Abstract
Incremental advances in our knowledge of how natural immune control of herpes simplex virus (HSV) develops have yielded insight as to why previous vaccine attempts have only been partially successful, however, our understanding of these pathways, particularly in humans, is still incomplete. Further elucidation of the innate immune events that are responsible for stimulating these effector responses is required to accurately inform vaccine design. An enhanced understanding of the mechanism of action of novel adjuvants will also facilitate the rational choice of adjuvant to optimise such responses. Here we review the reasons for the hitherto partial HSV vaccine success and align these with our current knowledge of how natural HSV immunity develops. In particular, we focus on the innate immune response and the role of dendritic cells in inducing protective T-cell responses and how these pathways might be recapitulated in a vaccine setting.
The case so far for developing a herpes simplex virus vaccine
Why do we need a HSV vaccine?
Development of a prophylactic vaccine for herpes simplex virus types 1 and 2 (HSV1 and 2) is a WHO-supported global public health priority because (1) genital herpes caused by HSV1/2 is now the commonest sexually transmitted infection and causes severe disease in neonates; (2) HSV1 is the leading cause of infectious blindness in western countries; (3) prior HSV2 infection leads to a two- to threefold increased risk of HIV infection globally.1 The synergy between HIV and HSV is not completely understood but >40% of HIV transmissions in sub-saharan Africa are estimated to occur in a setting of HSV2 infection.2 Daily suppressive antiviral therapy for HSV does not completely suppress viral shedding and had no impact on HIV acquisition,3 probably because of inadequate antiviral pharmacokinetics4 but a prophylactic HSV vaccine would most likely have a positive impact on the HIV epidemic.
HSV is a neurotropic virus that invades the skin and mucosal lining of the anogenital and oral tracts. HSV can penetrate into the upper layer, the epidermis, especially where the outermost, cornified layer is thin (labia, inner foreskin, facial lips), absent (rectum, endocervix, vagina) or traumatically destroyed. HSV productively infects the epidermal keratinocytes and Langerhans cells (LCs; a type of dendritic cell (DC)). It then enters cutaneous nerve endings and is transported along axons to a collection of nerve cells close to the spine, the dorsal root ganglion, to establish lifetime latent infection. After periodic reactivation, the virus is transported back along neurons to the mucosa where it causes recurrent lesions, or is shed asymptomatically.5 HSV1 causes oral herpes and initial (and occasional recurrent) genital herpes, whereas HSV2 causes initial and recurrent genital herpes.
What is the history of HSV vaccine clinical trials?
Progress in the development of vaccines for herpesviruses has been inconsistent. The live attenuated varicella virus Oka strain is the only human success although live attenuated vaccines for pseudorabies virus in pigs and Marek's disease in turkeys have also been successful. For 50 years, many attempts at HSV vaccine development have been unsuccessful. Live attenuated candidates were initially avoided because of carcinogenic fears and have now been replaced by specifically mutated attenuated viral candidates that are currently in clinical trials (such as HSV529).6 Other candidates include hybrid recombinant viruses, DNA vaccines and recombinant viral proteins.
Unlike live attenuated vaccines, recombinant protein vaccines require combination with an adjuvant to stimulate the immune system. Adjuvants enhance and direct the nature of the immune response, for example, towards T-cell or antibody responses or both. This is usually orchestrated through antigen-presenting cells, particularly DCs. The first partially effective HSV2 genital herpes vaccine candidate, Simplirix, consisted of a recombinant soluble viral surface protein, glycoprotein D2, and the adjuvant system AS04. Glycoprotein D2 is widely recognised by human populations, inducing both neutralising antibody and CD4 T cells7 and AS04 consists of alum and deacyl monophosphoryl lipid A (dMPL), extracted from the cell wall of Salmonella minnesota. Simplirix showed 74% efficacy but only in HSV1/2 seronegative women with long-term HSV2-infected partners.8 However, the subsequent Herpevac trial of Simplirix in randomly selected HSV1 and 2 seronegative women surprisingly showed efficacy against genital herpes caused by HSV1 (58%) but not HSV2 (only 20% efficacy).9 Thus cross-protection against HSV1 can be induced by this HSV2 gD vaccine. The better efficacy of the first trial may be attributed to subclinical exposure to the partner's genitally shed HSV2, priming a successful vaccine response. The efficacy of the novel adjuvant dMPL, a TLR4 agonist, was attributed to induction of CD4 Th1 patterns of immune response and also to neutralising antibody, but CD8 T-cell responses were not induced.10
Varicella zoster virus, which causes chicken pox and herpes zoster (shingles) is also an alphaherpesvirus, like HSV. The pathogenesis of the two viruses is similar. Recently a similarly formulated vaccine candidate for herpes zoster (Shingrix) was highly effective showing 97% efficacy, even in subjects >60 years of age.11 The vaccine consists of a single varicella glycoprotein and a similar adjuvant system, AS01B, which contains dMPL and QS21 formulated with liposomes. The saponin QS21 is derived from the bark of the soap bark tree (Quillaja saponaria). Enhanced vaccine-specific CD4 T-cell and humoral responses are elicited by this adjuvant, although again primary CD8 T cells are not stimulated.12
These trials demonstrate that substantial protection against HSV disease and herpes zoster can be induced by recombinant viral proteins combined with an adjuvant that induces the appropriate adaptive (T and B cell) immune response, by targeting innate immune antigen-presenting cells. So far with HSV this is partial.
Why only partial success so far?
The success of the Shingrix vaccine contrasts sharply with the partial success of the similarly formulated Simplirix vaccine. There are a number of potential reasons for the difference in efficacy, all of which highlight the need for understanding the immune pathways in natural varicella zoster virus and HSV infection to inform vaccine design. These include (1) differences in the immune response required to control the disease (although with these related viruses, the requirements are thought to be similar); (2) immunotherapy versus prophylaxis—herpes zoster is a reactivation disease, whereas the end point for the genital HSV trials was primary infection/disease, (3) differences in the action of the adjuvant. We will discuss these points with particular reference to HSV.
Both antibody and CD4 T-cell function were enhanced by the Simplirix vaccine but antibody correlated best with individual efficacy in the Herpevac trial.10 However, the Chiron vaccine candidate (gD/gB, MF59 adjuvant), which induced very high levels of neutralising antibody, was not efficacious.13 The induction of CD8 T cells may be required for success with both prophylactic and therapeutic HSV vaccines, and this has been supported by trials of candidate vaccines from Agenus and Genocea, discussed below.
The distinction between an immunotherapeutic vaccine and prophylactic vaccine is critical. Prophylactic vaccines (such as Simplirix) aim at preventing acquisition of a pathogen and thus must stimulate broad and durable immunity at all portals of pathogen entry, in the case of HSV, all mucosal surfaces. In order to generate a primary immune response, naive T cells require an antigen-specific signal and a second costimulatory signal (for example, CD80/86 ligation of CD27) to become activated into effector cells. DCs, which are relatively rare, are referred to as ‘professional' antigen-presenting cells as they (1) migrate to the lymph nodes where naive T and B cells reside and (2) are superior at providing the second activation signal. Thus, a successful prophylactic vaccine should stimulate the appropriate DCs. On the other hand, as herpes zoster is a reactivation disease, the Shingrix vaccine constitutes a therapeutic vaccine. Therapeutic vaccines aim to reduce recurrences or minimise disease severity and duration. Stimulating memory B and T cells is an easier task than stimulating primary lymphocytes as they are much more abundant, are more sensitive to and respond more vigorously to restimulation, and they can readily enter tissues during inflammation or indeed reside there. Critically, they do not require costimulation for activation so can be activated by a multitude of antigen-presenting cells including abundant keratinocytes, monocytes and inflammatory DCs (DCs arising from monocytes under inflammatory conditions). Memory lymphocytes are also more likely to be activated in the peripheral tissue where the disease occurs. Activation of memory T cells could account for much of the success of Shingrix over Simplirix.
A thorough understanding of the innate immune response that underpins a desired acquired response could go a long way to improving vaccine design. It is important to know (1) what type of immune response is desired and which pathogen epitopes/proteins are important, (2) which DCs to target to elicit the desired response, (3) how to target/activate those DCs and (4) how the selected adjuvant works.
Immune control of HSV
Innate immunity
An important component of the innate immune response to HSV is the production of type I interferons (IFN), which has been linked to protection against disease in both mouse models and humans.14 HSV can stimulate innate immune cells via toll-like receptor (TLR)2 and TLR9 directly via viral glycoprotein and viral DNA, respectively.15 This signalling results in the production of proinflammatory cytokines, including type I IFN (namely IFNα and β), which in turn stimulate the expression of multiple interferon-stimulated genes in surrounding cells. The collective action of these interferon-stimulated genes works to limit initial infection via functions including inhibition of viral protein expression, apopotosis and recruitment of immune cells. HSV can also stimulate type I IFN production from keratinocytes through various pattern recognition receptors.
Plasmacytoid DCs (PDCs) and natural killer cells (NK cells) are critical players in the innate immune response to HSV and their absence has been linked to enhanced susceptibility or exacerbated HSV disease.16, 17, 18 PDCs produce vast amounts of IFNα. They infiltrate recurrent HSV lesions at both early and late time points and reside at the dermo-epidermal junction, closely associated with activated T cells and NK cells.17, 19 Although their main role is IFNα production, they are capable of stimulating autologous T cells, namely CD8 T cells, in the absence of infection.19 This implies that they contribute to the development of adaptive immunity via cross-presentation, and indeed a subset of PDCs were recently found to upregulate CD8α, a marker associated with cross-presenting DCs in mice.20 Although their role in antigen presentation in vivo remains to be resolved, it is clear that PDCs do secrete high levels of antiviral IFNα and other cytokines to recruit NK cells, T and B cells to the infected site.
NK cells have two main roles in HSV innate immunity—to kill infected cells and produce IFNγ, which helps polarise a Th1 adaptive response.21 They are activated by DCs via cytokines and direct contact. In particular, activation by IFNα from PDCs or other TLR3-expressing cells22 is critical for their cytotoxic activity. Furthermore, NK cells can augment DC–T-cell responses and present antigen to T cells directly in vitro.23, 24 Eliciting NK cell activity may be an underappreciated facet of HSV vaccine design, yet enhanced NK cell cytotoxicity induced by vaccination has been linked to protection against herpes keratitis in mice.25
There is conflicting evidence on the absolute importance of NK cells and PDCs in HSV immunity, which may be in part accounted for by different animal models, different routes of infection and different sites of pathology (corneal versus mucosal versus systemic). However, the cumulative data indicate their essential role for early innate control of the virus, but that they are insufficient for full protection, which likely requires an adaptive immune response.
The role of T cells in control of HSV infection
There is increasing evidence that the important adaptive immune modalities in controlling HSV infection are neutralising antibody as well as both CD4 and CD8 T cells. A HSV vaccine will likely have to induce a stronger humoral and cellular immune response than is elicited by natural infection in order to prevent the establishment of latency, or in an immunotherapeutic setting, to prevent shedding/disease outbreaks. Neutralising antibodies were identified as a correlate of protection in the Simplirix trial.10 With the exception of herpes zoster vaccines, T-cell responses have not been identified as critical correlates of protection in humans, probably because candidate vaccines have either not sufficiently stimulated T-cell responses or the appropriate responses have not been measured. However, there is ample evidence of their importance in HSV immunity. The severity of HSV2 disease and/or shedding inversely correlates with the number of HSV-specific CD8 T cells in both immunocompetent and immunocompromised patients. This has been measured in both blood and HSV lesions.26, 27 In recurrent HSV genital lesions, CD4 T cells, monocytes and PDCs infiltrate first, followed days later by CD8 T cells,28 which coincides with viral clearance in the lesion.29 Both cytolytic activity and IFNγ production by T cells are important for clearance.28, 30 In humans, but not mice, HSV attempts to evade the immune system by downregulating MHC-I expression in infected keratinocytes. This is however reversed by IFNγ produced mainly by CD4 T cells, thus allowing CD8 T cells to recognise and kill infected keratinocytes.31 IFNγ also stimulates MHC-II expression on keratinocytes, allowing recognition by CD4 T cells.31 Thus, Th1 patterns of response are important for immune and vaccine control of HSV.
In primary HSV infections, which have almost exclusively been studied in mice, CD4 T cells are critically important in genital epithelial immunity, whereas CD8 T cells mostly have a role in clearing infection from neurons. In humans, CD4 T-cell help is critically important for optimal priming of HSV-specific CD8 T cells in both lymph nodes and tissues.32 Precisely how these T-cell responses are regulated in the lymph nodes and especially at the site of infection remains poorly understood although much ground has been made in mouse models.
After infection, HSV-specific memory CD8 T cells accumulate in the skin near sensory nerve endings in mice and humans. These cells rapidly control shedding of HSV from these nerve endings and infection of epithelial cells,33, 34 preventing the formation of new lesions.35, 36 Establishment of tissue resident memory CD8 T cells, especially of the αα phenotype,36 by vaccination could be effective at containing a primary HSV infection and preventing seeding of nerves that leads to latent infection in the dorsal root ganglion.37 However novel strategies, such as the use of topical chemokines may be needed to protect the full extent of the anogenital tract susceptible to HSV infection, as shown in mice by ‘prime and pull' strategies.35
The role of DCs in stimulating HSV immunity
DCs are essential for priming antigen-specific, naive CD4 and CD8 T cells. Classically, DCs take up a pathogen, become activated by pathogen-associated molecules such as cell wall components, lipoproteins or nucleic acids, and migrate to the draining lymph node where they present their antigens to and activate CD4 and CD8 T cells. Multiple phenotypically and functionally distinct DC subsets reside in the blood and peripheral tissues in mice and humans. In human skin, LCs are the major DC subtype populating the epidermis, whereas in the dermis, three major subtypes reside: CD141+/XCR1+ DCs, CD1a+/CD1c+ DCs and CD14+ DCs, the latter being distinct from the prevalent CD14+ macrophage population.38 Each subset has a tendency to polarise different T-cell responses39 with CD141+/XCR1+ DCs notably superior at stimulating CD8 T cells via antigen cross-presentation.40
In the case of HSV, the pathway to antigen presentation is complex involving multiple types of DCs. The viruse first infects LCs in the epidermis of mice and humans41, 42 but they are not the predominant DCs carrying HSV antigen out of skin nor presenting antigen to T cells in the draining lymph nodes. Instead, infected murine and human LCs undergo apoptosis and are taken up by bystander dermal DCs.41, 43, 44 In murine skin, these migratory dermal DCs carry HSV antigen out of skin and are essential for T-cell priming in the lymph nodes, together with XCR1+ lymph node-resident DCs. The migratory dermal DCs prime CD4 T cells45, 46 but CD8 T cells, at least in mice, are primed by cross-presentation from both migratory and lymph node-resident XCR1+ DCs (human CD141+ equivalents) that acquire antigen from the migratory DCs.47, 48 The contribution of migratory versus lymph node-resident DCs may also depend on the route of infection however, as Lee et al.49 showed that whereas migratory DCs were inefficient at priming T cells after epidermal infection with HSV, and perhaps acted as antigen ferries to the lymph node, they were in fact the most efficient DCs to prime CD4 and CD8 T cells after vaginal mucosal infection.
The role of various DCs may change again when T-cell priming at the peripheral site of infection, not lymph nodes, is considered. Macleod et al.50 observed that effector CD4 and CD8 T cells in mice were activated by different sets of antigen-presenting cells in the skin. Multiple epidermal and dermal DCs presented antigens to CD4 T cells, whereas CD8 T cells only responded to directly infected epidermal antigen-presenting cells including LCs and keratinocytes. It should be noted though that while HSV-specific CD8 T cells infiltrate into the epidermis and remain as tissue resident memory T cells in mice, in humans they do not, instead persisting in the dermis at the dermo-epidermal junction.27 Thus the antigen-presenting cells responsible for stimulating CD8 T cells in human skin may differ again.
The relative contributions of different DC subsets to stimulating T-cell subsets such as CD4 Th1, 2, 9 and 17, Tfh, Tregs and CD8 T cells in skin and lymph node is still being elucidated and the whole sequence of events remains unconfirmed in humans, especially in primary HSV infection. Murine models have been very useful in examining the route of infection of skin/mucosa after initial HSV infection, and subsequent immune events, but have limitations. True recurrent disease and shedding does not occur in mice. Murine skin is much thinner than human skin and does not show the same degree of stratification or the same distribution of immune cell subsets. As an example of the marked differences between humans and mice, HSV initially infects epidermal γδT cells in mice, but not in humans.51 To overcome these limitations, we have developed an ex vivo model of HSV infection in human foreskin explants and compared this with biopsies of primary HSV lesions from human genital tissue.
Evidence for a HSV antigen relay through epithelial DCs
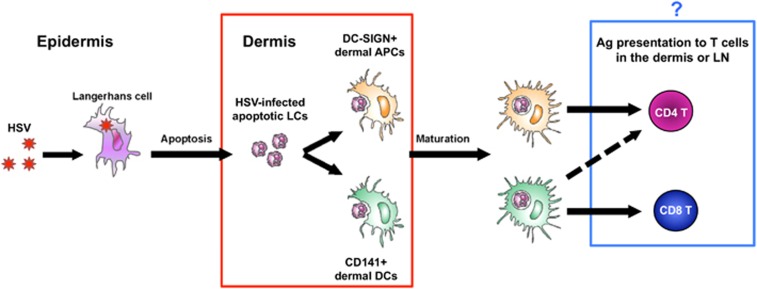
Using biopsies of initial genital herpes lesions and human foreskin explants, we recently confirmed that HSV is transferred from infected LCs in the epidermis to dermal DCs in human skin. We found that topically inoculated HSV1 or 2 was initially taken up by LCs in the epidermis and these LCs then underwent apoptosis while maturing and migrating to the dermis. In the upper dermis, apoptotic LCs expressing HSV1/2 were then taken up by both dermal CD141+ and DC-SIGN+ DCs in large clusters. We noted that CD141+ DCs in the cell clusters also upregulated the damaged cell receptor CLEC9A, which may mediate this process. Thus, HSV-infected human LCs undergo apoptosis and are taken up by different dermal DCs, which have the potential to present antigen to different T-cell subsets41 (Figure 1, red box).
Figure 1.
Relay of HSV through epithelial DCs may result in distinct pathways for stimulating CD4 and CD8 T cells. HSV initially infects LCs in the epidermis causing them to migrate into the dermis and apoptosis. Apoptotic, HSV-infected LCs are taken up by dermal CD141+ and DC-SIGN+ DC subsets that then mature (red box, known41) and have a potentially differential capacity to stimulate CD4 and CD8 T cells (blue box, unknown). CD141+ DCs have been demonstrated to be superior stimulators of CD8 T cells via cross-presentation but have the potential to also stimulate CD4 T cells (dashed arrow), whereas DC-SIGN+ DC subsets likely stimulate CD4 T cells.
The outcome of this HSV antigen relay in terms of T-cell stimulation is still to be elucidated but in human skin (although not necessarily at other sites), CD141+ DCs (equivalent to murine XCR1+ DCs) are more efficient than other dermal DC subsets at cross-presentation of exogenous antigens40 and may well prime CD8 T cells in the skin and lymph nodes. Presumably, other dermal DC subsets, including CD1a+ dermal DCs and CD14+/DC-SIGN+ dermal DCs, present exogenous HSV antigens directly to stimulate CD4 T cells, as in mice46 (Figure 1, blue box).
Some of the anomalies noted in mouse models may be explained by the HSV-epidermal-dermal DC relay described above, for example, the absence of LCs or dermal DCs bearing HSV DNA in lymph nodes49, 52 could be explained by DC processing of HSV antigens and DNA occurring en route to the lymph node after uptake of HSV-infected LCs. Indeed Puttur et al.51 found that HSV-infected LCs that did not undergo apoptosis, upregulated e-cadherin and were restricted in their migration out of the epidermis.
A number of critical questions remain in HSV immunology and are addressed in Table 1. These include what is the relative contribution of each DC subset (skin and lymph node) to T-cell priming in humans and which type of T-cell response does each DC prime? Does cross-priming occur in the skin? Why are there contradictory reports of the relative roles of migratory (dermal DCs) and resident DCs in stimulating T cells in lymph nodes? Is this just a matter of timing, depending on transfer of HSV antigen from a small number of migratory DCs to a larger number of resident DCs (that is, amplification)? And relevant to vaccine design, how critical is each step in this antigen relay for the stimulation of appropriate T- and B-cell responses? Are LCs essential in the process? Could they be bypassed by a HSV vaccine or should the epidermis be targeted?
Table 1. Critical questions remaining in HSV immunology.
| Critical questions in HSV immunology | Potential experimental approach |
|---|---|
| Relative contribution of different DCs to CD4 and CD8 T-cell priming/activation and polarisation (for example, Th1, 2, Tfh and so on) | Test the hypothesis that different dermal DC subsets mediate activation of different T-cell subsets and their polarisation in human models of HSV infection. |
| Does supplementary cross-priming of infiltrating CD8 T cells occur in skin, in addition to the lymph node (during primary infection)? | Look for responding CD8 T cells in situ in primary human HSV infection. |
| Role of migratory vs lymph node-resident DCs in T-cell priming. | This is still controversial in animal models and probably difficult to decipher in humans. |
| Which DCs in the HSV antigen relay are critical for T and B-cell responses? | Compare HSV responses in mice depleted for specific skin DC. For example, LC depleted (for example, huLang-DTA); CD103+ dermal DC depleted (human XCR1+ equivalent; for example, Batf3−/−); LC and CD11b+ dermal DC depleted with CSF1R antibody. |
| Do LCs or dermal DCs need to be targeted with a vaccine? | Compare vaccine responses for intradermal delivery versus epidermal delivery. |
Abbreviations: DCs, dendritic cells; HSV, herpes simplex virus.
Using knowledge of natural immunity to inform vaccine design
Key antigens for a HSV vaccine
HSV consists of a capsid enclosing the DNA genome, the tegument and an envelope containing glycoproteins including gB, gC, gD and gH/L. During HSV replication non-structural/enzymatic early proteins are expressed first, followed by late structural proteins. All are potential targets for CD4 and CD8 T cells. CD4 T cells mainly recognised late HSV structural proteins, especially gD and gB, consistent with vaccine studies,7 capsid protein VP5 and tegument protein UL49.53 In line with this, the majority of neutralising antibodies are directed towards gD and gB.54, 55 In contrast, CD8 T cells from all patients recognise a wide variety of viral proteins, including immediate early and early proteins.31, 56 Thus, vaccine candidates need to target CD4 and CD8 T-cell effectors via different repertoires of antigens and adjuvants.
HSV1 and 2 are very similar with highly related genomic sequences (83% nucleotide identity) and there is high serologic cross-reactivity between the viruses. However, although multiple T-cell epitopes have been defined, only a handful of cross-reactive epitopes in HSV1 and 2 have been identified. First, cross-reactive CD4 T-cell epitopes were defined in envelope glycoprotein gD57 and more recently, CD4 and CD8 cross-reactive epitopes from multiple proteins from HSV1, HSV2 and varicella zoster virus have been identified.58, 59 This latter finding raises the possibility of a human pan-alpha-herpesvirus vaccine. Theoretically, natural infection with varicella zoster virus could prime CD8 T cells that a HSV vaccine could boost, more easily than elicit. However, this remains to be appropriately tested. It is likely that novel ways of enhancing the magnitude of T-cell responses will be required including an adjuvant or inhibitory receptor blockade.
Prophylaxis versus immunotherapy
In the case of herpes zoster, memory T cells established in primary varicella zoster infection (and sustained by silent reactivation), although declining with age, might be readily amplified by Shingrix to control herpes zoster. This is likely to be an easier immunologic task than priming effective naive T cells to control a primary HSV infection, as mentioned above. In line with this, immunotherapy with a HSV vaccine consisting of long (35mer) peptides containing HSV epitopes together with heat shock protein Hsp70 and QS21 (HerpV from Agenus) induced specific CD4 and CD8 T-cell responses, correlating with a reduction in viral load and shedding.60 Moreover, a trial of the Genocea investigational vaccine candidate (GEN003), incorporating CD4 and CD8 T-cell-stimulating proteins (gD and ICP4, respectively) and the saponin-based adjuvant Matrix-M2, significantly reduced genital HSV2 shedding and genital herpes lesions over a 6-month period.61
Targeting critical DCs with a HSV vaccine for optimal cellular immunity
Given the sub-optimal performance of HSV vaccine candidates to date, a more directed approach specifically targeting and activating certain components of the immune system may be required to improve vaccine efficacy. As DCs have crucial roles in stimulating both humoral and cellular immune responses, targeting the right DCs with both antigen and adjuvant raises the possibility of enhancing and tailoring the immune response towards the desired outcome. In the case of HSV, three scenarios can be envisaged: (1) target epidermal LCs with appropriate antigens/adjuvants; (2) Bypass LCs and directly target the secondary dermal/lamina propria LCs; (3) Bypass the need for any migratory epithelial skin DCs for priming T- and B-cell responses DCs and target lymph node-resident DCs, via the lymphatics, as shown in several experimental models,62 However, migratory DCs do augment these responses and although LN-resident DCs may be able to mount an immune response more quickly than migratory DCs, this is likely not a high priority in a vaccine setting. The targeting of LCs or dermal DCs in the skin and mucosa with a vaccine can be accomplished through delivery of the vaccine into the direct vicinity of the LCs/DCs through epidermal/dermal/mucosal delivery devices such as microneedle arrays. Other approaches include combining the vaccine with an antibody to target the payload to a specific DC subset or an adjuvant that preferentially activates a particular subset of cells.
Dermal vaccine delivery devices
Microneedle devices are a developing drug delivery technology utilising an array of tiny projections that is briefly applied to the skin or mucosa to deliver vaccines into the dermis. Microneedles can be non-dissolvable or dissolvable and they have marked benefits over the traditional needle and syringe: microneedles eliminate the physical risks and discomfort of needle use and require little/no training to administer; they allow easy administration to mucosal surfaces; they are thermostable when coated with vaccine and most notably, allow for large dose reductions (up to almost 1000-fold) compared with intramuscular injection, without compromising efficacy.63, 64 It has been proposed that the increased potency is a result of enhanced DC targeting by delivery of the vaccine into their direct vicinity in the skin but this has not been confirmed. Microneedles are currently in clinical trials for influenza, polio and measles. Such a vaccine delivery device may be ideal for triggering the natural pathway to HSV immunity, that is, via epidermal and dermal DCs.
If LCs are a critical requirement in the antigen relay that leads to HSV immunity, the challenge of targeting them may be best overcome by delivery via a Nanopatch microneedle array. Most microneedle arrays deliver their payload deep into the dermis, whereas the Nanopatch is an optimised microneedle array that delivers antigen right at the dermo-epidermal junction resulting in efficient antigen uptake by LCs.64 This has been demonstrated with the Nanopatch delivery of a DNA vaccine for HSV in a mouse model.65
Targeting antigen to specific DC receptors
Targeting antigen specifically to DCs by conjugating it to antibodies against C-type lectin receptors expressed on DCs or by modifying the antigen to include the natural ligand of the C-type lectin receptor has resulted in enhanced cellular and humoral immune responses. A number of receptors that would be applicable for targeting dermal DCs have shown promise. DEC-205 is expressed fairly uniformly across all skin DC and macrophage subsets and DC-SIGN is restricted to macrophages and the small subset of CD14+ dermal DCs.66 When DEC-205 or DC-SIGN have been targeted with antibody-conjugated antigen or glycan modified antigen (for example, Lewis X structures for DC-SIGN) the result has been rapid endocytosis of the antigen and enhanced antigen presentation. When the antigen has been delivered in conjunction with an adjuvant, enhanced CD4 and CD8 T-cell responses as well as enhanced antibody responses have been demonstrated in mice.67, 68 However, in the absence of adjuvant, this has resulted in a tolerogenic or unresponsive state.69 Given their capacity for cross-presentation, CD141+ DCs have been targeted in the same ways via Clec9A and XCR1 (conjugated to antibody or XCL1). Several groups have indeed reported enhanced CD8 and CD4 T-cell responses, as well as efficient priming of follicular helper T cells resulting in boosted antibody responses70 even in the absence of adjuvant.71 In the presence of adjuvant, targeting XCR1 has resulted in protective immune responses in both viral and tumour models in mice, including mice expressing human XCR1.72
Targeting LCs in mice with long peptides via a langerin antibody has resulted in enhanced cross-presentation73 although in mice such LC cross-presentation was insufficient to prime CD8 T cells and in fact induced tolerance.74 This needs to be further tested with particulate antigens and in humans. Interestingly, Idoyaga et al.75 reported that in the presence of a strong DC stimulus such as CD40 ligand, targeting DEC-205, Clec9A or langerin, resulted in comparable Th1 and CTL responses.
Adjuvants for stimulating T-cell responses
A final, critical consideration in vaccine design is choosing an appropriate adjuvant. Alum, the adjuvant used in the majority of intramuscular vaccine formulations, cannot be used safely in the skin owing to reactogenicity.76 Furthermore, several novel adjuvants have been identified that stimulate superior immune responses to alum, which does not stimulate strong T-cell immunity. With increasing knowledge of the mode of action of different adjuvants, it should be possible to target particular DC subsets for activation thus tailoring the resulting immune response. Differences in efficacy between Shingrix (herpes zoster vaccine) and Simplirix (HSV vaccine) could potentially be partially attributed to the mode of action of their respective adjuvants. Although dMPL in both vaccines has been acknowledged for inducing strong Th1 CD4 immunity and boosting antibody titres, saponin-based adjuvants appear to be superior for inducing memory CD8 T-cell responses,60, 61 which may be protective against the reactivation disease herpes zoster. It should be remembered, however, that the requirements for priming memory CD8 T cells are less stringent than for naive CD8 T cells and noted that QS21-containing AS01B did not induce primary CD8 T-cell responses when used in prophylactic HSV,77 hepatitis B78 or malaria79 vaccine trials, unlike its effect in mice.80
Another promising candidate vaccine in preclinical trials is a trivalent HSV subunit vaccine, containing glycoprotein D2 (a primary target for neutralising antibodies and contains CD4 and CD8 epitopes), UL19 and UL25 (both are prevalent CD8 T-cell targets). When adjuvanted with stable oil in water emulsion and glucopyranosyl lipid A (GLA, a TLR4 agonist), this vaccine induced neutralising antibodies, Th1 polyfunctional CD4 T cells and CD8 T cells in mice and guinea pigs.81 This included priming naive polyfunctional CD8 T cells that were boosted by subsequent viral challenge, resulting in complete protection and prevention of latent infection in mice.
Where CD8 T cells are critical for immune control of a given pathogen, such adjuvants should be considered in vaccine design, and may be particularly relevant in a boost situation, but as discussed here, results from mouse models may not translate into humans. An important consideration for adjuvant selection, highlighting the difference between mice and men, is that murine XCR1+ DCs express TLR3, 4 and 9.82 Thus, TLR4 agonist adjuvants, including dMPL and GLA, may activate these cells and elicit enhanced naive CD8 T-cell responses in mice. This is unlikely to be duplicated in humans however, as human CD141+/XCR1+ DCs only express TLR383 and are unlikely to be stimulated by such components. A deeper understanding of the mechanism of action of these adjuvants is required. On this note, the liposomal cationic adjuvant formulation incorporates the TLR3 agonist poly(I:C) into liposomes in various iterations and strongly induces cytotoxic CD8 T-cell responses in mice to a range of antigens, including HIV, HPV and tuberculosis antigens.84, 85 CAF09 is the most potent of the series and is being optimised for testing in macaques. Stabilisation in liposomes prevents a non-specific systemic inflammatory response to polyI:C, one of the obstacles to using this TLR ligand as an adjuvant.84
Concluding remarks
The partial success of HSV vaccine trials with T- and B-cell adjuvants has stimulated development of diverse approaches with novel adjuvants and antigens. As the epidemiology of genital herpes is changing it is recognised that vaccine candidates must include HSV1/2 cross-reactive, immunodominant T-cell epitopes as well as neutralising antibody epitopes to both types. Increasing knowledge of the natural pathways of innate and adaptive immunity to primary herpes will illuminate key requirements to mimic in a vaccine. In particular, the specific skin/mucosal DC subsets to target in order to stimulate appropriate effector responses, including skin/mucosal CD8 T cells in addition to CD4 T cells and neutralising antibody, need to be defined. Furthermore, adjuvants that specifically activate these DCs in order to optimise these responses need to be defined. Finally, recruitment and activation of other critical innate cells, such as NK cells, should also be considered during adjuvant selection.
The authors declare no conflict of interest.
References
- Freeman EE, Weiss HA, Glynn JR, Cross PL, Whitworth JA, Hayes RJ. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS 2006; 20: 73–83. [DOI] [PubMed] [Google Scholar]
- Wald A, Link K. Risk of human immunodeficiency virus infection in herpes simplex virus type 2-seropositive persons: a meta-analysis. J Infect Dis 2002; 185: 45–52. [DOI] [PubMed] [Google Scholar]
- Celum C, Wald A, Lingappa JR, Magaret AS, Wang RS, Mugo N et al. Acyclovir and transmission of HIV-1 from persons infected with HIV-1 and HSV-2. N Engl J Med 2010; 362: 427–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffer JT, Swan DA, Corey L, Wald A. Rapid viral expansion and short drug half-life explain the incomplete effectiveness of current herpes simplex virus 2-directed antiviral agents. Antimicrob Agents Chemother 2013; 57: 5820–5829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham AL, Diefenbach RJ, Miranda-Saksena M, Bosnjak L, Kim M, Jones C et al. The cycle of human herpes simplex virus infection: virus transport and immune control. J Infect Dis 2006; 194 ((Suppl 1)): S11–S18. [DOI] [PubMed] [Google Scholar]
- Bernard MC, Barban V, Pradezynski F, de Montfort A, Ryall R, Caillet C et al. Immunogenicity, protective efficacy, and non-replicative status of the HSV-2 vaccine candidate HSV529 in mice and guinea pigs. PLoS ONE 2015; 10: e0121518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikloska Z, Cunningham AL. Herpes simplex virus type 1 glycoproteins gB, gC and gD are major targets for CD4 T-lymphocyte cytotoxicity in HLA-DR expressing human epidermal keratinocytes. J Gen Virol 1998; 79 Pt 2 353–361. [DOI] [PubMed] [Google Scholar]
- Stanberry LR, Spruance SL, Cunningham AL, Bernstein DI, Mindel A, Sacks S et al. Glycoprotein-D-adjuvant vaccine to prevent genital herpes. N Engl J Med 2002; 347: 1652–1661. [DOI] [PubMed] [Google Scholar]
- Belshe RB, Leone PA, Bernstein DI, Wald A, Levin MJ, Stapleton JT et al. Efficacy results of a trial of a herpes simplex vaccine. N Engl J Med 2012; 366: 34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belshe RB, Heineman TC, Bernstein DI, Bellamy AR, Ewell M, van der Most R et al. Correlate of immune protection against HSV-1 genital disease in vaccinated women. J Infect Dis 2014; 209: 828–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lal H, Cunningham AL, Godeaux O, Chlibek R, Diez-Domingo J, Hwang SJ et al. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med 2015; 372: 2087–2096. [DOI] [PubMed] [Google Scholar]
- Leroux-Roels I, Leroux-Roels G, Clement F, Vandepapeliere P, Vassilev V, Ledent E et al. A phase 1/2 clinical trial evaluating safety and immunogenicity of a varicella zoster glycoprotein e subunit vaccine candidate in young and older adults. J Infect Dis 2012; 206: 1280–1290. [DOI] [PubMed] [Google Scholar]
- Corey L, Langenberg AG, Ashley R, Sekulovich RE, Izu AE, Douglas JMJr et al. Recombinant glycoprotein vaccine for the prevention of genital HSV-2 infection: two randomized controlled trials. Chiron HSV Vaccine Study Group. JAMA 1999; 282: 331–340. [DOI] [PubMed] [Google Scholar]
- Chew T, Taylor KE, Mossman KL. Innate and adaptive immune responses to herpes simplex virus. Viruses 2009; 1: 979–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato A, Linehan MM, Iwasaki A. Dual recognition of herpes simplex viruses by TLR2 and TLR9 in dendritic cells. Proc Natl Acad Sci USA 2006; 103: 17343–17348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittan NA, Bergua A, Haupt S, Donhauser N, Schuster P, Korn K et al. Impaired plasmacytoid dendritic cell innate immune responses in patients with herpes virus-associated acute retinal necrosis. J Immunol 2007; 179: 4219–4230. [DOI] [PubMed] [Google Scholar]
- Lund JM, Linehan MM, Iijima N, Iwasaki A. Cutting Edge: plasmacytoid dendritic cells provide innate immune protection against mucosal viral infection in situ. J Immunol 2006; 177: 7510–7514. [DOI] [PubMed] [Google Scholar]
- Dalloul A, Oksenhendler E, Chosidow O, Ribaud P, Carcelain G, Louvet S et al. Severe herpes virus (HSV-2) infection in two patients with myelodysplasia and undetectable NK cells and plasmacytoid dendritic cells in the blood. J Clin Virol 2004; 30: 329–336. [DOI] [PubMed] [Google Scholar]
- Donaghy H, Bosnjak L, Harman AN, Marsden V, Tyring SK, Meng TC et al. Role for plasmacytoid dendritic cells in the immune control of recurrent human herpes simplex virus infection. J Virol 2009; 83: 1952–1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster P, Thomann S, Werner M, Vollmer J, Schmidt B. A subset of human plasmacytoid dendritic cells expresses CD8alpha upon exposure to herpes simplex virus type 1. Front Microbiol 2015; 6: 557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morandi B, Bougras G, Muller WA, Ferlazzo G, Munz C. NK cells of human secondary lymphoid tissues enhance T cell polarization via IFN-gamma secretion. Eur J Immunol 2006; 36: 2394–2400. [DOI] [PubMed] [Google Scholar]
- Vogel K, Thomann S, Vogel B, Schuster P, Schmidt B. Both plasmacytoid dendritic cells and monocytes stimulate natural killer cells early during human herpes simplex virus type 1 infections. Immunology 2014; 143: 588–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Osborne NR, Zeng W, Donaghy H, McKinnon K, Jackson DC et al. Herpes simplex virus antigens directly activate NK cells via TLR2, thus facilitating their presentation to CD4 T lymphocytes. J Immunol 2012; 188: 4158–4170. [DOI] [PubMed] [Google Scholar]
- Nandakumar S, Woolard SN, Yuan D, Rouse BT, Kumaraguru U. Natural killer cells as novel helpers in anti-herpes simplex virus immune response. J Virol 2008; 82: 10820–10831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu K, Dou J, Yu F, He X, Yuan X, Wang Y et al. An ocular mucosal administration of nanoparticles containing DNA vaccine pRSC-gD-IL-21 confers protection against mucosal challenge with herpes simplex virus type 1 in mice. VacciNE 2011; 29: 1455–1462. [DOI] [PubMed] [Google Scholar]
- Posavad CM, Koelle DM, Shaughnessy MF, Corey L. Severe genital herpes infections in HIV-infected individuals with impaired herpes simplex virus-specific CD8+ cytotoxic T lymphocyte responses. Proc Natl Acad Sci USA 1997; 94: 10289–10294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Koelle DM, Cao J, Vazquez J, Huang ML, Hladik F et al. Virus-specific CD8+ T cells accumulate near sensory nerve endings in genital skin during subclinical HSV-2 reactivation. J Exp Med 2007; 204: 595–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham AL, Turner RR, Miller AC, Para MF, Merigan TC. Evolution of recurrent herpes simplex lesions. An immunohistologic study. J Clin Invest 1985; 75: 226–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koelle DM, Posavad CM, Barnum GR, Johnson ML, Frank JM, Corey L. Clearance of HSV-2 from recurrent genital lesions correlates with infiltration of HSV-specific cytotoxic T lymphocytes. J Clin Invest 1998; 101: 1500–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbs ME, Strasser JE, Chu CF, Chalk C, Milligan GN. Clearance of herpes simplex virus type 2 by CD8+ T cells requires gamma interferon and either perforin- or Fas-mediated cytolytic mechanisms. J Virol 2005; 79: 14546–14554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikloska Z, Kesson AM, Penfold ME, Cunningham AL. Herpes simplex virus protein targets for CD4 and CD8 lymphocyte cytotoxicity in cultured epidermal keratinocytes treated with interferon-gamma. J Infect Dis 1996; 173: 7–17. [DOI] [PubMed] [Google Scholar]
- Greyer M, Whitney PG, Stock AT, Davey GM, Tebartz C, Bachem A et al. T Cell Help Amplifies Innate Signals in CD8(+) DCs for Optimal CD8(+) T Cell Priming. Cell Rep 2016; 14: 586–597. [DOI] [PubMed] [Google Scholar]
- Gebhardt T, Wakim LM, Eidsmo L, Reading PC, Heath WR, Carbone FR. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat Immunol 2009; 10: 524–530. [DOI] [PubMed] [Google Scholar]
- Khanna KM, Bonneau RH, Kinchington PR, Hendricks RL. Herpes simplex virus-specific memory CD8+ T cells are selectively activated and retained in latently infected sensory ganglia. Immunity 2003; 18: 593–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin H, Iwasaki A. A vaccine strategy that protects against genital herpes by establishing local memory T cells. Nature 2012; 491: 463–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Peng T, Johnston C, Phasouk K, Kask AS, Klock A et al. Immune surveillance by CD8alphaalpha+ skin-resident T cells in human herpes virus infection. Nature 2013; 497: 494–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay LK, Stock AT, Ma JZ, Jones CM, Kent SJ, Mueller SN et al. Long-lived epithelial immunity by tissue-resident memory T (TRM) cells in the absence of persisting local antigen presentation. Proc Natl Acad Sci USA 2012; 109: 7037–7042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haniffa M, Gunawan M, Jardine L. Human skin dendritic cells in health and disease. J Dermatol Sci 2015; 77: 85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klechevsky E, Morita R, Liu M, Cao Y, Coquery S, Thompson-Snipes L et al. Functional specializations of human epidermal langerhans cells and CD14+ dermal dendritic cells. Immunity 2008; 29: 497–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haniffa M, Shin A, Bigley V, McGovern N, Teo P, See P et al. Human tissues contain CD141hi cross-presenting dendritic cells with functional homology to mouse CD103+ nonlymphoid dendritic cells. Immunity 2012; 37: 60–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Truong NR, James V, Bosnjak L, Sandgren KJ, Harman AN et al. Relay of herpes simplex virus between Langerhans cells and dermal dendritic cells in human skin. PLoS Pathog 2015; 11: e1004812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprecher E, Becker Y. Skin Langerhans cells play an essential role in the defense against HSV-1 infection. Arch Virol 1986; 91: 341–349. [DOI] [PubMed] [Google Scholar]
- Bosnjak L, Miranda-Saksena M, Koelle DM, Boadle RA, Jones CA, Cunningham AL. Herpes simplex virus infection of human dendritic cells induces apoptosis and allows cross-presentation via uninfected dendritic cells. J Immunol 2005; 174: 2220–2227. [DOI] [PubMed] [Google Scholar]
- Jones CA, Fernandez M, Herc K, Bosnjak L, Miranda-Saksena M, Boadle RA et al. Herpes simplex virus type 2 induces rapid cell death and functional impairment of murine dendritic cells in vitro. J Virol 2003; 77: 11139–11149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hor J, Whitney PG, Zaid A, Brooks AG, Heath WR, Mueller SN. Spatiotemporally Distinct Interactions with Dendritic Cell Subsets Facilitates CD4+ and CD8+ T Cell Activation to Localized Viral Infection. Immunity 2015; 43: 554–565. [DOI] [PubMed] [Google Scholar]
- Zhao X, Deak E, Soderberg K, Linehan M, Spezzano D, Zhu J et al. Vaginal submucosal dendritic cells, but not Langerhans cells, induce protective Th1 responses to herpes simplex virus-2. J Exp Med 2003; 197: 153–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan RS, Waithman J, Bedoui S, Jones CM, Villadangos JA, Zhan Y et al. Migratory dendritic cells transfer antigen to a lymph node-resident dendritic cell population for efficient CTL priming. Immunity 2006; 25: 153–162. [DOI] [PubMed] [Google Scholar]
- Bedoui S, Whitney PG, Waithman J, Eidsmo L, Wakim L, Caminschi I et al. Cross-presentation of viral and self antigens by skin-derived CD103+ dendritic cells. Nat Immunol 2009; 10: 488–495. [DOI] [PubMed] [Google Scholar]
- Lee HK, Zamora M, Linehan MM, Iijima N, Gonzalez D, Haberman A et al. Differential roles of migratory and resident DCs in T cell priming after mucosal or skin HSV-1 infection. J Exp Med 2009; 206: 359–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macleod BL, Bedoui S, Hor JL, Mueller SN, Russell TA, Hollett NA et al. Distinct APC subtypes drive spatially segregated CD4+ and CD8+ T-cell effector activity during skin infection with HSV-1. PLoS Pathog 2014; 10: e1004303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puttur FK, Fernandez MA, White R, Roediger B, Cunningham AL, Weninger W et al. Herpes simplex virus infects skin gamma delta T cells before Langerhans cells and impedes migration of infected Langerhans cells by inducing apoptosis and blocking E-cadherin downregulation. J Immunol 2010; 185: 477–487. [DOI] [PubMed] [Google Scholar]
- Eidsmo L, Allan R, Caminschi I, van Rooijen N, Heath WR, Carbone FR. Differential migration of epidermal and dermal dendritic cells during skin infection. J Immunol 2009; 182: 3165–3172. [DOI] [PubMed] [Google Scholar]
- Koelle DM, Schomogyi M, McClurkan C, Reymond SN, Chen HB. CD4 T-cell responses to herpes simplex virus type 2 major capsid protein VP5: comparison with responses to tegument and envelope glycoproteins. J Virol 2000; 74: 11422–11425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns TM, Huang ZY, Gallagher JR, Lin Y, Lou H, Whitbeck JC et al. Patient-specific neutralizing antibody rsponses to herpes simplex virus are attributed to epitopes on gD, gB, or both and can be type specific. J Virol 2015; 89: 9213–9231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns TM, Huang ZY, Whitbeck JC, Ponce de Leon M, Lou H, Wald A et al. Dissection of the antibody response against herpes simplex virus glycoproteins in naturally infected humans. J Virol 2014; 88: 12612–12622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosken N, McGowan P, Meier A, Koelle DM, Sleath P, Wagener F et al. Diversity of the CD8+ T-cell response to herpes simplex virus type 2 proteins among persons with genital herpes. J Virol 2006; 80: 5509–5515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Taylor J, Sidney J, Mikloska Z, Bodsworth N, Lagios K et al. Immunodominant epitopes in herpes simplex virus type 2 glycoprotein D are recognized by CD4 lymphocytes from both HSV-1 and HSV-2 seropositive subjects. J Immunol 2008; 181: 6604–6615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu C, McCausland M, Sidney J, Duh FM, Rouphael N, Mehta A et al. Broadly reactive human CD8 T cells that recognize an epitope conserved between VZV, HSV and EBV. PLoS Pathog 2014; 10: e1004008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing L, Laing KJ, Dong L, Russell RM, Barlow RS, Haas JG et al. Extensive CD4 and CD8 T Cell Cross-Reactivity between Alphaherpesviruses. J Immunol 2016; 196: 2205–2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wald A, Koelle DM, Fife K, Warren T, Leclair K, Chicz RM et al. Safety and immunogenicity of long HSV-2 peptides complexed with rhHsc70 in HSV-2 seropositive persons. VacciNE 2011; 29: 8520–8529. [DOI] [PubMed] [Google Scholar]
- Van Wagoner N, Koltun W, Lucksinger G, Warren T, Tyring S, Bernstein D et al GEN-003 Phase 2 Interim Results: therapeutic Vaccine for Genital Herpes Significantly Reduces Viral Shedding and Genital Lesions. ID Week; 9 October, 2015; San Diego, CA, USA, 2015. https://idsa.confex.com/idsa/2015/webprogram/Paper52899.html.
- Gerner MY, Torabi-Parizi P, Germain RN. Strategically localized dendritic cells promote rapid T cell responses to lymph-borne particulate antigens. Immunity 2015; 42: 172–185. [DOI] [PubMed] [Google Scholar]
- Fernando GJ, Chen X, Primiero CA, Yukiko SR, Fairmaid EJ, Corbett HJ et al. Nanopatch targeted delivery of both antigen and adjuvant to skin synergistically drives enhanced antibody responses. J Control Release 2012; 159: 215–221. [DOI] [PubMed] [Google Scholar]
- Fernando GJ, Chen X, Prow TW, Crichton ML, Fairmaid EJ, Roberts MS et al. Potent immunity to low doses of influenza vaccine by probabilistic guided micro-targeted skin delivery in a mouse model. PLoS ONE 2010; 5: e10266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Kask AS, Crichton ML, McNeilly C, Yukiko S, Dong L et al. Improved DNA vaccination by skin-targeted delivery using dry-coated densely-packed microprojection arrays. J Control Release 2010; 148: 327–333. [DOI] [PubMed] [Google Scholar]
- Sandgren KJ, Smed-Sorensen A, Forsell MN, Soldemo M, Adams WC, Liang F et al. Human plasmacytoid dendritic cells efficiently capture HIV-1 envelope glycoproteins via CD4 for antigen presentation. J Immunol 2013; 191: 60–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boscardin SB, Hafalla JC, Masilamani RF, Kamphorst AO, Zebroski HA, Rai U et al. Antigen targeting to dendritic cells elicits long-lived T cell help for antibody responses. J Exp Med 2006; 203: 599–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SK, Stephani J, Schaefer M, Kalay H, Garcia-Vallejo JJ, den Haan J et al. Targeting glycan modified OVA to murine DC-SIGN transgenic dendritic cells enhances MHC class I and II presentation. Mol Immunol 2009; 47: 164–174. [DOI] [PubMed] [Google Scholar]
- Hawiger D, Inaba K, Dorsett Y, Guo M, Mahnke K, Rivera M et al. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J Exp Med 2001; 194: 769–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joffre OP, Sancho D, Zelenay S, Keller AM, Reis e Sousa C. Efficient and versatile manipulation of the peripheral CD4+ T-cell compartment by antigen targeting to DNGR-1/CLEC9A. Eur J Immunol 2010; 40: 1255–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahoud MH, Ahmet F, Kitsoulis S, Wan SS, Vremec D, Lee CN et al. Targeting antigen to mouse dendritic cells via Clec9A induces potent CD4 T cell responses biased toward a follicular helper phenotype. J Immunol 2011; 187: 842–850. [DOI] [PubMed] [Google Scholar]
- Hartung E, Becker M, Bachem A, Reeg N, Jakel A, Hutloff A et al. Induction of potent CD8 T cell cytotoxicity by specific targeting of antigen to cross-presenting dendritic cells in vivo via murine or human XCR1. J Immunol 2015; 194: 1069–1079. [DOI] [PubMed] [Google Scholar]
- Fehres CM, Duinkerken S, Bruijns SC, Kalay H, van Vliet SJ, Ambrosini M et al. Langerin-mediated internalization of a modified peptide routes antigens to early endosomes and enhances cross-presentation by human Langerhans cells. Cell Mol Immunol 2015. (e-pub ahead of print 12 October 2015; doi:10.1038/cmi.2015.87). [DOI] [PMC free article] [PubMed]
- Flacher V, Tripp CH, Mairhofer DG, Steinman RM, Stoitzner P, Idoyaga J et al. Murine Langerin+ dermal dendritic cells prime CD8+ T cells while Langerhans cells induce cross-tolerance. EMBO Mol Med 2014; 6: 1191–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idoyaga J, Lubkin A, Fiorese C, Lahoud MH, Caminschi I, Huang Y et al. Comparable T helper 1 (Th1) and CD8 T-cell immunity by targeting HIV gag p24 to CD8 dendritic cells within antibodies to Langerin, DEC205, and Clec9A. Proc Natl Acad Sci USA 2011; 108: 2384–2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Wu MX. Laser vaccine adjuvant for cutaneous immunization. Expert Rev Vaccines 2011; 10: 1397–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chlibek R, Smetana J, Pauksens K, Rombo L, Van den Hoek JA, Richardus JH et al. Safety and immunogenicity of three different formulations of an adjuvanted varicella-zoster virus subunit candidate vaccine in older adults: a phase II, randomized, controlled study. VacciNE 2014; 32: 1745–1753. [DOI] [PubMed] [Google Scholar]
- Leroux-Roels G, Van Belle P, Vandepapeliere P, Horsmans Y, Janssens M, Carletti I et al. Vaccine Adjuvant Systems containing monophosphoryl lipid A and QS-21 induce strong humoral and cellular immune responses against hepatitis B surface antigen which persist for at least 4 years after vaccination. VacciNE 2015; 33: 1084–1091. [DOI] [PubMed] [Google Scholar]
- Cummings JF, Spring MD, Schwenk RJ, Ockenhouse CF, Kester KE, Polhemus ME et al. Recombinant Liver Stage Antigen-1 (LSA-1) formulated with AS01 or AS02 is safe, elicits high titer antibody and induces IFN-gamma/IL-2 CD4+ T cells but does not protect against experimental Plasmodium falciparum infection. VacciNE 2010; 28: 5135–5144. [DOI] [PubMed] [Google Scholar]
- Didierlaurent AM, Collignon C, Bourguignon P, Wouters S, Fierens K, Fochesato M et al. Enhancement of adaptive immunity by the human vaccine adjuvant AS01 depends on activated dendritic cells. J Immunol 2014; 193: 1920–1930. [DOI] [PubMed] [Google Scholar]
- Odegard JM, Flynn PA, Campbell DJ, Robbins SH, Dong L, Wang K et al. A novel HSV-2 subunit vaccine induces GLA-dependent CD4 and CD8 T cell responses and protective immunity in mice and guinea pigs. VacciNE 2016; 34: 101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards AD, Diebold SS, Slack EM, Tomizawa H, Hemmi H, Kaisho T et al. Toll-like receptor expression in murine DC subsets: lack of TLR7 expression by CD8 alpha+ DC correlates with unresponsiveness to imidazoquinolines. Eur J Immunol 2003; 33: 827–833. [DOI] [PubMed] [Google Scholar]
- Jongbloed SL, Kassianos AJ, McDonald KJ, Clark GJ, Ju X, Angel CE et al. Human CD141+ (BDCA-3)+ dendritic cells (DCs) represent a unique myeloid DC subset that cross-presents necrotic cell antigens. J Exp Med 2010; 207: 1247–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordly P, Rose F, Christensen D, Nielsen HM, Andersen P, Agger EM et al. Immunity by formulation design: induction of high CD8+ T-cell responses by poly(I:C) incorporated into the CAF01 adjuvant via a double emulsion method. J Control Release 2011; 150: 307–317. [DOI] [PubMed] [Google Scholar]
- Korsholm KS, Hansen J, Karlsen K, Filskov J, Mikkelsen M, Lindenstrom T et al. Induction of CD8+ T-cell responses against subunit antigens by the novel cationic liposomal CAF09 adjuvant. VacciNE 2014; 32: 3927–3935. [DOI] [PubMed] [Google Scholar]