Cell division is often perceived to be a symmetric process by which one cell gives rise to two identical daughter cells. Throughout development, however, cell division can produce two daughter cells with different protein content, cell size, and developmental potential, that ultimately adopt distinct fates in a process termed asymmetric cell division1. One of the salient characteristics of the adaptive immune systems is the ability of a single antigen-specific T cell to proliferate and to differentiate into two distinct classes of daughter cells in responding to microbial infection, namely the terminal effector cells that mediate acute protection and the memory cells that provide long-term protective immunity. Work over the years has shed light on how this process occurs in vivo. One potential explanation is the asymmetric division of cytotoxic CD8 T cells. Specifically, the daughter cell proximal to the antigen-presenting cell (APC) is marked by higher expression of CD8 (CD8high) and is more likely to differentiate into effector-like T cells. The distal daughter cell expresses a lower level of CD8 (CD8low) and is more likely to differentiate into memory-like T cells2. Several transcription factors, including T-bet and TCF-1, have been shown to contribute to the phenotypic and functional differences between the daughter cells3,4. There is emerging evidence suggesting a role for PI3K signaling and nutrient sensing in dividing T cell fates4. Nevertheless, whether there exist metabolic programs that orchestrate asymmetric division in T cells and whether this process can be manipulated for therapeutic purposes remain incompletely understood.
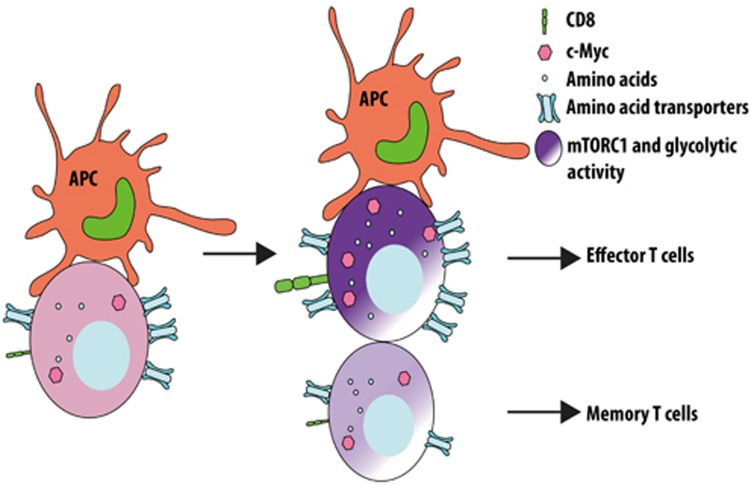
In a recent paper in Nature, Verbist et al.5 elegantly demonstrate that c-Myc, a major transcription factor that drives metabolic reprogramming necessary for T cell activation and proliferation6, is asymmetrically partitioned between the two daughter cells during the first division of activated T cells in mice5. Specifically, CD8high daughter cells have higher c-Myc compared with CD8low cells. Furthermore, c-Mychigh daughter cells are more activated and glycolytic, and thus more readily differentiate into terminal effector cells, whereas c-Myclow cells are more persistent and can proliferate upon secondary challenge (Figure 1). Mechanistically, the authors show that several amino acid transporters including CD98 and the neutral amino acid transporter SLC1A5 are more highly expressed in c-Mychigh daughter cells. This may contribute to the increased amino acid content, and thus the higher activity of mammalian target of rapamycin complex 1 (mTORC1) in these cells. Most importantly, depletion of the amino acid pool or inhibition of mTORC1 activity with rapamycin abolishes the asymmetric partitioning of c-Myc in daughter cells. Using T cell specific loss of Tsc1, which is a crucial negative regulator of mTORC1 activity, the authors further show that hyperactive mTORC1 signaling abrogates the asymmetric partitioning of c-Myc, as well as the proliferative potential differences between the two daughter cells. This genetic manipulation demonstrates the role of mTORC1 in mediating metabolic reprograming, which ultimately contributes to the distinct fates of two daughter cells. The findings from this study are consistent with a recent publication from the Powell group7. They demonstrated that the asymmetric partitioning of mTORC1 activity in activated CD8 T cells results in the generation of two daughter T cells with differential metabolic profiles and ultimately adopt distinct immune functions.
Figure 1.
Metabolic control of asymmetric cell division in CD8 T lymphocytes. Proximal daughter cells have higher intracellular amino acid concentrations associated with increased distribution of amino acid transporters and mTORC1 activity, as well as higher c-Myc levels. Proximal daughter cells differentiate readily into effector T cells whereas distal daughter cells have memory-forming potential.
The study adds to a growing number of reports demonstrating the crucial roles of metabolic reprogramming in T lymphocyte proliferation, differentiation, and function. The importance of nutrient sensing and amino acid concentrations in asymmetric cell division demonstrated from this study is especially intriguing and further highlights the intricate link between metabolism and the immune system. These findings reveal the exciting potential of manipulating the asymmetric division process to skew T cell responses in a direction that is beneficial for vaccine development or antitumor therapy. Nevertheless, they also raise the questions of what are the key players that control asymmetric distribution of mTORC1 activity and the various metabolic components such as amino acid transporters and what happen if this regulation is disturbed? It is known that complete loss or hyperactivation of mTORC1 activity is detrimental for the development of potent effector T cells or functional memory T cells, respectively8,9. Thus, the challenge remains to identify the regulatory elements that control the asymmetric partitioning of the metabolic machinery and to develop strategies that timely and precisely target the mTORC1-c-Myc axis in such a way that would yield the most therapeutic benefit.
References
- Knoblich JA. Nat Rev Mol Cell Biol 2001; 2:11–20. [DOI] [PubMed]
- Chang JT, Palanivel VR, Kinjyo I, et al. Science 2007; 315:1687–1691. [DOI] [PubMed]
- Chang JT, Ciocca ML, Kinjyo I, et al. Immunity 2011; 34:492–504. [DOI] [PMC free article] [PubMed]
- Lin WH, Adams WC, Nish SA, et al. Cell Rep 2015; 13:2203–2218. [DOI] [PMC free article] [PubMed]
- Verbist KC, Guy CS, Milasta S, et al. Nature 2016; 532:389–393. [DOI] [PMC free article] [PubMed]
- Wang R, Dillon CP, Shi LZ, et al. Immunity 2011; 35:871–882. [DOI] [PMC free article] [PubMed]
- Pollizzi KN, Sun IH, Patel CH, et al. Nat Immunol 2016 Apr 11. doi:10.1038/ni.3438
- Yang K, Shrestha S, Zeng H, et al. Immunity 2013; 39:1043–1056. [DOI] [PMC free article] [PubMed]
- Shrestha S, Yang K, Wei J, et al. Proc Natl Acad Sci USA 2014; 111:14858–14863. [DOI] [PMC free article] [PubMed]