Abstract
Human anisakiasis is a zoonosis acquired by eating raw or undercooked infected seafood. Herein, we report a case of acute dysentery caused by anisakiasis in a 64-year-old man in Malaysia. A colonoscopy was performed and a nematode larva was found penetrating the mucosa of the ascending colon. Bleeding was observed at the site of penetration. Y-shaped lateral epidermal cords were seen from the cross section of the worm, which is a prominent feature of Anisakis larva. Molecular analysis using polymerase chain reaction of cytochrome oxidase 2 (cox2) gene confirmed the specimen to be larva of Anisakis simplex.
Introduction
Anisakiasis is a zoonosis caused by the nematode belonging to the Anisakis genus.1 Two most common species to cause infection in humans are Anisakis simplex and Anisakis pegreffii.2 This parasite undergoes a complex life cycle that requires multiple hosts. In brief, the adult nematode that resides in the stomach of marine mammals lays unembryonated eggs which are shed through the host's feces. In water, the eggs become embryonated and the larvae mature into the second stage (L2). The free-swimming L2 larvae hatch and are consumed by crustaceans, where they develop into L3 larvae. When fish, squid, clams, or eels ingest the infected crustaceans, the larvae migrate to the new host's muscle tissues. Humans acquire the infection by eating raw or undercooked infected marine fish, squid, clams, or eels.3–5
After ingestion of the L3 larvae, humans can exhibit gastric, intestinal, extraintestinal, or allergic symptoms.3,4,6–8 Patients may present with epigastric pain, nausea, vomiting, symptoms of bowel obstruction, acute abdomen, or symptoms of allergic reaction such as urticaria, and angioedema.3,4,9 These manifestations are predominantly caused by the attachment, embedment, or penetration of the L3 larvae into the gastric and intestinal mucosa.10 The time to the onset of symptoms after ingestion of raw or undercooked infected marine animals vary from a few hours to several weeks.3,10
The larva cannot survive in humans and dies within a few weeks. Humans are an accidental host and larva cannot develop into adult in human tissue. Salting of marine fish does not kill the parasites. The parasites are killed if the fish is frozen at −20°C for a few days or cooking at 60°C.
Although anisakiasis cases were reported mainly in Japan in the past, this condition has now been diagnosed in many countries where eating of raw or undercooked seafood such as sushi and sashimi has become a trend. In Malaysia, with the burgeoning of Japanese restaurants, anisakiasis should be suspected in patients who give a history of ingesting raw or undercooked seafood and presenting with acute gastrointestinal symptoms.
Case Report
A 64-year-old man presented to a private hospital complaining of abdominal discomfort and passing stool with fresh blood in it. The day before presentation, the patient had brought an empurau fish (Tor tambroides) from Sarawak, Malaysian Borneo, to be cooked for dinner at a restaurant in Kuala Lumpur. In less than 30 minutes after ingesting the fish, he developed abdominal discomfort and passed out stool mixed with fresh blood twice. He also admitted to have eaten sushi 2 days before. The patient had a history of ischemic heart disease diagnosed a few years ago, and was started on clopidogrel, an antiplatelet drug.
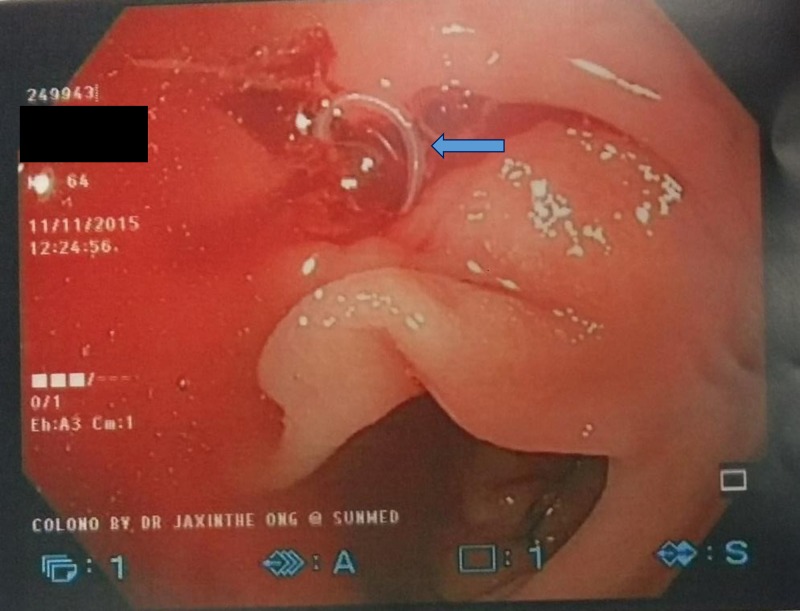
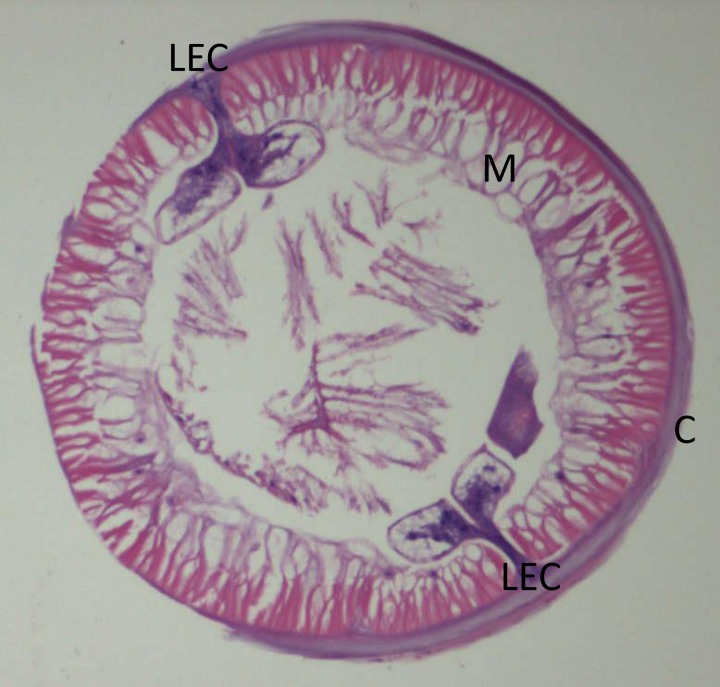
On examination, the patient was afebrile, had a blood pressure of 124/88 mmHg and a pulse rate of 82 beats per minute. Abdominal examination was unremarkable with no tenderness or guarding upon palpation. Blood test showed hemoglobin of 10.1 g/dL, total white cell count of 4.3 k/μL (neutrophils 47%, lymphocytes 40%, monocytes 9%, eosinophils 3%, and basophils 1%), platelet count of 162 k/μL, and erythrocyte sedimentation rate of 7 mm/hour. A colonoscopy was performed on the patient on the same day. A worm was observed burrowing into the mucosa of the ascending colon (Figure 1 ). There was blood oozing from the penetration site with blood clots forming around the worm. The whole worm was pulled out and sent to Parasite Southeast Asia Diagnostic (Para:SEAD) Laboratory, Department of Parasitology, Faculty of Medicine, University of Malaya, for identification. The specimen was examined under a stereomicroscope, and it showed a nematode larva measuring approximately 25 mm long, off-white in color, and moving actively (Figure 2 ). Histological examination of the cross sections of the worm showed the characteristic Y-shaped lateral epidermal cords which is diagnostic of Anisakis (Figure 3 ).
Figure 1.
Colonoscopy image of ascending colon. A worm (arrow) was observed burrowing into the mucosa with blood oozing from the penetration site.
Figure 2.
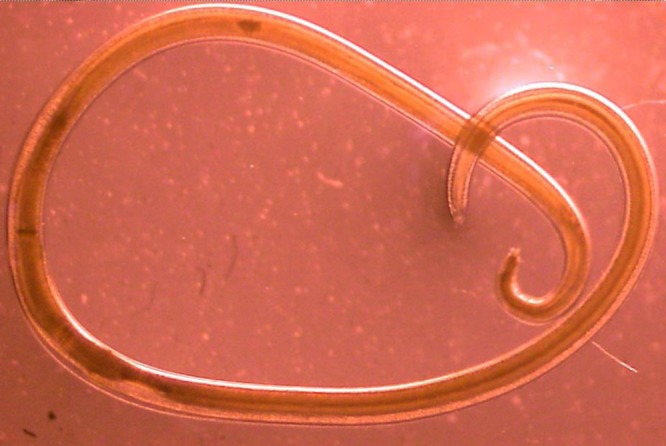
Stereomicroscope examination of Anisakis sp. larva. It has the common features of a nematode with vermiform, nonsegmented body. The mouth is located anteriorly with projections used in feeding.
Figure 3.
Hematoxylin-eosin stained cross section of Anisakis sp. larva. C = external cuticle; M = overlying muscle layer; LEC = Y-shaped lateral epidermal cords (×10 magnification).
For species-specific identification, the worm was therefore subjected to polymerase chain reaction targeting the mitochondrial cytochrome oxidase 2 (cox2) gene as described previously with minor modification.11 In brief, the larva was ground with a sterile pestle using mechanical vortex and the homogenate was then incubated overnight with proteinase K followed by genomic DNA extraction using a commercial kit. DNA amplification was performed and the positive amplicon was subjected to DNA sequencing. Homology search using the National Center for Biotechnology Information (NCBI) reference sequences with the Basic Local Alignment Search Tool confirmed the worm species as A. simplex. The sequence generated was then deposited in NCBI GenBank (accession no. KU257692).
During follow-up at 1 week, the patient was well and did not complain of any more rectal bleeding.
Discussion
After the first human infection reported in 1960 in the Netherlands, cases of anisakiasis have been described in other countries including Korea, Japan, the United Kingdom, Spain, Italy, France, Germany, the United States of America, and Egypt.12 Herein, we report, for the first time, a case of anisakiasis in Malaysia.
Misdiagnosis is common when dealing with anisakiasis due to the nonspecific symptoms of the disease. Diagnosis such as appendicitis, gastric ulcer, gastric tumor, cholecystitis, peritonitis, and Crohn's disease were frequently made before anisakiasis was confirmed.7,10 Furthermore, infection with this nematode can lead to a multitude of complications such as intestinal obstruction, eosinophilic enteritis, eosinophilic granuloma, and spontaneous rupture of the spleen which may further mask the underlying diagnosis.7,13,14
In the present case, the clue to the diagnosis was acquired when the patient gave a history of consuming sushi 2 days before clinical presentation at one of the Japanese restaurants in Kuala Lumpur. The empurau fish from Sarawak that he consumed 1 day before presentation is a freshwater fish and anisakiasis is associated with only marine fish. Since the patient was on clopidogrel, the attending physician was aware that the acute dysentery could also be due to the drug that the patient was taking. Gastrointestinal bleeding is an adverse effect of clopidogrel. On the basis of acute bleeding and the blood test on admission which revealed mild anemia, a colonoscopy was performed which showed bleeding at the site of worm penetration at the ascending colon.
Eosinophilia has been observed in less than half of the patients diagnosed with anisakiasis. In early diagnosis of gastrointestinal anisakiasis, eosinophil count is not useful.15 Eosinophil count was not raised in this patient. This may be explained by the fact that the larva had just started to burrow its way through the ascending colon and diagnosis was made early. Therefore, it may have been too early for the eosinophilic response.
Investigations that can aid clinicians in diagnosing anisakiasis include endoscopic examination that may provide visual evidence and removal of the worm for identification as has been performed in this patient. Imaging modalities such as barium studies or computed tomography scan may reveal narrowing of intestinal lumen. Serological studies have been used to detect Anisakis-induced IgE.4,6,16,17 This antibody, however, lacks specificity as a result of cross-reactivity with other nematode antigens. The serological test is not generally available and it is of limited benefit in early diagnosis. Nevertheless, there have been many instances where the diagnosis was only made after viewing histological sections from resected bowel after laparotomy.3,15,18 The most prominent morphological feature from a cross section of the nematode are the Y-shaped lateral epidermal cords.3,15 However, morphological identification is dependent on how well the worm has been conserved. Therefore, the molecular method has recently been used for the identification of Anisakis larvae.19,20 A very simple yet often overlooked aspect of diagnosis is acquiring history of ingestion of raw fish or seafood. This history is the only definite clue to the diagnosis.21
Unlike other helminths that infect humans, the Anisakis larvae do not respond to anthelmintics such as mebendazole, albendazole, or thiabendazole.22 The preferred treatment is the extraction of the worm via endoscopy.3 However, in cases of surgical complications, laparotomy or bowel resection may have to be performed.3,7,18 Corticosteroids are helpful to reduce the inflammatory response to the worm or when dealing with allergic reactions.10,23 Recently, conservative therapy has been found to be successful in treating intestinal anisakiasis.24 The conservative therapy that Shrestha and others suggest, requires the correct diagnosis of intestinal anisakiasis followed by close monitoring, the patient kept nil by mouth, insertion of a nasogastric tube, and analgesics for pain management.24 Patients are only allowed oral diet once the abdominal symptoms have subsided.
Culinary vacation and the introduction of food and cooking methods from foreign countries are becoming a trend worldwide. This trend may include eating raw or lightly cooked seafood. Anisakiasis, a seafood-borne disease that has never been seen in Malaysia is now reported for the first time. Therefore, clinicians in this part of the world should be aware of this diagnosis. Detailed history including dietary history has to be taken from patients with gastrointestinal symptoms. Thorough physical examination and investigation cannot be overemphasized.
Footnotes
Financial support: This work was supported by the University of Malaya High Impact Research Grant (UM.C/HIR/MOHE/MED/16) from the Ministry of Higher Education, Malaysia.
Authors' addresses: Amirah Amir, Romano Ngui, Wan Hafiz Wan Ismail, Yvonne A. L. Lim, Yee-Ling Lau, and Rohela Mahmud, Department of Parasitology, Faculty of Medicine, University of Malaya, Kuala Lumpur, Malaysia, E-mails: amirahamir@hotmail.my, skyromano@gmail.com, wanhafiz@ummc.edu.my, yvonne@ummc.edu.my, lauyeeling@um.edu.my, and rohela@ummc.edu.my. Kum T. Wong, Department of Pathology, Faculty of Medicine, University of Malaya, Kuala Lumpur, Malaysia, E-mail: wongkt@ummc.edu.my. Jaxinthe S. K. Ong, Sunway Medical Centre, Selangor, Malaysia, E-mail: jaxint@gmail.com.
References
- 1.Mattiucci S, Nascetti G. Advances and trends in the molecular systematics of anisakid nematodes, with implications for their evolutionary ecology and host-parasite co-evolutionary processes. Adv Parasitol. 2008;66:47–148. doi: 10.1016/S0065-308X(08)00202-9. [DOI] [PubMed] [Google Scholar]
- 2.Pozio E. Integrating animal health surveillance and food safety: the example of Anisakis. Rev Sci Tech. 2013;32:487–496. doi: 10.20506/rst.32.2.2246. [DOI] [PubMed] [Google Scholar]
- 3.Shweiki E, Rittenhouse DW, Ochoa JE, Punja VP, Zubair MH, Baliff JP. Acute small-bowel obstruction from intestinal anisakiasis after the ingestion of raw clams; documenting a new method of marine-to-human parasitic transmission. Open Forum Infect Dis. 2014;1:ofu087. doi: 10.1093/ofid/ofu087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bucci C, Gallotta S, Morra I, Fortunato A, Ciacci C, Iovino P. Anisakis, just think about it in an emergency! Int J Infect Dis. 2013;17:e1071–e1072. doi: 10.1016/j.ijid.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 5.Cho J, Lim H, Jung BK, Shin EH, Chai JY. Anisakis pegreffii larvae in sea eels (Astroconger myriaster) from the South Sea, Republic of Korea. Korean J Parasitol. 2015;53:349–353. doi: 10.3347/kjp.2015.53.3.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moreno-Ancillo A, Caballero MT, Cabanas R, Contreras J, Martin-Barroso JA, Barranco P, Lopez-Serrano MC. Allergic reactions to Anisakis simplex parasitizing seafood. Ann Allergy Asthma Immunol. 1997;79:246–250. doi: 10.1016/S1081-1206(10)63009-8. [DOI] [PubMed] [Google Scholar]
- 7.Valle J, Lopera E, Sanchez ME, Lerma R, Ruiz JL. Spontaneous splenic rupture and Anisakis appendicitis presenting as abdominal pain: a case report. J Med Case Reports. 2012;6:114. doi: 10.1186/1752-1947-6-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fernandez de Corres L, Audicana M, Del Pozo MD, Munoz D, Fernandez E, Navarro JA, Garcia M, Diez J. Anisakis simplex induces not only anisakiasis: report on 28 cases of allergy caused by this nematode. J Investig Allergol Clin Immunol. 1996;6:315–319. [PubMed] [Google Scholar]
- 9.Esteve C, Resano A, Diaz-Tejeiro P, Fernandez-Benitez M. Eosinophilic gastritis due to Anisakis: a case report. Allergol Immunopathol (Madr) 2000;28:21–23. [PubMed] [Google Scholar]
- 10.Sakanari JA, McKerrow JH. Anisakiasis. Clin Microbiol Rev. 1989;2:278–284. doi: 10.1128/cmr.2.3.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nadler SA, Hudspeth DS. Ribosomal DNA and phylogeny of the Ascaridoidea (Nemata: Secernentea): implications for morphological evolution and classification. Mol Phylogenet Evol. 1998;10:221–236. doi: 10.1006/mpev.1998.0514. [DOI] [PubMed] [Google Scholar]
- 12.Audicana MT, Kennedy MW. Anisakis simplex: from obscure infectious worm to inducer of immune hypersensitivity. Clin Microbiol Rev. 2008;21:360–379. doi: 10.1128/CMR.00012-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gomez B, Tabar AI, Tunon T, Larrinaga B, Alvarez MJ, Garcia BE, Olaguibel JM. Eosinophilic gastroenteritis and Anisakis. Allergy. 1998;53:1148–1154. doi: 10.1111/j.1398-9995.1998.tb03834.x. [DOI] [PubMed] [Google Scholar]
- 14.Nawa Y, Hatz C, Blum J. Sushi delights and parasites: the risk of fishborne and foodborne parasitic zoonoses in Asia. Clin Infect Dis. 2005;41:1297–1303. doi: 10.1086/496920. [DOI] [PubMed] [Google Scholar]
- 15.Caramello P, Vitali A, Canta F, Caldana A, Santi F, Caputo A, Lipani F, Balbiano R. Intestinal localization of anisakiasis manifested as acute abdomen. Clin Microbiol Infect. 2003;9:734–737. doi: 10.1046/j.1469-0691.2003.00660.x. [DOI] [PubMed] [Google Scholar]
- 16.van Thiel P, Kuipers FC, Roskam RT. A nematode parasitic to herring, causing acute abdominal syndromes in man. Trop Geogr Med. 1960;12:97–113. [PubMed] [Google Scholar]
- 17.Anadon AM, Rodriguez E, Garate MT, Cuellar C, Romaris F, Chivato T, Rodero M, Gonzalez-Diaz H, Ubeira FM. Diagnosing human anisakiasis: recombinant Ani s 1 and Ani s 7 allergens versus the UniCAP 100 fluorescence enzyme immunoassay. Clin Vaccine Immunol. 2010;17:496–502. doi: 10.1128/CVI.00443-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ito Y, Ikematsu Y, Yuzawa H, Nishiwaki Y, Kida H, Waki S, Uchimura M, Ozawa T, Iwaoka T, Kanematsu T. Chronic gastric anisakiasis presenting as pneumoperitoneum. Asian J Surg. 2007;30:67–71. doi: 10.1016/S1015-9584(09)60131-7. [DOI] [PubMed] [Google Scholar]
- 19.Mattiucci S, Paoletti M, Borrini F, Palumbo M, Palmieri RM, Gomes V, Casati A, Nascetti G. First molecular identification of the zoonotic parasite Anisakis pegreffii (Nematoda: Anisakidae) in a paraffin-embedded granuloma taken from a case of human intestinal anisakiasis in Italy. BMC Infect Dis. 2011;11:82. doi: 10.1186/1471-2334-11-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Umehara A, Kawakami Y, Araki J, Uchida A, Sugiyama H. Molecular analysis of Japanese Anisakis simplex worms. Southeast Asian J Trop Med Public Health. 2008;39:26. [Google Scholar]
- 21.Ido K, Yuasa H, Ide M, Kimura K, Toshimitsu K, Suzuki T. Sonographic diagnosis of small intestinal anisakiasis. J Clin Ultrasound. 1998;26:125–130. doi: 10.1002/(sici)1097-0096(199803/04)26:3<125::aid-jcu3>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 22.Plath F, Holle A, Zendeh D, Moller FW, Barten M, Reisinger EC, Liebe S. Anisakiasis of the stomach: a case report from Germany [in German] Z Gastroenterol. 2001;39:177–180. doi: 10.1055/s-2001-11150. [DOI] [PubMed] [Google Scholar]
- 23.Morlier D, Thiebault S, Dalcher G, Zeyer B, Muller J, Bader R. A rare etiology of acute occlusion of the small intestine: anisakiasis. Review of the literature apropos of a case [in French] Ann Gastroenterol Hepatol (Paris) 1989;25:99–103. [PubMed] [Google Scholar]
- 24.Shrestha S, Kisino A, Watanabe M, Itsukaichi H, Hamasuna K, Ohno G, Tsugu A. Intestinal anisakiasis treated successfully with conservative therapy: importance of clinical diagnosis. World J Gastroenterol. 2014;20:598–602. doi: 10.3748/wjg.v20.i2.598. [DOI] [PMC free article] [PubMed] [Google Scholar]