Abstract
Rationale
Ambient particulate matter (PM) is associated with acute exacerbations of airflow obstruction. Additionally, elderly individuals are more susceptible to increased functional morbidity following acute PM exposure.
Hypothesis/Objective
The purpose of the current study is to determine the aging effects of PM exposure on the responsiveness of airway smooth muscle in mice. We hypothesized that airway reactivity induced by methacholine (Mch) will increase with age in PM exposed mice.
Methods
Male C57BL/6 (B6) mice at 11, 39, 67, and 96 wks of age were exposed to either carbon black (CB concentration ~550 µg/m3) or room air (RA) for 3 hours on 3 consecutive days. One day after the last exposure, mice were anesthetized and airways resistance (Raw) was measured using forced oscillation at baseline and 1 minute after increasing half-log doses (0.1 to 30 mg/ml) of aerosolized Mch.
Results
Baseline Raw was significantly lesser in mice at 39, 67, and 96 wks compared with 11-wk old mice (p < 0.05). In RA exposed mice, an age-dependent decline in Mch-induced airway reactivity occurred in association with the highest Mch doses at ages 67 and 96 wks (p < 0.05). CB exposure caused a significant (p < 0.05) increase in Mch-induced Raw response in 67-wk old CB exposed mice compared with age-matched RA mice.
Conclusion
Our results show a progressive decrease in the Mch-induced Raw response with age in B6 mice. Overall, the effect of CB exposure resulted in significant increases in airway reactivity in middle-aged mice.
Introduction
Epidemiology studies have shown adverse effects of air pollution exposure, including particulate matter (PM), resulting in increased respiratory morbidity and mortality, {Bell, 2008 #5;Peng, 2008 #26;Samoli, 2008 #31;Zanobetti, 2009 #45}. Several subpopulations have been identified as susceptible groups to PM exposure, including asthmatics, children, and the elderly {Sacks, 2011 #135;Pope, 2000 #164}. In asthmatics and non-asthmatics, PM exposure has been associated with acute exacerbations in airflow obstruction due to bronchoconstriction {Widdicombe, 1962 #168;Rundell, 2005 #249}. Children and the elderly, in general, are considered susceptible to increased functional morbidity following PM exposure {Downs, 2007 #177;Scarlett, 1996 #157}. In naïve mice, PM exposure leads to increases in airway hyperreactivity (AHR) {Walters, 2001 #99;Lambert, 2003 #130}, but the aging effect on airway responsiveness to PM exposure in mice has not been previously studied.
Excluding the very young, most PM related disease mortalities tend to show a linear increase in susceptibility with age {Gouveia, 2000 #139}. Other studies have also found a general linear increase with age, but indicate that a plateau or decline in risk that occurs at about 65 years of age {Zeger, 2008 #46;Fung, 2005 #167}. This is consistent with the sigmoidal life expectancy tables when children under the age of 10 are excluded {Wilson, 1994 #137}. Based on this type of sigmoidal shape, one might expect the most susceptible older age group to be on the exponential part of the curve in the age range of 60 to 85 years old. Postulations as to why children and the elderly are considered susceptible is due to immature, developing organs for the young and a gradual decline in physiological function for older adults {Association, 2001 #140;Sacks, 2011 #135}. According to a report by the U.S. EPA, one specific change in function with age appears to be an increase in PM clearance time from the respiratory tract {EPA, 2009 #179}.
Four different profiles for the relationship between AHR and age have been described; (1) a positive correlation between airway hyperresponsiveness with increasing age {Bakke, 1991 #173;Britton, 1994 #174}, however, the youngest age included was 15 years of age, (2) a bimodal or u-shape response with the greatest airway reactivity at the oldest and youngest ages {Paoletti, 1995 #109;Hopp, 1985 #113}, (3) a negative correlation with age {Schwartz, 2002 #108}, and (4) no correlation with age {Trigg, 1990 #190}. In summary, the collective majority of results is inconsistent, but the bulk of the data suggests that AHR likely increases with age in adults.
To date, no single study has assessed the effect of age in adult male mice on AHR by comparing several age groups, or especially very old mice. Most studies on aging in mice that exist either focused more on development or baseline characteristics {McKenzie, 2010 #105;Huang, 2007 #202},and not on lung effects of air pollution. The only study that has assessed age differences in mice found no detectable changes in AHR for naïve female BALB/c mice, ages 6, 12, and 18 months {Busse, 2007 #51}. In male rats, the authors found a decline in Mch-induced constriction when comparing 14 to 29 mo old rats {Nagase, 1994 #244}. Missing from these studies of lung phenotype with age is the impact of airborne PM, and this is one of the primary endpoints of the present study.
The purpose of this study was to examine the effect of age as a potential risk modifier in AHR following PM exposure by focusing on age-exposure interactions. Since susceptibility to PM exposure generally increases with age, we hypothesized that these interactions feature an overall age-dependent decrease in AHR which is modified by a PM-induced increase in AHR. To test these hypotheses, male C57BL/6 (B6) mice from four age groups (ranging from 11 to 96 wks) were repeatedly exposed to carbon black (CB) for 3 hrs on 3 consecutive days. Measurements to assess changes in airway reactivity and lung parenchymal mechanical properties were performed one day later. Conceptually, the age groups used in this study represent late puberty, both early and late mid-life, and old age {Flurkey, 2007 #147}. The results of the study indicated that a robust age-exposure interaction was evident in middle-aged mice.
Methods
Animals
Male B6 mice at 11, 39, 67, and 96 wks of age were housed for most of their life in the Johns Hopkins School of Public Health animal facility. The mice were provided rodent chow and tap water ad libitum and housed on a 14-hr light/dark cycle. All experiments were conducted with approval from the Johns Hopkins University Medical Institutions Animal Care and Use Committee.
Carbon Black exposure protocol
Each mouse was exposed to either CB (~550 µg/m3) or room air (RA) for 3 hrs on 3 consecutive days. The exposure was conducted for a total of 16 separate times. During each 3-hr exposure, animals were individually confined to a Plexiglas holding chamber designed to reduce the potential stress of a nose-only inhalation device. The CB exposure methods for particle generation and exposure assessment were described previously {Hamade, 2008 #207}. Briefly, the CB (Regal 660; density 1.95 g/cm3; specific surface area 112 m2/g; empirical formula C910H34O10; composition 96.90% carbon, 1.42% oxygen, 0.30% hydrogen) was aerosolized by a Wright Dust Feeder (BGI, Inc., Waltham, MA). Particles were monitored with an Aerodynamic Particle Sizer (TSI, Shoreview, MN) for mass median aerodynamic diameter and count median diameter assessments. We used CB because it represented the carbon core of PM, and reduced the potential confounding effects due to chemical toxicity of other metals present in ambient PM. Additionally, exposure to PM with higher levels of elemental carbon have been shown to increase the risk for hospitalization in patient populations {Bell, 2009 #6}.
Respiratory measurements
The mice were allowed to recover for 24 hrs after the 3-day exposure to either CB or RA prior to measurement. Animals were anesthetized by intraperitoneal (i.p.) injection of ketamine (87.5 mg/kg, Ketathesia, Butler Animal Health Supply, Dublin, OH) and xylazine (17.5 mg/kg AnaSed Injectable, Lloyd Laboratories, Shenandoah, IA), and then the trachea was cannulated. After tracheotomy, the cannula was connected to a computer-controlled ventilator (FlexiVent, Montreal, Canada) while in the supine position, and ventilated at a rate of 150 breaths per minute with a tidal volume of 0.20 ml at 100% O2 on a positive end-expiratory pressure (PEEP) of 3 cmH2O. An intramuscular injection of succinylcholine (7.5 mg/kg, Quelicin, Hospira, Inc., Lake Forest, IL) was administered to each mouse to eliminate spontaneous respiratory movement that can affect the measurement of respiratory mechanics.
Each mouse was subjected to a deep inspiration at 30 cmH2O for 10 s ~1 min prior to baseline lung measurements. Mch-aerosol challenge was performed on each mouse following baseline measurements, which consisted of sequential doses of acetyl-beta-methacholine chloride (Sigma-Aldrich, St. Louis, MO) delivered for 10 s by aerosolization using an ultrasonic nebulizer on inspiration. Half-log doses of Mch were prepared by vortexing Mch in 0.9% PBS by serial dilution to obtain concentrations of 0.1, 0.3, 1, 3, 10, and 30 mg/ml. Lung measurements for total lung resistance (R), elastance (E), and compliance (C) were recorded 60 s after each sequential half-log dose of aerosolized Mch. The impedance of the respiratory system was measured, and then fitted by Flexivent software to a constant phase model {Hantos, 1992 #259} to provide measurements of airway resistance (Raw), tissue damping (G), and tissue elastance (H).
Statistical analysis
To analyze the Mch dose response curve for resistance and elastance, a piecewise, generalized least squares (GLS) model with weighted variance and an autoregressive correlation for repeated measures was used. Model parameters include age, exposure, their interaction, and dose of Mch. Mch ??dose?? was log-transformed (ln Mch) to generate a normalized distribution. A spline at 1 ln Mch (i.e. Mch at 3 mg/ml) was included since the dose-response curve was not linear, but instead followed an exponential function. The use of splines is similar to using other functions (e.g. sigmoidal or exponential) for assessing dose-response relationships {Aerts, 1994 #203;Verlato, 1996 #204}. The spline provides two slopes representing the reactive and nonreactive response. The second half of the linear regression (i.e the slope after Mch 3 mg/ml) represents the steepest part of the dose-response curve and has been characterized as reactivity {Aerts, 1994 #203}. Sample size for each age-exposure group ranged from 3 to 10 mice with a total sample size of 49 mice, yielding a total of 343 measurements (7 repeated doses of Mch per mouse). The GLS was performed with the R Statistical Computing Environment (version 2.13.0). Statistical significance was accepted at a p-value of 0.05 after performing a Wald test to compare the differences in slopes. Results were expressed as the mean slope with 95% confidence intervals (CI).
For analysis of other results, the data analysis was performed using Stata Statistical software release 11 (StataCorp, College Station, TX). A multiple linear regression was used to determine the change in baseline for age with a covariate for exposure for airway resistance and lung elastance. Mann-Whitney Rank sum test was used to determine differences between groups for all baseline lung parameters as well as weight and length measurements. Statistical significance was accepted at a p-value of 0.05 after a Bonferroni correction. A bootstrapped multiple linear regression was used to determine the trend with age for weight and length measurements. In the figures and tables, results were expressed as the mean ± standard error of the mean (SEM).
Results
As shown in table 1, there were no significant differences in weight or length between the groups of mice exposed to either CB or RA; however, there were significant increases in weight and length between age groups.
Table 1.
Body weights and lengths by age and exposure
| 11 weeks | 39 weeks | 67 weeks | 96 weeks | ||
|---|---|---|---|---|---|
|
Weight (g) (mean ± SEM) |
Room Air | 26.44 ± 0.563 | 33.47 ± 1.170# | 41.42 ± 2.921# | 48.38 ± 4.403# |
| Carbon Black | 25.46 ± 0.570 | 30.40 ± 1.075# | 34.34 ± 1.335# | 46.54 ± 3.090# | |
|
Length (cm) (mean ± SEM) |
Room Air | 9.23 ± 0.067 | 9.83 ± 0.120# | 10.22 ± 0.171# | 10.30 ± 0.247# |
| Carbon Black | 9.22 ± 0.053 | 9.65 ± 0.087# | 9.80 ± 0.144# | 10.27 ± 0.173# |
p-value <0.05 (Bonferroni corrected) when compared to 11 weeks.
In general, there was a significant (p < 0.01) decline in baseline Raw with age (figure 1a). Additionally, CB exposure led to an increase in Raw compared to controls (p < 0.01); however, only CB-exposed mice at 11 wks were significantly (p < 0.01) different from control mice.
Figure 1.
Baseline Airway Resistance (Raw) and Tissue Elastance (H) comparing age and carbon black differences. (A) Baseline airway resistance declines with age. At 11-wks, CB exposure results in a greater airway resistance. (B) Baseline tissue elastance also declines with age, however CB exposure results in a lower tissue elastance. Asterisk indicates significance at p < 0.05 for RA vs. CB exposure within each age, and the dagger indicates significance for each age compared to 11 weeks within exposure as determined by Mann-Whitney Rank Sum test.
Other baseline lung parameters, including H, showed similar exposure and age-dependent effects as Raw, although CB exposure resulted in a lower H at 11 wks compared to RA (figure 1b). All other lung parameters (R, E, and G) had similar trends as H, but R did not show an exposure effect at 11 wks (table 2).
Table 2.
Baseline values for the lung parameters displayed by age and exposure
|
Baseline
(mean ± SEM) |
|||||
|---|---|---|---|---|---|
| 11 weeks | 39 weeks | 67 weeks | 96 weeks | ||
| Raw | Room Air | 0.274±0.008 | 0.260±0.021 | 0.243±0.018 | 0.246±0.015 |
| Carbon Black | 0.311±0.008# | 0.249±0.011^ | 0.231±0.012^ | 0.222±0.018^ | |
| H | Room Air | 33.613±1.047 | 20.063±1.224^ | 24.298±4.017 | 20.735±1.407^ |
| Carbon Black | 29.888±0.660# | 20.161±1.022^ | 20.548±1.093^ | 18.721±1.885^ | |
| G | Room Air | 5.945±0.195 | 3.848±0.054^ | 4.414±0.922 | 3.128±0.101^ |
| Carbon Black | 5.299±0.152# | 3.738±0.169^ | 3.749±0.410 | 3.571±0.228^ | |
| R | Room Air | 0.655±0.015 | 0.469±0.012^ | 0.509±0.070 | 0.405±0.009^ |
| Carbon Black | 0.647±0.009 | 0.466±0.011^ | 0.440±0.030^ | 0.421±0.021^ | |
| E | Room Air | 32.473±0.983 | 20.619±1.106^ | 24.133±4.093 | 19.499±0.849^ |
| Carbon Black | 29.613±0.574 | 20.345±0.709^ | 20.419±3.663^ | 19.619±1.431^ | |
Significant difference from 11 weeks within exposure (p < 0.05)
Significant difference for CB compared to RA within age (p < 0.05)
With age in RA mice, the airways were significantly (p < 0.05) less responsive to Mch challenge as assessed from the slope of the dose-response curve ; however, this reactivity at 39 weeks was not significantly (p > 0.05) different from 11-wk old mice (figure 2a and 4a). For each unit increase in ln Mch, the change slope among age groups ranged from a significant (p < 0.01) increase of 0.601 (95% CI: 0.422 to 0.782) at 11 wks to an undetectable (p > 0.05) change of 0.192 (95% CI: −0.062 to 0.447) at 96 wks. These data indicate a significant (p < 0.05) decline in AHR (Raw) with increasing age (table 3).
Figure 2.
Reactivity slopes for airway resistance (Raw) and tissue elastance (H). Raw has an age-dependent decline in slope and CB exposure compared to RA results in a significant increase in Raw at 67 weeks (A). H also has an age-dependent decline in the slope; however, CB exposure has no effect on H (B). Data displayed as the mean slope ± SE. *p < 0.05 for RA vs. CB exposure within each age. #p < 0.05 for each age compared to 11 weeks within exposure. Sample size ranges from 3 to 10. H/ln Mch, the slope for tissue elastance as the natural log dose of Mch increases from 1 to 30 mg/mL; Raw/ln Mch, the slope for airway resistance as the natural log dose of Mch increases from 1 to 30 mg/mL.
Table 3.
Slopes for the lung parameters displayed by age and exposure
|
Slopes
(mean ± SEM) |
|||||
|---|---|---|---|---|---|
| 11 weeks | 39 weeks | 67 weeks | 96 weeks | ||
| Raw/lnMch | Room Air | 0.602±0.092* | 0.392±0.168* | 0.285±0.130*^ | 0.192 ± 0.130^ |
| Carbon Black | 0.539±0.092* | 0.619±0.145* | 0.641±0.118*# | 0.243±0.118*^ | |
| H/lnMch | Room Air | 15.499±1.993* | 7.755±3.638*^ | 5.019±2.818^ | 1.606±2.818^ |
| Carbon Black | 14.789±1.993* | 7.502±3.151* | 9.125±2.573* | 2.617±2.573^ | |
| G/lnMch | Room Air | 6.002±0.801* | 1.920±1.462^ | 1.843±1.133^ | 1.206±1.133^ |
| Carbon Black | 4.017±0.801* | 3.547±1.266* | 5.663±1.034*# | 0.943±1.034^ | |
| R/lnMch | Room Air | 1.378±0.138* | 0.581±0.252*^ | 0.524±0.195*^ | 0.268±0.195^ |
| Carbon Black | 0.934±0.138*# | 0.854±0.218* | 0.870±0.178* | 0.283±0.178^ | |
| E/lnMch | Room Air | 17.848±2.959* | 13.310±5.402* | 5.884±4.184^ | 4.369±4.184^ |
| Carbon Black | 19.773±2.959* | 13.547±4.678* | 18.138±3.820*# | 7.134±3.820^ | |
Slope significantly different from 0 (p < 0.05)
Significant difference from 11 weeks within exposure (p < 0.05)
Significant difference for CB compared to RA within age (p < 0.05)
In CB-exposed mice, there was a significant (p < 0.05) increase in AHR at 67 wks, compared with RA mice (figure 2b). For each unit increase in ln Mch, the change in the slope was 0.285 (95% CI: 0.031 to 0.539) at 67 wks for RA-exposed mice compared with 0.641 (95% CI: 0.409 to 0.873) for CB-exposed mice (table 3). These results suggested that an age-exposure interaction occurred at 67 wks of age. The results in CB-exposed mice were also noteworthy at 39 wks, in which the slope was 0.619 (95% CI: 0.334 to 0.903) compared with 0.392 (95% CI: 0.064 to 0.720) in RA-exposed mice.
The RA mice also showed a significant (p < 0.05) age-dependent decline in H, which was comparable to Raw for RA mice (figure 3a). However, there was no effect of CB exposure on H among the age groups (figure 3b), which differed from the Raw responses, especially in 67-wk old mice exposed to CB. The difference between Raw and H indicated that CB exposure affected functional aspects of the central airways without changing the elastic properties of the lung parenchyma.
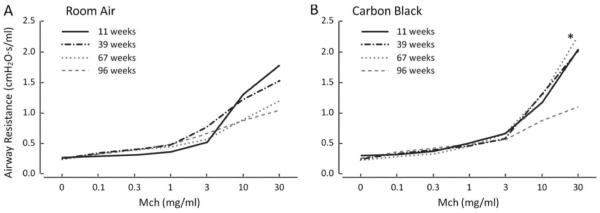
Figure 3.
Airway Resistance (Raw) displaying the average change as Mch dose increases. (A) Raw has an age-dependent decline with age, however CB exposure (B) results in a significant increase in Raw at 67 weeks. Asterisk indicates significance at a p < 0.05 when comparing RA to CB within age.
Other lung parameters for RA mice had the same age-dependent decline as seen in Raw (p < 0.05). However, unlike with Raw, there was no effect of CB exposure for R at 67 wks. Instead, mice exposed to CB at 11 wks showed a significant (p < 0.05) decline in R with a slope of 0.934 (95% CI: 0.663 to 1.204) relative to RA mice with a slope of 1.378 (95% CI: 1.108 to 1.649). The E and G parameters had the same CB exposure and age-dependent trends as demonstrated for Raw (table 3).
Overall, the effects of CB exposure were primarily limited to an increased Raw at the middle-age range. Exposure to CB did not affect other lung parameters, except at 11 wks for R and at 67 wks for E and G. Correlation analysis confirmed the absence of lung parenchymal and structural changes as a result of CB exposure because all mechanical properties of lung parenchymal function were weakly correlated with Raw, but highly correlated with R (supplementary table 1).
Discussion
The current study focused on age-exposure interactions as potential modifiers of airway responsiveness. The results indicated a decline in the Mch-induced AHR with age in naïve male C57Bl/6 mice, consistent published studies. Also, CB exposure had two major effects; (1) CB exposure increased baseline Raw in 11-wk old mice, and (2) there was an age-exposure interaction that occurred in 67-wk old mice resulting in greater airway responsiveness following CB exposure. Finally, 96-wk old mice demonstrated only modest changes in Raw with increasing dose of Mch, and CB-exposure did not result in a significant age-exposure interaction in this age group. These findings are unique in terms of their assessment of aging and PM interactions across several ages in mice.
The effects of PM exposure in the youngest mice (11-wks) demonstrated an increase in baseline responses of Raw before initiating Mch challenge that was not seen in any other age group. Under these conditions, the 3-day exposure may have led to increased Raw through increases in lung inflammation, mucus production, or epithelial cell hyperplasia {Tillie-Leblond, 2005 #255;Zhu, 1999 #256}.
The results also indicated an increase in AHR at 67 wks following CB exposure. Although the CB-induced increase in the responsiveness was specific to 67-wk old mice, combining age groups at 39 and 67 weeks also proved to be significantly different between RA and CB exposures, suggesting that increased sensitivity to CB exposure occurred as early as 39 wks of age. While AHR is a negative physiological response when associated with asthma and other respiratory diseases, bronchoconstriction has also been considered to play a role as a pulmonary defense mechanism. Bronchoconstriction as one of several possible pulmonary defense mechanisms may help to prevent toxins, gases, and other lung irritants from reaching the peripheral airways and the deeper parenchymal regions of the lung {Coleridge, 1994 #120}. The mechanism of increased deposition higher in the lungs following bronchoconstriction could be due to increased turbulent flow. Therefore, particle clearance might be enhanced because particles that deposit deeper in the lung take much longer to clear. For example, particles deposited in upper airways are cleared in 30 min from the trachea where as particles deposited in regions of the lung with ciliated epithelium are cleared in 24 hrs {Harada, 1985 #124}. Likewise, particles that reach the alveoli take even longer (days to weeks) to remove, since phagocytosis by alveolar macrophages is required before particles are cleared by way of the mucociliary escalator {Schlesinger, 1985 #123}. In a previous study focused on the effects of bronchoconstriction on particle deposition, the authors found differences in regional deposition of particles after induced bronchoconstriction, resulting in less deposition in the lower regions of the lung and faster particle clearance rates following bronchoconstriction {Svartengren, 1987 #250}.
Since nearly all of the mice in this experiment were considered healthy, an increase in AHR may be an indication of a pulmonary defense mechanism. Previous reviews have indicated that children and the elderly are considered to be more susceptible due to immature lungs in the young and a gradual decline in physiological lung function for older adults {Association, 2001 #140;Sacks, 2011 #135}. Such differences in the lung might explain why these subpopulations have compromised pulmonary defense responses to air pollution exposure. For example, in humans, male lungs continue to develop until the age of 18 {Postma, 2007 #136}. This same delay in lung development was also demonstrated in mice with interalveolar pore development continuing until about 3 months {Kawakami, 1984 #261;Ranga, 1980 #262}. According to some aging research in mice, B6 mice are estimated to be mature adults between 3 and 6 months {Flurkey, 2007 #147}. Therefore, it is possible that the lack of CB-induced AHR responses for 11-wk old mice seen in the current study might be a result of immature lung development, thus leaving the younger mice potentially more susceptible to adverse effects of PM exposure. As a corollary, the absence of CB-induced AHR in the 96-wk old mice may be due to a decline in the capacity of the airways to constrict, particularly since these responses did not differ from the RA exposed mice. Therefore, while an increase in AHR may be considered a marker for increased susceptibility to PM exposure in specific lung disease states, such as asthma, the absence of PM-induced AHR might also be an indication of increased susceptibility to adverse effects of PM exposure in the very young or very old, especially based on the parameters of this study which was looking at the acute effects of PM exposure in healthy, not chronically exposed mice.
One potential mechanism for the altered AHR in CB-exposed mice either pulmonary C-fibers or rapidly adapting receptors (RARs); also known as irritant receptors {Coleridge, 1994 #120}. Both have been shown to elicit a bronchoconstriction following exposure to various chemical lung irritants {Veronesi, 2001 #267}. The bronchoconstriction is likely mediated through cholinergic pathways or neuropeptide release, since enhanced AHR to histamine following ozone exposure has been shown to be partially eliminated by atropine pretreatment {Lee, 2001 #263}. One of the receptors that may play a role in the adaptation to environmental irritants by pulmonary neurons is TRPA1 (Transient Receptor Potential Anykrin 1) {Grace, 2011 #273}. Several neuropeptides that are known to be released by neurons following noxious stimuli include the tachykinins, substance P and neurokinin A (NKA), which also are potent inducers of a cascade of airway inflammatory responses and bronchoconstriction {Veronesi, 2001 #267}. Besides being released by the neurons, some inflammatory and epithelial cells are also known to release these tachykinins after exposure to irritants {Veronesi, 2001 #267}. Once released, these tachykinins act on neurokinin (NK1 or NK2) receptors on non-neural target cells, such that NKA, acting on NK2 receptors stimulates smooth muscle contraction {Veronesi, 2001 #267}. In general, the modification of CB exposure at the middle-age is likely through changes in one of these receptors or through modification of tachykinin release; therefore TRPA1 and NK2 receptors are likely involved in the modification of AHR following CB exposure.
The age-dependent decline in Mch-induced AHR was demonstrated by the 11-wk mice showing the greatest AHR while the 96-wk mice were the least responsive to Mch challenge. It has been proposed that the loss of sensitivity to Mch is related to age-dependent changes in muscarinic receptor subtypes and their subsequent responsiveness {Wills-Karp, 1993 #245}. Additionally, neural function decreases with age through changes in neuronal sensitivity and excitability. This suggests that the lack of response at age 96 wk could also be related to the overall decline neural and muscarinic function.
When comparing Raw to other lung parameters, increases in Raw reflect constriction in the conducting airways, whereas changes in H reflect modification of the lung parenchymal structure {Bates, 2003 #200}. Based on the results of this study, the effect of acute CB exposure at 67 wks appeared to be limited to the conducting airways without causing changes in the lung parenchyma. In a review by Cohen and Gold (1975) {Cohen, 1975 #126}, the authors indicated that deposition of particles in upper airways results in constriction without affecting compliance. This concept of particle deposition seems to be consistent with the current results in mice indicating that acute effects of CB exposure were related to changes in the upper airways compared to distal changes in the lung parenchyma.
The current results provide a cross-sectional understanding of the differential responses in AHR by age following PM exposure. The changes in AHR have not been well studied as a function of aging and PM exposure in animal models, but our work clearly demonstrates an age-dependent depression in AHR and a robust PM-induced increase in AHR through midlife. The age-dependent decline in Mch sensitivity in control mice may be related to changes in muscarinic receptor responsiveness. In concert, PM exposure may provoke vigorous lung defense mechanisms in midlife, which are absent in the oldest age group. Overall, these results provide unique insight into the difficulties and sometimes contradictions in the literature regarding the effects of PM exposure on morbidity and mortality as well as the different responses profiles that occur with age following bronchoconstriction challenges. In chronic or diseased states, the initial defensive functions of bronchoconstriction may be lost and contribute to increased morbidity and mortality following PM exposure. Therefore, future work regarding AHR and PM effects may benefit from trying to understand the chronic effects of PM exposure with age, with specific foxcus on how the chronic exposure modifies lung airway responses and defense mechanisms in the presence of airborne pollutants.
Supplementary Material
List of abbreviations
- PM
particulate matter
- CB
carbon black
- RA
room air
- Mch
Methacholine
- GLS
generalized least squares
- Raw
airway resistance
- H
tissue elastance
- G
tissue damping
- R
total airway resistance
- E
elastance
- C
compliance
References
- Aerts JG, Bogaard JM, Overbeek SE, Verbraak AF, Thio P. Extrapolation of methacholine log-dose response curves with a Cumulative Gaussian Distribution function. Eur Respir J. 1994;7(5):895–900. [PubMed] [Google Scholar]
- Association AL Urban air pollution and health inequities: a workshop report. Environ Health Perspect. 2001;109(Suppl 3):357–374. [PMC free article] [PubMed] [Google Scholar]
- Bakke PS, Baste V, Gulsvik A. Bronchial responsiveness in a Norwegian community. Am Rev Respir Dis. 1991;143(2):317–322. doi: 10.1164/ajrccm/143.2.317. [DOI] [PubMed] [Google Scholar]
- Bates JH, Irvin CG. Measuring lung function in mice: the phenotyping uncertainty principle. J Appl Physiol. 2003;94(4):1297–1306. doi: 10.1152/japplphysiol.00706.2002. [DOI] [PubMed] [Google Scholar]
- Bell ML, Ebisu K, Peng RD, Walker J, Samet JM, Zeger SL, Dominici F. Seasonal and regional short-term effects of fine particles on hospital admissions in 202 US counties, 1999-2005. Am J Epidemiol. 2008;168(11):1301–1310. doi: 10.1093/aje/kwn252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell ML, Ebisu K, Peng RD, Samet JM, Dominici F. Hospital admissions and chemical composition of fine particle air pollution. Am J Respir Crit Care Med. 2009;179(12):1115–1120. doi: 10.1164/rccm.200808-1240OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton J, Pavord I, Richards K, Knox A, Wisniewski A, Wahedna I, Kinnear W, Tattersfield A, Weiss S. Factors influencing the occurrence of airway hyperreactivity in the general population: the importance of atopy and airway calibre. Eur Respir J. 1994;7(5):881–887. [PubMed] [Google Scholar]
- Busse PJ, Zhang TF, Srivastava K, Schofield B, Li XM. Effect of ageing on pulmonary inflammation, airway hyperresponsiveness and T and B cell responses in antigen-sensitized and -challenged mice. Clin Exp Allergy. 2007;37(9):1392–1403. doi: 10.1111/j.1365-2222.2007.02775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AB, Gold WM. Defense mechanisms of the lungs. Annu Rev Physiol. 1975;37:325–350. doi: 10.1146/annurev.ph.37.030175.001545. [DOI] [PubMed] [Google Scholar]
- Coleridge HM, Coleridge JC. Pulmonary reflexes: neural mechanisms of pulmonary defense. Annu Rev Physiol. 1994;56:69–91. doi: 10.1146/annurev.ph.56.030194.000441. [DOI] [PubMed] [Google Scholar]
- Downs SH, Schindler C, Liu LJ, Keidel D, Bayer-Oglesby L, Brutsche MH, Gerbase MW, Keller R, Kunzli N, Leuenberger P, Probst-Hensch NM, Tschopp JM, Zellweger JP, Rochat T, Schwartz J, Ackermann-Liebrich U. Reduced exposure to PM10 and attenuated age-related decline in lung function. N Engl J Med. 2007;357(23):2338–2347. doi: 10.1056/NEJMoa073625. [DOI] [PubMed] [Google Scholar]
- EPA US . Integrated Science Assessment for Particulate Matter (Final Report) EPA/600/R-08/139F. U.S. Environmental Protection Agency; Washington, DC: 2009. [PubMed] [Google Scholar]
- Flurkey K, Currer JM, Harrison DE. Mouse Models in Aging Research. In: Fox JG, Davisson MT, Quimby FW, Barthold SW, Newcomer CE, Smith AL, editors. The Mouse in Biomedical Research. American College of Laboratory Animal Medicine (Elsevier); Burlington, MA: 2007. pp. 637–672. [Google Scholar]
- Fung KY, Luginaah I, Gorey KM, Webster G. Air pollution and daily hospitalization rates for cardiovascular and respiratory diseases in London, Ontario. Int J Environ Stud. 2005;62(6):677–685. doi: 10.1080/00207230500367879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouveia N, Fletcher T. Time series analysis of air pollution and mortality: effects by cause, age and socioeconomic status. J Epidemiol Community Health. 2000;54(10):750–755. doi: 10.1136/jech.54.10.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace MS, Belvisi MG. TRPA1 receptors in cough. Pulm Pharmacol Ther. 2011;24(3):286–288. doi: 10.1016/j.pupt.2010.11.002. [DOI] [PubMed] [Google Scholar]
- Hamade AK, Rabold R, Tankersley CG. Adverse cardiovascular effects with acute particulate matter and ozone exposures: interstrain variation in mice. Environ Health Perspect. 2008;116(8):1033–1039. doi: 10.1289/ehp.10689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantos Z, Daroczy B, Suki B, Nagy S, Fredberg JJ. Input impedance and peripheral inhomogeneity of dog lungs. J Appl Physiol. 1992;72(1):168–178. doi: 10.1152/jappl.1992.72.1.168. [DOI] [PubMed] [Google Scholar]
- Harada RN, Repine JE. Pulmonary host defense mechanisms. Chest. 1985;87(2):247–252. doi: 10.1378/chest.87.2.247. [DOI] [PubMed] [Google Scholar]
- Hopp RJ, Bewtra A, Nair NM, Townley RG. The effect of age on methacholine response. J Allergy Clin Immunol. 1985;76(4):609–613. doi: 10.1016/0091-6749(85)90783-3. [DOI] [PubMed] [Google Scholar]
- Huang K, Rabold R, Schofield B, Mitzner W, Tankersley CG. Age-dependent changes of airway and lung parenchyma in C57BL/6J mice. J Appl Physiol. 2007;102(1):200–206. doi: 10.1152/japplphysiol.00400.2006. [DOI] [PubMed] [Google Scholar]
- Kawakami M, Paul JL, Thurlbeck WM. The effect of age on lung structure in male BALB/cNNia inbred mice. Am J Anat. 1984;170(1):1–21. doi: 10.1002/aja.1001700102. [DOI] [PubMed] [Google Scholar]
- Lambert AL, Mangum JB, DeLorme MP, Everitt JI. Ultrafine carbon black particles enhance respiratory syncytial virus-induced airway reactivity, pulmonary inflammation, and chemokine expression. Toxicol Sci. 2003;72(2):339–346. doi: 10.1093/toxsci/kfg032. [DOI] [PubMed] [Google Scholar]
- Lee LY, Widdicombe JG. Modulation of airway sensitivity to inhaled irritants: role of inflammatory mediators. Environ Health Perspect. 2001;109(Suppl 4):585–589. doi: 10.1289/ehp.01109s4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie R, Burton MD, Royce SG, Tang ML. Age and sex influences on airway hyperresponsiveness. J Asthma. 2010;47(6):651–654. doi: 10.3109/02770901003692801. [DOI] [PubMed] [Google Scholar]
- Nagase T, Fukuchi Y, Teramoto S, Matsuse T, Orimo H. Mechanical interdependence in relation to age: effects of lung volume on airway resistance in rats. J Appl Physiol. 1994;77(3):1172–1177. doi: 10.1152/jappl.1994.77.3.1172. [DOI] [PubMed] [Google Scholar]
- Paoletti P, Carrozzi L, Viegi G, Modena P, Ballerin L, Di Pede F, Grado L, Baldacci S, Pedreschi M, Vellutini M, et al. Distribution of bronchial responsiveness in a general population: effect of sex, age, smoking, and level of pulmonary function. Am J Respir Crit Care Med. 1995;151(6):1770–1777. doi: 10.1164/ajrccm.151.6.7767519. [DOI] [PubMed] [Google Scholar]
- Peng RD, Chang HH, Bell ML, McDermott A, Zeger SL, Samet JM, Dominici F. Coarse particulate matter air pollution and hospital admissions for cardiovascular and respiratory diseases among Medicare patients. JAMA. 2008;299(18):2172–2179. doi: 10.1001/jama.299.18.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope CA., 3rd Epidemiology of fine particulate air pollution and human health: biologic mechanisms and who's at risk? Environ Health Perspect. 2000;108(Suppl 4):713–723. doi: 10.1289/ehp.108-1637679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma DS. Gender differences in asthma development and progression. Gend Med. 2007;4(Suppl B):S133–146. doi: 10.1016/s1550-8579(07)80054-4. [DOI] [PubMed] [Google Scholar]
- Ranga V, Kleinerman J. Interalveolar pores in mouse lung. Regional distribution and alterations with age. Am Rev Respir Dis. 1980;122(3):477–481. doi: 10.1164/arrd.1980.122.3.477. [DOI] [PubMed] [Google Scholar]
- Rundell KW, Spiering BA, Baumann JM, Evans TM. Bronchoconstriction provoked by exercise in a high-particulate-matter environment is attenuated by montelukast. Inhal Toxicol. 2005;17(2):99–105. doi: 10.1080/08958370590899479. [DOI] [PubMed] [Google Scholar]
- Sacks JD, Stanek LW, Luben TJ, Johns DO, Buckley BJ, Brown JS, Ross M. Particulate matter-induced health effects: who is susceptible? Environ Health Perspect. 2011;119(4):446–454. doi: 10.1289/ehp.1002255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samoli E, Peng R, Ramsay T, Pipikou M, Touloumi G, Dominici F, Burnett R, Cohen A, Krewski D, Samet J, Katsouyanni K. Acute effects of ambient particulate matter on mortality in Europe and North America: results from the APHENA study. Environ Health Perspect. 2008;116(11):1480–1486. doi: 10.1289/ehp.11345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarlett JF, Abbott KJ, Peacock JL, Strachan DP, Anderson HR. Acute effects of summer air pollution on respiratory function in primary school children in southern England. Thorax. 1996;51(11):1109–1114. doi: 10.1136/thx.51.11.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger RB. Effects of inhaled acids on respiratory tract defense mechanisms. Environ Health Perspect. 1985;63:25–38. doi: 10.1289/ehp.856325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz J, Schindler C, Zemp E, Perruchoud AP, Zellweger JP, Wuthrich B, Leuenberger P, Ackermann-Liebrich U. Predictors of methacholine responsiveness in a general population. Chest. 2002;122(3):812–820. doi: 10.1378/chest.122.3.812. [DOI] [PubMed] [Google Scholar]
- Svartengren M, Linnman L, Philipson K, Camner P. Regional deposition in human lung of 2.5 microM particles. Exp Lung Res. 1987;12(4):265–279. doi: 10.3109/01902148709062840. [DOI] [PubMed] [Google Scholar]
- Tillie-Leblond I, Gosset P, Tonnel AB. Inflammatory events in severe acute asthma. Allergy. 2005;60(1):23–29. doi: 10.1111/j.1398-9995.2005.00632.x. [DOI] [PubMed] [Google Scholar]
- Trigg CJ, Bennett JB, Tooley M, Sibbald B, D'Souza MF, Davies RJ. A general practice based survey of bronchial hyperresponsiveness and its relation to symptoms, sex, age, atopy, and smoking. Thorax. 1990;45(11):866–872. doi: 10.1136/thx.45.11.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verlato G, Cerveri I, Villani A, Pasquetto M, Ferrari M, Fanfulla F, Zanolin E, Rijcken B, de Marco R. Evaluation of methacholine dose-response curves by linear and exponential mathematical models: goodness-of-fit and validity of extrapolation. Eur Respir J. 1996;9(3):506–511. doi: 10.1183/09031936.96.09030506. [DOI] [PubMed] [Google Scholar]
- Veronesi B, Oortgiesen M. Neurogenic inflammation and particulate matter (PM) air pollutants. Neurotoxicology. 2001;22(6):795–810. doi: 10.1016/s0161-813x(01)00062-6. [DOI] [PubMed] [Google Scholar]
- Walters DM, Breysse PN, Wills-Karp M. Ambient urban Baltimore particulate-induced airway hyperresponsiveness and inflammation in mice. Am J Respir Crit Care Med. 2001;164(8):1438–1443. doi: 10.1164/ajrccm.164.8.2007121. Pt 1. [DOI] [PubMed] [Google Scholar]
- Widdicombe JG, Kent DC, Nadel JA. Mechanism of bronchoconstriction during inhalation of dust. J Appl Physiol. 1962;17:613–616. doi: 10.1152/jappl.1962.17.4.613. [DOI] [PubMed] [Google Scholar]
- Wills-Karp M. Age-related changes in pulmonary muscarinic receptor binding properties. Am J Physiol. 1993;265(2):L103–109. doi: 10.1152/ajplung.1993.265.2.L103. Pt 1. [DOI] [PubMed] [Google Scholar]
- Wilson DL. The analysis of survival (mortality) data: fitting Gompertz, Weibull, and logistic functions. Mech Ageing Dev. 1994;74(1-2):15–33. doi: 10.1016/0047-6374(94)90095-7. [DOI] [PubMed] [Google Scholar]
- Zanobetti A, Schwartz J. The effect of fine and coarse particulate air pollution on mortality: a national analysis. Environ Health Perspect. 2009;117(6):898–903. doi: 10.1289/ehp.0800108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeger SL, Dominici F, McDermott A, Samet JM. Mortality in the Medicare population and chronic exposure to fine particulate air pollution in urban centers (2000-2005) Environ Health Perspect. 2008;116(12):1614–1619. doi: 10.1289/ehp.11449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z, Homer RJ, Wang Z, Chen Q, Geba GP, Wang J, Zhang Y, Elias JA. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J Clin Invest. 1999;103(6):779–788. doi: 10.1172/JCI5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.