Abstract
Disseminated intravascular coagulation (DIC) is a frequent complication in sepsis that is associated with worse outcomes and higher mortality in patients. In addition to the uncontrolled generation of thrombi throughout the patient's vasculature, DIC often consumes large quantities of clotting factors leaving the patient susceptible to hemorrhaging. Owing to these complications, patients often receive anticoagulants to treat the uncontrolled clotting, often with mixed outcomes. This lack of success with the current array of anticoagulants can be partly explained by the fact that during sepsis clotting is often initiated by the immune system. Systemic inflammation has the capacity to activate and amplify coagulation and, as such, potential therapies for the treatment of sepsis-associated DIC need to address the interaction between inflammation and coagulation. Recent studies have suggested that platelets and neutrophil extracellular traps (NETs) are the key mediators of infection-induced coagulation. This review explores current anticoagulant therapies and discusses the development of future therapies to target platelet and NET-mediated coagulation.
Introduction
Systemic infection and inflammation is commonly associated with platelet aggregation and adhesion within the microvasculature. This systemic inflammation frequently results in sepsis and septic shock, conditions that involve uncontrolled inflammation, organ dysfunction and tissue damage. It is estimated that the number of severe, hospital-treated cases of sepsis approaches 19 million per year globally, and mortality is estimated to be ~30%. Although there is little reporting on sepsis in low- and middle-income countries, if the observed pattern of disease in North America is true across the globe, it is likely that there are up to five million deaths due to sepsis every year.1, 2, 3
Patients with sepsis frequently present with circulating platelet–leukocyte aggregates and are often thrombocytopenic.4, 5, 6 In fact, thrombocytopenia in septic patients is strongly associated with worse outcomes and higher mortality.7, 8 Moreover, patients with systemic infection often develop disseminated intravascular coagulation (DIC), a condition involving the systemic activation of the clotting cascade leading to fibrin deposition and thrombus formation throughout the vasculature, resulting in pulmonary embolism, multiorgan dysfunction syndrome and death.7, 8, 9, 10, 11 Paradoxically, not only is DIC associated with hypercoagulation, but, because of the consumption of coagulation factors and platelets, DIC also frequently results in severe bleeding events including hemorrhagic stroke,12 further complicating the treatment of this condition. Although DIC is frequently linked to infection, this condition can also occur in cancer, aneurysms and various traumas.13, 14 Regardless of the initiating insult, it is generally thought that DIC, and the damage it causes, is brought about through three broad mechanisms: increased systemic coagulation, decreased anticoagulative ability and a downstream effect of decreased ability for constructive coagulation (hemostasis).13, 14 Collectively, in septic patients these mechanisms result in a whole-body hypercoagulation and thus systemic thrombocytopenia and consumption of coagulation factors.10, 13, 14, 15
It is estimated that more than 80% of all sepsis patients have some degree of coagulopathy (either clinical or subclinical), and it is these patients who have a much higher mortality rate.16 Owing to the diverse nature of sepsis-inducing infections (virus versus bacteria; Gram positive versus Gram negative; pneumonia versus meningitis) treatments targeting the causative organisms have proven difficult. In addition, once systemic inflammation has been initiated, specifically treating the causative infection has little effect on the overall physiology of the patient. Currently, the standard treatments for mitigating septic shock are generalized, and therefore somewhat ineffective with regards to dealing with infection-induced coagulopathy. These generalized treatments include the use of antibiotics and corticosteroids, oxygen administration and fluid resuscitation with the overall goal to eliminate the infection and to maintain fluid homeostasis.16 Importantly, these treatments do not address the source of the coagulopathy. In fact, in cases of sepsis with DIC, fluid resuscitation could be more harmful than helpful as it could result in myocardial dysfunction or the formation of emboli and consequential infarction of other vessels.17 Thus, it is preferable to specifically treat DIC in order to mitigate coagulation-associated tissue pathogenesis and reduce risk of mortality in sepsis patients.
Coagulation involves sequential activation of a series of plasma proteases converging on the generation of activated thrombin. Thrombin facilitates the proteolytic cleavage of fibrinogen, leading to the formation of fibrin, a key component in the formation of thrombi, converting loose, dynamic platelet aggregates into stable platelet plugs that can obstruct the vasculature. Importantly, the activation of coagulation also feeds back onto the immune response. Serine proteases, such as thrombin, are able to cleave molecules known as protease-activated receptors (PARs) and recent work has shown a clear role for PAR activation in host immunity.18, 19 This crossover between coagulation and immunity highlights the need for strategies that modulate, but not completely inhibit immune-mediated coagulation.
Treating sepsis with anticoagulants
Over the past several decades, numerous attempts have been made and clinical trials conducted on the use of anticoagulants for the treatment of sepsis. These treatment strategies range from administration of purified or recombinant inhibitory proteins to administration of classic anticoagulants such as heparin, and are based on the observation that the balance between pro- and anticoagulant factors has been disturbed in septic patients (Figure 1a). Often septic patients, especially those with coagulopathy, present with reduced plasma levels of several key factors responsible for keeping coagulation in ‘check'.15, 20 This observation leads to the proposal that replacement of these depleted factors with exogenous proteins may help restore this balance in septic patients.
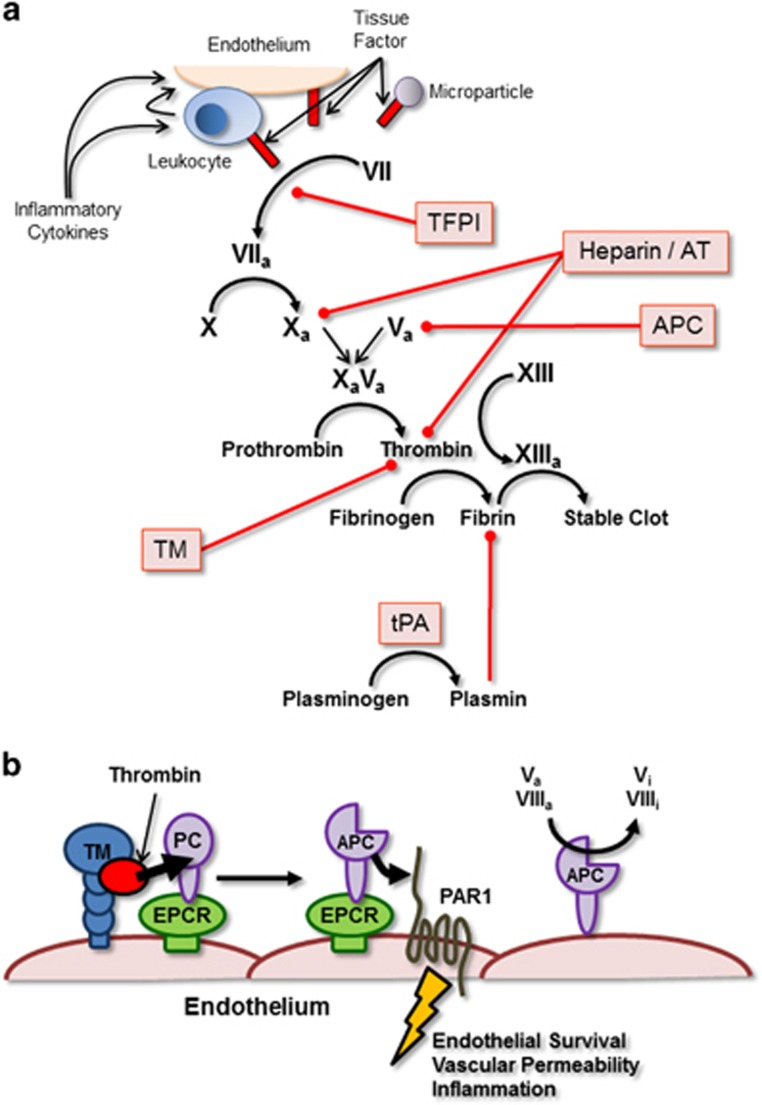
Figure 1.
Simplified schematic of the clotting cascade. (a) Simplified clotting cascade. Therapeutically administered anticoagulants are highlighted in red boxes and red lines illustrate the pathway targeted or protein blocked by the specific therapy. (b) Anticoagulant activity mediated by molecules on the surface of the vascular endothelium. TM-bound thrombin (red circle) is able to cleave and activate Protein C (PC) associated with EPCR. Activated Protein C (APC) can remain bound to the EPCR and subsequently cleave and activate, endothelial PARs resulting in anti-apoptotic signals, prevention of vascular permeability and inhibition of inflammation. APC that dissociates from EPCRs can remain associated with the endothelial surface and act to directly inhibit coagulation by inactivating factors Va and VIIIa resulting in the generation of Vi and VIIIi.
Antithrombin
Antithrombin (AT) is an endogenous protein produced by the liver that acts to directly bind and inhibit thrombin by forming thrombin–antithrombin complexes.15 Thrombin is the key protease at the apex of the coagulation cascade that facilitates the proteolytic conversion of fibrinogen to fibrin, the central protein component of thrombi. In addition, AT also inhibits a number of other clotting factors (Xa, IXa, VIIa, XIa and XIIa) and interacts with the vascular endothelium to modulate vascular tone and platelet activation.15 Early clinical trials studying the use of AT in septic patients showed some promise; however, this potential benefit was not observed once these small trials were scaled up into large, double-blinded, multicenter studies.10, 15, 21 Despite the overall lack of significant improvement in AT-treated patients when compared with controls, several subsequent studies of patient subgroups within the large clinical trial, specifically those with clinical evidence of DIC, reported improved outcomes following AT treatment in these patient groups.22 Moreover, recent studies in Japan specifically targeting AT therapy to septic patients with evidence of DIC have shown significant improvement in overall mortality.23 Fundamentally, AT may be of some benefit to select groups of septic patients.
Tissue factor pathway inhibitor
Tissue factor pathway inhibitor (TFPI) is an inhibitor of coagulation that is expressed by endothelial cells and targets the extrinsic pathway of coagulation by blocking the activation of Factor X by the Factor VIIa–Tissue Factor complex.15 In this way, TFPI helps maintain a hemostatic balance at the surface of the endothelium, preventing the inappropriate spontaneous activation of coagulation. In an effort to restore this balance in septic patients, several studies have looked at the effect of administering recombinant TFPI. Although early results were encouraging, larger randomized and controlled trials failed to demonstrate a significant protective effect of recombinant TFPI with regards to patient survival in severe sepsis.24 Importantly, administration of recombinant TFPI was associated with an increased bleeding risk in patients, a common and potentially dangerous side effect of many anticoagulant therapies.
Thrombomodulin
Thrombomodulin (TM) is a cell surface protein expressed by endothelial cells that binds thrombin, reduces coagulation and enhances activation of protein C (discussed below; Figure 1b). In addition, TM can attenuate inflammation by inhibiting complement and neutralizing High Mobility Group Box 1 (HMGB1), a pro-inflammatory protein released by both activated leukocytes and dead cells.10, 14, 15 It is this multifunctional role of TM, together with the observation that TM expression is often downmodulated in sepsis, that makes this molecule an attractive therapeutic target. Again, initial studies demonstrated a clear benefit in treating septic (and cancer) patients with TM, an effect that, although slightly diminished, remained statistically significant in larger randomized trials.25, 26 Importantly, TM administration appeared to have fewer adverse bleeding events associated with it when compared with other anticoagulant therapies.15, 25 Despite being statistically significant, the improvement in patient outcomes is minimal, suggesting that administration of recombinant TM is not a silver bullet with respect to treating DIC within the context of sepsis. Part of this lack of efficacy may be explained by the fact that endogenous TM is a cell surface protein that associates with other endothelial proteins and receptors (such as PARs and endothelial protein C receptors (EPCRs)). The therapeutically administered recombinant TM is a soluble protein that may simply not fully recapitulate these important protein–protein interactions.
Activated protein C
Part of the mechanism of TM-mediated inhibition of coagulation is through the activation of protein C. Protein C is bound by endothelial protein C receptors (EPCR) and is activated by TM-associated thrombin.27 The resultant activated protein C (APC) is then able to modify several different inflammation- and coagulation-associated pathways. APC inhibits clotting by inactivating factors Va and VIIIa, leading to reduced thrombin generation, is cytoprotective, stabilizes endothelial cell junctions inhibiting vascular leakage and degrades pro-inflammatory molecules such as extracellular histone, a key component of neutrophil extracellular traps (NETs). In early, limited trials on septic patients, significant improvements in outcomes following treatment with recombinant APC were observed. Importantly, these improvements were even more pronounced in patients presenting with evidence of DIC. Unfortunately, larger multicenter trials once again failed to confirm the protective effect of APC in sepsis and, in fact, these larger studies reported a significant increase in adverse bleeding events following treatment with recombinant APC.10, 15, 16, 28 Subgroup analysis demonstrated small but significant improvements in outcomes if APC was administered early or to patients with septic shock. Despite these small improvements in patient outcomes, and due in part to the reported increase in adverse bleeding events, recombinant APC is no longer available for clinical use, although plasma-derived protein C is still available in some countries.
Heparin
Interest in the use of heparin as an anticoagulant in sepsis has grown in recent years. Heparin, like many of the therapies discussed above, has multiple targets within both coagulation and inflammation, making it an appealing potential therapy. Within the coagulation cascade, heparin binds and activates AT, enhancing the ability of AT to bind and inhibit thrombin and other clotting factors. With respect to inflammation, heparin inhibits the activation of platelets, disrupts NETs and neutralizes extracellular histones.15 Much of the evidence for the use of heparin in the treatment of sepsis-associated DIC was initially provided by the control arms of other clinical trials (TM, AT and APC). Early trials designed to directly assess the effect of heparin treatment on the outcomes of septic patients yielded encouraging results with no significant increase in bleeding events.29 More recently, there has been mixed results from a series of clinical trials, although some of this variability can be attributed to differences in trial protocol and the type of heparin used (unfractionated versus low molecular weight heparin).13, 14, 15, 30 Given the lack of bleeding events and the early encouraging results, several large randomized and blinded studies are currently underway to assess the value of heparin as a therapeutic to treat sepsis.
Tissue plasminogen activator
Tissue plasminogen activator (tPA) serves to proteolytically cleave the proenzyme plasminogen resulting in the generation of plasmin, an enzyme that is able to breakdown fibrin-containing clots. Thus, rather than acting to limit the generation of thrombi, tPA instead acts to facilitate the clearance of clots once they are formed. Although recombinant tPA has been used frequently in the treatment of ischemic stroke, pulmonary embolism and myocardial infarct, this particular therapy has only seen limited use in infection-induced coagulopathies.31 Treatment of patients with pneumonia-associated coagulopathy or patients with meningitis-associated sepsis has proven successful, improving lung clearance and limiting systemic coagulation.32, 33 It is important to stress that this therapy has only been successfully applied to a limited number of sepsis cases. One reason for the limited use of tPA is the observation of hyperfibrinolysis in a number of patient groups with coagulopathy. Up to 20% of trauma patients develop serious bleeding events because of the reduced activity of thrombin-activated fibrinolysis inhibitor and plasminogen activator inhibitor 1 and the over activity of tPA.34 In these patients, despite increased activation of thrombin and fibrin deposition, the unchecked activity of tPA results in breakdown and clearance of these clots resulting in uncontrolled bleeding. Owing to this risk, recombinant tPA is not widely used in the treatment of inflammation-associated coagulopathies.
Anticoagulants as a treatment strategy
Although the use of each of the above approaches (along with other anticoagulants not listed) has yielded some positive results in the treatment of sepsis, at least in some patient subpopulations, none have proven to be a good and efficient ‘universal' treatment. Moreover, many of these approaches have demonstrated undesired side effects such as an increased frequency of bleeding events that prevents their use in many patients (trauma patients or those requiring surgery). As such, efforts are currently underway to identify new ways of approaching the problem of infection-associated coagulopathy. One aspect common to all therapies discussed above is that they target the terminal coagulation cascade, attempting to directly block thrombin generation/activity or enhance fibrin breakdown (Figure 1). That is, these therapies aim to inhibit all coagulation, regardless of the initiating pathway—extrinsic, intrinsic, inflammatory or infectious. Thus, these treatments do not only block infection-induced clotting but also prevent protective coagulation associated with hemostasis. Effective and safe therapies should aim to limit inappropriate coagulation while preserving the body's ability to clot in response to injury or surgery.
The common observation of at least some protection in subpopulations of septic patients following treatment with anticoagulants suggests that limiting coagulation has promise; however, the relatively limited success of this protection also suggests that we do not fully understand at least two important aspects of this treatment strategy. First, and beyond the scope of this review, is the fact that we are failing to identify the patient populations that would best benefit from anticoagulation therapy. A number of clinical trials have demonstrated that, whereas anticoagulant therapy failed to significantly improve outcomes of septic patients as a whole, treatment of those patients with severe sepsis or with evidence of DIC did appear to improve outcomes. The development of panels of biomarkers that can be rapidly screened early during a patient's presentation at a health center will greatly aid in the identification of those individuals who might best benefit from therapeutic administration of anticoagulants. Second, future therapies will need to be able to differentiate between pathogenic coagulation and hemostasis. Perhaps, the best strategy to achieve this second goal would be to target the infection-associated induction or the uncontrolled amplification of coagulation observed in sepsis as opposed to targeting the terminal effector activities of the coagulation cascade (Figure 2). In this way it may be possible to limit infection-induced clotting while preserving the ability of the host to maintain hemostasis in response to vascular injury (trauma and surgery). To accomplish this, one might consider targeting the interface between inflammation and coagulation with future treatment strategies in an effort to mitigate infection-induced coagulopathy.
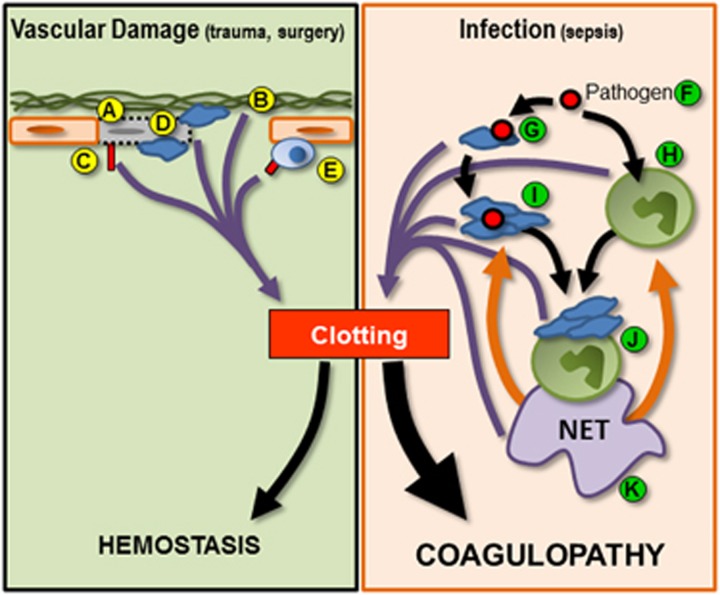
Figure 2.
Schematic illustrating mechanisms of initiation of hemostatic coagulation and infection-induced coagulopathy. Normal hemostasis (green box, left half of figure) can be initiated by (A) endothelial stress and damage. Endothelial death can result in the exposure of the subendothelial extracellular matrix (B), which can also initiate coagulation. Tissue factor expression by endothelial cells (C) and platelet recruitment to sites of damage (D) serve to activate/amplify coagulation. In addition, recruitment and activation of leukocytes such as monocytes can result in increased tissue factor expression (E), further driving coagulation. During infection (orange box, right half of figure), pathogens (F) serve to activate both platelets (G) and leukocytes such as neutrophils (H). Activated platelets can form circulating aggregates (I) or bind to the surface of neutrophils (J) inducing the release of NETs (K). Activated platelets, leukocytes, platelet–leukocyte aggregates and NETs feed into the clotting cascade (purple arrows), resulting in the inappropriate and uncontrolled systemic coagulation. In addition, NETs mediated a positive feedback loop within inflammation (orange arrows) further driving platelet and neutrophil activation and inducing the production of additional NETs. This uncontrolled amplification of clotting tips the balance away from hemostasis and toward systemic coagulopathy.
Coagulation and inflammation: a crossroadS
Inflammation and coagulation represent two overlapping and intertwined processes. The many interactions that occur between these pathways are not entirely surprising when one considers the evolutionary origin of each. In lower organisms (invertebrates) immunity and hemostasis are mediated by the same cell, the hemocyte.35 Possessing characteristics of both neutrophils and platelets, hemocytes are able to phagocytose invading pathogens and degranulate, releasing numerous immune mediators. Moreover, upon appropriate activation, hemocytes facilitate the ‘clotting' of hemolymph to either maintain hemostasis in the organism or to wall-off and contain an infection in an effort to limit pathogen dissemination. In much the same manner, coagulation in higher organisms (mammals) is thought to have a role in limiting pathogen dissemination, preventing the infection from using the bloodstream to rapidly spread through the body.
Platelets
Not only can infection and inflammation serve to activate coagulation, but coagulation can also feedback to amplify and expand inflammation. This bidirectional positive feedback is due in part to the fact that there are multiple points of overlap between these two processes. Perhaps, this overlap is best exemplified by the platelet. Platelets are well known for their role in hemostasis, adhering to the damaged endothelium and participating in the formation of a stable clot. In addition, platelets serve as potent amplifiers of the coagulation cascade,36, 37, 38 binding fibrinogen and von Willebrand factor, forming aggregates within the circulation that serve as platforms to support coagulation. Upon activation, platelets release molecules such as thromboxane A2 (TxA2) and ADP which, via autocrine or paracrine pathways, exponentially amplify platelet activation. Activated platelets also express phospholipids on their surface that serve as cofactors for coagulation proteins, bind microparticles from the circulation that express Factor VIIa–Tissue Factor, release molecules such as plasminogen activation inhibitor (PAI)-1 that prevent the breakdown of fibrin and express factor XIIIa, a clotting factor that facilitates the stabilization of fibrin-containing thrombi.
In addition to this classic role, responding to damaged vasculature and endothelial stress, systemic infection and inflammation can also directly trigger platelet aggregation and adhesion within the microvasculature. This platelet response to infection can be mediated either by direct recognition of the pathogen39 or in response to inflammatory stimuli generated by some other immune sentinel cell.40 Patients with sepsis frequently present with circulating platelet–leukocyte aggregates and are often thrombocytopenic.4, 5, 6 These platelet–leukocyte aggregates have the capacity to act as circulating platforms for thombi generation, producing clots that may become lodged in the microvasculature, obstructing blood flow and leading to tissue damage. In fact, platelet–leukocyte aggregates and thrombocytopenia in septic patients are associated with worse outcomes and higher mortality.7, 8 These observations strongly suggest that platelets directly contribute to infection-associated vascular disease and the development of intravascular coagulopathy.
Furthermore, in addition to driving coagulation during systemic infection (sepsis), platelets also interact with, and modify the activity of, various leukocyte populations within the bloodstream. Platelets express a variety of immune receptors, such as TLRs, which allow for direct recognition of pathogen-associated molecular patterns (PAMPs)39 and a diverse array of adhesion molecules that allow for interaction between platelets and a variety of immune cells. Platelets have been reported to directly modulate leukocyte activation, increasing adhesion molecule expression on the leukocyte, triggering neutrophil degranulation41 and enhancing phagocytosis.42 Moreover, platelets are the largest source of soluble CD40L (sCD40L), a molecule that has been shown to induce reactive oxygen species (ROS) production, activate macrophages and mediate optimal cytotoxic T-cell and B-cell activation.43 In addition, the large number of adhesion molecules on the platelet allow for the binding of leukocytes to activated platelets, amplifying cellular recruitment signals and facilitating leukocyte recruitment to areas of the vasculature devoid of, or with low levels of, classic adhesion molecules, thereby significantly enhancing the inflammatory immune response.
NETs
Binding and aggregation of activated platelets on the surface of adherent neutrophils is a key trigger for the release of NETs. NETs are diffuse extracellular structures of sticky, decondensed chromatin that is decorated with both nuclear (histone, HMGB1) and granular proteins (neutrophil elastase (NE), defensins, cathepsin G and myleoperoxidase (MPO)).44, 45 NETs serve to ensnare and kill pathogens such as bacteria and viruses, limiting pathogen dissemination from a site of infection.46 This inherent ability of NETs to kill pathogens also makes these structures extremely cytotoxic to host cells and tissues, damaging and killing endothelial cells and potentially exposing the subendothelium leading to the activation of the coagulation cascade.41
In addition to indirectly activating clotting through endothelial damage, recent reports have also indicated that various components of NETs can directly activate platelets, initiate thrombosis36, 47, 48, 49, 50 and inhibit fibrinolysis.49, 51, 52, 53 Contact between histones and NE on the NET with platelets and clotting factors has been shown to result in fibrin deposition, thrombus generation, cleavage of TFPI and inhibition of plasmin generation. At least some of this pro-coagulate activity is attributed to the extracellular histones that cover the NET. Purified histones have been shown to directly induce platelet activation and aggregation. Moreover, neutrophil proteases, such as NE and cathepsin G, have been shown to activate platelets through PARs,54, 55, 56 creating further potential for NETs to drive platelet aggregation and coagulopathy. Importantly, not only do NETs drive the activation of the coagulation cascade, but evidence also suggests that NETs may limit fibrinolysis. Extracellular histone has been shown to inhibit thrombomodulin,57 and in itself limit the generation of APC.
NETs not only activate platelets and coagulation, but they also appear to have a substantial role in augmenting and driving inflammation. Components of NETs, such as HMGB1 and DNA, are seen as damage associated molecular patterns and function to activate the innate immune system, supporting and augmenting vascular inflammation. The recognition of HMGB1 by TLR4 and RAGE on platelets, neutrophils, monocytes and macrophages results in cellular activation, increased recruitment to sites of inflammation and the release of pro-inflammatory immune mediators such as interleukin (IL)-6, IL-8 and tumor necrosis factor.58 In addition, HMGB1-mediated activation of neutrophils and platelets serves to enhance the production of NETs, further amplifying this inflammatory process. In these ways, HMGB1 is thought to act as a mediator of acute inflammation and to directly induce tissue pathology. Studies have demonstrated that direct administration of HMGB1 in animal models can recapitulate many of the inflammatory phenotypes observed during sepsis and that blockade of HMGB1 (through the administration of blocking antibodies) significantly lessens inflammation and tissue pathology.59, 60, 61 This direct link between HMGB1 and pathology has brought this molecule into focus as a potential therapeutic target in the treatment of sepsis.58, 62
These findings indicate that not only does pathogen-mediated platelet activation drive NET release but also demonstrate that NETs act to further enhance platelet activation and inflammation. This potent, positive feedback loop has the potential to greatly amplify infection-induced coagulation, supporting the proposal of a link between platelet activation, intravascular NETs and coagulopathy.57, 63, 64 The central role platelets/NETs have in the initiation/amplification of infection-induced coagulation makes these component interesting targets for potential future therapies aimed at limiting DIC in sepsis.
Novel therapeutic approaches to sepsis-associated DIC
An ideal therapy for the treatment of sepsis-associated DIC must aim to limit improper and excessive coagulation while preserving hemostatic functions of the clotting cascade. As discussed above, platelets and NETs make potentially interesting targets for the next-generation of therapeutics. Given that neither NETs, nor the immune receptors on platelets have a central role in the maintenance of hemostasis, it may be possible to uncouple infection-mediated coagulation from basic clotting in response to tissue or vascular injury (Figure 3). Importantly, new therapies that are able to stop the uncontrolled amplification of coagulation would serve not only to limit thrombosis and the associated vascular occlusions but would also prevent the depletion of clotting factors and, therefore, would also prevent the development of the consumptive coagulopathy often associated with sepsis.
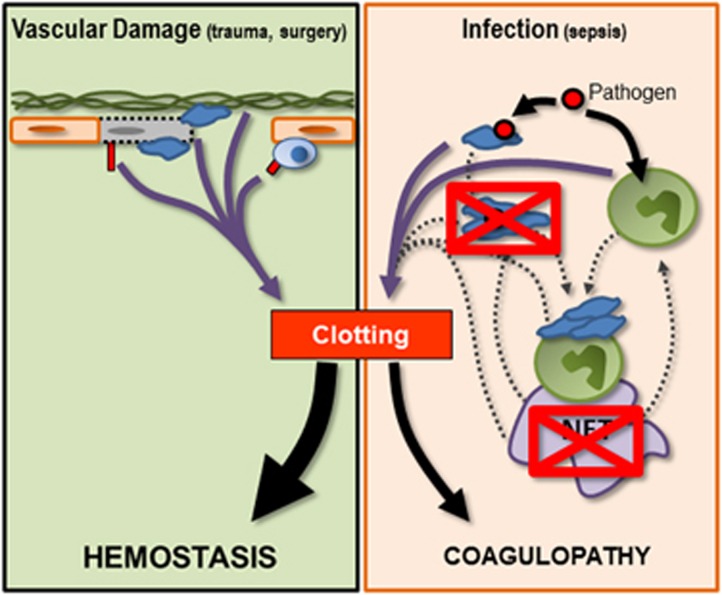
Figure 3.
Schematic illustrating the potential effects of antiplatelet or anti-NET treatments in infection-induced coagulopathy. Treatments (red crossed-out boxes) that act to limit pathogen-induced platelet aggregation or that block/degrade NETs may substantially limit the ability of infection-induced inflammation to drive coagulation. These therapies aim to limit the uncontrolled amplification of coagulation while protecting the ability of individual platelets to respond to pathogens or to vascular damage. By limiting platelet and NET-mediated amplification of coagulation during systemic inflammation, one might be able to restore balance to the coagulation system, preserving hemostasis while mitigating coagulopathy.
Targeting NETs
NETs are ultimately one of the key immune effector mechanisms that interact with and, potentially, drive the coagulation cascade and, as such, developing therapies that target these extracellular DNA structures may have some value in the treatment of infection-induced coagulopathy. From an evolutionary standpoint, NETs were developed to have an important role in the capture and killing of a wide variety of infectious organisms, limiting pathogen dissemination throughout the body. However, within the context of modern medicine, where septic patients rapidly receive broad-spectrum antibiotics upon admission to a health-care center, the role of NETs in combating the infection may no longer be essential. If infectious agents can be killed, or for the most part controlled, through the administration of antibiotics and antiviral therapies, NETs would no longer be needed to limit the spread of the pathogen. In these patients, the potential benefits of NETs are likely outweighed by the potential for tissue damage and inappropriate activation of the coagulation cascade. Indeed, studies looking at infections with organisms of relatively low pathogenesis (resulting in mild disease states) have demonstrated that NETs are dispensable and that either inhibiting the release of NETs, or breaking down of NETs in the vasculature after they were generated significantly reduced the observed tissue damage.65
Currently, a number of small molecules inhibitors for NETs are in development. These drugs aim to prevent the release on NETs from activated neutrophils, completely eliminating any potential NET-associated pathology. One group of molecules target Protein Arginine Deiminase 4 (PAD4), a critical protein required for the release of NETs. Studies in mice genetically deficient for PAD4 have demonstrated a near complete absence of NETs at sites of infection, this, despite normal neutrophil recruitment and activation.53 Although PAD4 inhibition is arguably the most effective approach at eliminating NETs altogether, this strategy does nothing to address NETs that had already been released and are present within tissues and the vasculature. As it is unlikely that a potential patient can be treated before developing sepsis, this therapeutic approach will only prevent the generation of additional NETs and will do nothing to mitigate the pathology of pre-existing NETs.
To address pre-existing NETs, one has to look to strategies that either block the active components associated with the NET or breakdown the NET itself. As discussed above, much of a NET's cytotoxicity and reported interactions with the coagulation system is mediated by the various NET-associated proteins such as histones and NE. The use of blocking antibodies against either whole nucleosomes or specific histones (that is, H4) has been shown to be effective in mitigating coagulopathy and pathology in animal models of sepsis.57, 63, 66 These approaches are particularly appealing as they leave the NET structure intact (allowing the NET to continue to catch pathogens) and can be used in patients once NETs have been released and are present in the vasculature. In addition, through antibody engineering, it may be possible to block these NET-associated proteins without fear of activating complement or engaging Fc receptors, thereby preventing the unintended exacerbation of inflammation in these patients.
In other studies, intravenous DNase has been used successfully to breakdown the DNA backbone of NETs. The use of this approach has been shown to mitigate tissue damage in some animal models of sepsis.65 Interestingly, subsequent studies have demonstrated that breakdown of the DNA backbone by DNase does not completely clear the NET from the vasculature but rather leaves significant quantities of histone and NE attached to the walls of the blood vessels.53 It should be noted that these studies examined two different models of systemic infection, and whereas DNase treatment was sufficient to prevent pathology in one model of infection (Escherichia coli), it was somewhat less effective in treating a different infection (Staphylococcus aureus). This variability in efficacy may reflect differences in the pathogens themselves or may reflect differences in the magnitude of the immune response and NET production in each model. Regardless of the reason for the differences in efficacy, DNase had a protective effect in both models and essentially remains an interesting option for the treatment of NET-induced coagulopathy.
Uncoupling coagulation and immunity
Potential therapies for the treatment of infection-induced DIC must be able to separate the immune functions of platelets from their role in coagulation. One potential advantage of targeting platelets is that numerous antiplatelet drugs have already been developed and are frequently used in the treatment of a wide variety of cardiovascular diseases. These drugs include cyclooxygenase inhibitors (acetylsalicylic acid), ADP receptor inhibitors (clopidogrel), thromboxane antagonists (terutroban), PAR antagonists (vorapaxar) and adhesion molecule inhibitors (tirofiban and abciximab). This pre-existing collection of antiplatelet drugs represents a potential pool of therapeutics for which efficacy and basic safety studies have already been completed, making their use in future clinical trials for sepsis and DIC easier. In addition, as there are large numbers of patients already receiving these treatments, retrospective chart studies may be able to shed some light on the potential effects of these drugs on coagulopathy in sepsis. These therapeutics represent a diverse spectrum of tools with the capacity to modify platelet function within the context of sepsis. Whereas some act to limit platelet activation and aggregation, others function to block paracrine and autocrine activation of platelets by soluble mediators synthesized and released from activated platelets. It is this latter category of drugs that are of particular interest.
Strategies to block paracrine signals such as prostaglandins, thromboxane A2 and ADP can serve to prevent uncontrolled amplification of platelet activation, reduce platelet aggregation and limit coagulation. This approach does not completely prevent the activation of individual platelets by the infection, but instead limits the magnitude of the platelet response. This functional ‘uncoupling' of platelet immunity from coagulation is of critical importance as it has been demonstrated that during some infections, complete inhibition of the platelet response cripples the immune system and the animal rapidly succumbs to the pathogen.67 During specific infections, such as those mediated by methicillin-resistant S. aureus, platelets directly recognize and bind the bacteria, shielding the host from the pathogen. Treatments that prevent this pathogen recognition and binding by platelets lead to significant endothelial cell death, vascular leakage, organ damage and death, highlighting the critical role platelets have in the host immune response.
A second advantage for targeting the platelet in sepsis is the fact that platelets not only interact with the coagulation cascade directly, but are also the principal inducers of NETs in the vasculature.65 Platelets binding to and aggregating on the surface of adherent neutrophils in the vasculature have been shown to trigger these neutrophils to release NETs. By itself, treatment strategies that limit platelet–neutrophil interactions have the potential to both mitigate coagulation and also to limit (or even eliminate) NET release. Prevention of NET release would serve to further limit coagulation and would prevent much of the NET-associated cytotoxicity and vascular damage.
Summary
Current anticoagulation therapies have shown limited success in the treatment of sepsis. Although some subsets of patients did experience a modest improvement in outcomes, in general, this therapeutic strategy has failed to dramatically improve the treatment of sepsis and infection-associated DIC. Although not a cure, it is this limited improvement in the outcomes of some patients that suggests that the strategy of targeting coagulation has merit.
The success of anticoagulation strategies might be improved if we are better able to target the ‘bad' and preserve the ‘good' coagulation, that is, if we are able to functionally uncouple infection-induced coagulopathy from hemostasis. Preservation of normal hemostasis would serve to limit bleeding events and would prevent bleeding complications seen in trauma and surgery. In addition, a more comprehensive understanding of how infection (and inflammation) drives coagulation will allow for the development of better and more precise treatment strategies. Much of the current evidence points at targeting platelets and NETs in an effort to block/limit infection-induced coagulation. Both platelets and NETs represent potent positive feedback loops that can drive and rapidly amplify coagulation. It is this uncontrolled amplification of clotting that poses the greatest risk to the patient, generating circulating thrombi and consuming clotting factors that may be needed for hemostasis. Antiplatelet and anti-NET therapies may act to limit this amplification of infection-induced coagulation, and, thus, can serve to achieve the overall goal of uncoupling inflammation from coagulation.
The authors declare no conflict of interest.
References
- Fleischmann C, Scherag A, Adhikari NK, Hartog CS, Tsaganos T, Schlattmann P et al. Assessment of global incidence and mortality of hospital-treated sepsis. Current estimates and limitations. Am J Respir Crit Care Med 2016; 193: 259–272. [DOI] [PubMed] [Google Scholar]
- Jawad I, Luksic I, Rafnsson SB. Assessing available information on the burden of sepsis: global estimates of incidence, prevalence and mortality. J Global Health 2012; 2: 010404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med 2008; 36: 296–327. [DOI] [PubMed] [Google Scholar]
- Gawaz M, Fateh-Moghadam S, Pilz G, Gurland HJ, Werdan K. Platelet activation and interaction with leucocytes in patients with sepsis or multiple organ failure. Eur J Clin Invest 1995; 25: 843–851. [DOI] [PubMed] [Google Scholar]
- Gawaz M, Dickfeld T, Bogner C, Fateh-Moghadam S, Neumann FJ. Platelet function in septic multiple organ dysfunction syndrome. Intensive Care Med 1997; 23: 379–385. [DOI] [PubMed] [Google Scholar]
- Kalsch T, Elmas E, Nguyen XD, Suvajac N, Klüter H, Borggrefe M et al. Endotoxin-induced effects on platelets and monocytes in an in vivo model of inflammation. Basic Res Cardiol 2007; 102: 460–466. [DOI] [PubMed] [Google Scholar]
- Levi M, Schultz M, van der PT. Sepsis and thrombosis. Semin Thromb Hemost 2013; 39: 559–566. [DOI] [PubMed] [Google Scholar]
- Spapen H. Liver perfusion in sepsis, septic shock, and multiorgan failure. Anat Rec (Hoboken) 2008; 291: 714–720. [DOI] [PubMed] [Google Scholar]
- Levi M, Ten Cate H. Disseminated intravascular coagulation. N Engl J Med 1999; 341: 586–592. [DOI] [PubMed] [Google Scholar]
- Semeraro N, Ammollo CT, Semeraro F, Colucci M. Sepsis-associated disseminated intravascular coagulation and thromboembolic disease. Mediterr J Hematol Infect Dis 2010; 2: e2010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isobe M, Uejima E, Seki M, Yamagishi Y, Miyawaki K, Yabuno K et al. Methicillin-resistant Staphylococcus aureus bacteremia at a university hospital in Japan. J Infect Chemother 18: 841–847. [DOI] [PubMed] [Google Scholar]
- Vergouwen MD, Schut ES, Troost D, van de BD. Diffuse cerebral intravascular coagulation and cerebral infarction in Pneumococcal meningitis. Neurocrit Care 2010; 13: 217–227. [DOI] [PubMed] [Google Scholar]
- Wada H, Matsumoto T, Yamashita Y. Diagnosis and treatment of disseminated intravascular coagulation (DIC) according to four DIC guidelines. J Intensive Care 2014; 2: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venugopal A. Disseminated intravascular coagulation. Indian J Anaesth 2014; 58: 603–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen KS, Sawheny E, Kinasewitz GT. Anticoagulant modulation of inflammation in severe sepsis. World J Crit Care Med 2015; 4: 105–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikura H, Nishida T, Murai A, Nakamura Y, Irie Y, Tanaka J et al. New diagnostic strategy for sepsis-induced disseminated intravascular coagulation: a prospective single-center observational study. Crit Care 2014; 18: R19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Bermejo FJ, Ruiz-Bailen M, Gil-Cebrian J, Huertos-Ranchal MJ. Sepsis-induced cardiomyopathy. Curr Cardiol Rev 2011; 7: 163–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weithauser A, Bobbert P, Antoniak S, Böhm A, Rauch BH, Klingel K et al. Protease-activated receptor-2 regulates the innate immune response to viral infection in a coxsackievirus B3-induced myocarditis. J Am Coll Cardiol 2013; 62: 1737–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoniak S, Owens AP 3rd, Baunacke M, Williams JC, Lee RD, Weithäuser A et al. PAR-1 contributes to the innate immune response during viral infection. J Clin Invest 2013; 123: 1310–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons J, Pittet JF. The coagulopathy of acute sepsis. Curr Opin Anaesthesiol 2015; 28: 227–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren BL, Eid A, Singer P, Pillay SS, Carl P, Novak I et al. Caring for the critically ill patient. High-dose antithrombin III in severe sepsis: a randomized controlled trial. JAMA 2001; 286: 1869–1878. [DOI] [PubMed] [Google Scholar]
- Wiedermann CJ, Kaneider NC. A systematic review of antithrombin concentrate use in patients with disseminated intravascular coagulation of severe sepsis. Blood Coagul Fibrinolysis 2006; 17: 521–526. [DOI] [PubMed] [Google Scholar]
- Gando S, Saitoh D, Ishikura H, Ueyama M, Otomo Y, Oda S et al. A randomized, controlled, multicenter trial of the effects of antithrombin on disseminated intravascular coagulation in patients with sepsis. Crit Care 2013; 17: R297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham E, Reinhart K, Opal S, Demeyer I, Doig C, Rodriguez AL et al. Efficacy and safety of tifacogin (recombinant tissue factor pathway inhibitor) in severe sepsis: a randomized controlled trial. JAMA 2003; 290: 238–247. [DOI] [PubMed] [Google Scholar]
- Saito H, Maruyama I, Shimazaki S, Yamamoto Y, Aikawa N, Ohno R et al. Efficacy and safety of recombinant human soluble thrombomodulin (ART-123) in disseminated intravascular coagulation: results of a phase III, randomized, double-blind clinical trial. J Thromb Haemost 2007; 5: 31–41. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Ramesh MK, Ernest D, LaRosa SP, Pachl J, Aikawa N et al. A randomized, double-blind, placebo-controlled, Phase 2b study to evaluate the safety and efficacy of recombinant human soluble thrombomodulin, ART-123, in patients with sepsis and suspected disseminated intravascular coagulation. Crit Care Med 2013; 41: 2069–2079. [DOI] [PubMed] [Google Scholar]
- Griffin JH, Zlokovic BV, Mosnier LO. Activated protein C: biased for translation. Blood 2015; 125: 2898–2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF, Lopez-Rodriguez A et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med 2001; 344: 699–709. [DOI] [PubMed] [Google Scholar]
- Jaimes F, De La Rosa G, Morales C, Fortich F, Arango C, Aguirre D et al. Unfractioned heparin for treatment of sepsis: a randomized clinical trial (The HETRASE Study). Crit Care Med 2009; 37: 1185–1196. [DOI] [PubMed] [Google Scholar]
- Liu XL, Wang XZ, Liu XX, Hao D, Jaladat Y, Lu F et al. Low-dose heparin as treatment for early disseminated intravascular coagulation during sepsis: a prospective clinical study. Exp Ther Med 2014; 7: 604–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaghi S, Eisenberger A, Willey JZ. Symptomatic intracerebral hemorrhage in acute ischemic stroke after thrombolysis with intravenous recombinant tissue plasminogen activator: a review of natural history and treatment. JAMA Neurol 2014; 71: 1181–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akol H, Boon E, van HF, van der HJ. Successful treatment of fulminant pneumococcal sepsis with recombinant tissue plasminogen activator. Eur J Intern Med 2002; 13: 389. [DOI] [PubMed] [Google Scholar]
- Zenz W, Muntean W, Gallistl S, Zobel G, Grubbauer HM. Recombinant tissue plasminogen activator treatment in two infants with fulminant meningococcemia. Pediatrics 1995; 96: 144–148. [PubMed] [Google Scholar]
- Maegele M, Schochl H, Cohen MJ. An update on the coagulopathy of trauma. Shock 2014; 41 (Suppl 1): 21–25. [DOI] [PubMed] [Google Scholar]
- Jiravanichpaisal P, Lee BL, Soderhall K. Cell-mediated immunity in arthropods: hematopoiesis, coagulation, melanization and opsonization. Immunobiology 2006; 211: 213–236. [DOI] [PubMed] [Google Scholar]
- Engelmann B, Massberg S. Thrombosis as an intravascular effector of innate immunity. Nat Rev Immunol 2013; 13: 34–45. [DOI] [PubMed] [Google Scholar]
- Esmon CT. The interactions between inflammation and coagulation. Br J Haematol 2005; 131: 417–430. [DOI] [PubMed] [Google Scholar]
- Levi M, van der PT, Schultz M. New insights into pathways that determine the link between infection and thrombosis. Neth J Med 2012; 70: 114–120. [PubMed] [Google Scholar]
- Ma AC, Kubes P. Platelets, neutrophils, and neutrophil extracellular traps (NETs) in sepsis. J Thromb Haemost 2008; 6: 415–420. [DOI] [PubMed] [Google Scholar]
- Jenne CN, Wong CH, Zemp FJ, McDonald B, Rahman MM, Forsyth PA et al. Neutrophils recruited to sites of infection protect from virus challenge by releasing neutrophil extracellular traps. Cell Host Microbe 2013; 13: 169–180. [DOI] [PubMed] [Google Scholar]
- Clark SR, Ma AC, Tavener SA, McDonald B, Goodarzi Z, Kelly MM et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med 2007; 13: 463–469. [DOI] [PubMed] [Google Scholar]
- Assinger A, Laky M, Schabbauer G, Hirschl AM, Buchberger E, Binder BR et al. Efficient phagocytosis of periodontopathogens by neutrophils requires plasma factors, platelets and TLR2. J Thromb Haemost 2011; 9: 799–809. [DOI] [PubMed] [Google Scholar]
- Elzey BD, Ratliff TL, Sowa JM, Crist SA. Platelet CD40L at the interface of adaptive immunity. Thromb Res 2011; 127: 180–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS et al. Neutrophil extracellular traps kill bacteria. Science 2004; 303: 1532–1535. [DOI] [PubMed] [Google Scholar]
- Borregaard N. Neutrophils, from marrow to microbes. Immunity 2010; 33: 657–670. [DOI] [PubMed] [Google Scholar]
- Yipp BG, Petri B, Salina D, Jenne CN, Scott BN, Zbytnuik LD et al. Infection-induced NETosis is a dynamic process involving neutrophil multitasking in vivo. Nat Med 2012; 18: 1386–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs TA, Brill A, Duerschmied D, Schatzberg D, Monestier M, Myers DD Jr et al. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci USA 2010; 107: 15880–15885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brill A, Fuchs TA, Savchenko AS, Thomas GM, Martinod K, De Meyer SF et al. Neutrophil extracellular traps promote deep vein thrombosis in mice. J Thromb Haemost 2012; 10: 136–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massberg S, Grahl L, von Bruehl ML, Manukyan D, Pfeiler S, Goosmann C et al. Reciprocal coupling of coagulation and innate immunity via neutrophil serine proteases. Nat Med 2010; 16: 887–896. [DOI] [PubMed] [Google Scholar]
- von Bruhl ML, Stark K, Steinhart A, Chandraratne S, Konrad I, Lorenz M et al. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J Exp Med 2012; 209: 819–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiaans SC, Wagener BM, Esmon CT, Pittet JF. Protein C and acute inflammation: a clinical and biological perspective. Am J Physiol Lung Cell Mol Physiol 2013; 305: L455–L466. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan V, DeGuzman F, Bao M, Hall SW, Leung LL, Phillips DR. A thrombin receptor function for platelet glycoprotein Ib-IX unmasked by cleavage of glycoprotein V. Proc Natl Acad Sci USA 2001; 98: 1823–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolaczkowska E, Jenne CN, Surewaard BG, Thanabalasuriar A, Lee WY, Sanz MJ et al. Molecular mechanisms of NET formation and degradation revealed by intravital imaging in the liver vasculature. Nat Commun 2015; 6: 6673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg JS, Wells PS, Kearon C, Anderson D, Crowther M, Weitz JI et al. Sensitivity and specificity of a rapid whole-blood assay for D-dimer in the diagnosis of pulmonary embolism. Ann Intern Med 1998; 129: 1006–1011. [DOI] [PubMed] [Google Scholar]
- Lappann M, Danhof S, Guenther F, Olivares-Florez S, Mordhorst IL, Vogel U. In vitro resistance mechanisms of Neisseria meningitidis against neutrophil extracellular traps. Mol Microbiol 2013; 89: 433–449. [DOI] [PubMed] [Google Scholar]
- Sambrano GR, Huang W, Faruqi T, Mahrus S, Craik C, Coughlin SR et al. Cathepsin G activates protease-activated receptor-4 in human platelets. J Biol Chem 2000; 275: 6819–6823. [DOI] [PubMed] [Google Scholar]
- Ammollo CT, Semeraro F, Xu J, Esmon NL, Esmon CT. Extracellular histones increase plasma thrombin generation by impairing thrombomodulin-dependent protein C activation. J Thromb Haemost 2011; 9: 1795–1803. [DOI] [PubMed] [Google Scholar]
- Wang H, Ward MF, Sama AE. Targeting HMGB1 in the treatment of sepsis. Expert Opin Ther Targets 2014; 18: 257–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JY, Park JS, Strassheim D, Douglas I, Diaz del Valle F, Asehnoune K et al. HMGB1 contributes to the development of acute lung injury after hemorrhage. Am J Physiol Lung Cell Mol Physiol 2005; 288: L958–L965. [DOI] [PubMed] [Google Scholar]
- Abraham E, Arcaroli J, Carmody A, Wang H, Tracey KJ. HMG-1 as a mediator of acute lung inflammation. J Immunol 2000; 165: 2950–2954. [DOI] [PubMed] [Google Scholar]
- Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science 1999; 285: 248–251. [DOI] [PubMed] [Google Scholar]
- Yang H, Wang H, Czura CJ, Tracey KJ. HMGB1 as a cytokine and therapeutic target. J Endotoxin Res 2002; 8: 469–472. [DOI] [PubMed] [Google Scholar]
- Semeraro F, Ammollo CT, Morrissey JH, Dale GL, Friese P, Esmon NL et al. Extracellular histones promote thrombin generation through platelet-dependent mechanisms: involvement of platelet TLR2 and TLR4. Blood 2011; 118: 1952–1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs TA, Bhandari AA, Wagner DD. Histones induce rapid and profound thrombocytopenia in mice. Blood 2011; 118: 3708–3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald B, Urrutia R, Yipp BG, Jenne CN, Kubes P. Intravascular neutrophil extracellular traps capture bacteria from the bloodstream during sepsis. Cell Host Microbe 2012; 12: 324–333. [DOI] [PubMed] [Google Scholar]
- Xu J, Zhang X, Pelayo R, Monestier M, Ammollo CT, Semeraro F et al. Extracellular histones are major mediators of death in sepsis. Nat Med 2009; 15: 1318–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong CH, Jenne CN, Petri B, Chrobok NL, Kubes P. Nucleation of platelets with blood-borne pathogens on Kupffer cells precedes other innate immunity and contributes to bacterial clearance. Nat Immunol 2013; 14: 785–792. [DOI] [PMC free article] [PubMed] [Google Scholar]