bangkok, Thailand. All patients were asked to undergo serology testing for Strongyloides IgG by indirect enzyme-linked immunosorbent assay (ELISA), and 3 days of stool collection for use in a simple smear along with formalin–ether concentration and agar plate techniques. Prevalence rates of strongyloidiasis were 5% by stool concentration technique, 5.4% by IgG-ELISA, and 6.7% by agar plate culture. Three of the eight strongyloidiasis cases in this study had hyperinfection syndrome. The tested risk factors of age, sex, occupation, and immunocompromising condition were not associated with Strongyloides infestation. Serology was only 42.9% sensitive (positive predictive value), but it was 96.3% specific (negative predictive value). In conclusion, prevalence rates of strongyloidiasis in this study were 5–7%. Although agar plate culture was the most sensitive technique, the other diagnostic methods might be alternatively used.
Introduction
Strongyloidiasis, an infection caused by Strongyloides stercolaris, is a common disease in tropical areas. Serious clinical syndromes, such as hyperinfection syndrome and disseminated strongyloidiasis, usually occur in immunocompromised populations. Typically, these include patients suffering from cell-mediated immune response defects, such as patients receiving corticosteroid therapy, immunosuppressive drugs, or chemotherapy, as well as patients with hematological malignancies, transplants, human immunodeficiency virus (HIV) infection, or human T-lymphotropic virus, type 1 (HTLV-1) infection.1
Diagnosing strongyloidiasis is problematic. Many diagnostic tests are available, but all have limitations. Culture techniques, such as the Harada Mori culture technique, polyethylene tube culture technique, and agar plate culture technique, along with methods used to detect larvae or adult worms are currently the clinical gold standards. However, all of these techniques are limited by the need for fresh stools, time consumed by the technique, high expense, and/or lack of availability in a general setting.2–4 Occasionally, stool concentration techniques have low sensitivities because S. stercolaris has a low parasite load and irregularly passes through stool2; repeated sampling is therefore required to increase sensitivity. Serology by Strongyloides IgG testing is also useful in diagnostic and follow-up testing of chronic strongyloidiasis in endemic areas, refugees from endemic areas and pretransplant patients.5–8 Although various serological tests for detecting strongyloidiasis exist (e.g., indirect immunofluorescence tests, enzyme-linked immunosorbent assays [ELISA], immunoblot tests, and indirect hemagglutination tests), they are all limited by cross-reactivity with other parasites, especially filarial infections.3 Moreover, few studies of serology diagnosis in immunocompromised patients have been published.9
The present study aimed to determine the prevalence of strongyloidiasis in patients with various types of immunocompromising conditions in an urban setting in Thailand, and to compare the detection rates of different methods.
Materials and Methods
The study was conducted in the Department of Medicine, Phramongkutklao Hospital, Bangkok, Thailand, from March 2010 to December 2010. Immunocompromised patients were defined as those receiving treatment with corticosteroids, immunosuppressive drugs, or chemotherapy; patients with hematological malignancies; organ transplantation patients; and HIV-infected patients who were symptomatic (having an acquired immune deficiency syndrome–defining illness) and/or had CD4+ cells < 200/mm3. We excluded patients younger than 18 years of age; patients who could not provide adequate specimens because of gastrointestinal (GI) bleeding; critically ill patients; and those who had received antiparasitic drugs within the 4 weeks before the study. All subjects gave written informed consent. The study was approved by the Ethics Committee of the Royal Thai Army Medical Department.
Sample collection and testing.
Patients were asked for three stool specimens produced on different days after admission. Stool samples were sent to the Department of Parasitology, Phramongkutklao College of Medicine within the same day of collection for fresh stool examination by the simple smear technique and for use in the formalin–ether concentration technique to detect any parasites, as well as use in the agar plate culture method to detect larvae of soil-transmitted helminths, Strongyloides, hookworm, and Trichostrongylus. The agar plate culture method used nutrient agar, which was incubated at 25°C (room temperature was about 30°C) for at least 3 days. The larvae were identified under a light microscope. In addition, modified acid fast bacilli and Trichrome staining, and polymerase chain reaction with primers specific for Blastocystis sp. were performed to identify other parasites. Blood samples were collected for S. stercolaris serology. The molecular weight cutoff antigen (< 30 kDa) in the present study was prepared by using a modified version of a technique previously described by Dekumyoy and others10 The Strongyloides infective larvae extract was filtered through an Ultrafree-MC centrifuge filter tube (Belford, MA; filter code: PLKT, 30 kDa) by centrifugation. In the previous study, the IgG-ELISA using this antigen had a sensitivity of 96.15% and a specificity of 78.44%. In the modified technique, the filtrates (< 30 kDa) were recentrifuged using a new filter tube following the same process. The filtrate was a partially purified antigen, which was determined by an IgG-ELISA. This test was 96% sensitive and 94% specific (P. Dekumyoy, unpublished data). The indirect ELISA was done by detection of Strongyloides-specific IgG at the Department of Helminthology, Faculty of Tropical Medicine, Mahidol University.
All cases of uncomplicated strongyloidiasis were treated with ivermectin, 200 mcg/day, once daily for two consecutive days. Severe forms of strongyloidiasis diseases (hyperinfection syndrome and disseminated strongyloidiasis) were treated with ivermectin, 200 mcg/day, once daily, for 2 weeks after the parasites were cleared.
Two-tailed Fisher's exact tests were used to define associations between selected factors and positive Strongyloides laboratory results. Epi Info v.3.5.3 (CDC, Atlanta, GA) was used for statistical analyses. A value of P < 0.05 was considered significant.
Results
We enrolled 135 patients, including 69 (51.1%) men and 66 (48.9%) women. Their mean age was 48.42 ± 17.23 years. Their mean body mass index was 21.52 ± 4.87 kg/m2. Their mean serum albumin level was 3.26 ± 0.8 g/dL. Their immunocompromising conditions included 54 who received corticosteroids (median dose: 30 mg/day, over a median period of 8 weeks), 53 receiving chemotherapy, 54 with hematologic malignancies, six organ transplantation recipients, 11 receiving immunosuppressive agents, and 20 with symptomatic HIV infections. Forty-nine patients had two or more immunocompromising conditions. Clinicopathological factors did not differ significantly between patients with and those without strongyloidiasis (Table 1).
Table 1.
Clinicopathological data of immunocompromised patients tested for strongyloidiasis
| Variable | Total | Strongyloides-positive (n) | % | P value* |
|---|---|---|---|---|
| Sex | 0.275 | |||
| Male | 69 | 6 | 8.7 | |
| Female | 66 | 2 | 3.0 | |
| Age | 0.690 | |||
| ≥ 60 years | 39 | 3 | 7.7 | |
| < 60 years | 96 | 5 | 5.2 | |
| Occupation | 1.000 | |||
| Soil exposure† | 46 | 3 | 6.5 | |
| No soil exposure | 89 | 5 | 5.6 | |
| HIV infection | 0.604 | |||
| Yes | 20 | 0 | 0.0 | |
| No | 115 | 8 | 7.0 | |
| Steroid treatment | 1.000 | |||
| Yes | 54 | 3 | 5.6 | |
| No | 81 | 5 | 6.2 | |
| Hematologic malignancy | 1.000 | |||
| Yes | 54 | 3 | 5.6 | |
| No | 81 | 5 | 6.2 | |
| Chemotherapy | 0.711 | |||
| Yes | 53 | 4 | 7.5 | |
| No | 82 | 4 | 4.9 | |
| Eosinophilia | 0.547 | |||
| Yes | 12 | 1 | 8.3 | |
| No | 119 | 7 | 5.9 | |
| ≥ 2 immunocompromised conditions | 0.710 | |||
| Yes | 49 | 2 | 4.1 | |
| No | 86 | 6 | 7.0 | |
HIV = human immunodeficiency virus.
Two-tailed Fisher's exact test.
For example, farmers, soldiers, and laborers.
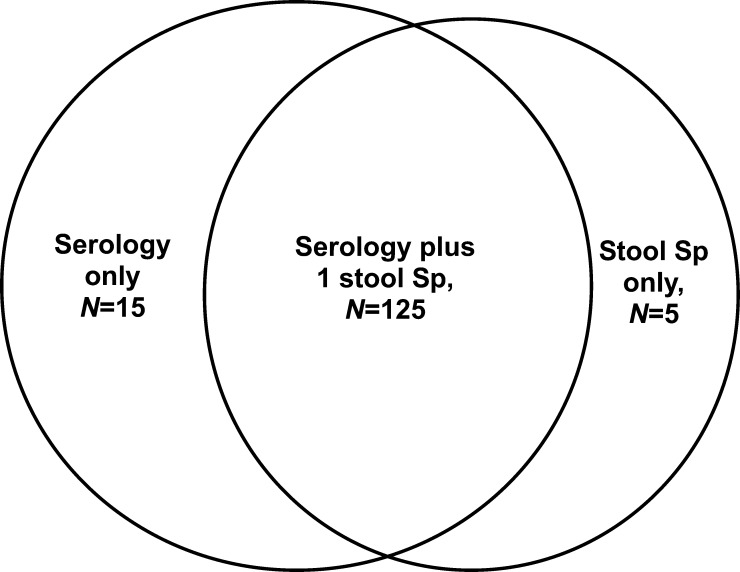
Serum samples were collected from 130 patients. We collected one stool sample each from 120 patients, two samples from 80 patients, and three samples from 46 patients (Figure 1 ). The prevalence rate of strongyloidiasis was 5%, 5.4%, or 6.7%, depending on whether it was assessed by using the stool concentration technique, the IgG-ELISA technique, or the agar plate culture technique. The use of three agar plate cultures for individual patients (the current gold standard) was 75% sensitive and 100% specific, and this method had a 100% positive predictive value (PPV) and a 98.3% negative predictive value (NPV). In contrast, serologic study by IgG-ELISA was only 42.9% sensitive and 96.3% specific, with a 42.9% PPV and a 96.3% NPV (Table 2).
Figure 1.
Numbers and intersection of stool and blood specimens. Sp = specimen.
Table 2.
Sensitivity, specificity, PPV, and NPV of agar plate culture and stool concentration tests of stool specimens obtained over 1, 2, or 3 days
| Methods | Positive/total | Cumulative | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|---|---|
| Agar × 1, n | 7/120 | 7 | 7/8 | 112/112 | 7/7 | 112/113 |
| % | 5.8 | 5.8 | 87.5 | 100.0 | 100.0 | 99.1 |
| Agar × 2, n | 5/80 | 7 | 7/8 | 112/112 | 7/7 | 112/113 |
| % | 6.3 | 5.8 | 87.5 | 100.0 | 100.0 | 99.1 |
| Agar × 3, n | 3/46 | 8* | 8/8 | 112/112 | 8/8 | 112/112 |
| % | 6.5 | 6.7 | 100.0 | 100.0 | 100.0 | 100.0 |
| Conc × 1, n | 6/120 | 6 | 6/8 | 112/112 | 6/6 | 112/114 |
| % | 5.0 | 5.0 | 75.0 | 100.0 | 100.0 | 98.2 |
| Conc × 2, n | 4/80 | 6 | 6/8 | 112/112 | 6/6 | 112/114 |
| % | 5.0 | 5.0 | 75.0 | 100.0 | 100.0 | 98.2 |
| Conc × 3, n | 2/46 | 6 | 6/8 | 112/112 | 6/6 | 112/114 |
| % | 4.3 | 5.0 | 75.0 | 100.0 | 100.0 | 98.2 |
| Serology test, n | 7/130 | – | 3/7 | 104/108 | 3/7 | 104/108 |
| % | 5.4 | – | 42.9 | 96.3 | 42.9 | 96.3 |
Agar = agar plate culture; Conc = stool concentration; NPV = negative predictive value; PPV = positive predictive value.
One additional case detected at the third agar plate culture.
Among the eight patients who were strongyloidiasis-positive, five had chronic strongyloidiasis and three had Strongyloides hyperinfection syndrome (SHS). We did not see any cases of disseminated strongyloidiasis, which is defined by the detection of Strongyloides larva outside of its life cycle.1
Of the three patients with SHS, two were receiving steroids and one had acute myeloid leukemia. All three had fever, and two had diarrhea. In two cases, the diagnoses of SHS was made by the presentation of Strongyloides larvae in the sputum examinations, and in the third case, this diagnosis was made by the presentation of massive amounts of Strongyloides larvae in the stool examination along with Salmonella septicemia. All three SHS cases had coinfections, two of which were Gram-negative bacteremia. One patient had both pulmonary nocardiosis and aspergillosis in addition to the finding of Strongyloides larvae. None of these patients had eosinophilia, nor could an association between eosinophilia and strongyloidiasis be demonstrated from the cohort as a whole (Table 1). Only 50% (four of eight) of the strongyloidiasis patients reported gastrointestinal symptoms, of which the most common symptom was abdominal pain.
Apart from strongyloidiasis, the other detected parasitic infestations in our subjects were Opisthorchis viverrini (0.8%), Entamoeba histolytica (4.2%; confirmed by the visualization, after Trichrome staining, of ingested red blood cells inside of a trophozoite), Giardia intestinalis (1.7%), and Blastocystis hominis (3.3%). Agar plate culture showed no larvae of other soil-transmitted helminths, hookworm, or Trichostrongylus spp.
Discussion
Globally, Thailand is a hotspot for strongyloidiasis.11 Variations in strongyloidiasis prevalence have been observed in different populations, times, and methods of diagnosis. Strongyloidiasis in Thailand has a high prevalence in the northeast and northern parts of the country.12 In 2000, the prevalence rate of strongyloidiasis in northeast Thailand with different methods of detection were 34.2% by the agar plate culture technique and 47.5% by modified unit-based ELISA.12 In 2004, a study of strongyloidiasis in northern Thailand found a prevalence of 15.9%, using both Kato and agar plate culture techniques.13 In the present study, the 6.7% prevalence found by using the agar plate technique was lower than that of the 2004 study in northern Thailand. This may be explained by the different population types assessed in the two studies. Our study was a hospital-based study of immunocompromised adult patients, set in the capital city, whereas the 2004 study was set in a general population in a rural village. Most of the patients in our study had nonagricultural occupations, most commonly administrative work (26.7%). Thus, strongyloidiasis was not associated with soil-exposure occupations in our study (Table 1).
Agar plate culture remained the test of choice in our study, which concords with previous studies in which the agar plate culture method had the best yield in general populations.4,14,15 However, unlike some other studies, ours failed to show increased yields with repeated specimens.16,17 Specifically, Hirata and others16 found that repeated agar plate culture examinations of three stool samples could increase the cumulative detection rate of the mild form of strongyloidiasis by approximately twice that of a single examination.
A meta-analysis study by Schar11 showed that sensitivities of serology testing in community- and hospital-based studies were 88–98% and 94–99%, respectively. In our study, serology was only 42.9% sensitive and 96.3% specific, with a 42.9% PPV and a 96.3% NPV. We observed some positive results from an anti-Strongyloides IgG-ELISA of samples from patients with negative results from stool-based examinations in our study, which could be explained by either previous infections or false positives. In 2001, Schaffel and others9 in Brazil studied the role of ELISAs in the diagnosis of strongyloidiasis in patients with hematologic malignancies, and they also found a low sensitivity of 68%, along with a slightly higher specificity at 89% when the Baermann–Moraes method was used as the gold standard for detecting Strongyloides larvae in three stool samples. The lower sensitivity of serology in the immunocompromised population may reflect a decreased level of antibody production in this population.3,9 Thus, serology-based tests are not a useful screening test for identifying strongyloidiasis in this population. Although serology provided a high specificity, it had a low PPV and a high NPV due to the low prevalence in our study population. However, the high NPV of serology suggests that it can be used as an alternative method to rule out or exclude strongyloidiasis in immunocompromised patients who have negative serology.9
Although the agar plate culture method remains the gold standard for diagnosis of strongyloidiasis in immunocompromised persons, it has the disadvantages of limited availability and a need for fresh stool. In contrast, some other methods can find other helminths and protozoa, whereas the agar plate culture can detect only Strongyloides spp., hookworm, and Trichostrongylus spp. Detection of other helminthiases is also useful in interpreting the results of antibody-detection assays. In addition, drawing blood for serology testing is more convenient than fecal collection in a hospital setting; we could collect 130 serum specimens, but were only able to collect three stool samples each from 46 patients. We recommend the use of multiple methods of Strongyloides detection to complement each other, especially for immunosuppressed patients.1,12
Risk factors for strongyloidiasis, such as chronic alcoholism, HIV infection, and malnutrition (body mass index < 18.5 kg/mm2 or serum albumin < 3 g/dL) were explored in the present study, but no risk factors were identified, which was dissimilar to previous reports (data not shown). Chronic alcoholism was found to be a high risk factor for Strongyloides infection in a study by Marques and others and in the meta-analysis study from Schar and others.11,18 This discrepancy between those studies and ours may be due to the fact that the immunocompromised patients in our population were mainly suffering from malignancies, and only three were active alcoholics.
Strongyloidiasis in HIV infection has been a controversial issue. Although disseminated strongyloidiasis was previously recognized as an opportunistic infection, it was removed from that list in 1987.19 Few studies have shown results about the risk of strongyloidiasis in HIV-infected patients, and corticosteroid use in severe Pneumocystis pneumonia has been suspected as a confounding factor.1,11 In Thailand, a hospital-based study from a northeastern province revealed that the prevalence of strongyloidiasis was significantly higher in HIV-positive patients than in HIV-negative patients; however, data regarding corticosteroid usage was not mentioned in that paper.20
There were limitations to the present study. First, the small sample size with a low prevalence of strongyloidiasis may have inadequate power to detect the different positive rates between tests. Second, as our study had few patients with each immunocompromising condition type, so the results may not be applicable for each disease. Although the ELISA test used in the present study was a noncommercial method, a previous study suggested its high sensitivity and specificity. However, using an in-house ELISA in the present study might limit the application of our findings in other setting. Finally, HTLV1 testing was not applied in our setting because HTLV1 is not endemic in Thailand.
Conclusion
The agar plate method had the highest detection rate for strongyloidiasis among patients with various types of immunocompromising conditions, whereas serology testing showed a low yield in this setting. Our finding supports those of previous studies, which found that strongyloidiasis is a problem in immunocompromised patients in tropical areas and that agar plate culture should be used as the standard for strongyloidiasis diagnosis. However, the burden of severe forms of strongyloidiasis in immunocompromised patients urges investigators to develop more sensitive tests.
ACKNOWLEDGMENTS
We thank all the patients and nurses who were involved in this study for their strong support and Sopon Iamsirithaworn for assisting us with the statistical analysis.
Footnotes
Financial support: This research was supported by funding from the Phramongkutklao College of Medicine and the Faculty of Tropical Medicine, Mahidol University. Its publication was supported by the Faculty of Tropical Medicine, Mahidol University.
Authors' addresses: Viravarn Luvira, Department of Clinical Tropical Medicine, Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand, E-mail: viravarn.luv@mahidol.ac.th. Kitti Trakulhun, Nirattar Chantawat, and Danabhand Phiboonbanakit, Division of Infectious Diseases, Department of Medicine, Phramongkutklao Hospital, Bangkok, Thailand, E-mails: kittitrak@gmail.com, bewty_038@hotmail.com, and padanabhand@gmail.com. Mathirut Mungthin and Tawee Naaglor, Department of Parasitology, Phramongkutklao College of Medicine, Bangkok, Thailand, E-mails: mathirut@hotmail.com and tawee_naaglor@yahoo.com. Wallop Pakdee and Paron Dekumyoy, Department of Helminthology, Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand, E-mails: wallop.pak@mahidol.ac.th and paron.dek@mahidol.edu.
References
- 1.Keiser PB, Nutman TB. Strongyloides stercoralis in the immunocompromised population. Clin Microbiol Rev. 2004;17:208–217. doi: 10.1128/CMR.17.1.208-217.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siddiqui AA, Berk SL. Diagnosis of Strongyloides stercoralis infection. Clin Infect Dis. 2001;33:1040–1047. doi: 10.1086/322707. [DOI] [PubMed] [Google Scholar]
- 3.Requena-Mendez A, Chiodini P, Bisoffi Z, Buonfrate D, Gotuzzo E, Munoz J. The laboratory diagnosis and follow up of strongyloidiasis: a systematic review. PLoS Negl Trop Dis. 2013;7:e2002. doi: 10.1371/journal.pntd.0002002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sato Y, Kobayashi J, Toma H, Shiroma Y. Efficacy of stool examination for detection of Strongyloides infection. Am J Trop Med Hyg. 1995;53:248–250. doi: 10.4269/ajtmh.1995.53.248. [DOI] [PubMed] [Google Scholar]
- 5.Biggs BA, Caruana S, Mihrshahi S, Jolley D, Leydon J, Chea L, Nuon S. Management of chronic strongyloidiasis in immigrants and refugees: is serologic testing useful? Am J Trop Med Hyg. 2009;80:788–791. [PubMed] [Google Scholar]
- 6.Loutfy MR, Wilson M, Keystone JS, Kain KC. Serology and eosinophil count in the diagnosis and management of strongyloidiasis in a non-endemic area. Am J Trop Med Hyg. 2002;66:749–752. doi: 10.4269/ajtmh.2002.66.749. [DOI] [PubMed] [Google Scholar]
- 7.Tomblyn M, Chiller T, Einsele H, Gress R, Sepkowitz K, Storek J, Wingard JR, Young JA, Boeckh MJ. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biol Blood Marrow Transplant. 2009;15:1143–1238. doi: 10.1016/j.bbmt.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Avery RK, Ljungman P. Prophylactic measures in the solid-organ recipient before transplantation. Clin Infect Dis. 2001;33:S15–S21. doi: 10.1086/320899. [DOI] [PubMed] [Google Scholar]
- 9.Schaffel R, Nucci M, Carvalho E, Braga M, Almeida L, Portugal R, Pulcheri W. The value of an immunoenzymatic test (enzyme-linked immunosorbent assay) for the diagnosis of strongyloidiasis in patients immunosuppressed by hematologic malignancies. Am J Trop Med Hyg. 2001;65:346–350. doi: 10.4269/ajtmh.2001.65.346. [DOI] [PubMed] [Google Scholar]
- 10.Dekumyoy P, Somtana K, Jantanawiwat P, Nuamtanong S, Sa-nguankiat S, Nuchfaong S, Janyapoon K, Chindanond D. Improved antigens for IgG-ELISA diagnosis of strongyloidiasis. Southeast Asian J Trop Med Public Health. 2002;33((Suppl 3)):53–59. [PubMed] [Google Scholar]
- 11.Schar F, Trostdorf U, Giardina F, Khieu V, Muth S, Marti H, Vounatsou P, Odermatt P. Strongyloides stercoralis: global distribution and risk factors. PLoS Negl Trop Dis. 2013;7:e2288. doi: 10.1371/journal.pntd.0002288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sithithaworn P, Srisawangwong T, Tesana S, Daenseekaew W, Sithithaworn J, Fujimaki Y, Ando K. Epidemiology of Strongyloides stercoralis in north-east Thailand: application of the agar plate culture technique compared with the enzyme-linked immunosorbent assay. Trans R Soc Trop Med Hyg. 2003;97:398–402. doi: 10.1016/s0035-9203(03)90069-1. [DOI] [PubMed] [Google Scholar]
- 13.Nontasut P, Muennoo C, Sa-nguankiat S, Fongsri S, Vichit A. Prevalence of Strongyloides in northern Thailand and treatment with ivermectin vs. albendazole. Southeast Asian J Trop Med Public Health. 2005;36:442–444. [PubMed] [Google Scholar]
- 14.Salazar SA, Gutierrez C, Berk SL. Value of the agar plate method for the diagnosis of intestinal strongyloidiasis. Diagn Microbiol Infect Dis. 1995;23:141–145. doi: 10.1016/0732-8893(95)00247-2. [DOI] [PubMed] [Google Scholar]
- 15.Khieu V, Schar F, Marti H, Sayasone S, Duong S, Muth S, Odermatt P. Diagnosis, treatment and risk factors of Strongyloides stercoralis in schoolchildren in Cambodia. PLoS Negl Trop Dis. 2013;7:e2035. doi: 10.1371/journal.pntd.0002035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirata T, Nakamura H, Kinjo N, Hokama A, Kinjo F, Yamane N, Fujita J. Increased detection rate of Strongyloides stercoralis by repeated stool examinations using the agar plate culture method. Am J Trop Med Hyg. 2007;77:683–684. [PubMed] [Google Scholar]
- 17.Steinmann P, Zhou XN, Du ZW, Jiang JY, Wang LB, Wang XZ, Li LH, Marti H, Utzinger J. Occurrence of Strongyloides stercoralis in Yunnan Province, China, and comparison of diagnostic methods. PLoS Negl Trop Dis. 2007;1:e75. doi: 10.1371/journal.pntd.0000075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marques CC, da Penha Zago-Gomes M, Goncalves CS, Pereira FE. Alcoholism and Strongyloides stercoralis: daily ethanol ingestion has a positive correlation with the frequency of Strongyloides larvae in the stools. PLoS Negl Trop Dis. 2010;4:e717. doi: 10.1371/journal.pntd.0000717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siegel MO, Simon GL. Is human immunodeficiency virus infection a risk factor for Strongyloides stercoralis hyperinfection and dissemination. PLoS Negl Trop Dis. 2012;6:e1581. doi: 10.1371/journal.pntd.0001581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pinlaor S, Mootsikapun P, Pinlaor P, Pipitgool V, Tuangnadee R. Detection of opportunistic and non-opportunistic intestinal parasites and liver flukes in HIV-positive and HIV-negative subjects. Southeast Asian J Trop Med Public Health. 2005;36:841–845. [PubMed] [Google Scholar]