In this review, McStay discusses recent findings regarding the morphology of active nucleolar organizer regions (NORs) and how they seed rapid nucleolar reformation after cell division.
Keywords: nucleolar organizer regions (NORs), nucleolus, ribosomal genes, upstream binding factor (UBF), human acrocentric chromosomes
Abstract
Nucleoli form around tandem arrays of a ribosomal gene repeat, termed nucleolar organizer regions (NORs). During metaphase, active NORs adopt a characteristic undercondensed morphology. Recent evidence indicates that the HMG-box-containing DNA-binding protein UBF (upstream binding factor) is directly responsible for this morphology and provides a mitotic bookmark to ensure rapid nucleolar formation beginning in telophase in human cells. This is likely to be a widely employed strategy, as UBF is present throughout metazoans. In higher eukaryotes, NORs are typically located within regions of chromosomes that form perinucleolar heterochromatin during interphase. Typically, the genomic architecture of NORs and the chromosomal regions within which they lie is very poorly described, yet recent evidence points to a role for context in their function. In Arabidopsis, NOR silencing appears to be controlled by sequences outside the rDNA (ribosomal DNA) array. Translocations reveal a role for context in the expression of the NOR on the X chromosome in Drosophila. Recent work has begun on characterizing the genomic architecture of human NORs. A role for distal sequences located in perinucleolar heterochromatin has been inferred, as they exhibit a complex transcriptionally active chromatin structure. Links between rDNA genomic stability and aging in Saccharomyces cerevisiae are now well established, and indications are emerging that this is important in aging and replicative senescence in higher eukaryotes. This, combined with the fact that rDNA arrays are recombinational hot spots in cancer cells, has focused attention on DNA damage responses in NORs. The introduction of DNA double-strand breaks into rDNA arrays leads to a dramatic reorganization of nucleolar structure. Damaged rDNA repeats move from the nucleolar interior to form caps at the nucleolar periphery, presumably to facilitate repair, suggesting that the chromosomal context of human NORs contributes to their genomic stability. The inclusion of NORs and their surrounding chromosomal environments in future genome drafts now becomes a priority.
The relationship between nucleolar organizer regions (NORs) and nucleoli was first established in the 1930s (Heitz 1931; McClintock 1934), but, for decades, the content of the former and the role of the latter remained mysterious. The era of molecular and cellular biology revealed that NORs contain tandem arrays of ribosomal gene (rDNA) repeats and that nucleoli are the sites of ribosome biogenesis. Biochemistry has revealed the inner workings of the nucleolus and the complexity of ribosome biogenesis (for review, see Pederson 2010). However, the genomic architecture of NORs and the chromosomal context in which they lie remains undetermined for most eukaryotes. The resulting void has placed limitations on our understanding of the fundamental mechanisms by which NORs orchestrate formation of the largest structure in the nucleus. In this review, I discuss recent findings regarding the morphology of active NORs and how they seed rapid nucleolar reformation after cell division. I review recent evidence uncovering potential roles for chromosomal context in the functioning of NORs and maintenance of the genomic stability of rDNA arrays. These studies establish, as a priority, the integration of NORs and their chromosomal surrounds into an updated human genome draft.
rDNA arrays and NORs
Ribosomal gene repeats are transcribed by RNA polymerase I (Pol I) to produce a preribosomal RNA (pre-rRNA) that undergoes modification and processing to remove external transcribed spacers (ETSs) and internal transcribed spacers (ITSs) to yield mature 18S, 5.8S, and 28S rRNAs (Fig. 1A). The pre-RNA ranges from 6.9 kb (35S) in yeast (Petes 1979) to ∼13 kb (47S) in mammals (Gonzalez and Sylvester 1995; Grozdanov et al. 2003). Pre-rRNA-coding regions are separated by intergenic spacers (IGSs) that vary in length from ∼2 kb in yeast to ∼30 kb in mammals. The IGS houses gene promoters and regulatory elements, such as spacer promoters and repetitive enhancer elements, which control pre-rRNA synthesis (Goodfellow and Zomerdijk 2013). The IGS also contains replication origins and replication fork barriers (RFBs) that prevent collisions between the replication and transcription machineries (Brewer et al. 1992; Kobayashi et al. 1992; Akamatsu and Kobayashi 2015). Finally, under certain circumstances, such as stress, loss of repressive chromatin modifications, replicative senescence, or aging, the IGS can be transcribed by RNA Pol II (Earley et al. 2010; Audas et al. 2012; Saka et al. 2013; Bierhoff et al. 2014).
Figure 1.
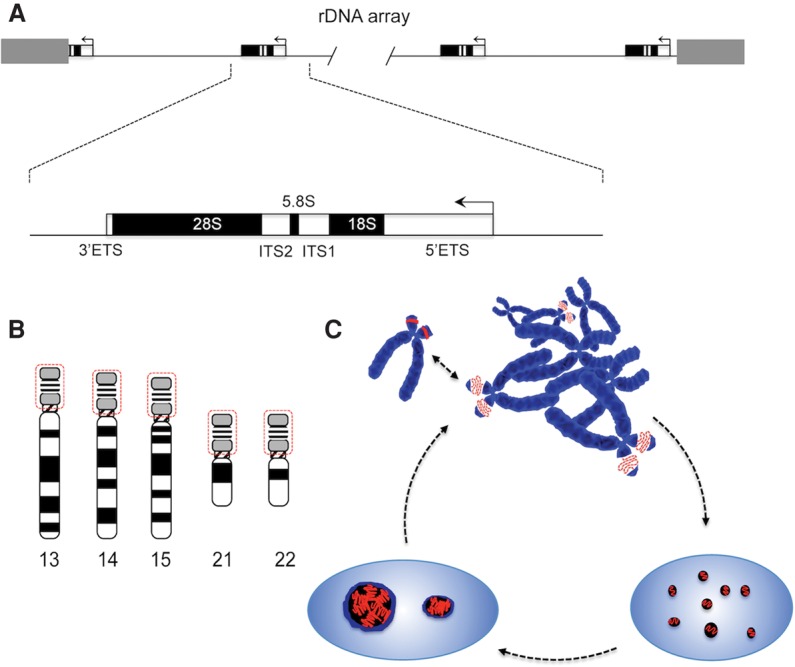
The location of NORs and the nucleolar cycle in human cells. (A) Schematic showing a human rDNA array expanded to show the pre-rRNA-coding sequences that are transcribed by RNA Pol I. The positions of mature rRNA-coding sequences, ETSs, and ITSs are indicated. (B) The locations of NORs on the acrocentric chromosome are indicated. The short arms, circled in red, are missing from the current genome draft GRCh38.p7. (C) During cell division, transcription ceases, and nucleoli disappear. NORs can be observed as achromatic gaps on DAPI-stained metaphase chromosomes due to undercondensation of rDNA (red dotted line). Silent NORs (solid red) fail to show this morphology and do not contribute to nucleolar formation. Transcription resumes in anaphase, and nucleoli form around individual active NORs. In most cell types, these then fuse, producing characteristic large nucleoli surrounded by heterochromatin.
NORs contain arrays of rDNA repeats organized in a head-to-tail fashion. The number of NORs present in the genome varies between species, and the rDNA content of NORs can vary between individuals of the same species and even between the cells of an individual (Stults et al. 2008, 2009). Surprisingly, molecular combing experiments suggest that ∼30% of human rDNA repeats are not organized in this canonical head-to-tail arrangement in human cell lines (Caburet et al. 2005). However, it should be pointed out that direct sequence evidence for these noncanonical rDNA repeats is unavailable. Throughout evolution, a recurring theme emerges. NORs are located close to regions of constitutive heterochromatin at either telomeres or centromeres (Nemeth and Langst 2011). In some cases, NORs are located on the short arms of so-called acrocentric chromosomes, sandwiched between centromeric and telomeric heterochromatin. One function of this constitutive heterochromatin is to isolate NORs from protein-coding genes. In the yeast Saccharomyces cerevisiae, there are up to 200 copies of a 9.1-kb repeat at a single NOR on chromosome XII (Petes 1979). In Arabidopsis thaliana, there are ∼700–800 copies of 10- to 10.5-kb rDNA repeats distributed between NORs located close to the telomeres on the top or north arms of chromosomes 2 and 4 (NOR2 and NOR4, respectively) (Copenhaver and Pikaard 1996). In Drosophila melanogaster, up to 600 rDNA repeats are distributed between NORs on the short arm of the entirely heterochromatic Y chromosome and in the centric heterochromatin of the X chromosome (Ritossa et al. 1966). In humans, ∼300 rDNA repeats are disturbed between NORs on the short arms of each of the five acrocentric chromosomes HSA13, HSA14, HSA15, HSA21, and HSA22 (Fig. 1B; Henderson et al. 1972). Variation in rDNA content of individual NORs can be observed by digesting chromosomes with restriction enzymes that lack recognition sites in the rDNA repeat. Products are analyzed by pulsed field gel electrophoresis (PFGE) followed by hybridization with rDNA probes (Sakai et al. 1995). The Pierce laboratory (Stults et al. 2008, 2009) has used this technique extensively to examine NORs in human cells. Using the restriction endonuclease EcoRV, they have revealed an enormous variability in NOR size, from 40 kb (one repeat) to 6 Mb (>130 repeats) (Stults et al. 2008, 2009). As we see below, this variability may impact on the ability to identify NORs and determine their activity status.
In mice, all chromosomes are acrocentric, and an estimated 200 rDNA repeats are distributed between NORs on the short arms of up to six mouse chromosomes, the identity of which varies from strain to strain (Britton-Davidian et al. 2012). C57 mice have NORs on both chromosome 12 and chromosome 15, while CBA/CaJ (CBA) mice have a NOR on chromosome 15 but not on chromosome 12, and 129P3/J (129P3) mice have a NOR on chromosome 12 but not on chromosome 15 (Strongin et al. 2014). It is possible that this variation between mouse strains may simply reflect differences in rDNA content of NORs between mouse strains rather than the absence or presence of NORs as such.
The Pol I transcription machinery
Eukaryotic rDNA promoters contain two regulatory elements important for directing transcription initiation: the core promoter and the upstream control element (Goodfellow and Zomerdijk 2013). Although the general layout of the rDNA promoter is conserved from yeast to humans, there is little sequence similarity between elements. Other than the coding sequences for 18S, 5.8S, and 28S mature rRNA, other rDNA sequences—including the IGS, ETSs, ITSs, and promoters—evolve rapidly. The Pol I machinery is dedicated solely to transcription of rDNA and is rapidly evolving. This is exemplified by incompatibilities between mouse and human machineries (McStay 2006).
Transcription by Pol I is dependent on a TBP-containing complex termed core factor (CF) in yeast, selectivity factor 1 (SL1) in humans, and TIF1B in mice. Yeast CF is a complex of TBP and at least three further proteins: Rrn6, Rrn7, and Rrn11. SL1 is a complex of TBP and at least four Pol I-specific TBP-associated factors (TAFIs) (Goodfellow and Zomerdijk 2013). SL1 is critical in the formation of a preinitiation complex (PIC) and binds in a highly sequence-specific manner to the core promoter element. Species specificity between mouse and human Pol I machineries resides in SL1.
A conserved feature of Pol I transcription machineries appears to be the involvement of a nucleolar-specific HMG-box-containing DNA-binding protein: Hmo1 in yeast and upstream binding factor (UBF; sometimes referred to as UBTF) in mammals. UBF was originally identified as a component of the mammalian PIC (Bell et al. 1988). It is characterized by an N-terminal dimerization domain, multiple HMG (high-mobility group) boxes, and a C-terminal acidic tail (Jantzen et al. 1990; McStay et al. 1991). Mammalian cells have two splice variants—UBF1 and UBF2—that are present in roughly equal proportions. UBF2 lacks 37 amino acids from the second HMG box (O'Mahony and Rothblum 1991) and apparently does not participate in PIC formation (Kuhn et al. 1994). In Xenopus and mice, UBF was also shown to bind to repetitive enhancer elements upstream of the gene promoter and stimulate transcription initiation (McStay et al. 1997). UBF was originally thought to be restricted to vertebrates, but it is now clear that it is present throughout metazoans—curious exceptions being Drosophila and Caenorhabditis elegans (Grob et al. 2011). Hmo1p, a yeast protein with little sequence similarity to UBF other than bearing a canonical HMG box, was identified as a bona fide Pol I transcription factor (Gadal et al. 2002). Surprisingly, human UBF1 can partially substitute for Hmo1 in vivo (Albert et al. 2013). More recently, general roles for UBF and Hmo1p in organizing chromatin across rDNA arrays have been revealed and are discussed below.
Recruitment of Pol I to PICs is mediated by another conserved component of the Pol I machinery, termed Rrn3 in yeast and humans and TIF-IA in mice (Goodfellow and Zomerdijk 2013). Rrn3/TIF-IA makes multiple protein–protein contacts with both CF/SL1 and Pol I. It is a primary target for signal transduction pathways responsible for the short-term regulation of rDNA transcription (Mayer and Grummt 2005).
Long-term regulation of ribosome biogenesis can involve epigenetic changes at rDNA promoters. Key players in the silencing of rDNA promoters in mammals are TTF1 (transcription termination factor 1) and NoRC (nucleolar chromatin remodeling complex), comprising TIP5 (TTF1-interacting protein 5) and ATPase Snf2H. (for review, see McStay and Grummt 2008). Interestingly, TIP5 (also known as BAZ2A) is overexpressed in prostate cancer and is involved in maintaining prostate cancer cell growth by directly interacting with EZH2 to maintain epigenetic silencing at Pol II transcribed genes repressed in metastasis (Gu et al. 2015). This is a somewhat paradoxical result, since up-regulation of ribosome biogenesis is a feature of most cancers. The Drosophila ortholog of TIP5, Toutatis, is also involved in the regulation of genes transcribed by Pol II (Emelyanov et al. 2012).
An often-quoted figure is that in mammalian cells, only ∼50% of rDNA repeats are actively transcribed at any given time (McStay and Grummt 2008; Goodfellow and Zomerdijk 2013). Transcriptionally active rDNA repeats are accessible to the cross-linking agent psoralen, whereas silent rDNA repeats are chromatinized and inaccessible (Conconi et al. 1989). Thus, the ratio of cross-linked (active) to noncross-linked (silent) repeats is 1:1 in most mammalian cell lines. The chromosomal distribution of these classes of repeat is discussed below.
The nucleolar cycle
In simple eukaryotes, including yeast, there is no breakdown of the nuclear membrane during mitosis. Nucleoli remain intact throughout this “closed” mitosis, and transcription is only momentarily inhibited during anaphase to ensure rDNA condensation and nucleolar segregation (Clemente-Blanco et al. 2009). In complex eukaryotes with an “open” mitosis, including humans, Pol I transcription is shut down, and nucleoli disappear during prophase. Beginning in telophase, Pol I transcription resumes, and nucleoli begin to reform around individual NORs, forming multiple small nucleoli (Savino et al. 2001). Nucleolar fusion then occurs, resulting in the formation of larger mature nucleoli containing multiple NORs (Fig. 1C). Due to their low DNA content, these mature nucleoli usually appear as dark regions in DAPI-stained cells. It appears that rDNA is the only DNA present within the nucleolar volume. A second characteristic of these mature nucleoli, particularly evident in human cells, is perinucleolar heterochromatin (PNH) (Nemeth and Langst 2011). In humans, this is derived from the heterochromatin located distal and proximal to NORs on the acrocentric short arms. The mechanism of nucleolar fusion is unknown but must involve very significant reorganization of as many as 10 chromosomal territories within the interphase nucleolus. Studies on the amplified nucleoli present in the large nucleus (germinal vesicle) of amphibian oocytes reveal that they behave like liquid droplets that can spontaneously coalesce in vitro (Brangwynne et al. 2011; Hyman et al. 2014). In contrast to the chromosome-tethered nucleoli of somatic cells, these amplified nucleoli form around episomal amplified rDNA repeats. It is tempting to speculate that the nucleolar fusion observed in human cells is biphasic. The first phase involves chromosome movement and the establishment of the PNH, and the second phase involves fusion of nucleolar material associated with individual NORs by a liquid-like behavior. In many human cancer cell lines, nucleoli exhibit an altered morphology—usually larger, more disorganized, and without a clearly visible PNH (Farley et al. 2015). This altered morphology appears to be reversible (Krystosek 1998). Finally, a number of non-NOR-bearing chromosomes and chromosomal domains that can associate with the PNH have been identified in nucleolar genomics and immuno-FISH experiments (for review, see Nemeth and Langst 2011; Padeken and Heun 2014).
Nucleoli of higher eukaryotes are comprised of three distinct subcompartments that can be seen in the electron microscope (Pederson 2010; Farley et al. 2015). The innermost fibrillar centers (FCs) are surrounded by dense fibrillar components (DFCs). FC/DFC compartments are in turn surrounded by granular components (GCs). Lower eukaryotic organisms typically lack FCs (Thiry and Lafontaine 2005). Current evidence suggests that ribosome biogenesis is a vectorial process, initiating in the FC and proceeding outward to the GC. Transcription of rDNA takes place at the interface between the FCs and DFCs. Pre-rRNA processing takes place within the DFCs, and ribosome subunit assembly occurs within the GCs followed by export of subunits to the cytoplasm for the final steps of maturation. In the light microscope, it is difficult to resolve FCs from the DFCs, but FCs/DFCs and GCs can be resolved easily. FCs/DFCs can be visualized with antibodies to UBF or fibrillarin (involved in cotranscriptional modification of pre-rRNA). The GCs can be stained with antibodies against late processing factors such as Nop52 (Savino et al. 2001). This nucleolar architecture is dependent on ongoing transcription (Hadjiolov 1985). Treatment of cells with low concentrations of actinomycin D (AMD), a DNA intercalating agent with specificity for G/C-rich rDNA, inhibits transcriptional elongation by Pol I. This results in a rapid reorganization of nucleolar structure involving the formation of caps at the nucleolar surface that comprise FC and at least some DFC proteins as well as rDNA (Sirri et al. 2008; Floutsakou et al. 2013). GCs are maintained in the nucleolar interior.
Nucleolar proteins are highly dynamic, rapidly exchanging between the nucleolus and nucleoplasm. Thus, nucleolar structure is generally considered to be a direct consequence of the process of ribosome biogenesis rather than being determined by some skeletal framework (Misteli 2001). Recently, however, evidence has been presented that RNA Pol II transcripts originating from intronic Alu elements are enriched in the human nucleolus and are required for its structural integrity (Caudron-Herger et al. 2015).
Morphology of NORs on metaphase chromosomes
NORs were first recognized on metaphase chromosomes due to their striking morphological appearance as achromatic gaps on stained chromosomes, sometimes referred to as secondary constrictions (primary constrictions being centromeres) (Heitz 1931; McClintock 1934). Electron tomography of human NORs has revealed that this morphology arises as a result of undercondensation of rDNA repeats within active NORs (Heliot et al. 1997). A second defining characteristic of active NORs in many if not all animals and plants is their ability to be selectively stained with silver nitrate (Goodpasture and Bloom 1975). Presumably, some strongly silver-staining proteins remain associated with these so-called AgNORs through metaphase. Such proteins may be causative in the undercondensation of DNA. The identity of these proteins in human cells began to be revealed when metaphase chromosomes were stained with antibodies to the Pol I transcription machinery. UBF, SL1, and TTF1 have been shown to associate with mitotic NORs (Roussel et al. 1993; Jordan et al. 1996; Sirri et al. 1999). Early indications that Pol I itself remained associated are not supported by live-cell imaging experiments (Leung et al. 2004). The list of proteins associated with mitotic NORs is expanding and now includes Treacle, the product of the TCOF1 gene that is mutated in Treacher Collins syndrome (Valdez et al. 2004); ATRX, mutated in α-thalassemia accompanied by X-linked mental retardation syndrome (Gibbons et al. 2000); and Sirt7, a mammalian homolog of yeast Sir2p, an NAD-dependent histone deacetylase, and an activator of Pol I transcription (Grob et al. 2009). A number of cell type-specific DNA-binding proteins with less well established links to rDNA or ribosome biogenesis also remain associated with NORs. These include pluripotency factors (Zentner et al. 2014), Runx2 (Young et al. 2007), and basonuclin (Tseng et al. 1999). It is possible that they exploit the open chromatin state of mitotic NORs as a segregation mechanism. At least two NOR-associated proteins, UBF and Treacle, have highly acidic domains that contribute to silver staining.
Silent human NORs that lack UBF and all the other Pol I-related factors are fully condensed and do not associate with nucleoli (McStay and Grummt 2008; Grob et al. 2014). In monochromosomal somatic cell hybrids with a full complement of mouse chromosomes and a single human acrocentric chromosome, human NORs are transcriptionally silent yet remain associated with mouse nucleoli (Sullivan et al. 2001). Interestingly, human NORs in this context retain UBF loading. Thus, fusion of a NOR into a larger nucleolus may not be entirely dependent on transcription of its rDNA.
UBF, a mitotic bookmark for active human NORs
The finding that UBF is more abundant than previously thought and that it binds extensively across human rDNA repeats throughout the cell cycle provided the initial evidence that it plays a more general role in organizing ribosomal gene chromatin (Roussel et al. 1993; O'Sullivan et al. 2002). These observations are underpinned by in vitro experiments showing that it had a very relaxed sequence specificity of DNA binding, binds to DNA in a cooperative manner, could bind to nucleosomal templates in vitro, and could compete with binding of linker histone H1 to a nucleosomal template (Putnam and Pikaard 1992; Copenhaver et al. 1994; Kermekchiev et al. 1997). Pseudo-NORs provide the first evidence that extensive UBF binding is directly responsible for the morphological appearance of active NORs (Mais et al. 2005). Pseudo-NORs were constructed by integrating megabase-scale synthetic UBF-binding site arrays into nonacrocentric chromosomes of human cells. During metaphase, pseudo-NORs appear as achromatic gaps, are positive in silver staining, and have a protein composition similar to that of endogenous active NORs. A causative role for UBF in establishing this morphology was revealed by siRNA-mediated depletion of UBF (Prieto and McStay 2007; Grob et al. 2014). Depletion of UBF from normal human cells results in a proportion of endogenous NORs losing UBF staining, becoming condensed, and losing their nucleolar association (Grob et al. 2014).
Pseudo-NORs lack promoters and pre-rRNA-coding sequences and thus do not progress to forming nucleoli during interphase. Nevertheless, they do form readily visible novel subnucleolar structures that are similar in protein composition to the FCs of mature nucleoli (Mais et al. 2005). Neo-NORs provided the final piece of data, linking UBF binding with nucleolar-forming capability (Grob et al. 2014). Neo-NORs contain UBF-binding site arrays interspersed with human promoters that drive expression of mouse pre-rRNA-coding sequences. Like pseudo-NORs, they adopt the morphology of active endogenous NORs. In contrast, however, they form apparently functional neonucleoli with visible FC/DFC and GC compartments. Neonucleoli are capable of assembling ribosomal subunits that can be found on polysomes, suggesting that they are functional. Thus, high-affinity UBF-binding sites coupled with rDNA transcription units are sufficient to form an NOR competent for nucleolar formation in human cells. Propagation of nucleoli through cell division in higher eukaryotes with an “open” mitosis is a staged process. NORs that were active in the previous interphase are bookmarked by UBF during mitosis (Grob et al. 2014; Grob and McStay 2014). This UBF-dependent bookmarking step ensures rapid reactivation of transcription in post-metaphase cells with pre-rRNA seeding recruitment of processing and assembly factors, resulting in nucleolar compartmentalization. Furthermore, it appears that this strategy has been conserved through evolution, as UBF is present across animal phyla (Grob et al. 2011). Yeast Hmo1p is similar to UBF in a number of respects. It binds extensively across rDNA and is involved in the establishment of an open chromatin state on active repeats (Wittner et al. 2011). As yeast nucleoli persist through metaphase, it is not entirely clear whether Hmo1p-dependent bookmarking is required.
NORs as units of regulation—a role for chromosomal context
The observation that NORs can exist in either active or silent states is further exemplified by the phenomenon of nucleolar dominance (McStay 2006; Tucker et al. 2010). In plant and animal interspecific hybrids and animal somatic cell hybrids, the NORs of one species can be dominant over the NORs of another. In a number of cases, dominance can be explained by incompatibilities between basal Pol I machineries and promoters across the two species involved. For example, mouse SL1 cannot form PICs on human promoters either in vitro or in the context of mouse monochromosomal somatic cell hybrids (Bell et al. 1990; Sullivan et al. 2001). Introduction of human TAFIs into mouse somatic cell hybrids can reactivate human NORs (Murano et al. 2014). Nucleolar dominance between more closely related species is more difficult to explain. Arabidopsis suecica is a naturally occurring hybrid of A. thaliana and Arabidopsis arenosa. In these plants, the A. thaliana rRNA genes are transcriptionally silent but can be reactivated by treatments or genetic changes that disrupt the heterochromatin on the silent NOR (Lawrence et al. 2004; Earley et al. 2006; Pontvianne et al. 2012). We can conclude that this model of nucleolar dominance does not involve incompatibilities in the transcription machineries and is maintained epigenetically by a combination of DNA methylation and appropriate histone modifications. However, the mechanism by which dominance is first established remained elusive.
More recent work from the Pikaard laboratory (Chandrasekhara et al. 2016) has established a role for chromosomal context in the preferential silencing of NORs. In A. thaliana strain Col-0, rDNA repeat subtypes (VAR1–4) contain minor DNA sequence variations. These subtypes were mapped to NOR2 and NOR4. A determination of the expression of various subtypes revealed that silenced and active rRNA gene subtypes are not intermingled. All silenced rRNA gene subtypes mapped to NOR2. All active rRNA gene subtypes mapped to NOR4 (Fig. 2A). One explanation for this is that the subtle gene sequence variation present in the repeats on NOR2 puts them at a competitive disadvantage. Another possibility is that the chromosomal context of NOR2 is important and that DNA sequences outside the rDNA array determine its activity. Intriguingly, the latter seems to be the case, since when repeats of the major rDNA variant (VAR1) that is silent in an NOR2 context are engineered into NOR4, they become active. It was proposed that sequences adjacent to the NOR on their centromere-proximal sides are likely important for the selective silencing of NOR2 (Fig. 2B). Interestingly, ∼60 kb immediately flanking NOR2 on its proximal side is composed of transposable elements and transposon remnants and appears to be packaged as transcriptionally repressed heterochromatin.
Figure 2.

Chromosomal context determines NOR activity status in A. thaliana. (A) The location of NORs (blue) on the top/north arms of chromosomes 2 and 4 (NOR2 and NOR4, respectively). In strain Col-0, NOR2 containing rDNA variants VAR1 and VAR3 is silent (red lettering). NOR4 containing VAR2 and VAR3 rDNA repeats is active (green lettering). In the strain ColSf-NOR4, NOR4 and adjacent sequences from strain Sf-2 (yellow) were introgressed (engineered) into the Col-0 genetic background. The SF-2 NOR4 (yellow) is comprised solely of VAR1 repeats. VAR1 repeats are active (green lettering) in the ColSf-NOR4 context. (B) A model proposing that a NOR inactivation center immediately proximal to NOR2 is responsible for its silencing (Chandrasekhara et al. 2016).
Further evidence for the importance of chromosomal context comes from study of chromosomal translocations in Drosophila. In addition to their roles in nucleolar formation and ribosome biogenesis, NORs on X and Y chromosomes are required for XY pairing during male meiosis (McKee and Karpen 1990). Analysis of two independent translocations involving the X chromosome and the small, mostly heterochromatic chromosome 4 reveals that the full function of the NOR on the X chromosome depends on not just its rDNA content but also chromosomal context (Briscoe and Tomkiel 2000). Despite flies bearing either translocation having the full complement of rDNA repeats, they exhibit an extreme “bobbed” (shortening and thinning of the bristles on the back of the head) phenotype, which is more typically associated with large deletions of the rDNA arrays. However, only one of the translocations disrupts XY pairing in meiosis. These results suggest that the X NOR has differing chromosomal context or cis-acting requirements for rDNA transcription and meiotic pairing. This finding is counter to a previous observation that a single rDNA transgene integrated into euchromatin could be transcribed, partially rescue a bobbed phenotype, and function in sex chromosome pairing (Karpen et al. 1988; McKee and Karpen 1990). Nevertheless, it was hypothesized that, in contrast to a single euchromatin-localized rDNA repeat, a specialized environment is required for an endogenous repetitive rDNA locus on the X chromosome. This specialized environment is disrupted by translocation, resulting in reduced expression (Briscoe and Tomkiel 2000).
Chromosomal context of human NORs
Human NORs are positioned on the short arms of the acrocentric chromosomes that still remain unsequenced and thus missing from the current human genome draft, GRCh38.p7. Seeking an understanding of the chromosomal context of human NORs and to identify potential NOR regulatory elements, my laboratory has begun to characterize the sequences on both proximal (centromeric) and distal (telomeric) sides of the rDNA arrays (Fig. 3A; Floutsakou et al. 2013). Building on earlier reports of sequences distal and proximal to the rDNA array on HSA21 and HSA22, respectively (Worton et al. 1988; Sakai et al. 1995; Gonzalez and Sylvester 1997), 207 kb of sequence immediately proximal and 379 kb distal to rDNA arrays have been reported recently (Floutsakou et al. 2013). Consensus proximal junction (PJ) and distal junction (DJ) sequences were constructed mostly from chromosome 21 BACs (bacterial artificial chromosomes). Comparison of these sequences with BACs and cosmids derived from the other acrocentrics revealed that the PJ and DJ sequences are, respectively, ∼95% and 99% identical between all five acrocentric chromosomes. Conservation of DJ sequences among the acrocentrics is consistent with frequent recombination between the rDNA arrays on each of the acrocentric chromosomes (Worton et al. 1988). However, conservation of PJ sequences suggests that there must also be frequent recombination events in the interval between the centromere and rDNA arrays. Proximal sequences are almost entirely segmentally duplicated, similar to the regions bordering centromeres. Consequently, they are unlikely to contain any specific elements that would regulate the activity of the linked NOR. In contrast, the distal sequence is predominantly unique to the acrocentric short arms and is dominated by a very large inverted repeat. Each arm of the inverted repeat is >100 kb, and they share an average sequence identity of 80%. There is a large (∼40-kb) block of a 48-base-pair (bp) satellite repeat, CER, at the distal end of the DJ (Fig. 3A). CER blocks are found distal to the rDNA on all acrocentric chromosomes, with additional pericentromeric blocks on chromosomes 14 and 22. Finally, there are two blocks of a novel 138-bp tandem repeat, ACRO138, present within the DJ.
Figure 3.
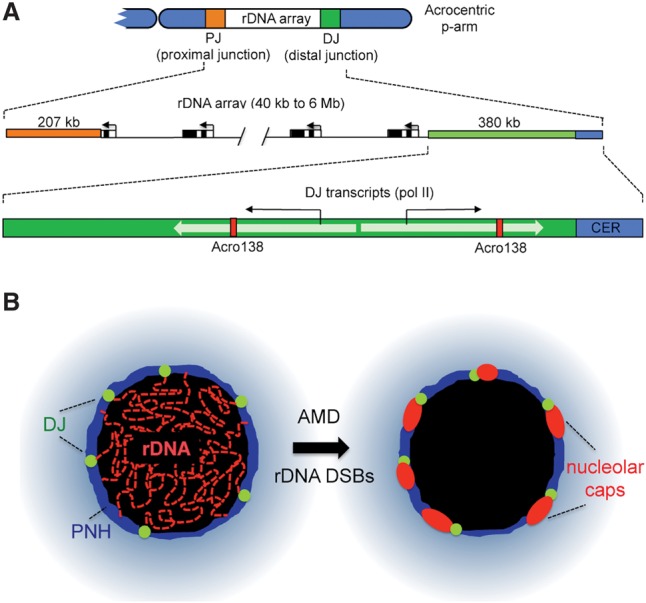
The chromosomal context of human NORs located on acrocentric short arms. (A) Schematic human acrocentric chromosome short arm showing the NOR (rDNA array), expanded below into rDNA repeats, and the PJ (orange) and DJ (green) regions. The DJ region is further expanded to show the location of inverted repeats (light green arrows), DJ promoters and transcripts, Acro138 repeat blocks (red), and CER satellite (blue). (B) Cartoon showing the transition from normal nucleolar organization to segregated nucleolar organization in response to AMD treatment or the introduction of rDNA double-strand breaks (DSBs). rDNA (red) retreats from the nucleolar interior (black) to the nucleolar periphery, forming caps adjacent to DJ sequences (green) that are embedded in PNH (dark blue) (Floutsakou et al. 2013; van Sluis and McStay 2015).
By inclusion of DJ sequences into a customized genome reference, ChIP-seq (chromatin immunoprecipitation [ChIP] combined with high-throughput sequencing) and RNA-seq (RNA sequencing) data sets from the ENCODE projects were used to build a chromatin and transcriptional profile across the DJ in a variety of human cell types (Floutsakou et al. 2013). The DJ has a complex chromatin landscape that is largely conserved among cell types. Each arm of the inverted repeat contains chromatin signatures for promoters as well as actively transcribed gene bodies. Spliced and polyadenylated Pol II transcripts that correspond to these DJ sequences can be readily detected. Transcripts appear not to contain significant ORFs and therefore fall into the class of nuclear long noncoding RNAs (lncRNAs) now implicated in establishing local chromatin states (Rinn 2014). Acro138 repeat blocks are in open chromatin and yield short transcripts possibly related to enhancer RNAs (eRNAs) (Lam et al. 2014).
The conservation of DJ sequence between the five human acrocentric chromosomes provides a unique opportunity to visualize NORs by FISH. Whereas the rDNA content of NORs can vary greatly, probing of human metaphase chromosome spreads with a DJ BAC results in signal that is consistent between NORs (Floutsakou et al. 2013). Using this probing scheme, it was observed that in most human cell lines analyzed, including multiple primary lines, at least one and sometimes as many as four of the NORs present have very little or no detectable rDNA (C van Vuuren and B McStay, unpubl.). Many studies have used silver staining of metaphase spreads prepared from stimulated human peripheral blood lymphocytes to determine how many NORs are active in normal human cells. The number of active NORs ranges from seven to 10, with an average of eight (Heliot et al. 2000). Possibly, NORs with low rDNA content are active but fall below a detection threshold in silver staining. At this point, it is worth considering the distribution of active versus silent rDNA repeats in humans and other mammals. If 50% of rDNA repeats are truly repressed, there are insufficient “silent” NORs to house them. We must conclude that active NORs are a mosaic of active and silent repeats.
DJ probes also reveal the distribution of NORs within the interphase nucleus. In cells with a normal karyotype, all 10 DJ signals can be clearly visualized. DJ signals associated with active NORs are embedded in the PNH, with their associated rDNA projecting into the nucleolar interior (Floutsakou et al. 2013). Thus, multiple NORs associated with larger mature nucleoli can be readily identified and enumerated. Interestingly, the nucleolar caps that result from the inhibition of Pol I transcription are located immediately adjacent to these DJ signals. Indeed, most nucleolar caps have a single DJ signal and correspond to an individual NOR (Fig. 3B). This suggests that DJ sequences may play a role in anchoring the linked rDNA array. Further evidence that this might be the case for this role came from the finding that ectopic DJ sequences integrated into metacentric chromosomes can still locate to the PNH (Floutsakou et al. 2013). DJ signals from silent NORs tend to be positioned elsewhere in the nucleus. The arrival of genome-editing technologies should facilitate future in-depth studies into the role of chromosomal context and DJ sequences in human NOR function.
Aging and the genomic stability of rDNA arrays
The highly repetitive nature of ribosomal gene arrays makes them prone to losing copies by homologous recombination (HR). Ribosomal gene arrays are the most unstable regions of the yeast genome (Kobayashi 2008). Yeast has evolved a gene amplification system to recover copy numbers (Fig. 4). A replication fork-blocking protein, Fob1p, blocks replication forks colliding head on with the transcription machinery by binding to a RFB located immediately downstream from the pre-rRNA-coding sequences (Brewer et al. 1992; Kobayashi et al. 1992). Fob1p binding to RFBs results in DNA double-strand breaks (DSBs) occurring at stalled forks (Weitao et al. 2003; Burkhalter and Sogo 2004; Kobayashi et al. 2004). These breaks are repaired by unequal sister chromatid exchange, resulting in amplification. Unequal crossover is promoted by Pol II transcripts originating at a promoter, E-pro, in the yeast IGS. IGS transcription dislodges the cohesin complex that holds sister chromatids in register, thereby promoting unequal crossovers (Kobayashi and Ganley 2005). Transcription from E-pro is in turn repressed by a histone deacetylase, Sir2p (Fritze et al. 1997). Ribosomal gene copy number maintenance involving both Fob1p and Sir2p has been shown to be a determinant of life span in yeast. The first links between rDNA and aging came from Guarente's group (Sinclair and Guarente 1997), who demonstrated that extrachromosomal rDNA circles (ERCs) accumulate in aging mother cells. It was suggested that ERCs arising from increased instability in the rDNA array could titrate some unknown factor required for cell cycle progression. Deletion of FOB1 stabilizes rDNA arrays and prolongs life span. Conversely, deletion of SIR2 increases ERC levels and shortens life span (Defossez et al. 1999; Takeuchi et al. 2003).
Figure 4.

Structure of the ribosomal DNA gene cluster in S. cerevisiae. The location of the rDNA cluster on chromosome XII is shown at the top, with the telomere (TEL) and centromere (CEN) indicated. A detailed view of an rDNA repeat unit is shown below. The 35S and 5S rRNA gene-coding regions are indicated, as is the rDNA origin of replication (rARS). The RFB (red box) is bound by Fob1p (pink). The locations of the 35S promoter and the bidirectional noncoding promoter E-pro (blue box), silenced by Sir2p, are indicated.
An “rDNA theory of aging” has been proposed, in which it is argued that it is the instability of the rDNA itself rather than the accumulation of ERCs that shortens life span (Kobayashi 2008). One attraction of the “rDNA theory of aging,” particularly when applying it to complex eukaryotes, is that there is little evidence for the presence of ERCs. Nevertheless, rDNA recombination has been observed in many eukaryotes, including humans (Killen et al. 2009; Stults et al. 2009). Currently, there is no evidence for involvement of mammalian SIR2 homologs in maintaining rDNA stability, although one homolog, Sirtuin 7, is localized in the nucleolus and is a positive regulator of Pol I transcription (Ford et al. 2006; Grob et al. 2009).
An RFB has also recently been described in the human rDNA repeat (Akamatsu and Kobayashi 2015), and IGS transcripts have been observed in humans and mice in response to various nucleolar stresses or replication senescence (Audas et al. 2012; Bierhoff et al. 2014). The degree to which the model proposed for yeast can be extended to human cells is an open question. Interestingly, replication stress on rDNA arrays has been linked to functional decline in aging mouse hematopoietic stem cells (Flach et al. 2014). The RecQ helicases RECQ4, WRN (mutated in Werner syndrome), and BLM (mutated in Bloom syndrome) are other good candidates for linking aging and rDNA instability, as they are involved in human progeria, and deletion of their yeast homolog, SGS1, results in rDNA instability (Bohr 2008; Bernstein et al. 2010). Moreover, cells from Blooms syndrome patients show high levels of recombination in their rDNA arrays (Killen et al. 2009).
Heterochromatin is a feature of both NORs and the chromosomal context within which they lie. A proportion of rDNA repeats within “active NORs” are in a silenced heterochromatic state (McStay and Grummt 2008), and the NOR itself is embedded in a heterochromatic chromosomal environment (Nemeth and Langst 2011). It appears that one or both of these forms of heterochromatin play a role in maintaining the genomic stability of rDNA repeats. In Drosophila, disruption of Su(var)3-9 or dcr-2 (dicer-2), responsible for H3K9me2 and involved in RNAi, respectively, globally disrupts heterochromatin, resulting in increased instability of the rDNA array and the appearance of ERCs (Peng and Karpen 2007). Loss of heterochromatin and increased recombination has also been associated with life span reduction in Drosophila (Larson et al. 2012). Human HCT116 cells, inactivated for DNMT1 and DNMT3b or treated with aza-dC, lack CpG methylation, another heterochromatic mark, and reactivate a large fraction of normally silent rRNA genes (Gagnon-Kugler et al. 2009). This is also associated with increased instability in rDNA arrays. While these studies clearly establish a role for heterochromatin in the genomic stability of rDNA, it is hard to tease apart the effects of globally disrupting heterochromatin, locally disrupting nucleolar-associated heterochromatin, and reactivating silent rDNA repeats.
DNA DSBs in human rDNA arrays
DNA DSBs, the most dangerous form of DNA lesion, initiate a complex DNA damage response. The kinase ATM (ataxia telangiectasia-mutated) is a key player in transducing DSB recognition into activation of cell cycle checkpoints and repair processes (Shiloh and Ziv 2013). Once activated by recruitment to DSBs, ATM phosphorylates the histone variant H2AX on Ser139 to yield γH2AX (Burma et al. 2001). In mammalian cells, two major pathways are used for repair: nonhomologous end-joining (NHEJ) and HR (Chapman et al. 2012). In NHEJ, the broken ends are minimally processed, aligned, and ligated together. Importantly, NHEJ does not require sequence complementarity and is error-prone. HR involves extensive DNA end resection to generate 3′ single-stranded overhangs. These invade double-stranded undamaged homologous DNA copies that template unscheduled DNA synthesis and accurate repair. Usually, the repair template is a sister chromatid. Thus, it is generally considered that accurate repair by HR is restricted to S and G2 phases of the cell cycle (Aylon et al. 2004), whereas error-prone NHEJ is predominant in G0/G1 cells (Lieber et al. 2003).
As human rDNA arrays show enhanced instability in cancer and aging diseases such as Bloom syndrome, a fuller understanding of how nucleoli respond to DSBs in human rDNA arrays has received much recent attention. Global introduction of DSBs into mouse cells by γ-irradiation or more local introduction into nucleoli by laser microirradiation results in inhibition of Pol I transcription mediated by ATM kinase (Kruhlak et al. 2007). Importantly, this study also showed that Pol I inhibition was restricted to nucleoli containing damaged DNA, suggesting that it was mediated locally. It should be pointed out, however, that similar experiments performed in human cells failed to show any inhibition of Pol I transcription following γ-irradiation (Moore et al. 2011). More surprisingly, it has been demonstrated that laser microirradiation-induced DSBs outside of nucleoli lead to an ATM-dependent pan-nuclear silencing of Pol I transcription (Ciccia et al. 2014; Larsen et al. 2014). The difficulty in interpreting such experiments is that we have no knowledge of the number or distribution of irradiation-induced DSBs. To study the response to rDNA DSBs within human cells in finer detail, recent studies have used CRISPR/Cas9 or the homing endonuclease I-PpoI to introduce DSBs into the rDNA repeats (Harding et al. 2015; van Sluis and McStay 2015; Warmerdam et al. 2016). The ability of I-PpoI to create rDNA DSBs in human cells had been established previously (Berkovich et al. 2007). All three of these recent studies demonstrate that introduction of DSBs into rDNA results in an ATM-dependent inhibition of Pol I transcription and movement of rDNA to nucleolar caps. Like the nucleolar caps observed in AMD-treated cells, rDNA DSB-induced caps are located immediately abutting DJ sequences in the PNH (van Sluis and McStay 2015). Revealing the regional specificity of this response, introduction of DSBs into NOR distal sequences by CRISPR/Cas9 did not result in either transcriptional inhibition or formation of nucleolar caps (van Sluis and McStay 2015). The study from our laboratory (van Sluis and McStay 2015) suggested that repair of DSBs was mediated by HR, as nucleolar caps contained γH2AX, BRCA1, RPA2, and Rad51. Further evidence for repair by HR was the detection of unscheduled DNA synthesis within nucleolar caps. Counter to the dogma that repair by HR is restricted to S and G2 phases of the cell cycle, these markers for HR were also observed within nucleolar caps in G1 cells (van Sluis and McStay 2015). The study from the Greenberg group (Harding et al. 2015) identified NHEJ as the predominant mode of DSB repair that was rapid and did not involve transcriptional inhibition. The presence of persistent breaks led to transcriptional inhibition and nucleolar reorganization. The final study from the Medema group (Warmerdam et al. 2016) suggested that HR inhibits break repair. Furthermore, they showed that HR required SMC5 (structural maintenance of chromosomes protein 5) a core component of the SMC5–SMC6 complex. SMC5–SMC6 has been implicated previously in repair of rDNA DSBs by HR in yeast (Torres-Rosell et al. 2007). The differences in these three studies regarding the repair pathway selection could be related to differences in the level and persistence of the DSBs. Perversely, genome-editing technologies involving HR may resolve the HR versus NHEJ repair pathway issue. By tagging a fraction of rDNA repeats with the target sequence for an exogenous nuclease, the level of rDNA DSBs can be more tightly controlled.
One common feature of these recent studies is the nucleolar reorganization in response to rDNA DSBs (Fig. 3B). Damaged and undamaged rDNA repeats move to form caps at the nucleolar periphery. It was argued that this movement stimulates repair by HR, as it increases the accessibility of DSBs to the repair machinery and provides a high local concentration of repair templates (van Sluis and McStay 2015). Importantly, repair by HR, even if templated in cis in G1 cells, does not necessarily involve loss of repeats. Synthesis-dependent strand annealing or dissolution of double Holliday junctions by branch migration can mediate noncrossover repair (Renkawitz et al. 2014). Interestingly, the BLM helicase is involved in branch migration, and its inactivation results in increased levels of unequal crossovers within rDNA arrays (Karow et al. 2000; Killen et al. 2009).
The movement of damaged rDNA repeats to an environment more permissive for repair has parallels with the HR-mediated repair of DSBs in heterochromatic repetitive DNA in Drosophila. Heterochromatin domains expand in response to the presence of DSBs. Proteins involved in resection (early HR) are recruited to DSBs within heterochromatin. In contrast, Rad51, which mediates strand invasion (late HR), associates only with DSBs that relocalize outside of the domain (Chiolo et al. 2011). It is not entirely clear what drives nucleolar reorganization in response to rDNA DSBs. An identical response is observed in response to AMD treatment, which does not induce DSBs or activate ATM (van Sluis and McStay 2015). This suggests that DSB-induced reorganization is a response to transcriptional inhibition resulting from activation of ATM rather than the presence DSBs or γH2AX. The nucleolar reorganization observed in response to rDNA DSBs suggests that the chromosomal context of rDNA arrays may contribute to their genomic stability.
NORs and a more complete human genome draft
Sometimes referred to as “a dirty little secret” (Callaway 2014), more than a decade after the official completion of the human genome project and multiple updates, many gaps still remain in the human genome. The largest of these are the short arms of all five acrocentric chromosomes. Undoubtedly, a major part of the reason for acrocentric short arms remaining unsequenced is the repeated nature of their DNA content. In addition to repeated rDNA, there are blocks of satellite repeats. A further complication arises from the fact that these five chromosome arms are highly similar in sequence. Recent work described in this review clearly undermines any suggestion that one can extrapolate the functioning of an NOR from the sequence of a single rDNA repeat. I predict that there are many mysteries about NORs yet to be revealed and suggest that complete descriptions of their genomic architectures are long overdue. Lack of genomic information for NORs and their surrounds is common across animal phyla. In mice, for example, there is not a single description of the sequences either immediately distal or proximal to rDNA. Consequently, comparative biology, a powerful tool in identifying conserved regulatory mechanisms, cannot be exploited. Single-molecule long-read sequencing technologies should pave the road to completion.
Having the full DNA sequence for the each of the human acrocentric short arms will advance our understanding of how NORs function and how the genomic stability of rDNA arrays is maintained. Furthermore, as we enter the era of population-wide genome sequencing, inclusion of these sequences in future genome drafts would significantly advance our capability for discovering and exploring links between NORs, human disease, and aging.
Physicists infer the existence of “dark matter” to explain enigmatic properties of the universe. By analogy, biologists will fully understand the biology of nucleoli only when NORs and surrounding sequences—genomic “dark matter”—are characterized and included in an updated genome draft.
Acknowledgments
I thank Carol Duffy and the reviewers for valuable comments on the manuscript and acknowledge the Sciences Foundation Ireland-Health Research Board-Wellcome Trust Biomedical Research Partnership (Investigator Award 106199/Z/14/Z) for funding work in my laboratory.
Footnotes
Article is online at http://www.genesdev.org/cgi/doi/10.1101/gad.283838.116.
References
- Akamatsu Y, Kobayashi T. 2015. The human RNA polymerase I transcription terminator complex acts as a replication fork barrier that coordinates the progress of replication with rRNA transcription activity. Mol Cell Biol 35: 1871–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert B, Colleran C, Leger-Silvestre I, Berger AB, Dez C, Normand C, Perez-Fernandez J, McStay B, Gadal O. 2013. Structure-function analysis of Hmo1 unveils an ancestral organization of HMG-box factors involved in ribosomal DNA transcription from yeast to human. Nucleic Acids Res 41: 10135–10149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audas TE, Jacob MD, Lee S. 2012. Immobilization of proteins in the nucleolus by ribosomal intergenic spacer noncoding RNA. Mol Cell 45: 147–157. [DOI] [PubMed] [Google Scholar]
- Aylon Y, Liefshitz B, Kupiec M. 2004. The CDK regulates repair of double-strand breaks by homologous recombination during the cell cycle. EMBO J 23: 4868–4875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell SP, Learned RM, Jantzen HM, Tjian R. 1988. Functional cooperativity between transcription factors UBF1 and SL1 mediates human ribosomal RNA synthesis. Science 241: 1192–1197. [DOI] [PubMed] [Google Scholar]
- Bell SP, Jantzen HM, Tjian R. 1990. Assembly of alternative multiprotein complexes directs rRNA promoter selectivity. Genes Dev 4: 943–954. [DOI] [PubMed] [Google Scholar]
- Berkovich E, Monnat RJ Jr, Kastan MB. 2007. Roles of ATM and NBS1 in chromatin structure modulation and DNA double-strand break repair. Nat Cell Biol 9: 683–690. [DOI] [PubMed] [Google Scholar]
- Bernstein KA, Gangloff S, Rothstein R. 2010. The RecQ DNA helicases in DNA repair. Annu Rev Genet 44: 393–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierhoff H, Dammert MA, Brocks D, Dambacher S, Schotta G, Grummt I. 2014. Quiescence-induced lncRNAs trigger H4K20 trimethylation and transcriptional silencing. Mol Cell 54: 675–682. [DOI] [PubMed] [Google Scholar]
- Bohr VA. 2008. Rising from the RecQ-age: the role of human RecQ helicases in genome maintenance. Trends Biochem Sci 33: 609–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brangwynne CP, Mitchison TJ, Hyman AA. 2011. Active liquid-like behavior of nucleoli determines their size and shape in Xenopus laevis oocytes. Proc Natl Acad Sci 108: 4334–4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer BJ, Lockshon D, Fangman WL. 1992. The arrest of replication forks in the rDNA of yeast occurs independently of transcription. Cell 71: 267–276. [DOI] [PubMed] [Google Scholar]
- Briscoe A Jr, Tomkiel JE. 2000. Chromosomal position effects reveal different cis-acting requirements for rDNA transcription and sex chromosome pairing in Drosophila melanogaster. Genetics 155: 1195–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton-Davidian J, Cazaux B, Catalan J. 2012. Chromosomal dynamics of nucleolar organizer regions (NORs) in the house mouse: micro-evolutionary insights. Heredity (Edinb) 108: 68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhalter MD, Sogo JM. 2004. rDNA enhancer affects replication initiation and mitotic recombination: Fob1 mediates nucleolytic processing independently of replication. Mol Cell 15: 409–421. [DOI] [PubMed] [Google Scholar]
- Burma S, Chen BP, Murphy M, Kurimasa A, Chen DJ. 2001. ATM phosphorylates histone H2AX in response to DNA double-strand breaks. J Biol Chem 276: 42462–42467. [DOI] [PubMed] [Google Scholar]
- Caburet S, Conti C, Schurra C, Lebofsky R, Edelstein SJ, Bensimon A. 2005. Human ribosomal RNA gene arrays display a broad range of palindromic structures. Genome Res 15: 1079–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaway E. 2014. ‘Platinum’ genome takes on disease. Nature 515: 323. [DOI] [PubMed] [Google Scholar]
- Caudron-Herger M, Pankert T, Seiler J, Nemeth A, Voit R, Grummt I, Rippe K. 2015. Alu element-containing RNAs maintain nucleolar structure and function. EMBO J 34: 2758–2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekhara C, Mohannath G, Blevins T, Pontvianne F, Pikaard CS. 2016. Chromosome-specific NOR inactivation explains selective rRNA gene silencing and dosage control in Arabidopsis. Genes Dev 30: 177–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman JR, Taylor MR, Boulton SJ. 2012. Playing the end game: DNA double-strand break repair pathway choice. Mol Cell 47: 497–510. [DOI] [PubMed] [Google Scholar]
- Chiolo I, Minoda A, Colmenares SU, Polyzos A, Costes SV, Karpen GH. 2011. Double-strand breaks in heterochromatin move outside of a dynamic HP1a domain to complete recombinational repair. Cell 144: 732–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccia A, Huang JW, Izhar L, Sowa ME, Harper JW, Elledge SJ. 2014. Treacher Collins syndrome TCOF1 protein cooperates with NBS1 in the DNA damage response. Proc Natl Acad Sci 111: 18631–18636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemente-Blanco A, Mayan-Santos M, Schneider DA, Machin F, Jarmuz A, Tschochner H, Aragon L. 2009. Cdc14 inhibits transcription by RNA polymerase I during anaphase. Nature 458: 219–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conconi A, Widmer RM, Koller T, Sogo JM. 1989. Two different chromatin structures coexist in ribosomal RNA genes throughout the cell cycle. Cell 57: 753–761. [DOI] [PubMed] [Google Scholar]
- Copenhaver GP, Pikaard CS. 1996. RFLP and physical mapping with an rDNA-specific endonuclease reveals that nucleolus organizer regions of Arabidopsis thaliana adjoin the telomeres on chromosomes 2 and 4. Plant J 9: 259–272. [DOI] [PubMed] [Google Scholar]
- Copenhaver GP, Putnam CD, Denton ML, Pikaard CS. 1994. The RNA polymerase I transcription factor UBF is a sequence-tolerant HMG-box protein that can recognize structured nucleic acids. Nucleic Acids Res 22: 2651–2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defossez PA, Prusty R, Kaeberlein M, Lin SJ, Ferrigno P, Silver PA, Keil RL, Guarente L. 1999. Elimination of replication block protein Fob1 extends the life span of yeast mother cells. Mol Cell 3: 447–455. [DOI] [PubMed] [Google Scholar]
- Earley K, Lawrence RJ, Pontes O, Reuther R, Enciso AJ, Silva M, Neves N, Gross M, Viegas W, Pikaard CS. 2006. Erasure of histone acetylation by Arabidopsis HDA6 mediates large scale gene silencing in nucleolar dominance. Genes Dev 20: 1283–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earley KW, Pontvianne F, Wierzbicki AT, Blevins T, Tucker S, Costa-Nunes P, Pontes O, Pikaard CS. 2010. Mechanisms of HDA6-mediated rRNA gene silencing: suppression of intergenic Pol II transcription and differential effects on maintenance versus siRNA-directed cytosine methylation. Genes Dev 24: 1119–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emelyanov AV, Vershilova E, Ignatyeva MA, Pokrovsky DK, Lu X, Konev AY, Fyodorov DV. 2012. Identification and characterization of ToRC, a novel ISWI-containing ATP-dependent chromatin assembly complex. Genes Dev 26: 603–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farley KI, Surovtseva Y, Merkel J, Baserga SJ. 2015. Determinants of mammalian nucleolar architecture. Chromosoma 124: 323–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flach J, Bakker ST, Mohrin M, Conroy PC, Pietras EM, Reynaud D, Alvarez S, Diolaiti ME, Ugarte F, Forsberg EC, et al. 2014. Replication stress is a potent driver of functional decline in ageing haematopoietic stem cells. Nature 512: 198–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floutsakou I, Agrawal S, Nguyen TT, Seoighe C, Ganley AR, McStay B. 2013. The shared genomic architecture of human nucleolar organizer regions. Genome Res 23: 2003–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford E, Voit R, Liszt G, Magin C, Grummt I, Guarente L. 2006. Mammalian Sir2 homolog SIRT7 is an activator of RNA polymerase I transcription. Genes Dev 20: 1075–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritze CE, Verschueren K, Strich R, Easton Esposito R. 1997. Direct evidence for SIR2 modulation of chromatin structure in yeast rDNA. EMBO J 16: 6495–6509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadal O, Labarre S, Boschiero C, Thuriaux P. 2002. Hmo1, an HMG-box protein, belongs to the yeast ribosomal DNA transcription system. EMBO J 21: 5498–5507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnon-Kugler T, Langlois F, Stefanovsky V, Lessard F, Moss T. 2009. Loss of human ribosomal gene CpG methylation enhances cryptic RNA polymerase II transcription and disrupts ribosomal RNA processing. Mol Cell 35: 414–425. [DOI] [PubMed] [Google Scholar]
- Gibbons RJ, McDowell TL, Raman S, O'Rourke DM, Garrick D, Ayyub H, Higgs DR. 2000. Mutations in ATRX, encoding a SWI/SNF-like protein, cause diverse changes in the pattern of DNA methylation. Nat Genet 24: 368–371. [DOI] [PubMed] [Google Scholar]
- Gonzalez IL, Sylvester JE. 1995. Complete sequence of the 43-kb human ribosomal DNA repeat: analysis of the intergenic spacer. Genomics 27: 320–328. [DOI] [PubMed] [Google Scholar]
- Gonzalez IL, Sylvester JE. 1997. Beyond ribosomal DNA: on towards the telomere. Chromosoma 105: 431–437. [DOI] [PubMed] [Google Scholar]
- Goodfellow SJ, Zomerdijk JC. 2013. Basic mechanisms in RNA polymerase I transcription of the ribosomal RNA genes. Subcell Biochem 61: 211–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodpasture C, Bloom SE. 1975. Visualization of nucleolar organizer regions in mammalian chromosomes using silver staining. Chromosoma 53: 37–50. [DOI] [PubMed] [Google Scholar]
- Grob A, McStay B. 2014. Construction of synthetic nucleoli and what it tells us about propagation of sub-nuclear domains through cell division. Cell Cycle 13: 2501–2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grob A, Roussel P, Wright JE, McStay B, Hernandez-Verdun D, Sirri V. 2009. Involvement of SIRT7 in resumption of rDNA transcription at the exit from mitosis. J Cell Sci 122: 489–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grob A, Colleran C, McStay B. 2011. UBF an essential player in maintenance of active NORs and nucleolar formation. In The nucleolus (ed. Olson EOJ), pp. 83–103. Springer, New York, NY. [Google Scholar]
- Grob A, Colleran C, McStay B. 2014. Construction of synthetic nucleoli in human cells reveals how a major functional nuclear domain is formed and propagated through cell division. Genes Dev 28: 220–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grozdanov P, Georgiev O, Karagyozov L. 2003. Complete sequence of the 45-kb mouse ribosomal DNA repeat: analysis of the intergenic spacer. Genomics 82: 637–643. [DOI] [PubMed] [Google Scholar]
- Gu L, Frommel SC, Oakes CC, Simon R, Grupp K, Gerig CY, Bar D, Robinson MD, Baer C, Weiss M, et al. 2015. BAZ2A (TIP5) is involved in epigenetic alterations in prostate cancer and its overexpression predicts disease recurrence. Nat Genet 47: 22–30. [DOI] [PubMed] [Google Scholar]
- Hadjiolov AA. 1985. The nucleolus and ribosome biogenesis. Springer-Verlag, Wien, New-York. [Google Scholar]
- Harding SM, Boiarsky JA, Greenberg RA. 2015. ATM dependent silencing links nucleolar chromatin reorganization to DNA damage recognition. Cell Rep 13: 251–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitz E. 1931. Die ursache der gesetzmässigen zahl, lage, form und grosse pflanzlicher nukleolen. Planta 12: 775–844. [Google Scholar]
- Heliot L, Kaplan H, Lucas L, Klein C, Beorchia A, Doco-Fenzy M, Menager M, Thiry M, O'Donohue MF, Ploton D. 1997. Electron tomography of metaphase nucleolar organizer regions: evidence for a twisted-loop organization. Mol Biol Cell 8: 2199–2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heliot L, Mongelard F, Klein C, O'Donohue MF, Chassery JM, Robert-Nicoud M, Usson Y. 2000. Nonrandom distribution of metaphase AgNOR staining patterns on human acrocentric chromosomes. J Histochem Cytochem 48: 13–20. [DOI] [PubMed] [Google Scholar]
- Henderson AS, Warburton D, Atwood KC. 1972. Location of ribosomal DNA in the human chromosome complement. Proc Natl Acad Sci 69: 3394–3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman AA, Weber CA, Julicher F. 2014. Liquid-liquid phase separation in biology. Annu Rev Cell Dev Biol 30: 39–58. [DOI] [PubMed] [Google Scholar]
- Jantzen HM, Admon A, Bell SP, Tjian R. 1990. Nucleolar transcription factor hUBF contains a DNA-binding motif with homology to HMG proteins. Nature 344: 830–836. [DOI] [PubMed] [Google Scholar]
- Jordan P, Mannervik M, Tora L, Carmo-Fonseca M. 1996. In vivo evidence that TATA-binding protein/SL1 colocalizes with UBF and RNA polymerase I when rRNA synthesis is either active or inactive. J Cell Biol 133: 225–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karow JK, Constantinou A, Li JL, West SC, Hickson ID. 2000. The Bloom's syndrome gene product promotes branch migration of holliday junctions. Proc Natl Acad Sci 97: 6504–6508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpen GH, Schaefer JE, Laird CD. 1988. A Drosophila rRNA gene located in euchromatin is active in transcription and nucleolus formation. Genes Dev 2: 1745–1763. [DOI] [PubMed] [Google Scholar]
- Kermekchiev M, Workman JL, Pikaard CS. 1997. Nucleosome binding by the polymerase I transactivator upstream binding factor displaces linker histone H1. Mol Cell Biol 17: 5833–5842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killen MW, Stults DM, Adachi N, Hanakahi L, Pierce AJ. 2009. Loss of Bloom syndrome protein destabilizes human gene cluster architecture. Hum Mol Genet 18: 3417–3428. [DOI] [PubMed] [Google Scholar]
- Kobayashi T. 2008. A new role of the rDNA and nucleolus in the nucleus—rDNA instability maintains genome integrity. Bioessays 30: 267–272. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Ganley AR. 2005. Recombination regulation by transcription-induced cohesin dissociation in rDNA repeats. Science 309: 1581–1584. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Hidaka M, Nishizawa M, Horiuchi T. 1992. Identification of a site required for DNA replication fork blocking activity in the rRNA gene cluster in Saccharomyces cerevisiae. Mol Gen Genet 233: 355–362. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Horiuchi T, Tongaonkar P, Vu L, Nomura M. 2004. SIR2 regulates recombination between different rDNA repeats, but not recombination within individual rRNA genes in yeast. Cell 117: 441–453. [DOI] [PubMed] [Google Scholar]
- Kruhlak M, Crouch EE, Orlov M, Montano C, Gorski SA, Nussenzweig A, Misteli T, Phair RD, Casellas R. 2007. The ATM repair pathway inhibits RNA polymerase I transcription in response to chromosome breaks. Nature 447: 730–734. [DOI] [PubMed] [Google Scholar]
- Krystosek A. 1998. Repositioning of human interphase chromosomes by nucleolar dynamics in the reverse transformation of HT1080 fibrosarcoma cells. Exp Cell Res 241: 202–209. [DOI] [PubMed] [Google Scholar]
- Kuhn A, Voit R, Stefanovsky V, Evers R, Bianchi M, Grummt I. 1994. Functional differences between the two splice variants of the nucleolar transcription factor UBF: the second HMG box determines specificity of DNA binding and transcriptional activity. EMBO J 13: 416–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam MT, Li W, Rosenfeld MG, Glass CK. 2014. Enhancer RNAs and regulated transcriptional programs. Trends Biochem Sci 39: 170–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen DH, Hari F, Clapperton JA, Gwerder M, Gutsche K, Altmeyer M, Jungmichel S, Toledo LI, Fink D, Rask MB, et al. 2014. The NBS1–Treacle complex controls ribosomal RNA transcription in response to DNA damage. Nat Cell Biol 16: 792–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson K, Yan SJ, Tsurumi A, Liu J, Zhou J, Gaur K, Guo D, Eickbush TH, Li WX. 2012. Heterochromatin formation promotes longevity and represses ribosomal RNA synthesis. PLoS Genet 8: e1002473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence RJ, Earley K, Pontes O, Silva M, Chen ZJ, Neves N, Viegas W, Pikaard CS. 2004. A concerted DNA methylation/histone methylation switch regulates rRNA gene dosage control and nucleolar dominance. Mol Cell 13: 599–609. [DOI] [PubMed] [Google Scholar]
- Leung AK, Gerlich D, Miller G, Lyon C, Lam YW, Lleres D, Daigle N, Zomerdijk J, Ellenberg J, Lamond AI. 2004. Quantitative kinetic analysis of nucleolar breakdown and reassembly during mitosis in live human cells. J Cell Biol 166: 787–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieber MR, Ma Y, Pannicke U, Schwarz K. 2003. Mechanism and regulation of human non-homologous DNA end-joining. Nat Rev Mol Cell Biol 4: 712–720. [DOI] [PubMed] [Google Scholar]
- Mais C, Wright JE, Prieto JL, Raggett SL, McStay B. 2005. UBF-binding site arrays form pseudo-NORs and sequester the RNA polymerase I transcription machinery. Genes Dev 19: 50–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer C, Grummt I. 2005. Cellular stress and nucleolar function. Cell Cycle 4: 1036–1038. [DOI] [PubMed] [Google Scholar]
- McClintock B. 1934. The relatioship of a particular chromosomal element to the development of the nucleoli in Zea Mays. Zeit ZellforschMikAnat 21: 294–328. [Google Scholar]
- McKee BD, Karpen GH. 1990. Drosophila ribosomal RNA genes function as an X–Y pairing site during male meiosis. Cell 61: 61–72. [DOI] [PubMed] [Google Scholar]
- McStay B. 2006. Nucleolar dominance: a model for rRNA gene silencing. Genes Dev 20: 1207–1214. [DOI] [PubMed] [Google Scholar]
- McStay B, Grummt I. 2008. The epigenetics of rRNA genes: from molecular to chromosome biology. Annu Rev Cell Dev Biol 24: 131–157. [DOI] [PubMed] [Google Scholar]
- McStay B, Frazier MW, Reeder RH. 1991. xUBF contains a novel dimerization domain essential for RNA polymerase I transcription. Genes Dev 5: 1957–1968. [DOI] [PubMed] [Google Scholar]
- McStay B, Sullivan GJ, Cairns C. 1997. The Xenopus RNA polymerase I transcription factor, UBF, has a role in transcriptional enhancement distinct from that at the promoter. EMBO J 16: 396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misteli T. 2001. The concept of self-organization in cellular architecture. J Cell Biol 155: 181–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore HM, Bai B, Boisvert FM, Latonen L, Rantanen V, Simpson JC, Pepperkok R, Lamond AI, Laiho M. 2011. Quantitative proteomics and dynamic imaging of the nucleolus reveal distinct responses to UV and ionizing radiation. Mol Cell Proteomics 10: M111.009241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murano K, Okuwaki M, Momose F, Kumakura M, Ueshima S, Newbold RF, Nagata K. 2014. Reconstitution of human rRNA gene transcription in mouse cells by a complete SL1 complex. J Cell Sci 127: 3309–3319. [DOI] [PubMed] [Google Scholar]
- Nemeth A, Langst G. 2011. Genome organization in and around the nucleolus. Trends Genet 27: 149–156. [DOI] [PubMed] [Google Scholar]
- O'Mahony DJ, Rothblum LI. 1991. Identification of two forms of the RNA polymerase I transcription factor UBF. Proc Natl Acad Sci 88: 3180–3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Sullivan AC, Sullivan GJ, McStay B. 2002. UBF binding in vivo is not restricted to regulatory sequences within the vertebrate ribosomal DNA repeat. Mol Cell Biol 22: 657–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padeken J, Heun P. 2014. Nucleolus and nuclear periphery: velcro for heterochromatin. Curr Opin Cell Biol 28: 54–60. [DOI] [PubMed] [Google Scholar]
- Pederson T. 2010. The nucleolus. Cold Spring Harb Perspect Biol 3: a000638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng JC, Karpen GH. 2007. H3K9 methylation and RNA interference regulate nucleolar organization and repeated DNA stability. Nat Cell Biol 9: 25–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petes TD. 1979. Yeast ribosomal DNA genes are located on chromosome XII. Proc Natl Acad Sci 76: 410–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontvianne F, Blevins T, Chandrasekhara C, Feng W, Stroud H, Jacobsen SE, Michaels SD, Pikaard CS. 2012. Histone methyltransferases regulating rRNA gene dose and dosage control in Arabidopsis. Genes Dev 26: 945–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieto JL, McStay B. 2007. Recruitment of factors linking transcription and processing of pre-rRNA to NOR chromatin is UBF-dependent and occurs independent of transcription in human cells. Genes Dev 21: 2041–2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam CD, Pikaard CS. 1992. Cooperative binding of the Xenopus RNA polymerase I transcription factor xUBF to repetitive ribosomal gene enhancers. Mol Cell Biol 12: 4970–4980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renkawitz J, Lademann CA, Jentsch S. 2014. Mechanisms and principles of homology search during recombination. Nat Rev Mol Cell Biol 15: 369–383. [DOI] [PubMed] [Google Scholar]
- Rinn JL. 2014. lncRNAs: linking RNA to chromatin. Cold Spring Harb Perspect Biol 6: a018614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritossa FM, Atwood KC, Lindsley DL, Spiegelman S. 1966. On the chromosomal distribution of DNA complementary to ribosomal and soluble RNA. Natl Cancer Inst Monogr 23: 449–472. [PubMed] [Google Scholar]
- Roussel P, Andre C, Masson C, Geraud G, Hernandez VD. 1993. Localization of the RNA polymerase I transcription factor hUBF during the cell cycle. J Cell Sci 104: 327–337. [DOI] [PubMed] [Google Scholar]
- Saka K, Ide S, Ganley AR, Kobayashi T. 2013. Cellular senescence in yeast is regulated by rDNA noncoding transcription. Curr Biol 23: 1794–1798. [DOI] [PubMed] [Google Scholar]
- Sakai K, Ohta T, Minoshima S, Kudoh J, Wang Y, de Jong PJ, Shimizu N. 1995. Human ribosomal RNA gene cluster: identification of the proximal end containing a novel tandem repeat sequence. Genomics 26: 521–526. [DOI] [PubMed] [Google Scholar]
- Savino TM, Gebrane-Younes J, De Mey J, Sibarita JB, Hernandez-Verdun D. 2001. Nucleolar assembly of the rRNA processing machinery in living cells. J Cell Biol 153: 1097–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiloh Y, Ziv Y. 2013. The ATM protein kinase: regulating the cellular response to genotoxic stress, and more. Nat Rev Mol Cell Biol 14: 197–210. [PubMed] [Google Scholar]
- Sinclair DA, Guarente L. 1997. Extrachromosomal rDNA circles—a cause of aging in yeast. Cell 91: 1033–1042. [DOI] [PubMed] [Google Scholar]
- Sirri V, Roussel P, Hernandez-Verdun D. 1999. The mitotically phosphorylated form of the transcription termination factor TTF-1 is associated with the repressed rDNA transcription machinery. J Cell Sci 112: 3259–3268. [DOI] [PubMed] [Google Scholar]
- Sirri V, Urcuqui-Inchima S, Roussel P, Hernandez-Verdun D. 2008. Nucleolus: the fascinating nuclear body. Histochem Cell Biol 129: 13–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strongin DE, Groudine M, Politz JC. 2014. Nucleolar tethering mediates pairing between the IgH and Myc loci. Nucleus 5: 474–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stults DM, Killen MW, Pierce HH, Pierce AJ. 2008. Genomic architecture and inheritance of human ribosomal RNA gene clusters. Genome Res 18: 13–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stults DM, Killen MW, Williamson EP, Hourigan JS, Vargas HD, Arnold SM, Moscow JA, Pierce AJ. 2009. Human rRNA gene clusters are recombinational hotspots in cancer. Cancer Res 69: 9096–9104. [DOI] [PubMed] [Google Scholar]
- Sullivan GJ, Bridger JM, Cuthbert AP, Newbold RF, Bickmore WA, McStay B. 2001. Human acrocentric chromosomes with transcriptionally silent nucleolar organizer regions associate with nucleoli. EMBO J 20: 2867–2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi Y, Horiuchi T, Kobayashi T. 2003. Transcription-dependent recombination and the role of fork collision in yeast rDNA. Genes Dev 17: 1497–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiry M, Lafontaine DL. 2005. Birth of a nucleolus: the evolution of nucleolar compartments. Trends Cell Biol 15: 194–199. [DOI] [PubMed] [Google Scholar]
- Torres-Rosell J, Sunjevaric I, De Piccoli G, Sacher M, Eckert-Boulet N, Reid R, Jentsch S, Rothstein R, Aragon L, Lisby M. 2007. The Smc5–Smc6 complex and SUMO modification of Rad52 regulates recombinational repair at the ribosomal gene locus. Nat Cell Biol 9: 923–931. [DOI] [PubMed] [Google Scholar]
- Tseng H, Biegel JA, Brown RS. 1999. Basonuclin is associated with the ribosomal RNA genes on human keratinocyte mitotic chromosomes. J Cell Sci 112: 3039–3047. [DOI] [PubMed] [Google Scholar]
- Tucker S, Vitins A, Pikaard CS. 2010. Nucleolar dominance and ribosomal RNA gene silencing. Curr Opin Cell Biol 22: 351–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez BC, Henning D, So RB, Dixon J, Dixon MJ. 2004. The Treacher Collins syndrome (TCOF1) gene product is involved in ribosomal DNA gene transcription by interacting with upstream binding factor. Proc Natl Acad Sci 101: 10709–10714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Sluis M, McStay B. 2015. A localized nucleolar DNA damage response facilitates recruitment of the homology-directed repair machinery independent of cell cycle stage. Genes Dev 29: 1151–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warmerdam DO, van den Berg J, Medema RH. 2016. Breaks in the 45S rDNA lead to recombination-mediated loss of repeats. Cell Rep 14: 2519–2527. [DOI] [PubMed] [Google Scholar]
- Weitao T, Budd M, Hoopes LL, Campbell JL. 2003. Dna2 helicase/nuclease causes replicative fork stalling and double-strand breaks in the ribosomal DNA of Saccharomyces cerevisiae. J Biol Chem 278: 22513–22522. [DOI] [PubMed] [Google Scholar]
- Wittner M, Hamperl S, Stockl U, Seufert W, Tschochner H, Milkereit P, Griesenbeck J. 2011. Establishment and maintenance of alternative chromatin states at a multicopy gene locus. Cell 145: 543–554. [DOI] [PubMed] [Google Scholar]
- Worton RG, Sutherland J, Sylvester JE, Willard HF, Bodrug S, Dube I, Duff C, Kean V, Ray PN, Schmickel RD. 1988. Human ribosomal RNA genes: orientation of the tandem array and conservation of the 5′ end. Science 239: 64–68. [DOI] [PubMed] [Google Scholar]
- Young DW, Hassan MQ, Pratap J, Galindo M, Zaidi SK, Lee SH, Yang X, Xie R, Javed A, Underwood JM, et al. 2007. Mitotic occupancy and lineage-specific transcriptional control of rRNA genes by Runx2. Nature 445: 442–446. [DOI] [PubMed] [Google Scholar]
- Zentner GE, Balow SA, Scacheri PC. 2014. Genomic characterization of the mouse ribosomal DNA locus. G3 4: 243–254. [DOI] [PMC free article] [PubMed] [Google Scholar]


