Abstract
Rationale:
Maternal obesity pre-programmes offspring to develop obesity and associated cardiovascular disease. Perivascular adipose tissue (PVAT) exerts an anti-contractile effect on the vasculature, which is reduced in hypertension and obesity.
Objective:
The objective of this study was to determine whether maternal obesity pre-programmes offspring to develop PVAT dysfunction in later life.
Methods:
Female Sprague–Dawley rats were fed a diet containing 10% (control) or 45% fat (high fat diet, HFD) for 12 weeks prior to mating and during pregnancy and lactation. Male offspring were killed at 12 or 24 weeks of age and tension in PVAT-intact or -denuded mesenteric artery segments was measured isometrically. Concentration–response curves were constructed to U46619 and norepinephrine.
Results:
Only 24-week-old HFD offspring were hypertensive (P<0.0001), although the anti-contractile effect of PVAT was lost in vessels from HFD offspring of each age. Inhibition of nitric oxide (NO) synthase with 100 μM l-NMMA attenuated the anti-contractile effect of PVAT and increased contractility of PVAT-denuded arteries (P<0.05, P<0.0001). The increase in contraction was smaller in PVAT-intact than PVAT-denuded vessels from 12-week-old HFD offspring, suggesting decreased PVAT-derived NO and release of a contractile factor (P<0.07). An additional, NO-independent effect of PVAT was evident only in norepinephrine-contracted vessels. Activation of AMP-activated kinase (with 10 μM A769662) was anti-contractile in PVAT-denuded (P<0.0001) and -intact (P<0.01) vessels and was due solely to NO in controls; the AMPK effect was similar in HFD offspring vessels (P<0.001 and P<0.01, respectively) but was partially NO-independent.
Conclusions:
The diminished anti-contractile effects of PVAT in offspring of HFD dams are primarily due to release of a PVAT-derived contractile factor and reduced NO bioavailability.
Introduction
Obesity is a major health problem worldwide in both adults and children. Alarmingly, 42 million children under the age of five are either overweight or obese.1 Maternal obesity can influence offspring health, the so-called ‘foetal programming of disease'.2, 3, 4 Human and animal studies have shown that offspring of obese mothers and dams fed high fat diet (HFD) during pregnancy develop obesity, glucose intolerance and cardiovascular dysfunction.4, 5, 6, 7, 8 However, the mechanism by which maternal obesity programmes offspring to develop cardiometabolic disorders is currently unknown.
Resistance artery dysfunction has a major role in the progression of cardiovascular disease and obesity-related insulin resistance.9 Vascular contractility is regulated by the endothelium; in health, the endothelium releases both vasodilator and vasoconstrictor agents (primarily nitric oxide (NO) and endothelin-1, respectively); an imbalance in their actions due to endothelial dysfunction can lead to the development of hypertension.10 Several studies have demonstrated that if rats and mice are fed an obesogenic diet during pregnancy and lactation, then despite their weaning onto a standard rodent chow, the offspring also display endothelial dysfunction,4, 11, 12 suggesting that this too results from foetal programming. Recently, perivascular adipose tissue (PVAT) has been recognised as an endocrine organ that secretes a number of bioactive proteins (adipokines; reviewed by Almabrouk et al.13) which can modulate vascular tone.14 PVAT exerts an anti-contractile effect in healthy subjects through the release of PVAT-derived relaxant factors.15 Animal studies have demonstrated PVAT-induced vasodilation in numerous vascular beds15, 16, 17, 18, 19 and have identified several vasorelaxant factors, including adiponectin and NO.20 However, obesity results in oxidative stress, hypoxia and inflammation of the adipose tissue leading to abnormal adipokine production.21 Animal studies have linked an increased PVAT mass with diminished anti-contractile effect.22, 23, 24 In rat mesenteric arteries, this is associated with reduced of adiponectin, increased reactive oxygen species formation, macrophage infiltration into PVAT and reduced NO bioavailability.23 However, the effect of maternal obesity on PVAT regulation of resistance artery tone in offspring is currently unknown.
AMP-activated protein kinase (AMPK) maintains energy homoeostasis25 and has a key role in glucose, lipid and protein metabolism.26 These processes are dysregulated in obesity and metabolic disorders, conditions in which AMPK activity is also reduced.27 Thus, an impaired AMPK pathway may be a contributory factor to endothelial dysfunction in obese rats. Moreover, endothelial dysfunction in obese rats is alleviated by AMPK activation, which increases phosphorylation (and thus activation) of endothelial NO synthase (eNOS).28 Weston et al.29 concluded that AMPK has a role downstream of adiponectin receptor activation in PVAT-dependent hyperpolarisation of myocytes, and thus reduced AMPK activation could also modify the PVAT effect on vascular contractility in offspring.
We hypothesised that foetal programming, induced by maternal obesity, would cause vascular changes in offspring that resulted not only from endothelial dysfunction but also from PVAT dysfunction. Thus, the aim of the present study was to identify any such changes in endothelial or PVAT control of vascular tone with particular focus on the involvement of NO and AMPK.
Methods
Animals
All animal procedures complied with the United Kingdom Animals (Scientific Procedures) Act 1986. Animals were housed under a 12 h light/12 h dark cycle and provided with food and water ad libitum. Female Sprague–Dawley rats (185–200 g, Charles River, Harlow, UK) were fed an obesogenic, 45% fat diet (824018, SDS Diets, Witham, UK) (HFD) or a control 10% fat diet (824050, SDS diets) for a 12-week period. Rats were then mated and continued on their respective diets during pregnancy and lactation. At weaning, male offspring were provided with 10% fat diet (824050, SDS diets) until they were killed at either 12 or 24 weeks of age. Body weight was recorded fortnightly in the dams and weekly in the offspring. This study utilized 12 or 24-week-old offspring of control dams (12/24wCO) and 12/24-week-old offspring of HFD dams (12/24wHFDO).
Blood pressure
Systolic and diastolic blood pressure were measured in conscious rats using a CODA tail cuff blood pressure monitoring system30 (Kent Scientific, Torrington, CT, USA).
Blood glucose measurement
Fasting blood samples were taken from the lateral tail vein of conscious rats for determination of plasma insulin levels (Rat Insulin ELISA, Alpco, Salem, NH, USA) and blood glucose concentration (automatic blood glucose monitor, Contour, Bayer Consumer Care AG, Basel, Switzerland).
Adipocyte size measurement
PVAT adipocyte sizes were determined as described previously.31
Wire myography
Animals were fasted overnight and killed by CO2 inhalation followed by mechanical disruption of the diaphragm. On the experimental day, epididymal fat pads were weighed. The mesenteric vascular bed was isolated and placed in ice-cold physiological salt solution. Second order mesenteric arteries with surrounding PVAT (+PVAT) or cleaned of all fat and connective tissue (−PVAT) were mounted on 40-μm wires in a small vessel wire myograph and equilibrated in physiological salt solution at 37 °C (gassed with 95% air and 5% CO2) for 30 min. Vessels were then normalised using a standardised technique32 and again equilibrated for 30 min. Chart 5 Pro Software (ADInstruments, Oxford, UK) was used to record and measure changes in the vessel tension.
Pharmacological modulation
Cumulative concentration–response curves to U46619 (9,11-dideoxy-9a,11a-methanoepoxy prostaglandin F2a, R&D Systems, Abingdon, UK) and norepinephrine bitartrate (Sigma-Aldrich, Gillingham, UK) were constructed (10 nmol l−1–3 μmol l−1 and 1 nmol l−1–30 μmol l−1, respectively). Where appropriate, arteries were incubated with the AMPK activator—A769662 (6,7-dihydro-4-hydroxy-3-(2′-hydroxy[1,1′-biphenyl]-4-yl)-6-oxo-thieno[2,3-b]pyridine-5—carbonitrile, 10 μM, R&D Systems) for an hour and/or NOS inhibitor—l-NMMA acetate (NG-monomethyl-l-arginine, 100 μM, R&D Systems) for 45 min. Functional endothelial integrity was assessed by relaxation to acetylcholine (10 μM, Sigma- Aldrich) in arteries pre-contracted with U46619.
Tissue lysates
PVAT-denuded arteries and PVAT were snap-frozen in liquid nitrogen and stored at −80 °C. Tissue lysates were then prepared with RIPA buffer (Sigma-Aldrich) supplemented with protein protease inhibitors (Roche, Mannheim, Germany) and phosphatase inhibitors (Roche) using a glass:glass homogeniser, rotated at 4 °C for 30 min and then centrifuged (21 910 g, 5 min, 4 °C). Protein concentration was measured using a Bradford assay.33 The supernatants were divided into aliquots and stored at −80 °C. On the experimental day, one volume of sample buffer (320 mM Tris, 5% SDS, 25% glycerol, 5% β-mercaptoethanol, 1% bromophenol blue) was added to four volumes of the tissue lysates.
Western blot
Equal amounts of proteins (40 μg) were separated by stain-free SDS-polyacrylamide gel electrophoresis (7.5%), and then transferred to mini low fluorescence polyvinylidene difluoride membranes (Bio-Rad Laboratories, Hemel Hempstead, UK). The blots were visualised using Chemidoc MP Imaging system (Bio-Rad Laboratories), then the membrane was blocked with 3.5% (w/v) bovine serum albumin in TBS-T (10 mM Tris pH8, 150 mM NaCl, 0.1% Tween 2.0) for an hour at room temperature before exposure to primary antibody (anti-eNOS, 1:200, Santa Cruz Biotechnology, Dallas, TX, USA; anti-peNOSser1177, 1:200, Santa Cruz Biotechnology; anti-AMPKα, 1:500, Cell Signalling (Hitchin, UK); anti-pAMPKα, 1:500, Cell Signalling; 4 °C, overnight) and then horseradish peroxidase-conjugated secondary antibody (goat anti-rabbit, 1:10 000, Jackson ImmunoResearch Laboratories, West Grove, PA, USA) for an hour at room temperature. Chemiluminescence was performed with Clarity Western ECL Substrate (Bio-Rad Laboratories) and detected using Chemidoc MP Imaging system. Blots were quantified using ImageLab 5.2.1. (Bio-Rad Laboratories) software and protein expression was normalised to the total protein of the sample as previously described.34
Data analysis
If the standardized differences between groups are similar to those in our previous studies, we expected that samples sizes of 8–10 would be sufficient to demonstrate significant differences in each experimental protocol with a power of 90% at a 5% level of confidence.
Data are presented as mean±s.e.m. unless otherwise stated. Data were expressed as percentage contraction to 60 mM K+ PSS for U46619 dose responses and curves were fitted using non-linear regression analysis.
Statistical analysis for the wire myograph experiments was performed using GraphPad Prism (v6, GraphPad software, La Jolla, CA, USA) with repeated measures two-way analysis of variance test followed by Bonferroni post hoc test for multiple comparisons. Cardiometabolic parameters were analysed using GraphPad Prism (v6, GraphPad software) with an unpaired t-test, whereas protein expression was analysed using a one-sample t-test. Researchers were not blinded to animal grouping.
Results
Maternal characteristics
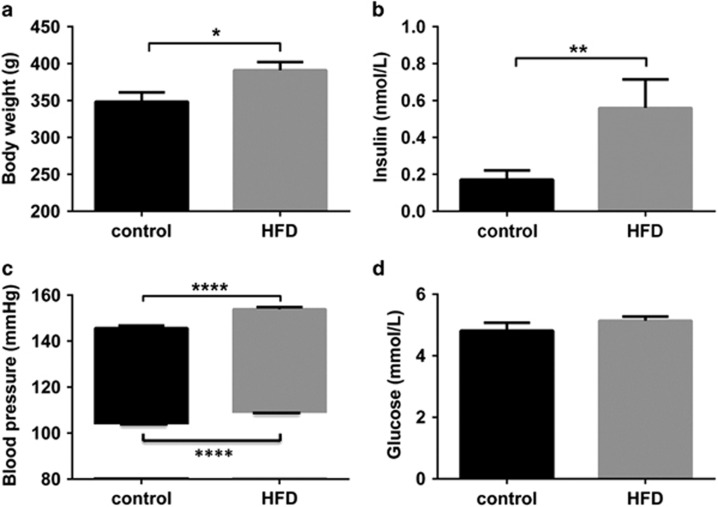
At the time of mating, body weight, insulin, and systolic and diastolic blood pressure were all significantly increased in HFD females compared with controls although glucose remained unchanged (Figure 1). All 6 control rats had uncomplicated pregnancies, whereas only 8 out of 15 rats fed a HFD successfully mated and two of these died as a result of pregnancy-related complications. There was no difference in litter sizes between the 6 control (median 15 pups per dam, range 14–17) and 6 HFD (median 15 pups per dam, range 13–15) dams.
Figure 1.
Cardiometabolic profile of dams. Body weight (a), serum insulin (b) and blood pressure (c) were increased in HFD (grey bars) dams compared with controls (black bars) prior to pregnancy but the serum glucose concentration (d) was similar. Data are expressed as mean±s.e.m., n=6, *P<0.05, **P<0.01, ****P<0.0001, unpaired t-test.
Offspring characteristics
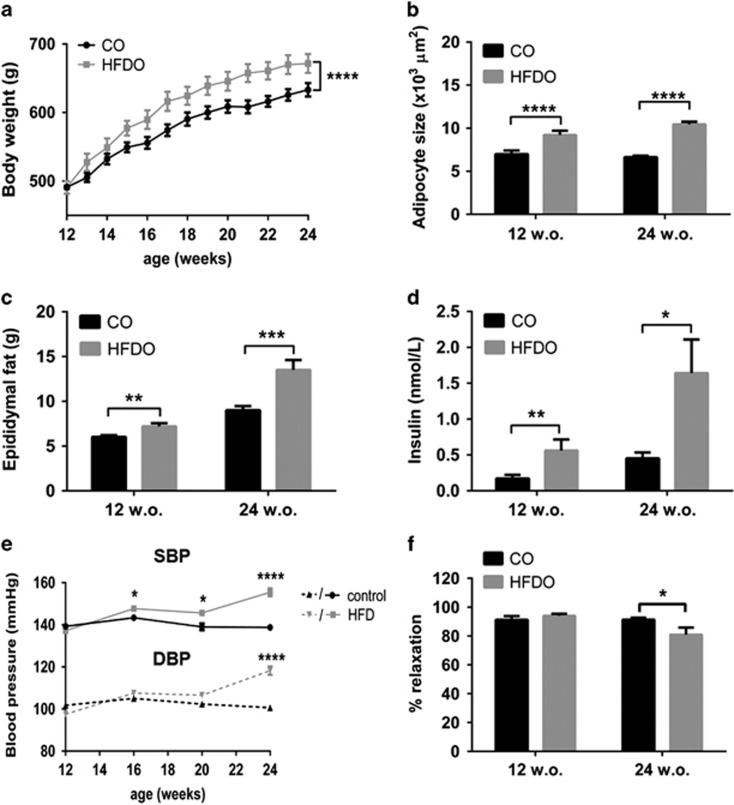
Body weight of HFD offspring was similar to that of controls at 12 weeks of age (12wHFDO) but was significantly increased at 24 weeks (24wHFDO) (P<0.0001, n=21–23, Figure 2a) and adipocytes were significantly larger in HFDO of both ages (each P<0.0001, n=10, Figure 2b). Nevertheless, epididymal fat pad weights were increased in both 12wHFDO (P<0.01, n=21–23) and 24wHFDO (P<0.001, n=14–23) (Figure 2c) and the plasma insulin concentration was also increased in both 12wHFDO (P<0.01, n=8–16) and 24wHFDO (P<0.05, n=10–13, Figure 2d). Both systolic and diastolic blood pressure remained unchanged between the two groups at 12 weeks of age. However, systolic blood pressure was elevated from week 16 onwards in the HFDO and both systolic and diastolic blood pressure were also increased in 24wHFDO (P<0.0001, n=8–12, Figure 2e). In comparison with age-matched controls, U46619-contracted mesenteric arteries from 24wHFDO rats relaxed less to 10 μM acetylcholine (P<0.05, n=8, Figure 2f), although this was not evident in vessels from 12wHFDO.
Figure 2.
Cardiometabolic characteristics of offspring. Body weight (a) was increased in 24-week-old offspring of HFD dams (HFDO). Adipocytes (b) were larger in both 12- and 24-week-old offspring of HFD dams. Epididymal fat pads (c) and insulin (d) were increased in 12- and 24-week-old offspring of HFD dams compared with their respective controls (CO). Systolic blood pressure (e) was significantly increased from week 16 onwards in offspring of HFD dams, whereas diastolic blood pressure (e) was significantly increased in 24-week-old offspring of HFD dams. U46619 pre-constricted arteries from 24-week-old offspring of HFD dams relaxed less to 10 μM acetylcholine compared with control vessels (f), but this was not evident in vessels from 12-week-old offspring of HFD dams. Data are expressed as mean±s.e.m., n=12–23, *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001, unpaired t-test/two-way analysis of variance with Bonferroni post hoc test.
The anti-contractile effect of PVAT is lost in offspring of HFD dams
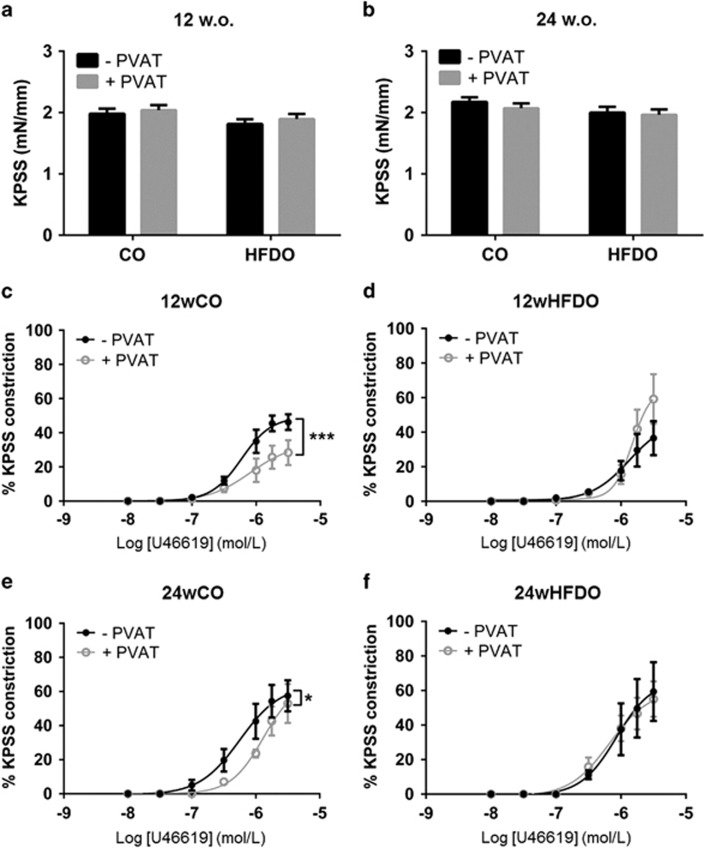
The constriction to 60 mM K+ PSS was similar in PVAT-denuded and PVAT-intact vessels from 12wCO and 12wHFDO as well as 24wCO and 24wHFDO (all n=8, Figures 3a and b).
Figure 3.
The anti-contractile effect of PVAT is lost in offspring of HFD dams. The contractions to 60 mM K+ PSS were similar in PVAT-denuded and PVAT-intact vessels from (a) 12- and (b) 24-week-old offspring. PVAT reduced contractions to U46619 in vessels from (c) 12- and (e) 24-week-old offspring of control dams but not in arteries from (d) 12- and (f) 24-week-old offspring of HFD dams. Data are expressed as mean±s.e.m., n=8, *P<0.05, ***P<0.001, one-way/two-way analysis of variance.
The presence of PVAT reduced contractions to U46619 in vessels from control offspring at both 12 (Figure 3c) and 24 (Figure 3e) weeks of age (P<0.001 and P<0.05, respectively, n=8). This effect was lost in both 12wHFDO and 24wHFDO (Figures 3d and f). When vessels were contracted with norepinephrine, the effect of PVAT was evident in 12wCO and 24wCO arteries and also in 12wHFDO arteries but was totally lacking in 24wHFDO vessels (Supplementary Figure 1).
NO contributes to the anti-contractile effect of PVAT in offspring of control dams
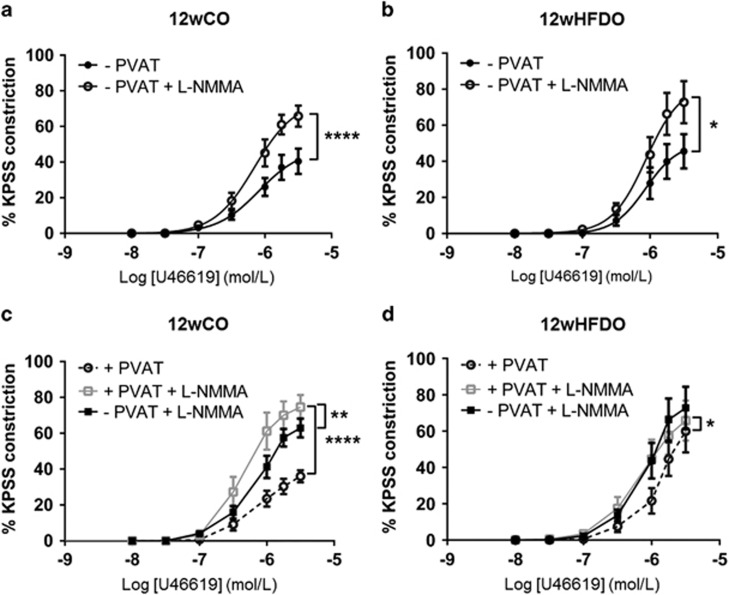
Inhibition of NOS with l-NMMA (100 μM) increased contractility to U46619 in both PVAT-denuded (Figure 4a) and PVAT-intact (Figure 4c) arteries from 12wCO (each P<0.0001, n=8). Moreover, PVAT was still able to exert an anti-contractile effect against norepinephrine following NOS inhibition in 12wCO and 12wHFDO arteries (Supplementary Figure 2).
Figure 4.
NO contributes to the anti-contractile effect of PVAT. NOS inhibition with l-NMMA increased contractions to U46619 in arteries with and without PVAT from 12-week-old offspring of control (a and c) and HFD (b and d) dams, which abolished the anti-contractile effect of PVAT. Data are expressed as mean±s.e.m., n=8, *P<0.05, **P<0.01, ****P<0.0001, two-way analysis of variance.
NOS inhibition led to a similar increase in contractility to U46619 in PVAT-denuded control and 12wHFDO vessels (Figures 4a and b). However, when PVAT was present, the increase in the U46619-induced (3 μmol l−1) constriction following incubation with l-NMMA (5.7±16.1%, n=8) was smaller in the 12wHFDO vessels than in the controls (38.6±7.5%, n=8; P<0.05, Figures 4c and d). Although eNOS expression was, in fact, increased in PVAT from 12wHFDO compared with controls (P<0.01, n=4, Supplementary Figure 3). There was a trend towards reduced peNOS expression compared with controls, but this did not achieve statistical significance (P=0.31, n=4, Supplementary Figure 3). There was no significant difference in eNOS and peNOS expression in mesenteric arteries from 12-week-old offspring after PVAT removal (Supplementary Figure 3).
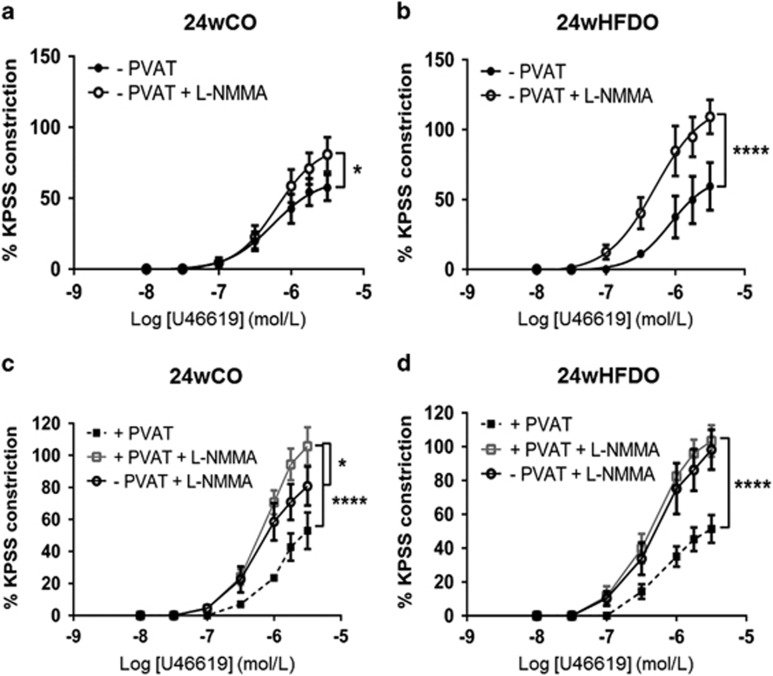
Inhibition of NOS increased contractility of PVAT-denuded vessels from both 24wCO and 24wHFDO but was more pronounced in the latter (Figures 5a and b, Supplementary Figure 4). This effect was also observed when 24wCO and 24HFDO arteries were constricted with norepinephrine (Supplementary Figure 5). In the presence of PVAT, contractions to U46619 were similar in control and HFD vessels following NOS inhibition with l-NMMA (Figures 5c and d). The proportion of eNOS that was phosphorylated was not significantly different in PVAT-denuded arteries, but it was decreased in PVAT (Supplementary Figure 6) from 24wHFDO compared with their respective controls. The relative expression of eNOS was decreased in PVAT, but it was increased in PVAT-denuded arteries from 24wHFDO compared with their respective controls (Supplementary Figure 6).
Figure 5.
NO synthase inhibition increases contractions in 24-week-old offspring of HFD dams. NOS inhibition with 100 μM l-NMMA increased contractions to U46619 in PVAT-denuded vessels from 24-week-old offspring of control (a) and HFD (b) dams but was more pronounced in the latter. It also led to an increase in contractions in PVAT-intact mesenteric arteries isolated from 24-week-old offspring of control (c) and HFD (d) dams, which abolished the anti-contractile effect of PVAT. Data are expressed as mean±s.e.m., n=8, *P<0.05, ****P<0.0001, two-way analysis of variance.
AMPK activation reduces vascular contractility
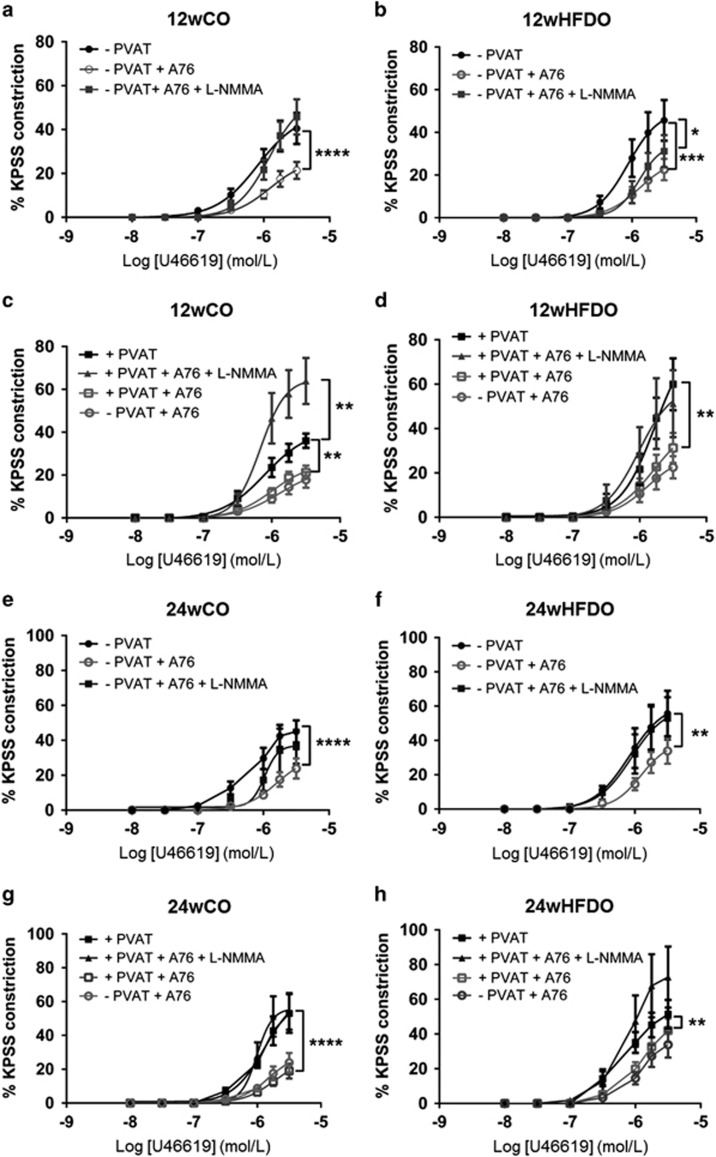
AMPK activation with A769662 reduced the contractile response to U46619 in PVAT-denuded mesenteric artery segments from both 12wCO (P<0.0001, n=8, Figure 6a) and 12wHFDO (P<0.001, n=8, Figure 6b). Although, the anti-contractile effect of the AMPK activator was lost following inhibition of NOS in 12wCO vessels constricted with U46619 (Figure 6a), it was still present in 12wHFDO (P<0.05, n=8, Figure 6b). AMPK also reduced contractions to U46619 in PVAT-intact vessels from both 12wCO and 12wHFDO (P<0.01, n=8, Figures 6c and d). NOS inhibition prevented the anti-contractile effects both of AMPK and of PVAT against tone induced by U46619 in 12wCO and 12wHFDO (Figures 6c and d). AMPK activation also led to a reduction in contractility of PVAT-denuded and PVAT-intact vessels, constricted with noradrenaline from 12wCO and 12wHFDO (Supplementary Figure 7). However, in the presence of the NOS inhibitor, AMPK was no longer able to relax vessels constricted with norepinephrine (Supplementary Figure 7).
Figure 6.
AMPK reduces vascular contraction in NO-dependent manner in control offspring. AMPK activation with 10 μM A769662 (A76) reduced vascular contractions to U46619 in arteries without PVAT from 12- and 24-week-old offspring of control (a) and HFD (b) dams; this was abolished in the presence of NOS inhibitor (100 μM, l-NMMA) in the control (a) but not in HFD (b) vessels. AMPK activation also reduced contractions to U46619 in PVAT-intact arteries from control (c) and HFD (d) vessels, whereas in the presence of l-NMMA, it led to a large increase in contraction in offspring of control (c) but not in offspring of HFD (d) dams. A76 also reduced vascular contractions to U46619 in arteries without (a and b) and with (c and d) PVAT from 24-week-old offspring of control (e and g) and HFD (f and h) dams in a NO-dependent manner. Data are expressed as mean±s.e.m., n=8, *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001, two-way analysis of variance.
AMPK activation reduced the contractile response to U46619 in PVAT-denuded arteries from both 24wCO (Figure 6e) and 24wHFDO (Figure 6f) (P<0.0001 and P<0.01, respectively, n=8); the anti-contractile effect was lost in the presence of NOS inhibitor (Figures 6e and f). AMPK activation also induced vasorelaxation in PVAT-intact vessels from both 24wCO and 24wHFDO (P<0.0001 and P<0.01, respectively, n=8, Figures 6g and h). AMPK activation was no longer effective in PVAT-intact arteries from both 24wCO and 24wHDFO incubated with the NOS inhibitor (Figures 6g and h). The vasorelaxant effect of AMPK was also observed in PVAT-intact and PVAT-denuded vessels, constricted with norepinephrine, from 24wCO and 24wHFDO (Supplementary Figure 8).
The relative expressions of AMPK and phosphorylated AMPK (pAMPK) were increased in PVAT from 12wHFDO compared with their respective controls (Supplementary Figure 9). There was no difference in AMPK and pAMPK expression in PVAT from 24wHFDO compared with their respective controls (Supplementary Figure 9).
Discussion
This study investigated the effect of a maternal HFD on PVAT regulation of resistance artery tone in offspring. The novel finding of this study was that the anti-contractile effect of PVAT was lost in offspring of HFD dams at both 12 and 24 weeks of age. The lost anti-contractile properties of PVAT could be due in part to a reduction in PVAT-derived NO bioavailability but also to the presence of an unidentified contractile factor in 12wHFDO. However, at 24 weeks of age, both vascular and PVAT-derived NO are altered in HFDO. Vascular NO was increased in 24wHFDO, whereas PVAT-derived NO appeared to be reduced. There was no difference in AMPK-induced modulation of vascular tone between CO and HFDO at both ages. The loss of the PVAT effect in 12wHFDO was particularly interesting because it occurred prior to any measurable change in blood pressure.
Obesity has detrimental effects on health; it can lead to the development of insulin resistance, the metabolic syndrome, type 2 diabetes mellitus and increased blood pressure; all are major risk factors for cardiovascular disease.35 Maternal obesity can be effectively induced in various rodents by high fat or high sucrose feeding;36 however, it can lead to infertility and pregnancy-related complications.4, 37, 38 This was evident in the present study in which all 6 control rats had uncomplicated pregnancies, whereas only 8 out of 15 rats fed the HFD successfully mated, with two dying near term as a result of pregnancy-related complications (triggered by in utero deaths). The HFD resulted in a significantly increased body weight, both before and during pregnancy, compared with controls and an increase in plasma insulin levels, suggesting that these rats had developed insulin resistance. Dams fed the HFD also displayed an increased systolic and diastolic blood pressure as typically seen in human obesity.39
The maternal environment can also influence the offspring's long-term health, so-called ‘foetal programming of disease'. Numerous studies have shown that maternal obesity increases the offspring's adiposity and has negative effects on cardiometabolic health (reviewed by Drake and Reynolds40). In the present study, blood pressure and body weight were similar in 12wCO and 12wHFDO. However, systolic blood pressure was elevated from week 16 onwards in the HFDO and progressively increased with age. Diastolic blood pressure was also significantly increased in 24wHFDO. This is consistent with previous studies, which also found that the cardiometabolic status of offspring of obese dams changes at 24 weeks of age (increased body weight and blood pressure).4, 41, 42 Despite weaning onto standard chow diet (10% fat), adiposity and insulin levels were increased in HFDO at both 12 and 24 weeks of age, again consistent with previous findings.4 Thus, in the present study, the offspring of the HFD dams demonstrated clear evidence of pre-programmed cardiometabolic dysfunction.
PVAT exerts anti-contractile effects on vessels in healthy subjects through the release of PVAT-derived relaxant factors,17, 24 but this anti-contractile effect is reduced in hypertension43, 44 and obesity.15 Therefore, the present study hypothesised that maternal obesity might pre-programme offspring not only to develop endothelial dysfunction as previously described4 but also PVAT dysfunction later on in life.
Our study showed that PVAT reduced contractions to U46619 and to norepinephrine in mesenteric arteries isolated from 12wCO and 24wCO rats and that maternal diet could influence PVAT function in offspring. Thus, the anti-contractile effect of PVAT was lost in 24wHFDO arteries constricted with norepinephrine and was abolished in mesenteric arteries from 12wHFDO and 24wHFDO contracted with U46619. Nevertheless, after NOS inhibition, there was still clear evidence for an anti-contractile effect of PVAT when HFDO vessels were contracted with norepinephrine, suggesting that norepinephrine, but not U46619, stimulated the release of an additional PVAT-derived vasorelaxant factor. Over-nutrition during either pregnancy or lactation alone is able to pre-programme hypertension in offspring.42 Therefore, in the present study, the observed changes in offspring PVAT effects could be a result of pre-programming in utero or be due to over-nutrition during lactation.
The anti-contractile properties of PVAT were lost in 12wHFDO in which plasma insulin levels were increased but the rats were not overweight. The endothelial function was also preserved in 12wHFDO but was reduced in 24wHFDO vessels compared with controls. Thus, metabolic and PVAT dysfunction preceded obesity, endothelial dysfunction and hypertension in HFDO. In 24wHFDO, endothelial and PVAT dysfunctions were against a background of obesity and hypertension. Nevertheless, PVAT dysfunction may contribute to the progression of cardiometabolic disorders as it occurs prior to obesity and hypertension in HFDO.
PVAT induces an anti-contractile effect through the release of NO.20 In the present study, NOS inhibition with l-NMMA caused a greater increase in U46619-induced contractions in the presence than in the absence of PVAT in both 12wCO and 24wCO. This suggests that the anti-contractile effect of PVAT in control offspring is NO-dependent at both ages, consistent with the reported role of PVAT-derived NO in mouse mesenteric arteries.20 The detection of eNOS and peNOS expression in PVAT provided further support in favour of PVAT as a source of NO. However, although protein expression of eNOS was maintained in arteries from 12wHFDO, following incubation with l-NMMA, the increase in contraction was lower in the PVAT-intact arteries from 12wHFDO compared with those from 12wCO. These data indicate a possible reduction in the PVAT-derived NO bioavailability could be responsible for the loss of anti-contractile effect of PVAT in 12wHFDOs. However, PVAT was still able to exert an anti-contractile effect to norepinephrine when 12wCO and 12wHFDO vessels were incubated with the NOS inhibitor. This suggests that NO is not the sole factor responsible for mediating the anti-contractile effect of PVAT to norepinephrine in vessels from 12-week-old offspring. Adiponectin might be the additional vasorelaxant factor as it is a downstream effector of β3-adrenoceptor activation29 and the anti-contractile effect of PVAT is lost in mice following adiponectin gene deletion.20
Although contractions to U46619 were similar in 24wCO and 24wHFDO PVAT-denuded vessels, they were significantly enhanced in 24wHFDO vessels after incubation with l-NMMA. This suggests an increased basal release of NO, which must compensate either for loss of other endothelium-derived vasodilator factors or for an increased contractile effect of the endothelium in the 24wHFDO vessel (possibly due to enhanced release of contractile prostanoids;45 examination of this was beyond the scope of the present study). Unlike in the −PVAT vessels, there was no difference in response to U46619 in PVAT-intact vessels after NOS inhibition. Nevertheless, it was noticeable that in the presence of PVAT, the 24wCO and 24wHFDO vessel responses to U46619 were similar to those of −PVAT vessels from 24wHFDO and that in all three, the responses were greater than those in −PVAT 24wCO. This may be an indication that the release of NO, which normally counter-balances the effects of a PVAT-derived contractile factor (both in control and HFD offspring vessels) is increased in the 24wHFDO vessels to offset an increased release of the unidentified contractile factor. It was therefore expected that expression of eNOS in the endothelium might be enhanced to account for the increased NO. Indeed, in comparison with controls, eNOS expression was increased in PVAT-denuded or -intact mesenteric arteries from 24wHFDO, but the total expression of phosphorylated (active) eNOS was unchanged (with a trend towards a reduction). Thus, some other factor, such as reduced NO degradation, must be responsible for the elevation of NO.
Obesity leads to impaired release of constricting and dilating factors from both endothelium and PVAT.46, 47 In the present study, there was no evidence of endothelium dysfunction in the HFDO although there was a lack of anti-contractile effect of mesenteric artery PVAT in the 24wHFDO, which could be partially due to the release of an unidentified contractile factor from the artery together with reduced PVAT-derived NO bioavailability.
AMPK phosphorylates eNOS at ser1177, which leads to eNOS activation and increased NO bioavailability, which then reduces vascular tone.48, 49 Activation of AMPK can also induce vasorelaxation through stimulation of myocyte sarcoendoplasmic reticulum calcium transport ATPase (SERCA) and large conductance calcium-activated potassium channels (BKCa) channel activity, which each result in a lowering of intracellular calcium.29, 50 The active (phosphorylated) form of AMPK was shown to be reduced in the skeletal muscle and heart of fetuses from sheep fed a HFD during pregnancy.51, 52 Therefore, in the present study, the effect of AMPK activation on the vascular contractility in offspring was also investigated. AMPK activation with A769662 resulted in a reduction in vessel contractility to both U46619 and norepinephrine that was partially NO-mediated in 12wCO and 24wCO and thus AMPK could contribute to the anti-contractile effect of PVAT by increasing NO release from the PVAT. In PVAT-denuded vessels from 12wHFDO, the AMPK activator was still able to induce the vasorelaxation against both U46619 and norepinephrine contractions in the presence of l-NMMA. This NO-independent relaxant effect of A769662 was possibly due to a direct effect on the vascular smooth muscle. Although AMPK activity is reduced in heart and skeletal muscle of sheep fetuses when the mother is fed a HFD during pregnancy,51, 52 in the present study, AMPK expression and phosphorylated AMPK expression were increased in PVAT from 12wHFDO compared with their respective controls. This could account for the preserved AMPK-induced modulation of vascular tone in HFDO. AMPK also exerted a partially NO-dependent vasorelaxation of PVAT-intact and PVAT-denuded mesenteric arteries from 24wHFDO, constricted with either U46619 or norepinephrine. Thus, it appears that maternal obesity does not affect the ability of AMPK to induce vasorelaxation in offspring mesenteric arteries even when PVAT is dysfunctional.
The current study has clearly demonstrated that maternal obesity in rats adversely affects vascular responses in offspring as young as 12 weeks old by reducing the anti-contractile effect of PVAT. The subsequent enhanced contractility to U46619 was in part due to enhanced release of an unidentified contractile factor but also due partially to loss of NO. Nevertheless, the AMPK signalling was intact in the obese dam offspring. Thus, AMPK activators, such as metformin (which upregulates eNOS phosphorylation and increases NO bioavailability in the vasculature53), may be therapeutically useful for maintaining PVAT function and opposing the development of pre-programmed hypertension. Importantly, translating these data to the human situation suggests that maternal obesity may already have pre-programmed the development of hypertension in young adults, highlighting the importance of educating young children of obese mothers about lifestyle changes and/or the use of treatments to minimise the consequence of the perinatal damage that has already occurred.
Acknowledgments
This study was funded by the British Heart Foundation (FS/12/68/30006).
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on International Journal of Obesity website (http://www.nature.com/ijo)
Supplementary Material
References
- World Health Organisation. 2015, http://www.who.int/mediacentre/factsheets/fs311/en.
- Koletzko B, Brands B, Poston L, Godfrey K, Demmelmair H. Early nutrition programming of long-term health. Proc Nutr Soc 2012; 71: 371–378. [DOI] [PubMed] [Google Scholar]
- Poston L. Maternal obesity, gestational weight gain and diet as determinants of offspring long term health. Best Pract Res Clin Endocrinol Metab 2012; 26: 627–639. [DOI] [PubMed] [Google Scholar]
- Samuelsson AM, Matthews PA, Argenton M, Christie MR, McConnell JM, Jansen EH et al. Diet-induced obesity in female mice leads to offspring hyperphagia, adiposity, hypertension, and insulin resistance: a novel murine model of developmental programming. Hypertension 2008; 51: 383–392. [DOI] [PubMed] [Google Scholar]
- Fraser A, Tilling K, Macdonald-Wallis C, Sattar N, Brion MJ, Benfield L et al. Association of maternal weight gain in pregnancy with offspring obesity and metabolic and vascular traits in childhood. Circulation 2010; 121: 2557–2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamun AA, O'Callaghan M, Callaway L, Williams G, Najman J, Lawlor DA. Associations of gestational weight gain with offspring body mass index and blood pressure at 21 years of age: evidence from a birth cohort study. Circulation 2009; 119: 1720–1727. [DOI] [PubMed] [Google Scholar]
- Oken E. Maternal and child obesity: the causal link. Obstet Gynecol Clin North Am 2009; 36: 361–36x. [DOI] [PubMed] [Google Scholar]
- Shankar K, Harrell A, Liu X, Gilchrist JM, Ronis MJ, Badger TM. Maternal obesity at conception programs obesity in the offspring. Am J Physiol Regul Integr Comp Physiol 2008; 294: R528–R538. [DOI] [PubMed] [Google Scholar]
- Muris DM, Houben AJ, Schram MT, Stehouwer CD. Microvascular dysfunction: an emerging pathway in the pathogenesis of obesity-related insulin resistance. Rev Endocr Metab Disord 2013; 14: 29–38. [DOI] [PubMed] [Google Scholar]
- Feletou M, Vanhoutte PM. Endothelium-derived hyperpolarizing factor: where are we now? Arterioscler Thromb Vasc Biol 2006; 26: 1215–1225. [DOI] [PubMed] [Google Scholar]
- Khan IY, Taylor PD, Dekou V, Seed PT, Lakasing L, Graham D et al. Gender-linked hypertension in offspring of lard-fed pregnant rats. Hypertension 2003; 41: 168–175. [DOI] [PubMed] [Google Scholar]
- Torrens C, Ethirajan P, Bruce KD, Cagampang FR, Siow RC, Hanson MA et al. Interaction between maternal and offspring diet to impair vascular function and oxidative balance in high fat fed male mice. PLoS One 2012; 7: e50671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almabrouk TA, Ewart MA, Salt IP, Kennedy S. Perivascular fat, AMP-activated protein kinase and vascular diseases. Br J Pharmacol 2014; 171: 595–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aghamohammadzadeh R, Withers S, Lynch F, Greenstein A, Malik R, Heagerty A. Perivascular adipose tissue from human systemic and coronary vessels: the emergence of a new pharmacotherapeutic target. Br J Pharmacol 2012; 165: 670–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenstein AS, Khavandi K, Withers SB, Sonoyama K, Clancy O, Jeziorska M et al. Local inflammation and hypoxia abolish the protective anticontractile properties of perivascular fat in obese patients. Circulation 2009; 119: 1661–1670. [DOI] [PubMed] [Google Scholar]
- Dubrovska G, Verlohren S, Luft FC, Gollasch M. Mechanisms of ADRF release from rat aortic adventitial adipose tissue. Am J Physiol Heart Circ Physiol 2004; 286: H1107–H1113. [DOI] [PubMed] [Google Scholar]
- Gollasch M, Dubrovska G. Paracrine role for periadventitial adipose tissue in the regulation of arterial tone. Trends Pharmacol Sci 2004; 25: 647–653. [DOI] [PubMed] [Google Scholar]
- Guzik TJ, Marvar PJ, Czesnikiewicz-Guzik M, Korbut R. Perivascular adipose tissue as a messenger of the brain-vessel axis: role in vascular inflammation and dysfunction. J Physiol Pharmacol 2007; 58: 591–610. [PubMed] [Google Scholar]
- Soltis EE, Cassis LA. Influence of perivascular adipose tissue on rat aortic smooth muscle responsiveness. Clin Exp Hypertens A 1991; 13: 277–296. [DOI] [PubMed] [Google Scholar]
- Lynch FM, Withers SB, Yao Z, Werner ME, Edwards G, Weston AH et al. Perivascular adipose tissue-derived adiponectin activates BK(Ca) channels to induce anticontractile responses. Am J Physiol Heart Circ Physiol 2013; 304: H786–H795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achike FI, To NH, Wang H, Kwan CY. Obesity, metabolic syndrome, adipocytes and vascular function: A holistic viewpoint. Clin Exp Pharmacol Physiol 2011; 38: 1–10. [DOI] [PubMed] [Google Scholar]
- Ma L, Ma S, He H, Yang D, Chen X, Luo Z et al. Perivascular fat-mediated vascular dysfunction and remodeling through the AMPK/mTOR pathway in high-fat diet-induced obese rats. Hypertens Res 2010; 33: 446–453. [DOI] [PubMed] [Google Scholar]
- Marchesi C, Ebrahimian T, Angulo O, Paradis P, Schiffrin EL. Endothelial nitric oxide synthase uncoupling and perivascular adipose oxidative stress and inflammation contribute to vascular dysfunction in a rodent model of metabolic syndrome. Hypertension 2009; 54: 1384–1392. [DOI] [PubMed] [Google Scholar]
- Szasz T, Webb RC. Perivascular adipose tissue: more than just structural support. Clin Sci (Lond) 2012; 122: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carling D, Mayer FV, Sanders MJ, Gamblin SJ. AMP-activated protein kinase: nature's energy sensor. Nat Chem Biol 2011; 7: 512–518. [DOI] [PubMed] [Google Scholar]
- Hardie DG. AMPK: a key regulator of energy balance in the single cell and the whole organism. Int J Obes (Lond) 2008; 32 (Suppl 4): S7–S12. [DOI] [PubMed] [Google Scholar]
- Ruderman NB, Carling D, Prentki M, Cacicedo JM. AMPK, insulin resistance, and the metabolic syndrome. J Clin Invest 2013; 123: 2764–2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng G, Long Y, Yu YR, Li MR. Adiponectin directly improves endothelial dysfunction in obese rats through the AMPK-eNOS Pathway. Int J Obes (Lond) 2010; 34: 165–171. [DOI] [PubMed] [Google Scholar]
- Weston AH, Egner I, Dong Y, Porter EL, Heagerty AM, Edwards G. Stimulated release of a hyperpolarizing factor (ADHF) from mesenteric artery perivascular adipose tissue: involvement of myocyte BKCa channels and adiponectin. Br J Pharmacol 2013; 169: 1500–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng M, Whitesall S, Zhang Y, Beibel M, D'Alecy L, DiPetrillo K. Validation of volume-pressure recording tail-cuff blood pressure measurements. Am J Hypertens 2008; 21: 1288–1291. [DOI] [PubMed] [Google Scholar]
- Aghamohammadzadeh R, Greenstein AS, Yadav R, Jeziorska M, Hama S, Soltani F et al. Effects of bariatric surgery on human small artery function: evidence for reduction in perivascular adipocyte inflammation, and the restoration of normal anticontractile activity despite persistent obesity. J Am Coll Cardiol 2013; 62: 128–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulvany MJ, Halpern W. Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ Res 1977; 41: 19–26. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976; 72: 248–254. [DOI] [PubMed] [Google Scholar]
- Gilda JE, Gomes AV. Stain-free total protein staining is a superior loading control to beta-actin for western blots. Anal Biochem 2013; 440: 186–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bult MJ, van DT, Muller AF. Surgical treatment of obesity. Eur J Endocrinol 2008; 158: 135–145. [DOI] [PubMed] [Google Scholar]
- White CL, Purpera MN, Morrison CD. Maternal obesity is necessary for programming effect of high-fat diet on offspring. Am J Physiol Regul Integr Comp Physiol 2009; 296: R1464–R1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segovia SA, Vickers MH, Gray C, Reynolds CM. Maternal obesity, inflammation, and developmental programming. Biomed Res Int 2014; 2014: 418975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen J, Vestergaard M, Wisborg K, Kesmodel U, Secher NJ. Pre-pregnancy weight and the risk of stillbirth and neonatal death. BJOG 2005; 112: 403–408. [DOI] [PubMed] [Google Scholar]
- Kotsis V, Stabouli S, Bouldin M, Low A, Toumanidis S, Zakopoulos N. Impact of obesity on 24-hour ambulatory blood pressure and hypertension. Hypertension 2005; 45: 602–607. [DOI] [PubMed] [Google Scholar]
- Drake AJ, Reynolds RM. Impact of maternal obesity on offspring obesity and cardiometabolic disease risk. Reproduction 2010; 140: 387–398. [DOI] [PubMed] [Google Scholar]
- Howie GJ, Sloboda DM, Kamal T, Vickers MH. Maternal nutritional history predicts obesity in adult offspring independent of postnatal diet. J Physiol 2009; 587 (Pt 4): 905–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan IY, Dekou V, Douglas G, Jensen R, Hanson MA, Poston L et al. A high-fat diet during rat pregnancy or suckling induces cardiovascular dysfunction in adult offspring. Am J Physiol Regul Integr Comp Physiol 2005; 288: R127–R133. [DOI] [PubMed] [Google Scholar]
- Galvez-Prieto B, Somoza B, Gil-Ortega M, Garcia-Prieto CF, de Las Heras AI, Gonzalez MC et al. Anticontractile effect of perivascular adipose tissue and leptin are reduced in hypertension. Front Pharmacol 2012; 3: 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C, Su LY, Lee RM, Gao YJ. Alterations in perivascular adipose tissue structure and function in hypertension. Eur J Pharmacol 2011; 656: 68–73. [DOI] [PubMed] [Google Scholar]
- Somlyo AP, Somlyo AV. Signal transduction by G-proteins, rho-kinase and protein phosphatase to smooth muscle and non-muscle myosin II. J Physiol 2000; 522 (Pt 2): 177–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Alfonso MS, Gil-Ortega M, Garcia-Prieto CF, Aranguez I, Ruiz-Gayo M, Somoza B. Mechanisms of perivascular adipose tissue dysfunction in obesity. Int J Endocrinol 2013; 2013: 402053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauricio MD, Aldasoro M, Ortega J, Vila JM. Endothelial dysfunction in morbid obesity. Curr Pharm Des 2013; 19: 5718–5729. [DOI] [PubMed] [Google Scholar]
- Chen ZP, Mitchelhill KI, Michell BJ, Stapleton D, Rodriguez-Crespo I, Witters LA et al. AMP-activated protein kinase phosphorylation of endothelial NO synthase. FEBS Lett 1999; 443: 285–289. [DOI] [PubMed] [Google Scholar]
- Morrow VA, Foufelle F, Connell JM, Petrie JR, Gould GW, Salt IP. Direct activation of AMP-activated protein kinase stimulates nitric-oxide synthesis in human aortic endothelial cells. J Biol Chem 2003; 278: 31629–31639. [DOI] [PubMed] [Google Scholar]
- Schneider H, Schubert KM, Blodow S, Kreutz CP, Erdogmus S, Wiedenmann M et al. AMPK dilates resistance arteries via activation of SERCA and BKCa channels in smooth muscle. Hypertension 2015; 66: 108–116. [DOI] [PubMed] [Google Scholar]
- Wang J, Ma H, Tong C, Zhang H, Lawlis GB, Li Y et al. Overnutrition and maternal obesity in sheep pregnancy alter the JNK-IRS-1 signaling cascades and cardiac function in the fetal heart. FASEB J 2010; 24: 2066–2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu MJ, Han B, Tong J, Ma C, Kimzey JM, Underwood KR et al. AMP-activated protein kinase signalling pathways are down regulated and skeletal muscle development impaired in fetuses of obese, over-nourished sheep. J Physiol 2008; 586: 2651–2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis BJ, Xie Z, Viollet B, Zou MH. Activation of the AMP-activated kinase by antidiabetes drug metformin stimulates nitric oxide synthesis in vivo by promoting the association of heat shock protein 90 and endothelial nitric oxide synthase. Diabetes 2006; 55: 496–505. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.