Abstract
The insulin-like growth factor (IGF) signaling system plays a critical role in tumorigenesis, highlighting the potential of targeting IGF-1R as an anti-cancer therapy. Although multiple anti-IGF-1R monoclonal antibody (mAb) drugs have been developed, challenges remain in the validation of the therapeutic effects and understanding the molecular mechanism of these mAbs. Herein, we conducted a study to validate the effect of Figitumumab (CP), an anti-IGF-1R mAb, in a panel of non-small cell lung cancer (NSCLC) cell lines. We found all tested cell lines were sensitive to CP, and CP could block IGF-1R and the downstream PI3K/AKT pathway activation. Unexpectedly, we found CP could activate ERK signaling pathway in IGF-1R kinase independent manner, which we further verified was mainly mediated by β-arrestin2. We also investigated the anti-tumor effect of metformin alone as well as its combination with CP to target NSCLC. Metformin could target IGF-1R signaling pathway by attenuating PI3K/AKT and MEK/ERK signaling pathways and down-regulating IGF-1R. Finally, we found that combining metformin with CP could further induce IGF-1R down-regulation and was more effective to target NSCLC cells. Our data suggests the combining of metformin with CP has additive therapeutic value against NSCLC.
The insulin-like growth factor (IGF) signaling system plays critical roles in tumor cell proliferation, apoptosis, malignant transformation, angiogenesis and cell motility1,2,3,4. In lung cancer, over-expression of IGF-1 and/or IGF-1R was associated with poor prognosis and survival5,6,7,8. IGF-1R targeting therapy has become a highly attractive area in anti-cancer drug development during the last decade9. Antibodies against IGF-1R were designed to specifically block ligand-induced receptor activation by completing with ligands, and thus induce receptor internalization/degradation and cell signaling abrogation10. Several preclinical and clinical studies have demonstrated the efficacy of these anti-IGF-1R mAbs in cancer therapy11,12; however, challenging remains because some anti-IGF-1R mAbs failed to shown similar therapeutic effects in clinical trials, with hyperglycemia being one of the most frequent side effects13,14,15.
CP (Figitumumab, CP-751,871), a monoclonal anti-IGF-1R antibody, has been shown to suppressed tumor initiation and progression in some preclinical studies16,17,18. Phase I, II trials on CP showed some promising results, with well-tolerance and mild adverse events19,20. As to NSCLC, Phase II trial showed CP was safe and effective, but a phase III trial in advanced NSCLC with CP showed significantly more side effects and less efficacy, which unfortunately resulted in the discontinuation of the trial21. In order to decide whether we should resume the clinical trial on CP, it is important for us to better understand the molecular mechanism of CP, which might help us to stratify the NSCLC patients and minimize its side effects. In addition, it is critical to study the combination of CP with other drugs that could potentially enhance its therapeutic effects against NSCLC, and thus could encourage the enrollment of patients into the trial.
Metformin (1, 1-dimethylbiguanide) is drawing increasing attention for its potential anti-neoplastic effects. Several clinic studies have observed intriguing results that metformin is associated with risk and/or mortality in many cancer types, including lung cancer22,23. Meanwhile, increasing experimental data have revealed metformin’s anti-cancer properties including inhibiting cancer cell proliferation, migration, invasion and metastasis24,25,26. Since many cancer cells are characterized with a constitutive high glucose uptake rate27, the “calorie restriction mimetic” activities of metformin are deemed to among the factors that contribute to its inhibitory effects on cancer growth and development28,29,30. Therefore, metformin is incorporated into current studies for cancer cell metabolism therapeutic approaches. At the molecular level, the activation of LKB1/AMPK/mTOR pathway and the inhibition of insulin-induced bio-cellular activities are investigated to exert its anti-neoplastic effects31.
Beyond the above, the emerging effects of metformin on IGF system capture our attention. Werner et al. has demonstrated that metformin could down-regulate the IGF-1R signaling pathway in a panel of uterine serous carcinoma cell lines, leading to the inhibition of cell proliferation and migration regardless of the p53 status32. In another study, metformin is reported to diminish the phosphorylation of a series of receptor tyrosine kinase (RTKs) and the down-stream PI3/AKT/mTOR and Ras/Raf/MEK/ERK pathways in lung tissues in an AMPK independent pattern in liver IGF-1–deficient (LID) mice model with NNK-induced lung carcinogenesis33.
β-arrestins are documented to function as E3 ubiquitin ligase adaptors thus mediate ubiquitylation of seven-transmembrane receptors (7TMRs), which result in receptor endocytosis, recycling or degradation34,35. Recent studies have broadened the functional scope of β-arrestins in receptor internalization to the field of RTKs, such as TGF-β and IGF-1R36,37. The concept of “biased agonist” has cut a figure in the field of 7TMRs, and biased ligands and receptors have been identified that preferentially signal through either G protein- or β-arrestin-biased pathways34. Biased agonists give intriguing possibility of directing cellular signaling with unprecedented precision and specificity and facilitate the development of new types of drugs with fewer side effects35,38.
Our group aims at testing the sensitivity of NSCLC cells to CP and further testing the effect of CP on signaling pathways and receptor down-regulation. In particular, we discussed the role of β-arrestins in regulating cell signaling and therapeutic effect. Furthermore, we tested the therapeutic enhancement effect of metformin when combining with CP to treat NSCLC. We summarized the theoretical basis that support our hypothesis as following: (1) the caloric restriction effect and other anti-neoplastic mechanisms make metformin good choice in cancer therapy (2) anti-IGF-1R mAbs targeting therapy is promising approach against cancer, including NSCLC (3) inhibition effects on IGF-1R signaling system highlighting metformin as a candidate as an adjuvant in anti-IGF-1R system therapy (4) anti-IGF-1R mAb targeting therapy may benefit from metformin for its hypoglycemic property by reducing side effects. In summary our results help to better understand the mechanism and side effects of CP when using it to target NSCLC and we demonstrated the potential of combining CP with metformin as a novel NSCLC therapeutic strategy.
Results
Sensitivity to CP treatment in NSCLC cell lines
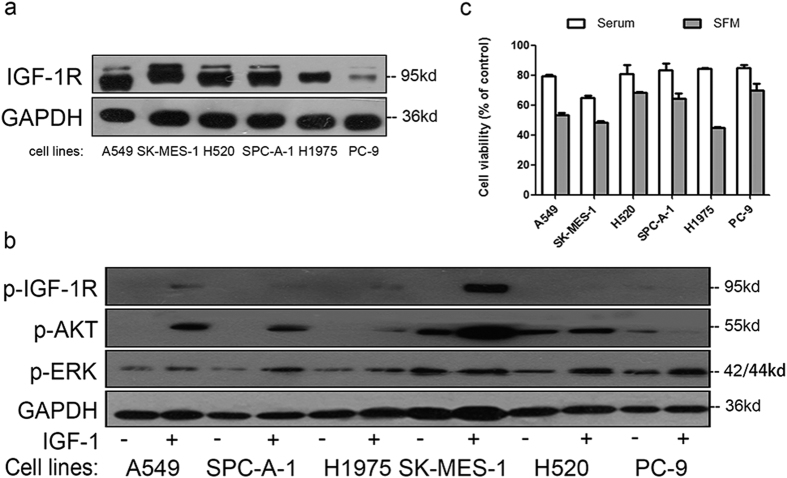
Firstly, we investigated the IGF-1R expression level in a panel of NSCLC cell lines, including A549, SK-MES-1, H520, SPC-A-1, H1975 and PC-9 (Fig. 1a). IGF-1R was expressed in all tested NSCLC cell lines, although the exact expression level was variable, with A549 and PC-9 displaying the highest and lowest levels, respectively. We then stimulated these cells with IGF-1, a complete IGF-1R agonist. As shown in Fig. 1b, IGF-1 could induce IGF-1R phosphorylation and activation of downstream RAS/RAF/MEK/ERK and PI3K/AKT signaling pathways in most cell lines, and the level of activation was generally correlated with the amount of IGF-1R in the cell. These results suggested that IGF-1R signaling pathway was responsive in all the cell lines we used.
Figure 1. IGF-1R expression and sensitivity to CP treatment in NSCLC cell lines.
(a) NSCLC cell lysates were prepared and IGF-1R were detected by WB. (b) Cells were starved for 12h and stimulated with 50 ng/ml IGF-1 for 10 min. p-IGF-1R, p-ERK, p-AKT and GAPDH were detected by WB. (c) Cells were treated with 100 ng/ml of CP for 48 h with presence or absence of serum. Cell viability was tested through MTT. The number of viable cells following CP treatment was presented as percentage of untreated cells. Data were presented as mean ± S.E.M.
Subsequently, we investigated the sensitivity of these NSCLC cells to anti-IGF-1R mAb CP (100 ng/mL CP, whose molar concentration is 10-fold less than 50 ng/mL IGF-1). As demonstrated in Fig. 1c, cell viability decreased in all NSCLC cell lines after CP treatment, ranging from 64.7% in SK-MES-1 to 85.0% in PC-9 under serum added medium conditions, and from 45.0% in H1975 and 70.1% in PC-9 under serum free medium (SFM) conditions. CP was originally designed to compete with ligands for binding of IGF-1R, thus preventing the ligand-induced signaling pathway activation. This idea was supported by the inhibitory effect of CP seen in NSCLC cell lines in serum-added medium (Fig. 1c). However, it was surprising to see that CP had more inhibitory effects in cells under SFM conditions. It is possible that besides being an IGF-1 competitor, CP might have another IGF-1R dependent mechanism to inhibit cell proliferation and/or promote cell death.
β-Arrestins regulate IGF-1 but not CP-induced IGF-1R down-regulation
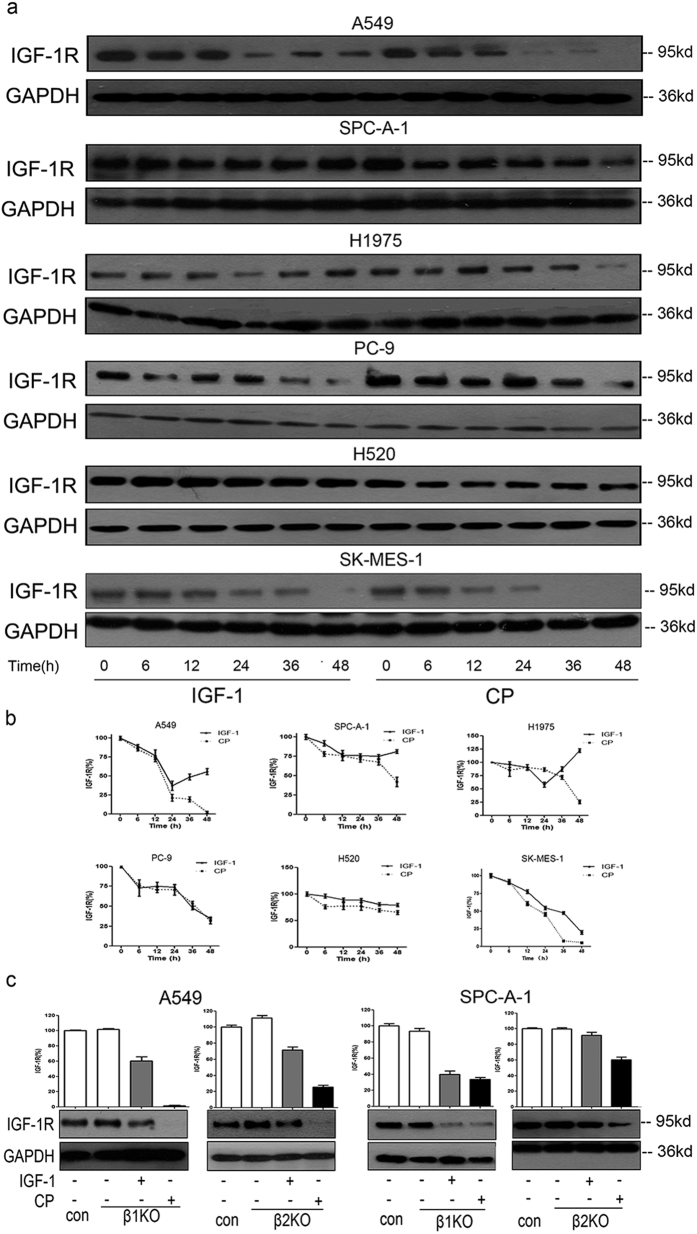
Multiple RTK-targeting mAbs were documented to have a delayed effect on the down-regulation of corresponding receptors. We processed a series of experiments to verify the receptor down-regulation property of CP and investigated its possible mechanisms. In order to get the pure effects of CP without the competition of IGF-1 or other growth factors that are normally presented in serum, all experiments were performed in SFM condition. As shown in Fig. 2a,b, both IGF-1 and CP could induce IGF-1R down-regulation in a time-dependent manner. The IGF-1R down-regulation effect was stronger and more sustained after CP treatment in most cell lines, especially at later time points, while in multiple IGF-1 treated cells (A549, H1975 and SPC-A-1) the expression of IGF-1R rebounded at around 48h. Since the molar concentration of CP was 10-fold less than that of IGF-1, our data suggested that CP is more effective in inducing IGF-1R down-regulation.
Figure 2. CP could induce IGF-1R down-regulation.
(a) Cells were starved for 12 hs, then treated with 50 ng/ml IGF-1 or 100 ng/ml CP for a serious of time points (0, 6, 12, 24, 36, 48 h). Cell lysates were prepared and the level of IGF-1R was detected WB. (b) Intensity of the bands for IGF-1R at different time points in Fig. 2a were quantified by densitometry, normalized to GAPDH, and displayed as a percentage of the intensity at 0 h. Data were presented as mean ± S.E.M. (c) A549 and SPC-A-1 cells were knocked down for either β-arrestin 1 or β-arrestin 2. Cells were then starved for 12 h, treated with 100 ng/ml CP or 50 ng/ml IGF-1 for 24 h. Cells transfected with scrambled siRNA as control. IGF-1R and GAPDH were detected via WB. The intensity of the bands was presented as mean ± S.E.M.
We tried to investigate if β-arrestins regulate CP-induced IGF-1R down-regulation. We used siRNA to knock-down (KD) specific β-arrestin isoform (β-arrestin1 and β-arrestin2) in A549 and SPC-A-1 cell lines. As shown in Fig. 2c, both β-arrestin1 and β-arrestin2 are involved in IGF-1-induced receptor down-regulation in A549 cells, while β-arrestin2 is the main isoform that regulates IGF-1-induced receptor down-regulation in SPC-A-1 cells; however, neither β-arrestin1 KD nor β-arrestin2 KD could rescue CP-induced IGR-1R down-regulation. These data implies that CP-induced IGF-1R endocytosis and down-regulation is β-arrestins independent and thus is different from that of IGF-1.
CP could induce β-arrestin–dependent ERK activation and inhibiting ERK activation could increase the therapeutic effect of CP against NSCLC
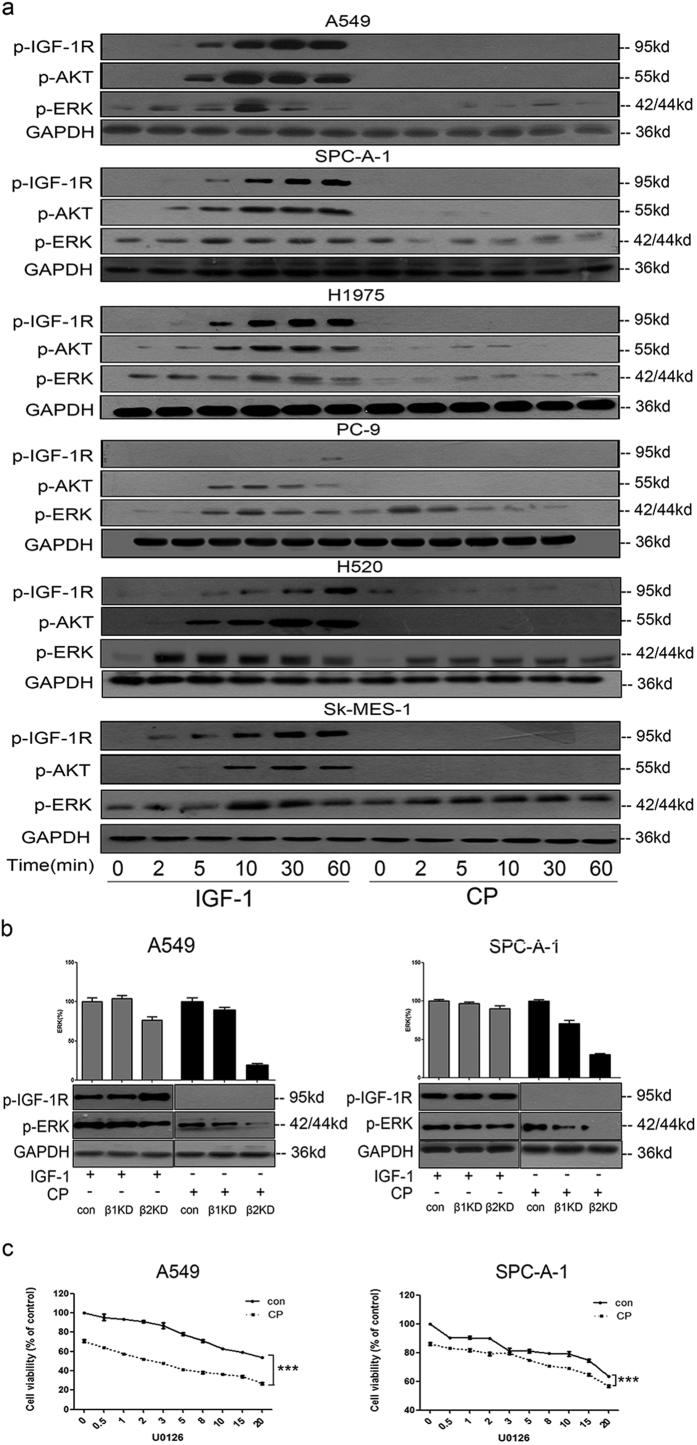
We further investigated the effects of CP on IGF-1R signaling pathways in NSCLC cells and compared it with IGF-1 stimulation (Fig. 3a). As a full agonist, IGF-1 could induce IGF-1R phosphorylation and downstream signaling pathways activation within 5 min. The activation effects of IGF-1 showed in a time depended pattern, peaking around 10 to 30 min and lasting for at least 60 min. In contrast, p-IGF-1R and p-AKT were undetectable after CP treatment. To our surprise, CP could induce ERK phosphorylation in most NSCLC cell lines, although the ERK activation level was still lower than that induced by IGF-1. Since IGF-1R was kept un-phosphorylated (under the sensitivity of Western Blot) under CP treatment, it suggested that there might be another IGF-1R kinase independent mechanism to mediate the phosphorylation of ERK.
Figure 3. CP could induce β-arrestin–dependent ERK activation and inhibiting ERK activation could increase the therapeutic effect of CP.
(a) Cells were starved for 12 h and treated with 50 ng/ml IGF-1 or 100 ng/ml CP for a series of time points (0, 2, 5, 10, 30, 60 min). Cell lysates were analyzed via WB for p-IGF-1R, p-AKT, p-ERK and GAPDH. (b) A549 and SPC-A-1 cells were knocked down for either β-arrestin1 or β-arrestin 2. Cells were then starved for 12 h, treated with 100 ng/ml CP or 50 ng/ml IGF-1 for 10 min. Cells transfected with scrambled siRNA as controls. p-IGF-1R, p-ERK and GAPDH were detected via WB. The intensity of the bands was presented as mean ± S.E.M. (c) A549 and SPC-A-1 cells were treated with U0126 (0, 0.5, 1, 2, 3, 5, 8, 10, 15, 20 μM) for 60 min, and then incubated with or without 100 ng/ml CP for 48 h. Cell viability was tested via MTT. The number of viable cells following treatment is presented as percentage of the untreated cells. Data were presented as mean ± S.E.M.
There have been several studies demonstrating the novel role of β-arrestins to regulate the G protein–coupled receptors (GPCRs) signaling pathway39. We investigated the hypothesis that β-arrestins play a role in IGF-1R-independent ERK activation. As shown in Fig. 3b, knockdown of either β-arrestin 1 or β-arrestin 2 did not interfere the IGF-1 induced ERK phosphorylation, suggesting different mechanisms of downstream pathways activation between IGF-1R and GPCRs. Intriguingly, knockdown of β-arrestin 1 moderately decreased CP-induced ERK phosphorylation while knockdown of β-arrestin 2 almost completely inhibited the CP-stimulated activation of ERK (Fig. 3b). Our results suggested that CP could induce IGF-1R phosphorylation independent activation of ERK, and this process is mediated through β-arrestins, mainly β-arrestin 2.
Due to the surprising ERK activation from CP treatment, we suspected that it might compromise the therapeutic effect of CP to target NSCLC. We then tried to use U0126, a MEK inhibitor, to block ERK activation. U0126 was added 60 min prior to CP treatment in A549 and SPC-A-1 cell lines. As shown in Fig. 3c, U0126 treatment could further inhibit the proliferation of CP-treated NSCLC cells, with higher U0126 having more significant inhibition effect. These results suggested that accidental ERK activation might be one of the main side effects of CP, and combing CP with ERK inhibitors could potentially enhance the efficacy of CP therapy to target NSCLC.
Anti-proliferation effect of metformin alone and in combination with CP in NSCLC cell lines
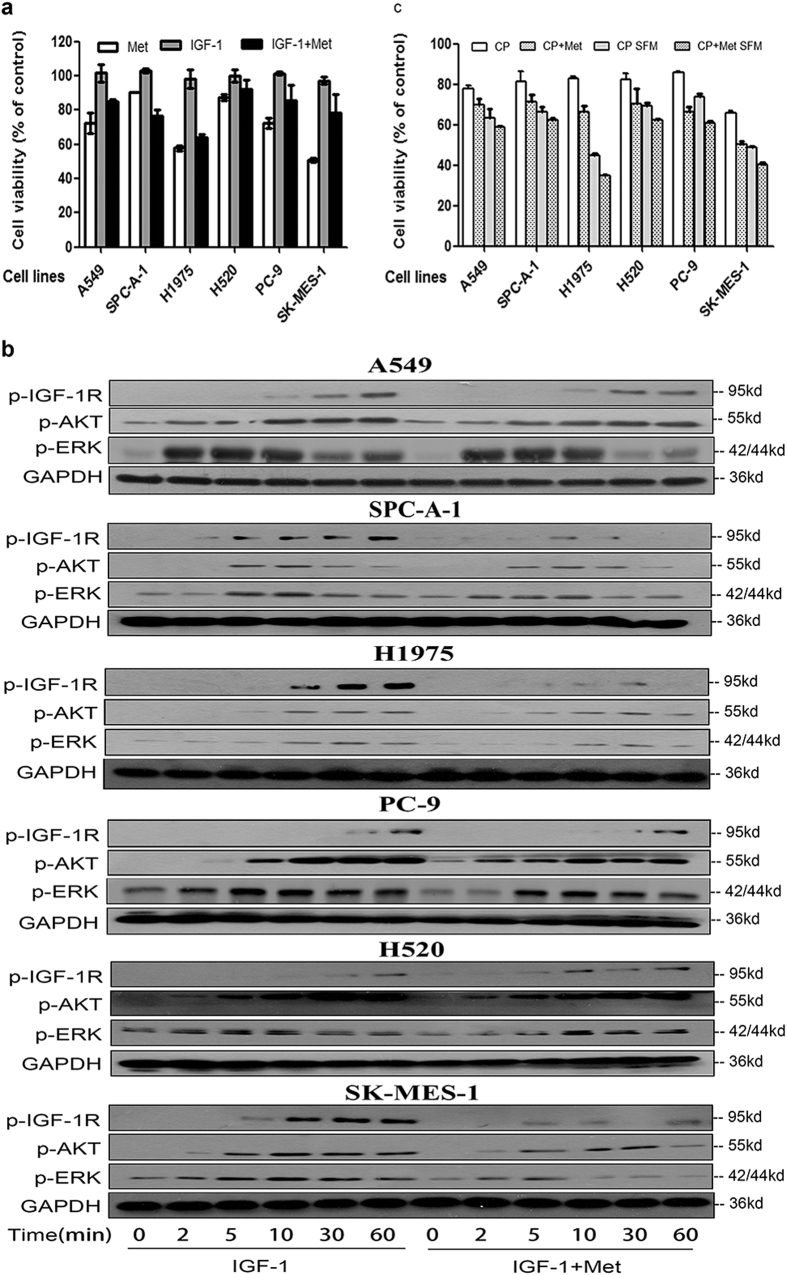
Having shown that CP could be potentially used as a drug to target NSCLC and its side effect of accidental ERK activation, we then tried to investigate if CP could coordinate with metformin to treat NSCLC. Firstly, we investigated the inhibition effects of metformin alone on a panel of NSCLC cell lines. As shown in Fig. 4a, cell viability decreased in all NSCLC cell lines that were tested, ranging from 50.6% in SK-MES-1 to 90.5% in SPC-A-1 cell lines. In addition, in most cell lines IGF-1 could partially rescue the growth inhibitory effects of metformin, implying that simultaneously inhibiting IGF-1R might further improve the anti-tumor effect of metformin. We further tested the effects of metformin on the IGF-1R signaling pathway. As demonstrated in Fig. 4b, metformin could significantly attenuate the IGF-1 induced activation of IGF-1R, AKT and ERK in a panel of NSCLC cell lines, which could at least partially explain the antitumor effects of metformin in Fig. 4a.
Figure 4. Metformin inhibits NSCLC through IGF-1R signaling pathway.
(a) Cells were treated 48h with metformin (3 mM) alone, IGF-1 (50 ng/ml) alone, or IGF-1 plus metformin. Cell viability was tested via MTT. The number of viable cells following treatments is presented as percentage of untreated cells. Data were presented as mean ± S.E.M. (b) After 12 h starvation, A549 and SPC-A-1 cells were treated with or without 3 mM metformin for 1 h. Cells were then treated with 50 ng/ml IGF-1 for a series of time points. Cell lysates were analyzed via WB for p-IGF-1R, p-AKT, p-ERK and GAPDH. (c) Cells were pre-treated with 3 mM Metformin for 1 h, and then 100 ng/ml CP was added with presence or absence of serum. MTT was conducted to test cell viability after 48 h of incubation. The number of viable cells following treatment is presented as percentage of untreated cells. Data were presented as mean ± S.E.M.
Having demonstrated that both metformin and CP target IGF-1R signaling pathway, the next question is whether combining CP and metformin could have additive effects on NSCLC cell lines. Consistent with previous results, CP could effectively inhibit cell proliferation in NSCLC cell lines and CP is more effective in SFM condition than serum-added conditions (Fig. 4c). In addition, the combination of metformin with CP could further decrease the cell survival, with cell viability ranging from 50.6% in SK-MES-1 to 71.5% in SPC-A-1 under serum added conditions while ranging from 34.8% in H1975 to 62.3% in H520 under the SFM conditions. Our result suggested that CP and metformin have additive therapeutic effects to target NSCLC cell lines. Even though both CP and metformin target IGF-1R signaling pathway, the combination might further inhibit IGF-1R activation, and/or either metformin or CP have additional anti-tumor effects that are not overlapped with each other.
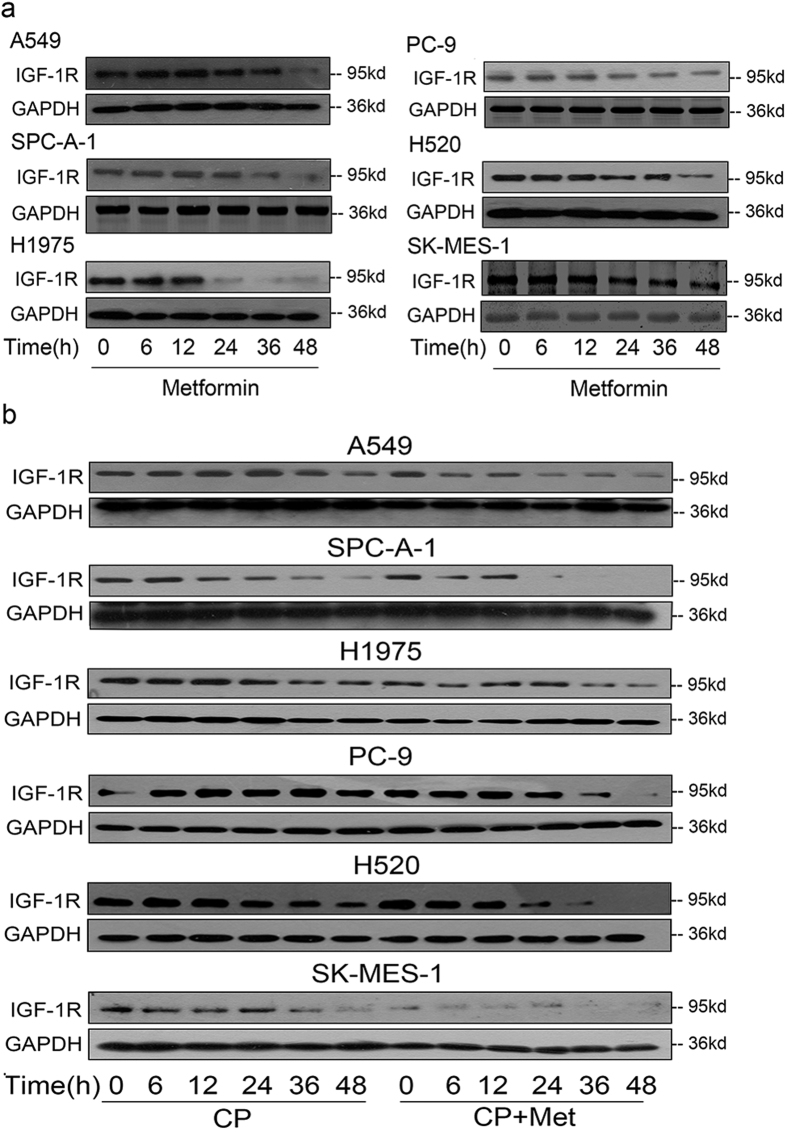
Metformin could promote the CP-induced IGF-1R down-regulation
To further elucidate the additive therapeutic effects of CP and metformin, we tried to check the IGF-1R expression level after drug treatment in NSCLC cell lines. As shown in Fig. 5a, a time-dependent down-regulation of IGF-1R was seen in all the tested cell lines, which was also seen under CP treatment (Fig. 2a). We then assessed the receptor down-regulation after combination metformin and CP treatment. As demonstrated in Fig. 5b, CP plus metformin could further down-regulate IGF-1R compared with CP only group. Therefore, the additive IGF-1R down-regulation could at least partially explain the additive therapeutic effect of combining CP and metformin to target NSCLC.
Figure 5. Metformin could promote the CP-induced IGF-1R down-regulation.
(a) 3 mM Metformin was added to cells for a series of time points (0, 6, 12, 24, 36, 48 h). The expression level of IGF-1R and GAPDH were detected through WB. (b) Cells were starved for 12h, 3mM Metformin was added to cells for 1h, then 100 ng/ml CP was added for a series of time points (0, 6, 12, 24, 36, 48 h). The expression level of IGF-1R and GAPDH were detected through WB.
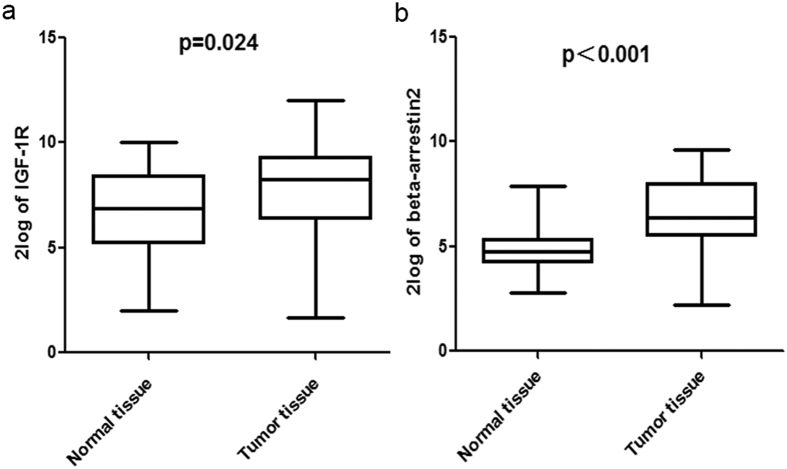
IGF -1R and β-arrestin 2 are highly expressed in NSCLC tumor tissues
Based on the above results, we recognized the importance of IGF -1R and the corresponding signaling pathway in NSCLC. And the role of β-arrestins in receptor down-regulation and signaling transduction implied the potential of β-arrestins as a new target. We tested mRNA levels of IGF -1R and β-arrestin2 in tumor tissues and the corresponding normal tissues of 35 patients from Shandong Provincial Hospital Affiliated to Shandong University by qRT-PCR. The clinical characters of the patients are described in Table 1. As shown in Fig. 6a, the expression of IGF -1R mRNA was higher in NSCLC tumors than the normal tissue (p = 0.024), confirming the critical role of IGF -1R in carcinogenesis. And β-arrestin2 mRNA level was higher in tumor tissues than normal tissues (p < 0.001) (Fig. 6b), suggesting the active of β-arrestin2 in NSCLC.
Table 1. The characters of the patients.
| Parameters | Patients (number) |
|---|---|
| Gender | |
| Male | 27 |
| Female | 8 |
| Age | |
| <60 | 10 |
| ≥60 | 25 |
| Smoking history | |
| No | 8 |
| Yes | 27 |
| Histotype | |
| Adenocarcinoma | 14 |
| Squamous carcinoma | 21 |
| Pathological tumor stage | |
| T1/2 | 31 |
| T3 | 4 |
| Lymph node metastasis | |
| Negative | 19 |
| Positive | 16 |
Figure 6. IGF -1R and β-arrestin 2 mRNA expression in NSCLC tumor tissues.
(a) IGF -1R mRNA expression levels in tumor tissues and normal tissues. Data was shown as 2log of the values. (b) β-arrestin 2 mRNA expression levels in tumor tissues and normal tissues. Data was shown as 2log of the values.
Discussion
In the current study, we tested the effects of CP on a panel of NSCLC cell lines and all tested cell lines were sensitive to CP treatment in both serum-added and serum-free medium. On one hand, the inhibition of cell proliferation in serum-added medium verified that CP could block IGF-1R signaling by competing with IGF-1. On the other hand, the proliferation inhibition effects observed in serum-free medium implied that there might have IGF-1R phosphorylation independent mechanisms that account for the therapeutic effect of CP.
We verified IGF-1R down-regulation effect of CP in NSCLC cells and our data showed the roles of β-arrestins in IGF-1 and CP induced IGF-1R down-regulation are different. β-arrestins serves as E3 ligase adaptors to mediate ubiquitylation of 7TMRs and thus scaffold receptor endocytosis and traffick intracellular itinerary of the receptors, and this pattern was also observed in IGF-1R that β-arrestin1 participated in IGF-1R ubiquitylation and down-regulation39,40. The exact role of β-arrestin1 and β-arrestin2 seems to be different depending on different cell types and receptors34. Also, there’s study illustrating that there are different ubiquitylation sites on β-arrestin2 which would govern its interaction with different receptors and subsequent internalization and downstream signaling41.
We investigated the effects of CP on IGF-1R signaling pathway and unexpectedly found CP could provoke ERK activation without IGF-1R phosphorylation, which might complain some of the side effects observed in CP clinical trials. Furthermore, we found that CP-induced ERK activation was mediated through β-arrestin2. Based on above, we would like to consider CP as a β-arrestin-biased agonist to IGF-1R. β-arrestins have been shown to scaffold with c-Src and lead to subsequent ERK1/2 activation of in β2AR42, and massive in vitro and in vivo data support β-arrestins as a signaling transducer34,43,44,45. Moreover, the β-arrestins-dependent process was reported to be temporally slower onset, which is consistent with our data that CP-induced p-ERK was both weaker and slower than that of IGF-1. Signaling of the two biased arms is pharmacologically distinct, that is, one biased signaling pathway may translate into favorable physiological effects whereas the other appears to result in unbeneficial results46,47. This notion highlights the potential to improve therapeutic outcomes by preferring or avoiding specific signaling arms. When it comes to CP-induced signal activation in NSCLC, the β-arrestin2-biased ERK signaling could be inhibited by U0126, suggesting the potential of controlling the biased signaling to enhance therapeutic effect of CP in the future. A related experiment was done by Leong et al., who demonstrated that the inhibition of MEK signaling with U0126 might help to overcome IGF-1R/IR resistance in vitro and in vivo in colorectal cancer48. Although several studies have demonstrated that β-arrestins served as scaffolding proteins to regulate specific components of the MAPK cascade, their exact molecular functions vary depending on specific agonists, receptors and cell types49,50. The details of the CP-induced ERK signaling need to be further investigated.
We then assessed the anti-proliferation effects of metformin on a panel of NSCLC cell lines, and our results suggested that the anti-tumor effect of metformin was at least partially though the inhibition of IGF-1R signaling pathway. We also found that metformin could down-regulate IGF-1R and attenuate IGF-1R phosphorylation and the downstream PI3K/AKT and Ras/Raf/MEK/ERK pathway activation after IGF-1 stimulation, which further echoed our hypothesis. Our finding is agreed with previous studies that treatment with metformin inhibit cell viability via inhibition of AKT signaling in glioblastoma51. Meanwhile, there are studies demonstrating the effect of metformin on ERK signaling pathway33,52. Because recent studies on metformin mainly focus on AMPK signaling pathway25,31, our findings help to strengthen the role of metformin on IGF-1R signaling pathway, making metformin a promising candidate in anti-cancer therapy. Actually, there have been studies reporting the use of metformin to improve the clinic prognosis in patients who underwent regular chemotherapy with esophageal adenocarcinoma, rectal cancer and other cancers53,54. Some other studies also investigated the synergistic anti-cancer effects of metformin when combining with BRAF or other tyrosine kinase inhibitors in cell lines and animal models15,55. Herein, we tested the combination of metformin with CP treatment and we observed an additive anti-cancer effects when targeting NSCLC cell lines, which we think is partially mediated through IGF-1R down-regulation and signaling attenuation.
Conclusion
Although CP can block IGF-1R downstream PI3K/AKT signaling pathway through ligand competition, CP could also provoke an IGF-1R phosphorylation independent ERK activation mediated via β-arrestin2, which may probably influence its therapeutic effect. CP can down-regulate IGF-1R, which would contribute to the therapeutic benefits. The adding of U0126 and metformin may enhance the therapeutic effect of CP to target NSCLC.
Materials and Methods
Reagents
IGF-1Rβ, p-IGF-1β, p-Erk1/2, p-Akt were purchased from Cell Signaling Technology. β-arrestin1 and β-arrestin2 were from Abcam. GAPDH was from Santa Cruz Biotechnology. U0126 was from Calbiochem. IGF-1 was from Sigma. Figitumumab was provided by Pfizer.
Cell Culture
A549, PC-9, H520, SPC-A-1, NCI-H1975 and SK-MES-1 cell lines were purchased from ATCC, SK-MES-1 was cultured in EMEM medium, the other cell lines were cultured in RIPM H1640 medium supplemented with 10% fetal bovine serum (FBS), and grown in an atmosphere of 5% CO2 /95% humidified air at 37 °C. Cell culture media was from Thermo Scientific Hyclone and FBS was obtained from Life Technologies Gibco.
Transfection
The siRNA of β-arrestin-1(ccaguccaaauggaaagcutt)(257) and β-arrestin-2(cgagccuucugugcuaaautt) (731) were obtained from GenePharma (Shanghai, China). For the silencing of β-arrestin-1 or β-arrestin-2, transient transfection of siRNAs (30nM) targeting human β-arrestin-1 or β-arrestin-2, negative control (scrambled sequence), was performed using LipofectamineRNAiMAX (Invitrogen) according to the manufacturer’s protocol. Each knockdown experiment described herein was detected for specific reduced expression of β-arrestins (75–90%) 48 h later.
Western Blot Analysis
Protein samples were dissolved in lithium dodecyl sulfate (LDS) sample buffer (Invitrogen) and equal amounts of samples were separated by SDS/PAGE. We have described the detail protocol previously56.
Densitometry Analysis
Band intensity was measured by ImageJ 1.63 (US National Institutes of Health, Bethesda, Maryland, USA,) and displayed relative to band intensity of the stated loading control.
Cell Viability Assay/Cell proliferation assay
Cell proliferation was measured with MTT. Cells were incubated in 96-well tissue culture plates, and after appropriate treatment time, MTT was added to a final concentration of 1 mg/mL, and the reaction mixture was incubated for 4 hours at 37 °C. The resulting crystals were dissolved in DMSO and the absorbance was read at 560 nm. Each experiment was repeated thrice, with cells without any stimulation as controls.
Patient tissues and qRT-PCR
The frozen tissues were from patients with primary NSCLC who had undergone a surgical resection in Shandong Provincial Hospital. This study was reviewed and approved by the Ethical Committee of Shandong Provincial Hospital, and written informed consent was given by participants. All experiments were performed in accordance with relevant guidelines and regulations. Total RNA was obtained from NSCLC frozen tumor tissues and the corresponding pare-neoplastic normal tissues, pericarcinous tissues of lung cancer and cell lines using TRIzol reagent (Invitrogen), according to the manufacturer’s instructions. RNA concentration was determined spectrophoto metrically, and integrity was checked by gel electrophoresis. mRNA was converted into cDNA using PrimeScript RT reagent Kit with gDNA Eraser (Takara, Japan). Afterwards, each cDNA was amplified with SYBR Premix Ex Taq (TliRNaseH Plus, Takara, Japan) and analyzed by Roche LightCycler 480II.
Statistical Analysis
Statistical analysis was done using the Student t test or two-way ANOVA to correct for multiple comparisons, as appropriated. All statistical tests were two-sided and were done using SPSS software 19.0 (SPSS Inc., Chicago, IL).
Additional Information
How to cite this article: Cao, H. et al. Metformin Enhances the Therapy Effects of Anti-IGF-1R mAb Figitumumab to NSCLC. Sci. Rep. 6, 31072; doi: 10.1038/srep31072 (2016).
Acknowledgments
The work was supported by Provincial Science and Technology Development Planning of Shandong (2012GG0021836), National Natural Science Foundation of China (81301728), and Key Project of Natural Science Foundation of Shandong Province (ZR2013HZ001). We thank Pfizer for generously providing Figitumumab.
Footnotes
Author Contributions H.C., H.S., J.D. and Q.L. conceived and designed the experiments. H.C., W.D., X.Q., J.X. and L.Z., performed the experiments. H.C. and X.Q. analyzed the data. H.C., W.D., X.Q., Q.L. and J.D. wrote the main manuscript text. All the authors reviewed and modified the manuscript.
References
- Furstenberger G. & Senn H. J. Insulin-like growth factors and cancer. Lancet Oncol 3, 298–302 (2002). [DOI] [PubMed] [Google Scholar]
- Werner H. & Bruchim I. The insulin-like growth factor-I receptor as an oncogene. Arch Physiol Biochem 115, 58–71, 10.1080/13813450902783106 (2009). [DOI] [PubMed] [Google Scholar]
- Samani A. A., Yakar S., LeRoith D. & Brodt P. The role of the IGF system in cancer growth and metastasis: overview and recent insights. Endocr Rev 28, 20–47, 10.1210/er.2006-0001 (2007). [DOI] [PubMed] [Google Scholar]
- Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer 8, 915–928, 10.1038/nrc2536 (2008). [DOI] [PubMed] [Google Scholar]
- Badzio A. et al. Increased insulin-like growth factor 1 receptor protein expression and gene copy number in small cell lung cancer. J Thorac Oncol 5, 1905–1911, 10.1097/JTO.0b013e3181f38f57 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H. et al. Association between circulating levels of IGF-1 and IGFBP-3 and lung cancer risk: a meta-analysis. Plos One 7, e49884, 10.1371/journal.pone.0049884 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa M. et al. Clinical significance of IGF1R expression in non-small-cell lung cancer. Clin Lung Cancer 13, 136–142, 10.1016/j.cllc.2011.10.006 (2012). [DOI] [PubMed] [Google Scholar]
- Kim J. S. et al. Prognostic impact of insulin receptor expression on survival of patients with nonsmall cell lung cancer. Cancer 118, 2454–2465, 10.1002/cncr.26492 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gualberto A. & Pollak M. Emerging role of insulin-like growth factor receptor inhibitors in oncology: early clinical trial results and future directions. ONCOGENE 28, 3009–3021, 10.1038/onc.2009.172 (2009). [DOI] [PubMed] [Google Scholar]
- Sachdev D. & Yee D. Disrupting insulin-like growth factor signaling as a potential cancer therapy. MOL CANCER THER 6, 1–12, 10.1158/1535-7163.mct-06-0080 (2007). [DOI] [PubMed] [Google Scholar]
- Schoffski P. et al. An open-label, phase 2 study evaluating the efficacy and safety of the anti-IGF-1R antibody cixutumumab in patients with previously treated advanced or metastatic soft-tissue sarcoma or Ewing family of tumours. Eur J Cancer 49, 3219–3228, 10.1016/j.ejca.2013.06.010 (2013). [DOI] [PubMed] [Google Scholar]
- Rajan A. et al. Cixutumumab for patients with recurrent or refractory advanced thymic epithelial tumours: a multicentre, open-label, phase 2 trial. Lancet Oncol 15, 191–200, 10.1016/s1470-2045(13)70596-5 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bono J. S. et al. Phase II Randomized Study of Figitumumab plus Docetaxel and Docetaxel Alone with Crossover for Metastatic Castration-Resistant Prostate Cancer. Clin Cancer Res, 10.1158/1078-0432.ccr-13-1869 (2014). [DOI] [PubMed] [Google Scholar]
- Becerra C. R. et al. Figitumumab in patients with refractory metastatic colorectal cancer previously treated with standard therapies: a nonrandomized, open-label, phase II trial. Cancer Chemoth Pharm 73, 695–702, 10.1007/s00280-014-2391-2 (2014). [DOI] [PubMed] [Google Scholar]
- Morgillo F. et al. Synergistic effects of metformin treatment in combination with gefitinib, a selective EGFR tyrosine kinase inhibitor, in LKB1 wild-type NSCLC cell lines. Clin Cancer Res 19, 3508–3519, 10.1158/1078-0432.ccr-12-2777 (2013). [DOI] [PubMed] [Google Scholar]
- Ii M. et al. The efficacy of IGF-I receptor monoclonal antibody against human gastrointestinal carcinomas is independent of k-ras mutation status. Clin Cancer Res 17, 5048–5059, 10.1158/1078-0432.ccr-10-3131 (2011). [DOI] [PubMed] [Google Scholar]
- Kurmasheva R. T. et al. The insulin-like growth factor-1 receptor-targeting antibody, CP-751,871, suppresses tumor-derived VEGF and synergizes with rapamycin in models of childhood sarcoma. Cancer Res 69, 7662–7671, 10.1158/0008-5472.can-09-1693 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen B. D. et al. Combination therapy enhances the inhibition of tumor growth with the fully human anti-type 1 insulin-like growth factor receptor monoclonal antibody CP-751,871. Clin Cancer Res 11, 2063–2073, 10.1158/1078-0432.ccr-04-1070 (2005). [DOI] [PubMed] [Google Scholar]
- Lacy M. Q. et al. Phase I, pharmacokinetic and pharmacodynamic study of the anti-insulinlike growth factor type 1 Receptor monoclonal antibody CP-751,871 in patients with multiple myeloma. J Clin Oncol 26, 3196–3203, 10.1200/jco.2007.15.9319 (2008). [DOI] [PubMed] [Google Scholar]
- Olmos D. et al. Safety, pharmacokinetics, and preliminary activity of the anti-IGF-1R antibody figitumumab (CP-751,871) in patients with sarcoma and Ewing’s sarcoma: a phase 1 expansion cohort study. Lancet Oncol 11, 129–135, 10.1016/s1470-2045(09)70354-7 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scagliotti G. V. et al. Randomized, phase III trial of figitumumab in combination with erlotinib versus erlotinib alone in patients with nonadenocarcinoma nonsmall-cell lung cancer. Ann Oncol 26, 497–504, 10.1093/annonc/mdu517 (2015). [DOI] [PubMed] [Google Scholar]
- Lund S. S. Metformin and mortality. Diabetologia 56, 937–938, 10.1007/s00125-013-2843-y (2013). [DOI] [PubMed] [Google Scholar]
- Lou Z. et al. Bortezomib induces apoptosis and autophagy in osteosarcoma cells through mitogen-activated protein kinase pathway in vitro. J Int Med Res 41, 1505–1519, 10.1177/0300060513490618 (2013). [DOI] [PubMed] [Google Scholar]
- Bao B. et al. Metformin inhibits cell proliferation, migration and invasion by attenuating CSC function mediated by deregulating miRNAs in pancreatic cancer cells. Cancer Prev Res 5, 355–364, 10.1158/1940-6207.capr-11-0299 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerezo M. et al. Metformin blocks melanoma invasion and metastasis development in AMPK/p53-dependent manner. Mol Cancer Ther 12, 1605–1615, 10.1158/1535-7163.mct-12-1226-t (2013). [DOI] [PubMed] [Google Scholar]
- Zhu P. et al. Metformin Selectively Targets Tumor Initiating Cells in erbB-2 Overexpressing Breast Cancer Models. Cancer Prev Res, 10.1158/1940-6207.capr-13-0181 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D. & Weinberg R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674, 10.1016/j.cell.2011.02.013 (2011). [DOI] [PubMed] [Google Scholar]
- Colman R. J. et al. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science 325, 201–204, 10.1126/science.1173635 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercken E. M., Carboneau B. A., Krzysik-Walker S. M. & de Cabo R. Of mice and men: the benefits of caloric restriction, exercise, and mimetics. Ageing Res Rev 11, 390–398, 10.1016/j.arr.2011.11.005 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zannella V. E. et al. Reprogramming metabolism with metformin improves tumor oxygenation and radiotherapy response. Clin Cancer Res 19, 6741–6750, 10.1158/1078-0432.ccr-13-1787 (2013). [DOI] [PubMed] [Google Scholar]
- Zakikhani M., Dowling R., Fantus I. G., Sonenberg N. & Pollak M. Metformin is an AMP kinase-dependent growth inhibitor for breast cancer cells. Cancer Res 66, 10269–10273, 10.1158/0008-5472.can-06-1500 (2006). [DOI] [PubMed] [Google Scholar]
- Sarfstein R. et al. Metformin downregulates the insulin/IGF-I signaling pathway and inhibits different uterine serous carcinoma (USC) cells proliferation and migration in p53-dependent or -independent manners. Plos One 8, e61537, 10.1371/journal.pone.0061537 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn B. J. et al. Inhibition of lung tumorigenesis by metformin is associated with decreased plasma IGF-I and diminished receptor tyrosine kinase signaling. Cancer Prev Res 6, 801–810, 10.1158/1940-6207.capr-13-0058-t (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla A. K., Xiao K. & Lefkowitz R. J. Emerging paradigms of beta-arrestin-dependent seven transmembrane receptor signaling. Trends Biochem Sci 36, 457–469, 10.1016/j.tibs.2011.06.003 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter E., Ahn S., Shukla A. K. & Lefkowitz R. J. Molecular mechanism of beta-arrestin-biased agonism at seven-transmembrane receptors. Annu Rev Pharmacol 52, 179–197, 10.1146/annurev.pharmtox.010909.105800 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W. et al. Beta-arrestin 2 mediates endocytosis of type III TGF-beta receptor and down-regulation of its signaling. Science 301, 1394–1397, 10.1126/science.1083195 (2003). [DOI] [PubMed] [Google Scholar]
- Girnita L. et al. Beta-arrestin and Mdm2 mediate IGF-1 receptor-stimulated ERK activation and cell cycle progression. J Biol Chem 282, 11329–11338, 10.1074/jbc.M611526200 (2007). [DOI] [PubMed] [Google Scholar]
- Whalen E. J., Rajagopal S. & Lefkowitz R. J. Therapeutic potential of beta-arrestin- and G protein-biased agonists. Trends Mol Med 17, 126–139, 10.1016/j.molmed.2010.11.004 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy S. K. & Lefkowitz R. J. beta-Arrestin-mediated receptor trafficking and signal transduction. Trends Pharmacol Sci 32, 521–533, 10.1016/j.tips.2011.05.002 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H. et al. beta-Arrestin-biased agonism as the central mechanism of action for insulin-like growth factor 1 receptor-targeting antibodies in Ewing’s sarcoma. P Natl Acad Sci USA 109, 20620–20625, 10.1073/pnas.1216348110 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy S. K. & Lefkowitz R. J. Receptor-specific ubiquitination of beta-arrestin directs assembly and targeting of seven-transmembrane receptor signalosomes. J Biol Chem 280, 15315–15324, 10.1074/jbc.M412418200 (2005). [DOI] [PubMed] [Google Scholar]
- Luttrell L. M. et al. Beta-arrestin-dependent formation of beta2 adrenergic receptor-Src protein kinase complexes. Science 283, 655–661 (1999). [DOI] [PubMed] [Google Scholar]
- McDonald P. H. et al. Beta-arrestin 2: a receptor-regulated MAPK scaffold for the activation of JNK3. Science 290, 1574–1577 (2000). [DOI] [PubMed] [Google Scholar]
- Beaulieu J. M. et al. An Akt/beta-arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell 122, 261–273, 10.1016/j.cell.2005.05.012 (2005). [DOI] [PubMed] [Google Scholar]
- Schmid C. L. & Bohn L. M. Serotonin, but not N-methyltryptamines, activates the serotonin 2A receptor via a ss-arrestin2/Src/Akt signaling complex in vivo. J Neurosci 30, 13513–13524, 10.1523/jneurosci.1665-10.2010 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters R. W. et al. beta-Arrestin1 mediates nicotinic acid-induced flushing, but not its antilipolytic effect, in mice. J Clin Invest 119, 1312–1321, 10.1172/jci36806 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple G. et al. 3-(1H-tetrazol-5-yl)-1,4,5,6-tetrahydro-cyclopentapyrazole (MK-0354): a partial agonist of the nicotinic acid receptor, G-protein coupled receptor 109a, with antilipolytic but no vasodilatory activity in mice. J Med Chem 51, 5101–5108, 10.1021/jm800258p (2008). [DOI] [PubMed] [Google Scholar]
- Flanigan S. A. et al. Overcoming IGF1R/IR resistance through inhibition of MEK signaling in colorectal cancer models. Clin Cancer Res 19, 6219–6229, 10.1158/1078-0432.ccr-13-0145 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luttrell L. M. et al. Activation and targeting of extracellular signal-regulated kinases by beta-arrestin scaffolds. P Natl Acad Sci USA 98, 2449–2454, 10.1073/pnas.041604898 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott M. G. et al. Cooperative regulation of extracellular signal-regulated kinase activation and cell shape change by filamin A and beta-arrestins. Mol Cell Biol 26, 3432–3445, 10.1128/mcb.26.9.3432-3445.2006 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurth R. et al. Metformin selectively affects human glioblastoma tumor-initiating cell viability: A role for metformin-induced inhibition of Akt. Cell Cycle 12, 145–156, 10.4161/cc.23050 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ning J. & Clemmons D. R. AMP-activated protein kinase inhibits IGF-I signaling and protein synthesis in vascular smooth muscle cells via stimulation of insulin receptor substrate 1 S794 and tuberous sclerosis 2 S1345 phosphorylation. Mol Endocrinol 24, 1218–1229, 10.1210/me.2009-0474 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner H. D. et al. Metformin use and improved response to therapy in esophageal adenocarcinoma. Acta Oncol 52, 1002–1009, 10.3109/0284186x.2012.718096 (2013). [DOI] [PubMed] [Google Scholar]
- Skinner H. D. et al. Metformin use and improved response to therapy in rectal cancer. Cancer Med-US 2, 99–107, 10.1002/cam4.54 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan P. et al. Phenformin enhances the therapeutic benefit of BRAF(V600E) inhibition in melanoma. P Natl Acad Sci USA 110, 18226–18231, 10.1073/pnas.1317577110 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T. et al. Antitumor effects and molecular mechanisms of figitumumab, a humanized monoclonal antibody to IGF-1 receptor, in esophageal carcinoma. Sci Rep-UK 4, 6855, 10.1038/srep06855 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]