Abstract
Gnathostomiasis is a zoonotic parasitosis endemic in many Asian and some Latin American countries. Most human infections are caused by Gnathostoma spinigerum in Asia and Gnathostoma binucleatum in the Americas, and recently, imported cases have been increasing among travelers returning from endemic regions. Confirmation of the clinical diagnosis relies largely on serologic tests, with a G. spinigerum-antigen-based immunoblot currently being the diagnostic method of choice. However, we repeatedly experienced that sera from patients with clinically suspected American gnathostomiasis gave negative results in this assay. Therefore, we used homologous methods to prepare G. spinigerum- and G. binucleatum-antigen-based immunoblot assays, and evaluated the cross-reactivity of the two assays. The results show incomplete cross-reactivity between the two assays: the G. spinigerum-antigen-based immunoblot apparently only detects Asian gnathostomiasis caused by G. spinigerum, whereas the G. binucleatum-antigen-based immunoblot is apparently capable of detecting American as well as Asian gnathostomiasis.
Introduction
Human gnathostomiasis is an endemic foodborne parasitic zoonosis in many Asian and in some Latin America countries.1 Gnathostoma species are parasitic nematodes following a three-host life cycle with a copepod as first intermediate host harboring the parasite's second-stage larva, a freshwater fish as second intermediate host harboring the third-stage larvae (AL3), and a carnivorous mammal as definitive host in which the parasite reaches maturity. Humans become infected with advanced AL3 by eating raw or poorly cooked freshwater fish (or another second-intermediate host). In humans, however, the parasites are unable to mature further, but instead migrate, still in their AL3 stage, through human tissues, causing a larva migrans syndrome (Figure 1 ).
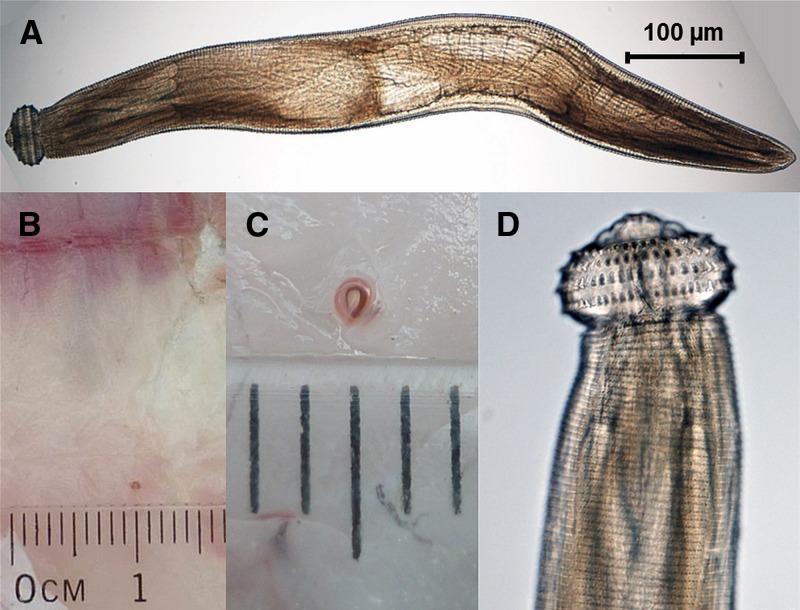
Figure 1.
Morphological characteristics of Gnathostoma larvae: (A) advanced third-stage larvae (AL3) of Gnathostoma binucleatum, (B, C) encysted AL3 larva in the muscle of freshwater fish, and (D) characteristic cephalic region of Gnathostoma spp. covered by transverse rows of cuticular spines and the adjacent body covered with transverse rows of flat spines.
The parts of the world with the highest incidence of gnathostomiasis are those where raw fish is part of the standard or traditional cuisine. The most common clinical presentation and hallmark of human gnathostomiasis are intermittent subcutaneous swellings or serpiginous creeping eruptions that may appear anywhere on the body (cutaneous larva migrans syndrome). The larvae are capable of migrating with a speed of up to 1 cm/hour and, if untreated, for many years through the host's body.2 When migrating deeper, larvae may penetrate and migrate through muscles and internal organs causing visceral larva migrans syndrome. In rare cases, invasion of the central nervous system (both the brain and spinal cord) can lead to neurological sequelae or even be fatal,3 although such cases have almost exclusively been reported from Thailand.4
The diagnosis of human gnathostomiasis is mainly based on the patient's history (living in or traveling to endemic regions, dietary intake history) and the clinical symptoms. A definitive diagnosis requires identification of the nematode in a tissue specimen, but this is feasible only in cases of cutaneous or other superficial migration of the parasite; therefore, serology is usually the method of choice to confirm the clinical diagnosis. The currently most widely used serological assay in Asia and Europe is an immunoblot (Western blot), testing patients' sera against a crude antigen preparation of Gnathostoma spinigerum AL3. The detection of a G. spinigerum-specific 24-kDa band in this assay is considered diagnostic of gnathostomiasis.5,6 In the absence of commercially available test kits, serological testing is currently restricted to several research institutions that offer diagnostic services (e.g., Hospital for Tropical Diseases, Mahidol University, Bangkok, Thailand; Swiss Tropical and Public Health Institute [Swiss TPH], Basel, Switzerland).
When testing sera of patients presenting with larva migrans syndrome for gnathostomiasis in serological assays, cross-reactivity to other tissue-invasive helminths (e.g., Anisakis, Angiostrongylus, Strongyloides, and Toxocara) as well as Gnathostoma species specificity demands consideration. Especially, the latter is problematic, as the cross-reactivity pattern among the six currently known zoonotic Gnathostoma species is largely unknown (Table 1).
Table 1.
Human-pathogenic Gnathostoma species, their geographic distribution, and their definitive animal hosts7
| Human-pathogenic Gnathostoma species | Geographic distribution | Definitive animal hosts |
|---|---|---|
| Gnathostoma spinigerum* | China, India, Japan, southeast Asia | Wild and domestic cats and dogs |
| Gnathostoma binucleatum* | Central and South America | Wild and domestic cats and dogs |
| Gnathostoma doloresi | Japan, southeast Asia | Wild and, probably, domestic pigs |
| Gnathostoma hispidum | Australia, Central America, China, Japan, Korea, southeast Asia | Wild and domestic pigs |
| Gnathostoma malaysiae | Japan, southeast Asia | Rats |
| Gnathostoma nipponicum | Korea, Japan | Weasels |
Most human infections are caused by G. spinigerum in Asia and G. binucleatum in Latin America.
Although G. spinigerum has long been recognized to cause the majority of human infections in Asia, the species responsible for the majority of human cases in the Americas was only more recently identified. The first cases of human gnathostomiasis in the Americas were described in 1970 in Mexico8 and in 1979 in Ecuador.9 Although early morphological studies of larvae obtained by biopsy from patients in Mexico and Ecuador described the larvae to be similar to those of G. spinigerum,9–12 later molecular genetic analysis revealed that human gnathostomiasis in Mexico and Ecuador is probably caused exclusively by the morphologically very similar species Gnathostoma binucleatum. Furthermore, it appears that G. spinigerum is not naturally present in the American continents, although it was recently detected in live Asian swamp eels that were legally imported for sale in ethnic food markets in the United States.13–16
Over the years, we repeatedly noticed that sera from patients with suspected American gnathostomiasis (including a series of Peruvian patients seen at the Department for Infectious Diseases, Tropical Medicine and Dermatology, Universidad Peruana Cayetano Heredia, Lima, Peru, of which some were even confirmed by excisional biopsy) tested negative in our G. spinigerum-antigen-based immunoblot (Marti, H, unpublished data). As we suspected incomplete cross-reactivity among Gnathostoma spp. to account for this failure, the reference laboratory for human parasitic diseases at the Swiss TPH developed, in analogy to the established in-house G. spinigerum-antigen-based immunoblot, a G. binucleatum-antigen-based immunoblot, and evaluated the reaction patterns of reference sera in both assays.
Materials and Methods
Advanced AL3 of G. spinigerum were isolated from liver tissue of Asian swamp eels (Synbranchidae: Monopterus albus) caught in the vicinity of Vientiane, Lao People's Democratic Republic. (Note: the immunoblot prepared from crude antigen of these larvae has been part of the serodiagnostic panel offered by the reference laboratory for human parasitic diseases at the Swiss TPH, since 2002.) AL3 of G. binucleatum were isolated from muscle tissue of the freshwater fish “Huanchiche”/tiger fish (Erythrinidae: Hoplias microlepis) caught in rice fields in the vicinity of Samborondon, Ecuador. After microscope-aided preparation from host tissue, larvae were stored in normal saline solution at −18°C for several days until shipment on dry ice and storage at −80°C at the Swiss TPH pending further processing. To confirm the species of the Gnathostoma larvae, internal transcribed spacer (ITS-2) gene sequences of two randomly selected larvae from each location were individually determined according to published protocols.13,14,17 Antigen extraction from larvae, antigen preparation, and immunoblotting were performed according to published protocols.5,6 We cross-tested positive and negative clinical reference sera as well as three sera from patients with suspected American gnathostomiasis in both assays. The three patients' sera were sent to the Swiss TPH reference laboratory for human parasitic diseases from the United States, Chile, and Sweden. The U.S. American patient developed the typical clinical and histological hallmarks of cutaneous gnathostomiasis after consuming “ceviche” (a traditional Latin American raw fish dish) in Belize and Guatemala [approximately 6 months before onset of symptoms], and in the United States [approximately 2–3 weeks before onset of symptoms]). Unfortunately, the specific travel and food history of the Chilean and Swedish patients were not available.
Ethics.
Fish and eels used in the study were bought dead at local fish markets. Approval for the use of dead fish was obtained from the Ethics Committee (Cómite Institutional de Ética para el Uso de Animales) at the University Peruana Cayetano Heredia: CEIA-60114. The serum samples used in this study were sent to the Swiss TPH for diagnostic purposes. After routine diagnostic, residual sera were routinely stored anonymized in the serum bank of the Diagnostic Center of the Swiss TPH for research purposes. Approval for the use of these anonymized sera for the evaluation of serodiagnostic tests was obtained from the Ethics Committee for northwest and central Switzerland: EKNZ-UBE-15/22. In addition, we obtained written informed consent from the U.S. patient as the serum sample was, unlike the Chilean and Swedish serum samples, not anonymized, and the patient thus traceable.
Results
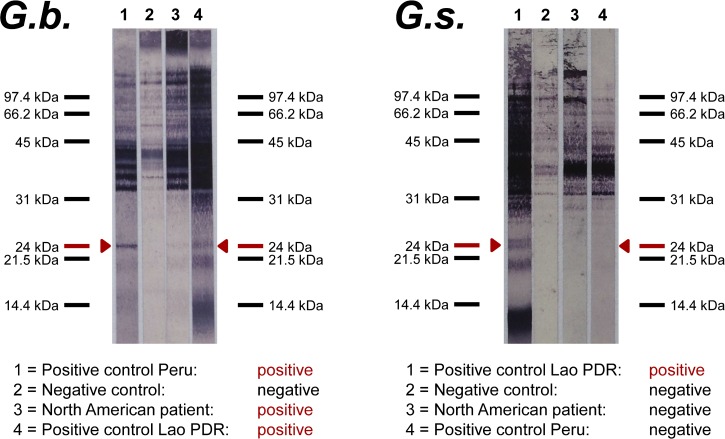
ITS-2 sequencing of AL3 obtained from Laotian swamp eels showed 99% similarity to the published G. spinigerum reference sequence (GenBank accession Z97175), and the ITS-2 sequences of Ecuadorian larvae was 100% identical to the published G. binucleatum ITS-2 reference sequence (GenBank accession Z79072), thus confirming the two different species as antigen sources used for the immunoblot preparations. The results of the G. spinigerum- and the G. binucleatum-antigen-based western blots were as follows: The G. spinigerum-antigen-based immunoblot showed the diagnostic 24-kDa band when tested with the reference serum of a Laotian patient with clinical gnathostomiasis, whereas it gave a negative result with the reference serum of a Peruvian patient with clinical gnathostomiasis, and it did not react with the sera of the U.S. American, the Chilean, and the Swedish patients. In contrast, the G. binucleatum-antigen-based immunoblot showed the diagnostic 24-kDa band when tested with the reference sera of the Laotian and the Peruvian patients, and also reacted with the sera of the U.S. American, the Chilean, and the Swedish patients. Figure 2 shows the cross-reaction patterns of the two assays tested with the Laotian and Peruvian reference sera and the U.S. patient's serum. For clarity, we refrained from integrating the immunoblot images of the Chilean and Swedish patient into Figure 2: both immunoblots show the same cross-reactivity and intensity patterns as did the U.S. patient. The alignment of all the blot images was unfeasible as the sera were tested on different dates with different running times, thus deviating in the scaling of the standard molecular weight ladders.
Figure 2.
Results of the Gnathostoma binucleatum (G.b.)- and Gnathostoma spinigerum (G.s.)-antigen-based western blots.
Discussion
Gnathostoma spinigerum and G. binucleatum cause most cases of human gnathostomiasis in Asia and the Americas, respectively. For the diagnosis of G. spinigerum infections, a G. spinigerum-AL3-antigen-based immunoblot, showing excellent sensitivity and specificity,5 is currently the diagnostic method of choice. This assay is available in reference laboratories in Asia and Europe.2 However, the sensitivity and specificity of this assay in patients infected with American Gnathostoma spp. remain unclear.
In the Americas, most reported cases of human gnathostomiasis are from Mexico, where local studies have successfully evaluated somatic and excretory-secretory antigens of G. binucleatum AL3 in immunoblot assays to diagnose human gnathostomiasis.18,19 No study has ever evaluated the usage of G. binucleatum AL3 in immunoblot assays in Ecuador or Peru, where human gnathostomiasis occurs.20 However, a Gnathostoma doloresi antigen-based enzyme-linked immunosorbent assay (ELISA) has successfully been used to diagnose gnathostomiasis in Ecuadorian patients in the past.21 In the published literature, we found only one study investigating the cross-reactivity of serological assays with regard to Asian and American gnathostomiasis: Ishiwata and colleagues tested sera of patients with clinical gnathostomiasis from Mexico, where G. binucleatum is endemic, and from Japan, where G. doloresi predominates, using ELISAs and immunoblots prepared from adult-worm extracts of G.spinigerum, Gnathostoma hispidum, and G. doloresi.22 The authors concluded that each of the three adult-worm extracts was equally useful to diagnose gnathostomiasis by ELISA and immunoblot, regardless of the causative species. However, we found no published study that investigated the cross-reactivity of serological assays analogously prepared from AL3 extracts.
Therefore, we developed, in parallel to the established G. spinigerum-AL3-antigen-based immunoblot, a G. binucleatum-AL3-antigen-based immunoblot and evaluated the cross-reactivity pattern between and within both assays. Although our current data are limited, and the performance of the two immunoblots needs to be further validated using sera from larger cohorts, two important issues are offered for discussion. First, the G. spinigerum-antigen-based immunoblot fails to detect South American gnathostomiasis, presumably caused by G. binucleatum. This observation is of considerable clinical importance, considering that this assay is currently the diagnostic test of choice offered by reference laboratories in Asia and Europe, and the fact that many physicians ordering this test may not be aware of the different Gnathostoma spp. endemic in Asia and the Americas. Second, we found that an analogously prepared G. binucleatum-AL3-antigen-based immunoblot is apparently capable of detecting American as well as Asian gnathostomiasis. Although these preliminary results are promising, the G. binucleatum-AL3-antigen-based immunoblot still needs to be validated before the assay can be implemented in routine diagnostics. As done with the G. spinigerum-AL3-antigen-based immunoblot in the past, we are currently evaluating the assay's reactivity with sera from patients with other confirmed tissue-invasive nematode infections to assess the assay's specificity.
In recent years, gnathostomiasis has emerged as an important differential diagnosis in international travelers presenting with larva migrans syndrome7 after returning from endemic as well as previously unknown endemic regions.23 We would like to highlight that the currently most widely used G. spinigerum-based serodiagnostic assay should be interpreted with caution if a patient is likely to be infected with a non-spinigerum Gnathostoma species, especially with G. binucleatum in the Americas.
Footnotes
Authors' addresses: Andreas Neumayr, Beatrice Nickel, and Hanspeter Marti, Medical Services and Diagnostic, Swiss Tropical and Public Health Institute, Basel, Switzerland, E-mails: andreas.neumayr@unibas.ch, beatrice.nickel@unibas.ch, and hanspeter.marti@unibas.ch. Jose Ollague, Dermatopathology Section, Universidad Central del Ecuador, Guayaquil, Ecuador, E-mail: dr_ollague@hotmail.com. Francisco Bravo and Eduardo Gotuzzo, Instituto de Medicina Tropical Alexander von Humboldt, Universidad Peruana Cayetano Heredia, Lima, Peru, E-mails: fbravopuccio@gmail.com and eduardo.gotuzzo@upch.pe. Pedro Jimenez, Research Department, Foundation for Marine Coastal Management and Research (FEMM), Guayaquil, Ecuador, E-mail: peterjoe01@yahoo.es. Scott Norton, Department of Dermatology, Children's National Medical Center, Washington, DC, E-mail: scottanorton@gmail.com. Pham Ngoc Doanh, Institute of Ecology and Biological Resources, Vietnam Academy of Science and Technology, Hanoi, Vietnam, E-mail: pndoanh@iebr.ac.vn. Yukifumi Nawa, Research Division, Faculty of Medicine, Khon Kaen University, Khon Kaen, Thailand, E-mail: yukinawa@kku.ac.th. Yoichiro Horii, Department of Veterinary Sciences, Faculty of Agriculture, University of Miyazaki, Miyazaki, Japan, E-mail: horii@cc.miyazaki-u.ac.jp.
References
- 1.Nawa Y. Historical review and current status of gnathostomiasis in Asia. Southeast Asian J Trop Med Public Health. 1991;22((Suppl)):217–219. [PubMed] [Google Scholar]
- 2.Herman JS, Chiodini PL. Gnathostomiasis, another emerging imported disease. Clin Microbiol Rev. 2009;22:484–492. doi: 10.1128/CMR.00003-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schmutzhard E, Boongird P, Vejjajiva A. Eosinophilic meningitis and radiculomyelitis in Thailand, caused by CNS invasion of Gnathostoma spinigerum and Angiostrongylus cantonensis. J Neurol Neurosurg Psychiatry. 1988;51:80–87. doi: 10.1136/jnnp.51.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Katchanov J, Sawanyawisuth K, Chotmongkoi V, Nawa Y. Neurognathostomiasis, a neglected parasitosis of the central nervous system. Emerg Infect Dis. 2011;17:1174–1180. doi: 10.3201/eid1707.101433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nopparatana C, Setasuban P, Chaicumpa W, Tapchaisri P. Purification of Gnathostoma spinigerum specific antigen and immunodiagnosis of human gnathostomiasis. Int J Parasitol. 1991;21:677–687. doi: 10.1016/0020-7519(91)90079-m. [DOI] [PubMed] [Google Scholar]
- 6.Tapchaisri P, Nopparatana C, Chaicumpa W, Setasuban P. Specific antigen of Gnathostoma spinigerum for immunodiagnosis of human gnathostomiasis. Int J Parasitol. 1991;21:315–319. doi: 10.1016/0020-7519(91)90033-4. [DOI] [PubMed] [Google Scholar]
- 7.Diaz JH. Gnathostomiasis: an emerging infection of raw fish consumers in Gnathostoma nematode-endemic and nonendemic countries. J Travel Med. 2015;22:318–324. doi: 10.1111/jtm.12212. [DOI] [PubMed] [Google Scholar]
- 8.Pelaez D, Perez-Reyes R. Gnathostomiasis in America [in Spanish] Rev Latinoam Microbiol. 1970;12:83–91. [PubMed] [Google Scholar]
- 9.Ollague W, Ollague J, Guevara de Veliz A. Eosinophilic migratory nodular panniculitis (human gnathostomiasis in Ecuador). 1st finding of the parasite in South America [in Spanish] Med Cutan Ibero Lat Am. 1982;10:73–78. [PubMed] [Google Scholar]
- 10.Ogata K, Nawa Y, Akahane H, Diaz Camacho SP, Lamothe-Argumedo R, Cruz-Reyes A. Short report: gnathostomiasis in Mexico. Am J Trop Med Hyg. 1998;58:316–318. doi: 10.4269/ajtmh.1998.58.316. [DOI] [PubMed] [Google Scholar]
- 11.Ollague W. Gnathostomiasis (nodular migratory eosinophilic panniculitis) J Am Acad Dermatol. 1985;13:835–836. doi: 10.1016/s0190-9622(85)80412-6. [DOI] [PubMed] [Google Scholar]
- 12.Ollague W, Ollague J, Guevara de Veliz A, Penaherrera S. Human gnathostomiasis in Ecuador (nodular migratory eosinophilic panniculitis). First finding of the parasite in South America. Int J Dermatol. 1984;23:647–651. doi: 10.1111/j.1365-4362.1984.tb01224.x. [DOI] [PubMed] [Google Scholar]
- 13.Almeyda-Artigas RJ, Bargues MD, Mas-Coma S. ITS-2 rDNA sequencing of Gnathostoma species (Nematoda) and elucidation of the species causing human gnathostomiasis in the Americas. J Parasitol. 2000;86:537–544. doi: 10.1645/0022-3395(2000)086[0537:IRSOGS]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 14.Bertoni-Ruiz F, Lamothe-Argumedo R, García-Prieto L, Osorio-Sarabia D, León-Régagnon V. Systematics of the genus Gnathostoma (Nematoda: Gnathostomatidae) in the Americas. Rev Mex Biodivers. 2011;82:453. [Google Scholar]
- 15.Razo L. Gnathostoma and gnathostomiasis in Ecuador. Southeast Asian J Trop Med Public Health. 2004;35:92–96. [Google Scholar]
- 16.Cole RA, Choudhury A, Nico LG, Griffin KM. Gnathostoma spinigerum in live Asian swamp eels (Monopterus spp.) from food markets and wild populations, United States. Emerg Infect Dis. 2014;20:634–642. doi: 10.3201/eid2004.131566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Campista-Leon S, Delgado-Vargas F, Landa A, Willms K, Lopez-Moreno HS, Mendoza-Hernandez G, Rios-Sicairos J, Bojorquez-Contreras AN, Diaz-Camacho SP. Identification of immunodominant peptides from Gnathostoma binucleatum. Am J Trop Med Hyg. 2012;87:888–896. doi: 10.4269/ajtmh.2012.12-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zambrano-Zaragoza JF, Duran-Avelar Mde J, Messina-Robles M, Vibanco-Perez N. Characterization of the humoral immune response against Gnathostoma binucleatum in patients clinically diagnosed with gnathostomiasis. Am J Trop Med Hyg. 2012;86:988–992. doi: 10.4269/ajtmh.2012.11-0741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alvarez P, Morales A, Bravo F. Gnathostomiasis, experiencia en una practica privada en Lima‐Peru. Folia dermatol Peru. 2011;22:67. [Google Scholar]
- 21.Mimori T, Tada I, Kawabata M, Ollague WL, Calero GH, De Chong YF. Immunodiagnosis of human gnathostomiasis in Ecuador by skin test and ELISA using Gnathostoma doloresi antigen. Jpn J Trop Med Hyg. 1987;15:191–196. [Google Scholar]
- 22.Ishiwata K, Camacho SP, Ogata K, Nakamura-Uchiyama F, Hiromatsu K, Nawa Y. Evaluation of the antigenic similarities of adult-worm extracts from three Gnathostoma species, using sera from Mexican and Japanese patients with Gnathostoma infections. Ann Trop Med Parasitol. 2003;97:629–637. doi: 10.1179/000349803225001490. [DOI] [PubMed] [Google Scholar]
- 23.Herman JS, Wall EC, van-Tulleken C, Godfrey-Faussett P, Bailey RL, Chiodini PL. Gnathostomiasis acquired by British tourists in Botswana. Emerg Infect Dis. 2009;15:594–597. doi: 10.3201/1504.081646. [DOI] [PMC free article] [PubMed] [Google Scholar]