Abstract
Aim:
Recent studies show that the extract of a Chinese herb Polygalae Radix exerts cognition-enhancing actions in rats and humans. The aim of this study was to characterize the pharmacological profiles of active compounds extracted from Polygalae Radix.
Methods:
Two fractions P3 and P6 and two compounds PTM-15 and polygalasaponin XXXII (PGS32) were prepared. Neuroprotective effects were evaluated in primary cortical neurons exposed to high concentration glutamate, serum deficiency or H2O2. Anti-dementia actions were assessed in scopolamine-induced amnesia in mice using step-through avoidance tests and channel water maze tests. After conducting the channel water maze tests, TrkB phosphorylation in mouse hippocampus was detected using Western blotting. Long-term potentiation (LTP) was induced in the dentate gyrus in adult rats; PGS32 (5 μL 400 μmol/L) was injected into the lateral cerebral ventricle 20 min after high frequency stimulation (HFS).
Results:
Compared to the fraction P6, the fraction P3 showed more prominent neuroprotective effects in vitro and cognition-enhancing effects in scopolamine-induced amnesia in mice. One active compound PGS32 in the fraction P3 exerted potent cognition-enhancing action: oral administration of PGS32 (0.125 mg·kg−1·d−1) for 19 days abolished scopolamine-induced memory impairment in mice. Furthermore, PGS32 (0.5 and 2 mg·kg−1·d−1) significantly stimulated the phosphorylation of TrkB in the hippocampus. Intracerebroventricular injection of PGS32 significantly enhanced HFS-induced LTP in the dentate gyrus of rats.
Conclusion:
PGS32 attenuates scopolamine-induced cognitive impairments in mice, suggesting that it has a potential for the treatment of cognitive dysfunction and dementia.
Keywords: Polygalae Radix, polygalasaponin XXXII, triterpenoid saponin, neuroprotection, scopolamine, dementia, tyrosine kinase B, LTP
Introduction
Neurodegenerative diseases are a group of chronic, progressive disorders characterized by the gradual loss of neurons in discrete areas of the central nervous system1. These groups of diseases include highly prevalent diseases, such as Parkinson's disease and Alzheimer's disease, and other rare diseases, including prion diseases (also known as transmissible spongiform encephalopathies), spinocerebellar ataxia, amyotrophic lateral sclerosis and Huntington's disease2,3. As age-related diseases, neurodegenerative diseases are becoming an increasing burden as the human population ages, with approximately 5.1 million households affected by Alzheimer's disease, and almost 300 000 people of age 65 years or older afflicted with Parkinson's disease in the US as of 20104.
Traditional therapies for neurodegenerative diseases enhance cognition and improve the quality of life of patients, but they do not alter the natural course or the ultimate outcome of these diseases5. Using herbal treatments in neurodegenerative disease therapies has generated unprecedented levels of interest because of the multi-function, multi-target characteristics of these treatments6. Many researchers have developed new compounds or active extracts from herbal materials for the treatment of neurodegenerative disorders.
Polygalae Radix, a well-known herb listed in the Chinese Pharmacopoeia (2015 edition), has been prescribed as a tonic, antipsychotic, expectorant and detumescent for thousands of years in traditional Chinese medicine. Modern pharmacological studies have already demonstrated that Polygalae Radix extract exhibits a wide variety of effects, such as hypotensive7, anti-inflammatory8, anti-depressive9, anti-oxidative and anti-apoptotic10 effects, cognitive improvements and cerebral protection11. The Polygalae Radix extract BT-11 has been shown to have memory-enhancing effects in rats with scopolamine-induced amnesia12 and cognition-improving effects in two randomized, double-blind, placebo-controlled comparison studies, one in healthy adults and one in elderly humans13,14. However, the biologically active components of Polygalae Radix extract remains unclear, and additional preclinical studies are necessary to develop more effective monomers from Polygalae Radix extracts.
In the present study, we attempted to screen for polygala saponin monomers with neuroprotective and anti-dementia effects and to further clarify our understanding of their underlying mechanisms.
Materials and methods
Drugs and reagents
Glutamate, nerve growth factor (NGF, purity >97%), MTT, huperzine A (Hup-A), poly-D-lysine (PLL), and cytarabine (Ara-C) were purchased from Sigma Chemical Company Sigma-Aldrich (St Louis, MO, USA). Scopolamine hydrobromide was purchased from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). Donepezil was provided by the Department of Chemosynthesis, Institute of Materia Medica, Chinese Academy of Medical Sciences and Peking Union Medical College (Beijing, China). Dulbecco's modified Eagle's medium/nutrient mixture F-12 (DMEM/F12), Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum (FBS), and horse serum (ES) were purchased from Gibco BRL (New York, NY, USA). Anti tyrosine kinase B (TrkB) and phosphorylated TrkB (p-TrkB) antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Other materials were purchased from other commercial sources.
Compound preparation
The compounds were prepared as previously described15. Fraction Fc-2 and fraction Fc-3 were P3, while Fraction Fc-7 was P6. Fraction Fc-2 was separated by preparative medium pressure liquid chromatography and eluted with MeOH-H2O to obtain nine fractions (Fc2-1 to Fc2-9), and then PTM-15 and polygala saponin XXXII (PGS32) were further separated from Fc2-8 by semi-preparative high pressure liquid chromatography (MeOH-H2O 70:30+0.05% CF3COOH, 7 mL/min). PGS32 was first isolated from the root of Polygala japonica Houtt by Zhang et al in 199616.
Primary cortical neuron culture and treatment
Primary cortical neurons were prepared from rat embryos at 17±19 d gestation as previously described17. Cerebral neocortices were removed and cultured in DMEM/F12 containing 10% ES and 10% FBS. Cells were plated in PLL-coated 96-well plates at 1×106/mL for toxicity experiments. Then, plates were incubated at 37 °C in a humidified atmosphere with 5% CO2. Two days later, 10 μg/mL Ara-C was added to the cultures to inhibit the outgrowth of various cells for 48 h, including glial cells, mechanocytes, and nerve stem cells. The primary cortical neurons were cultured for another seven days, and they were then used for treatment. The media were changed every two days. Nerve growth factor was used as the positive control.
Glutamate at a concentration of 10 mmol/L was used as an inducer for neurotoxicity in primary cortical neurons. The test compounds were added to each of the treated groups at concentrations of 1, 10, and 100 μg/mL. Twenty-four hours after treatment, 10 μL MTT (5 mg/mL) was added to each well, and cells were cultured in the incubator for 4 h. Then, 100 μL solubilization solution [10% sodium dodecyl sulfate (SDS) in 0.01 mol/L HCl] was added to each well of the 96-well plate. The plate was incubated overnight at 37 °C, and the optical absorbance of the cells was measured at 570 nm with a microtiter plate reader.
We conducted MTT assays to measure the survival rate of primary cortical neurons injured by serum deficiency. The original culture media were replaced by media containing 0.5% FBS and 0.5% ES, with or without Polygalae Radix-derived compounds. The cells in the control group were treated with complete culture media but no compounds. The cells were cultured for another 24 h. Then, an MTT assay was performed to measure the survival rate of primary cortical neurons.
PC12 cell culture and treatment
PC12 cells were cultured in DMEM supplemented with 5% ES and 5% FBS. The media were changed every 2 days, and cells were seeded on PLL-coated plates at 5×104/mL. After 24 h subculture, cells were transferred to culture media containing 100 μmol/L H2O2 with or without Polygalae Radix-derived compounds. After 24 h, the MTT assay was performed to measure the survival rates of PC12 cells.
Animals and drug treatment
Male Kunming mice (18–20 g), C57BL/6J mice (18–20 g) and Wistar rats (230–260 g) were provided by the Experimental Animal Center of the Chinese Academy of Medical Sciences. They were housed in a room under temperature and light control (23 °C, 12 h-light cycle) and had free access to food and water. All animal experiments were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No 80–23), and the animal study was approved by the Animal Care Committee of the Chinese Academy of Medical Sciences and the Peking Union Medical College.
Eighty-four Kunming mice were divided into 7 groups as follows: control group, model group, 7.5 mg/kg P3 group, 30 mg/kg P3 group, 7.5 mg/kg P6 group, 30 mg/kg P6 group, and 5 mg/kg donepezil group (the last of which was the positive control group). The experiment lasted for 16 days. P3, P6 and donepezil were suspended in double-distilled water (DDW) and orally administered (ig) to the mice (0.1 mL/10 g) every day. The same volume of DDW was given to the control group and the model group. During the behavioral tests from the 10th day onward, the P3, P6, donepezil and model groups were injected with scopolamine (5 mg/kg d 10–d 11 for step-through avoidance tests, 2 mg/kg d 13–d 16 for channel water maze tests, ip, suspended in saline) 30 min prior to the test, whereas the control group was injected with the same volume of normal saline.
Eighty-four C57BL/6J mice were divided into seven groups as follows: control group, model group, PTM-15 5 mg/kg group, PTM-15 20 mg/kg group, PGS32 5 mg/kg group, PGS32 20 mg/kg group and positive control group. PTM-15 and PGS32 were suspended in DDW and administered orally (ig) to mice at doses of 5 or 20 mg/kg. Donepezil (5 mg/kg) was suspended in DDW and orally administered (ig) to the positive control group. The same volumes (0.1 mL/10 g) of DDW were given to the control group and model group. The experiment lasted for 13 days. The PTM-15, PGS32, donepezil and model groups were injected with scopolamine (2 mg/kg d 10–d 13, ip, suspended in saline) 30 min prior to the channel water maze test, whereas the control group was injected with the same volume of normal saline.
Seventy-two C57BL/6J mice were divided into six groups as follows: control group, model group, PGS32 groups (0.125, 0.5, or 2 mg/kg), and positive control group. PGS32 at different concentrations were suspended in DDW and administered orally (ig) to mice. Hup-A (0.05 mg/kg) was suspended in DDW and orally administered (ig) to the positive control group. The same volume (0.1 mL/10 g) of DDW was given to the control and model groups. The experiment lasted for 19 days. The PGS32, Hup-A, and model groups were injected with scopolamine (2 mg/kg, d 16–d 19, ip, suspended in saline) 30 min prior to the channel water maze test, whereas the control group was injected with the same volume of normal saline.
Step-through avoidance test
The step-through avoidance tests were conducted following procedures described previously with minor modification18. The apparatus contained a chamber with two compartments (dark/light), which were connected by a guillotine door. The dark compartment contained a stainless steel grid floor that could deliver electric shocks. During the acquisition trial, each mouse was placed in the light compartment. After 180 s of habituation, the guillotine door was raised, and the initial latency time of each mouse to enter the dark compartment was recorded. Immediately after the mouse entered the dark compartment, the guillotine door was closed, and an electric shock was delivered. Five seconds later, the mouse was removed and returned to its home cage. Twenty-four hours after training, the latency time was measured following the same procedures in the acquisition trial, but no electric shock was delivered. The number of times that the mouse entered the dark side was recorded as the number of errors. The latency time to enter the dark compartment was recorded up to 300 s.
Channel water maze test
The channel water maze test was performed as previously described with minor modifications19. A channel water maze (73 cm×42 cm×30 cm) made of black glass was designed by the Pharmacological Institute of the Chinese Academy of Medical Sciences. The tank was filled with water to a depth of 20 cm and maintained at 22±2 °C. There were four impasses and a platform at the terminal in the water maze. Mice could avoid drowning by climbing onto the platform.
On the first day of training, starting point A was cut off by a black bar. Each mouse was placed on the platform at the terminal for 15 s, and then it was placed on A with its head facing the bar. The mouse was allowed to swim freely twice for 2 min during each training session. The latency time to find the platform and the number of errors in which the mouse swam into impasse 1 were recorded. Any mouse that failed to find the platform after 2 min was guided to the platform, where it was placed for 15 s. On the second day of training, starting point B was cut off, and each mouse was placed on B; the latency time to find the platform and the number of errors in which the mouse swam into impasse 1, 2, or 3 were recorded. On the third day of training, each mouse was placed on C. The latency time to find the platform and the number of errors in which the mouse swam into impasse 1, 2, 3, or 4 were recorded. The fourth day was the testing day. Each mouse was placed on C; the latency time to find the platform and the number of errors in which the mouse swam into impasse 1, 2, 3, or 4 were recorded.
Western blotting
After channel water maze testing, six mice from each group were decapitated, and hippocampus samples from the mice were homogenized thoroughly and then lysed in RIPA lysis buffer [50 mmol/L Tris (pH 7.4), 150 mmol/L NaCl, 1% NP40, 0.5% sodium deoxycholate and 0.1% SDS]. Protein concentrations were measured with a BCA kit (Applygen Technologies, Beijing, China). The sample lysates were solubilized in SDS sample buffer, separated by SDS-PAGE, and transferred to a PVDF membrane as previously described20. The membrane was blocked with 3% BSA and incubated with primary antibody, followed by horseradish peroxidase-conjugated secondary antibody. Detection was performed using an enhanced ECL plus detection system. The relative density of the bands was analyzed by Gelpro 32 software.
Electrophysiological assays
Twelve Wistar rats (230−260 g), 3 months old, were randomly assigned to either the control group (n=6) or the PGS32 group (n=6). Recordings of evoked potentials, intracerebroventricular (icv) drug delivery, data collection and analysis were made as previously described21. In brief, the amplitude of the population spike (PS) was employed to assess the excitation level of the granular cell population in the dentate gyrus. The baseline was recorded for 30 min before long-term potentiation (LTP) was induced by high frequency stimulation [HFS: 10 bursts of 5 stimuli (at 100 Hz, 0.2 ms duration, 200 ms interburst interval)]. Twenty minutes after HFS, 5 μL 400 μmol/L PGS32 (icv, final concentration of 1 μmol/L) was injected into each test subject, while the same volume 0.4% DMSO-Normal Saline was injected into each control rat, and the intensity of LTP was recorded for another hour.
Statistical analyses
Data are expressed as the mean±SEM as indicated. The data were analyzed using Graph Pad Prism 5.0 (San Diego, CA, USA) and the SPSS 16.0 software (Chicago, IL, USA). Group differences in PS amplitude in the electrophysiological assays were analyzed using two-way analysis of variance (ANOVA) with repeated measures. Group differences in cytotoxicity assays and cognitive behavior tests were evaluated using one-way ANOVA followed by Duncan's multiple-range test. The accepted level of significance was set at P<0.05.
Results
Neuroptotective effects of P3 and P6 in in vitro models
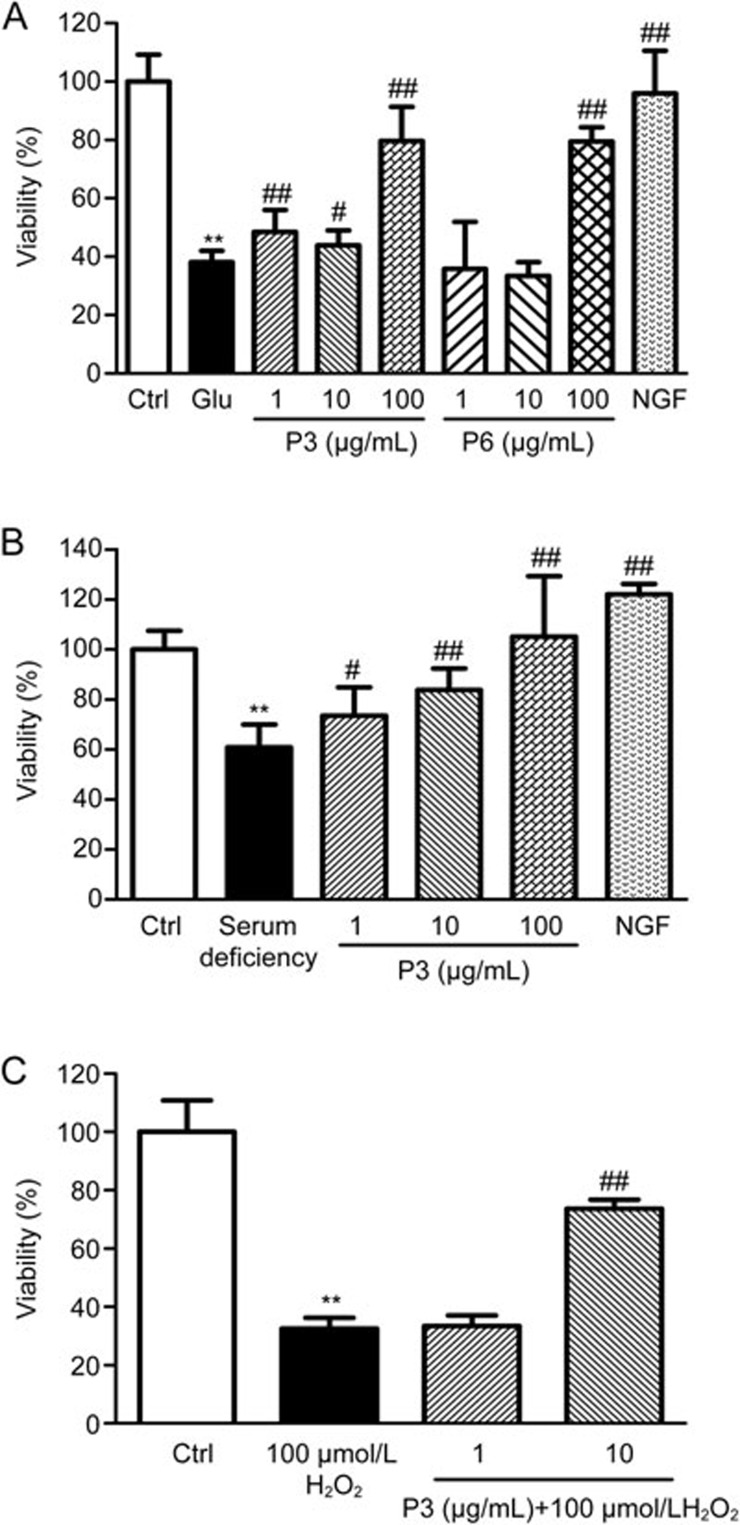
Figure 1A illustrates that treatment with 10 mmol/L glutamate resulted in a decrease in cell viability. P3 and P6 protected against the effects of glutamate on primary cortical neurons. P3 (1, 10, 100 μg/mL) significantly increased the cell survival rate (P<0.01, P<0.05, P<0.01), while P6 showed neuroprotective effects only at the concentration of 100 μg/mL (P<0.01). As shown in Figure 1B, P3 significantly increased the survival rate of primary cortical neurons under serum deficient conditions and did so in a dose-dependent manner (1 μg/mL, P<0.05; 10 μg/mL, P<0.01; 100 μg/mL, P<0.01). Figure 1C showed that the viability of PC12 cells exposed to 100 μmol/L H2O2 for 24 h was less than the viability in the normal group (P<0.01), and P3 showed neuroprotective effects against H2O2-induced PC12 cell death at a concentration of 10 μg/mL (P<0.01).
Figure 1.
(A) Effects of P3 and P6 on the survival rate of primary cortical neurons injured by glutamate. **P<0.01 vs Ctrl. #P<0.05, ##P<0.01 vs Glu. (B) Effects of P3 on the survival rate of primary cortical neurons injured by serum deficiency. **P<0.01 vs Ctrl. #P<0.05, ##P<0.01 vs serum deficiency. (C) Effects of P3 on the survival rate of PC12 cells injured by H2O2. Mean±SEM. n=9–11. **P<0.01 vs Ctrl. ##P<0.01 vs 100 μmol/L H2O2.
Effects of P3 and P6 on learning and memory performance in a scopolamine-induced amnesia model in mice
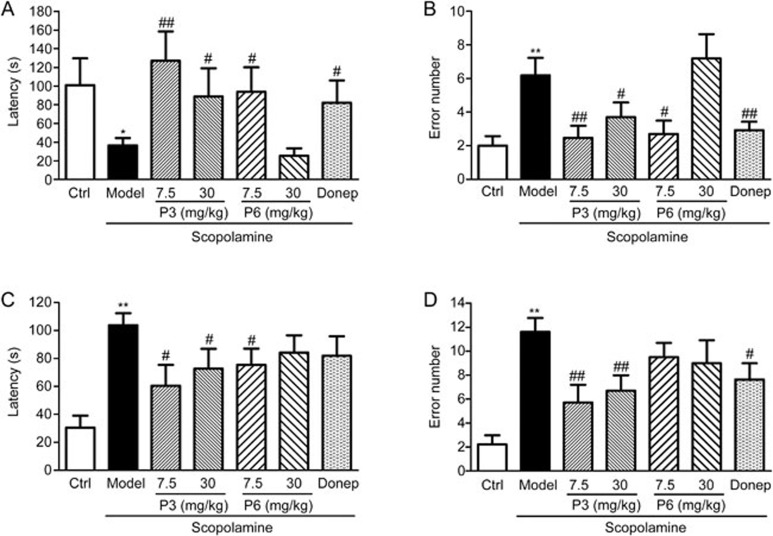
Increased latency times may serve as indicators of memory enhancement, whereas decreased latency times indicate memory impairment in the step-through avoidance test. No significant differences in step-through latency time were observed between groups during the acquisition trials (data not shown). During the retention trials, the control group mice hesitated to re-enter the dark compartment, but the model group mice re-entered the dark compartment more frequently (P<0.01). The model group mice showed shorter latency times (P<0.05). In both the 7.5 and the 30 mg/kg P3-treated groups, mice showed longer latency times and fewer error trials, while P6 only reversed scopolamine-induced memory impairments at a dose of 7.5 mg/kg. Additionally, similar patterns were observed following administration of 5 mg/kg donepezil, an acetylcholinesterase inhibitor that was used as a positive control (Figure 2A and 2B).
Figure 2.
(A) Effects of P3 and P6 on the latency time tested 24 h after training in the step-through passive avoidance test. Mean±SEM. n=11–12. (B) Effects of P3 and P6 on the error numbers tested 24 h after training in the step-through passive avoidance test. Mean±SEM. n=11–12. (C) Effects of P3 and P6 on the latency time after training in the channel water maze test. Mean±SEM. n=9–11. (D) Effects of P3 and P6 on the error numbers tested 24 h after training in the channel water maze test. Mean±SEM. n=9–11. *P<0.05, **P<0.01 vs Ctrl. #P<0.05, ##P<0.01 vs Model.
In the channel water maze test, the latency time for P3 and P6 treated mice to reach the platform significantly decreased compared with the mice in the model group, and the mice treated with P3 7.5 mg/kg had the best performance (Figure 2C). The number of error trials in the model group was higher than in the control group (P<0.01). The total numbers of errors declined significantly only in the P3-treated group (Figure 2D). These results suggest that P3 showed better neuroprotective and cognition-enhancing effects than did P6. Hence, we chose P3 for our further research.
Effects of PTM-15 and PGS32 on learning and memory performance in the scopolamine-induced amnesia model in mice
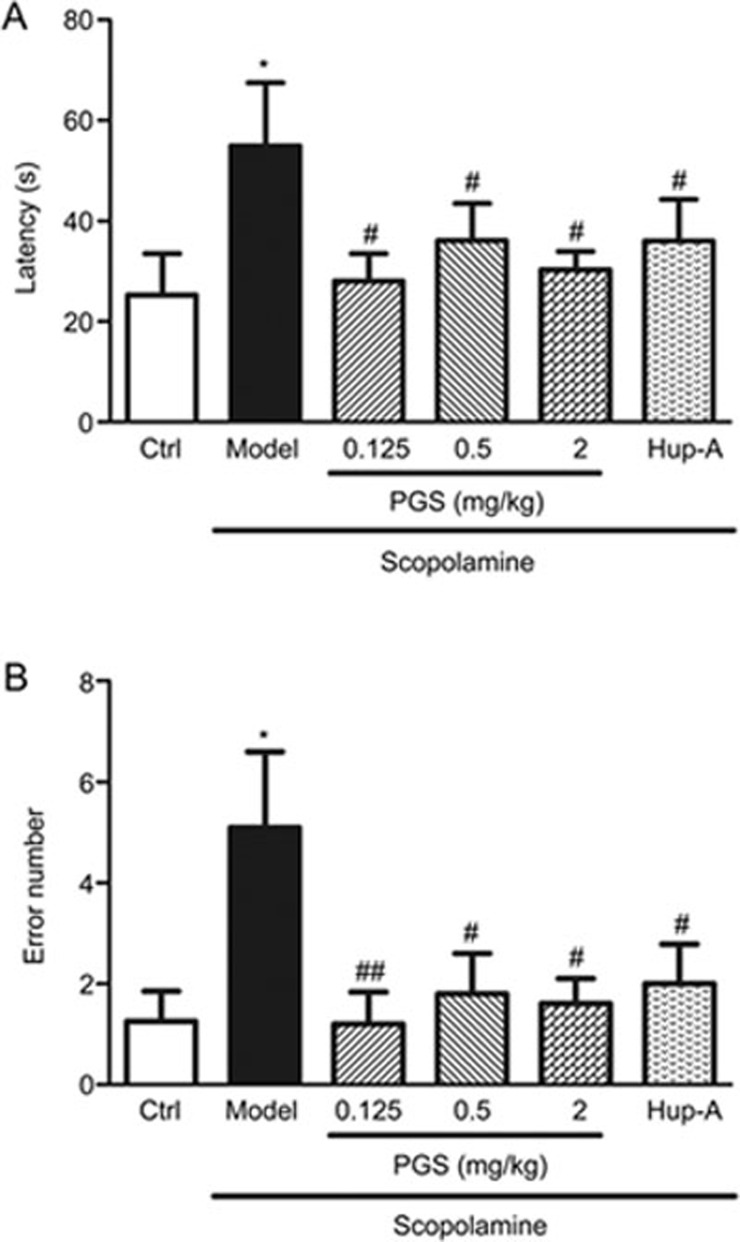
PTM-15 and PGS32 were further separated from P3. In the channel water maze test, we observed that the latency time and number of errors in mice treated by the administration of scopolamine (1 mg/kg) increased significantly compared to those of vehicle-treated control mice (P<0.01, P<0.01). Furthermore, the increased latency times and error numbers induced by the administration of scopolamine were significantly reversed by treatment with 5 mg/kg PGS32 (Figure 3). These results demonstrated that PGS32 attenuated scopolamine-induced learning and memory impairments in mice. In our behavioral test, the 20 mg/kg PGS32 treatment was not as effective as the 5 mg/kg treatment; these results suggested that a lower dose of PGS32 might be more effective. Thus, we designed a new experiment with a concentration gradient of 0.125, 0.5, and 2 mg/kg.
Figure 3.
Effects of PTM-15 and PGS32 on scopolamine-induced learning and memory deficits in the channel water maze test. (A) Effects of PTM-15 and PGS32 on the latency time tested 24 h after training in the channel water maze test. (B) Effects of PTM-15 and PGS32 on the error numbers tested 24 h after training in the channel water maze test. Mean±SEM. n=10–12. **P<0.01 vs Ctrl. ##P<0.01 vs Model.
Effects of PGS32 on learning and memory performance in the scopolamine-induced amnesia model in mice
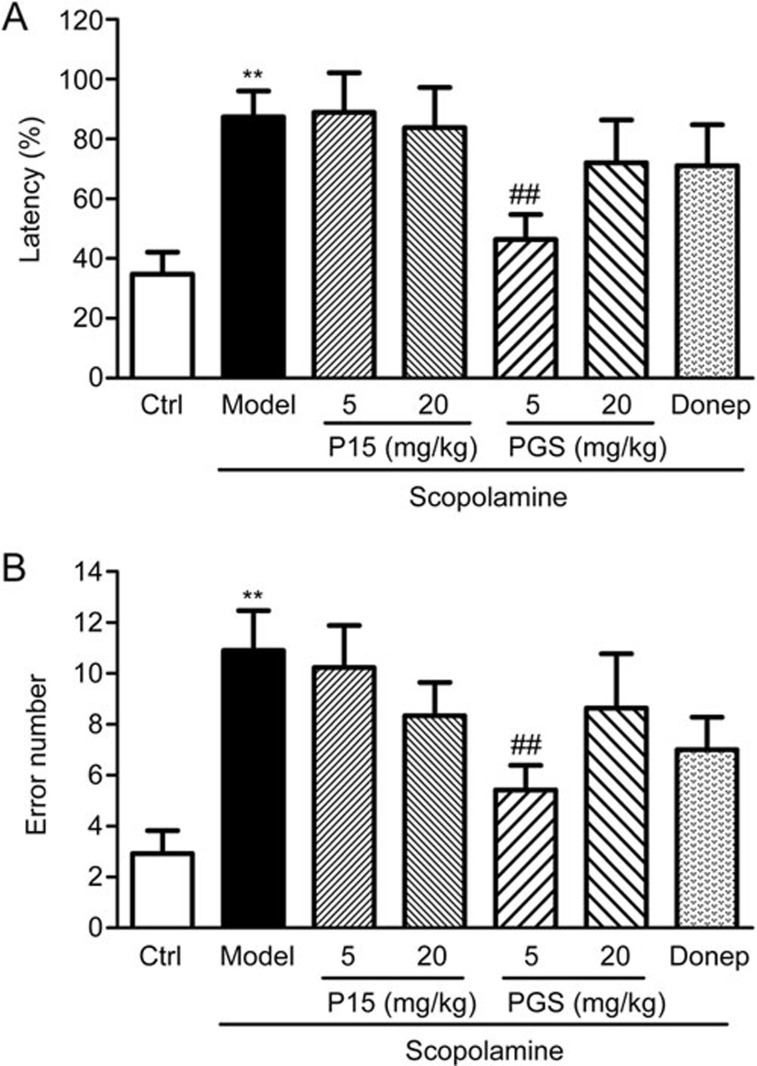
In the channel water maze test, we observed that the latency times and error numbers in mice treated with scopolamine (2 mg/kg) increased significantly compared to the vehicle-treated control mice (P<0.05, P<0.05). The latency times to reach the platform of PGS32 treated mice decreased significantly compared with the model group, and the total error numbers declined significantly in the PGS32-treated group. PGS32 (0.125, 0.5, and 2 mg/kg) treatment reversed the memory impairments induced by scopolamine. Furthermore, Hup-A treatment showed similar cognition-improving effects (Figure 4).
Figure 4.
Effects of PGS32 (0.125–2 mg/kg) on scopolamine-induced learning and memory deficits in the channel water maze test. (A) Effects of PGS32 on the latency time tested 24 h after training in the channel water maze test. (B) Effects of PGS32 on the error times tested 24 h after training in the channel water maze test. Mean±SEM. n=10–12. *P<0.05 vs Ctrl. #P<0.05, ##P<0.01 vs Model.
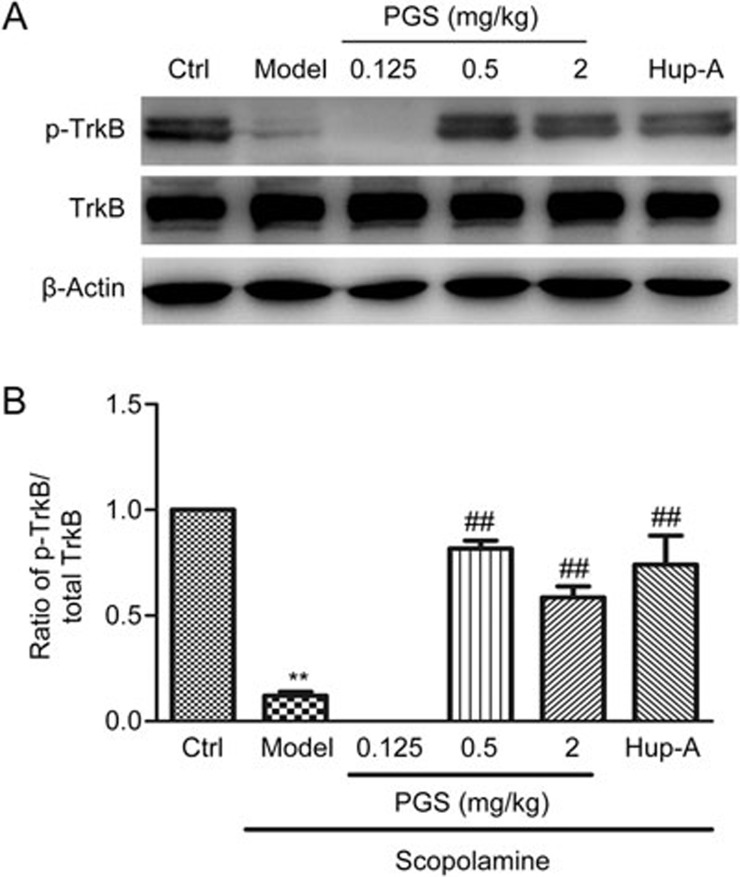
Effects of PGS32 on TrkB and p-TrkB in the hippocampal lysates of mice following the channel water maze test
The activation of TrkB by BDNF mediates intracellular signal transduction in which the phosphorylation of TrkB plays a pivotal role. Western blotting results showed that the level of p-TrkB was significantly decreased in the scopolamine-treated mice compared with the control group, whereas there was no significant difference in total TrkB expression between the groups. PGS32 (0.5 or 2 mg/kg) and Hup-A treatment significantly up-regulated p-TrkB compared with the model group (Figure 5).
Figure 5.
Effects of PGS32 (0.125–2 mg/kg) on the levels of phosphorylated TrkB and TrkB in the hippocampal lysates of mice following the channel water maze test. (A) Representative immunoblots of the hippocampal lysates of the mice. (B) The ratio of p-TrkB/t-TrkB after PGS32 treatment. Mean±SEM. n=6. **P<0.01 vs Ctrl. ##P<0.01 vs model.
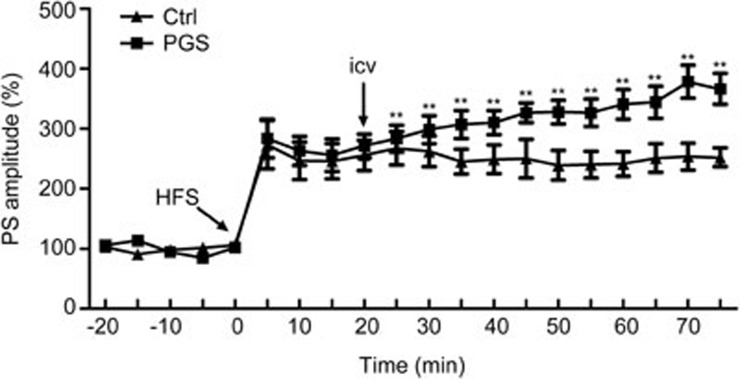
Effects of PGS32 on long-term potentiation induced by high-frequency stimulation in the dentate gyrus of anesthetized rats
LTP was induced by high frequency stimulation after a stable baseline of 20 min. Before treatment, there were no significant differences in HFS-induced PS amplitudes between these two groups (Control: 273%±40%; PGS32: 283%±32%). PGS32 or vehicle (DMSO) was injected into the lateral cerebral ventricle of rats 20 min after HFS. Compared with the control group, the PS amplitude of the PGS32 treatment group was enhanced significantly (P<0.01). PS amplitudes increased to 310%±20% within 20 min after PGS32 application, and at 40 min, 50 min, 55 min after PGS32 treatment, PS amplitudes increased to 340%±24%, 378%±27%, and 366%±26%, respectively. These results suggest that PGS32 significantly enhanced hippocampal LTP. Therefore, PGS32 may promote synaptic plasticity in the hippocampus (Figure 6).
Figure 6.
Effects of PGS32 on long-term potentiation induced by 10 bursts of 5 stimuli (100 Hz, 0.2 ms stimulus duration, 200 ms interburst interval) in the dentate gyrus of anesthetized rats. The baseline was recorded for 30 min before HFS. Twenty minutes after HFS-induced LTP, PGS32 1 μmol/L or 0.4% icy dimethyl sulfoxide (DMSO) was administered intracerebroventricularly, and the intensity of LTP was recorded for another hour. Mean±SEM. n=6. **P<0.01 vs Ctrl.
Discussion
In the current study, we demonstrated that P3 not only has neuroprotective effects on excitotoxicity, oxidative stress and serum deficiency induced damage but also has cognition-improving effects in scopolamine-induced amnesia model mice. Moreover, we separated P3 to yield PGS32, and we discovered that PGS32 reversed scopolamine-induced amnesia in mice at a dose of 0.125 mg/kg. These results suggest that PGS32 might be one of the active monomers in Polygalae Radix.
Glutamate and ROS can contribute to neuronal damage22. Compounds ameliorating these types of damage may be effective in treating neurodegenerative diseases23. We used MTT assays to screen for neuroprotective compounds. We discovered that P3 protected primary cortical neurons against serum deficiency and glutamate-induced damage and PC12 cells against H2O2-induced death. Several researchers have demonstrated that Polygalae Radix extract provides neuroprotection of PC12 cells against 6-hydroxydopamine (6-OHDA)-induced damage and of primary cortical neurons against Aβ25–35-induced cell damage24,25. These results suggest that P3 may be effective in the treatment of various neurodegenerative diseases.
Learning and memory impairments are primary clinical symptoms observed in all types of dementia26. We used a scopolamine-induced amnesia model to further investigate the efficacy of P3. As a muscarinic receptor antagonist, scopolamine causes profound memory impairments by antagonizing muscarinic cholinergic receptors in the central cholinergic systems27. Donepezil is a selective reversible inhibitor of acetylcholinesterase; Hup-A, an alkaloid isolated from the Chinese folk medicine Huperzia serrata, is a reversible and selective inhibitor of AChE5,28. These improve cognitive deficits in a broad range of animal models and have been used in treating Alzheimer's disease in the clinic. Avoidance step-through tests and channel water maze tests were used to evaluate learning and memory ability in rodents. We discovered that P3 showed cognition-enhancing effects in scopolamine-induced amnesia model in mice. To accelerate these developments, we separated polygala saponin monomers from P3. Of these polygala saponin monomers, PGS32 (1 μmol/L) showed neuroprotective effects against serum deficiency and glutamate induced damage in PC12 cells15; PGS32 (0.125, 0.5, and 2 mg/kg) treatments reversed the learning and memory impairments induced by scopolamine. We focused on PGS32 because it was more potent in vivo and in vitro, and its underlying mechanisms are discussed below.
Polygalae radix extract can protect against N-methyl D-aspartate (NMDA) neurotoxicity29 and induce the expression of brain-derived neurotrophic factor (BDNF)30, which is involved in the modulation of synaptic transmission and plasticity31. Activation of TrkB by BDNF results in the phosphorylation of several tyrosine residues and subsequently the stimulation of intracellular signal transduction pathways, such as the MAPK, PI3K and phospholipase C-γ pathways32. Phosphorylation of TrkB is associated with the recruitment of pro-survival signaling pathways that lead to long-term neuroprotection and improved cognition33. We have demonstrated previously that PGS32 can activate ERK and CREB and further increase the expression of BDNF34. In this study, we found that PGS32 up-regulated the phosphorylation of TrkB. It was reported that compared with the vehicle control rats, p-TrkB was significantly decreased in the scopolamine-infused rats35. Galantamine, a cholinergic stimulator used as a positive control, was shown to restore p-TrkB levels and improved cognitive functioning in AD-like rodent models in another study, which is inconsistent with our results. These results suggest that PGS32 may improve learning and memory by enhancing the BDNF/TrkB/MAPK cascade.
BDNF/TrkB signaling plays a fundamental role in both developmental and adult synaptic plasticity36. LTP of synaptic transmission in the hippocampus is the primary experimental model for investigating the synaptic basis of learning and memory in vertebrates37. Electrophysiological LTP assays are widely used in screening candidate cognition-improving drugs. Learning and memory can be studied at many levels, from the molecular to the behavioral. For our cognitive function test, which was a relatively long-term experiment, we chose oral administration to mimic the practical application of pharmaceutical preparations in the clinic. Oral administration is a mild and easy route for drug delivery. For the LTP measurement, we focused on the immediate impact of PGS32 on basic synaptic transmission to investigate the underlying mechanisms of its cognition improving effects, and we chose intracerebroventricular administration. Our previous studies have shown that PGS32 induces LTP in anesthetized adult rats, but the mechanisms of LTP maintenance remain unclear34. In this study, we used HFS to further understand the underlying mechanisms of the LTP enhancement of PGS32. The intensity of LTP after PGS32 injection was significantly enhanced compared to the control group after HFS. These results suggest that PGS32 not only can induce LTP but also can maintain LTP. PGS32 may promote synaptic plasticity in the hippocampus. Although we demonstrated that PGS32 has the ability to improve learning and memory and to promote hippocampal LTP, further studies in other animal models of dementia are necessary to confirm the protective effects of PGS32.
In summary, we have validated that PGS32, a triterpene saponin from Polygalae Radix, can ameliorate memory impairments induced by scopolamine in mice. The mechanisms by which PGS32 improves memory dysfunction appear to involve the protection of neurons from glutamate and ROS damage, the up-regulation of the phosphorylation of TrkB and the maintenance of LTP. These results suggest that PGS32, an active constituent of Polygalae Radix, may be a competitive candidate for the treatment of neurodegenerative disorders.
Author contribution
Heng ZHOU analyzed the data and wrote the paper; Wei XUE and Shi-feng CHU performed the research; Zhen-zhen WANG provided assistance with experimental methods and revised the paper; Dong-ming ZHANG and Chuang-jun LI extracted the PGS32; Yi-na JIANG, Lin-ming LUO and Piao LUO took part in the research; Gang LI provided comments and technical support; Nai-hong CHEN designed the study and revised the paper.
Acknowledgments
The authors thank Dr Yan HUANG (Beijing Institute of Pharmacology and Toxicology, Beijing, China) for his technical assistance. This work was supported by the National Natural Science Foundation of China (No 81173578, 81273629, 81373997, 81303250, 21272278, 81260650, and 81560685), the National Mega-project for Innovative Drugs (No 2012ZX09301002-004, 2012ZX09103101-006, and 2012ZX09301002-001), the Program for Changjiang Scholars and Innovative Research Team of the University (PCSIRT) (No IRT1007), the Beijing Natural Science Foundation (No 7142115), and the Beijing Key Laboratory of New Drug Mechanisms and Pharmacological Evaluation Study (No BZ0150).
References
- Gao HM, Hong JS. Why neurodegenerative diseases are progressive: uncontrolled inflammation drives disease progression. Trends Immunol 2008; 29: 357–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radi E, Formichi P, Battisti C, Federico A. Apoptosis and oxidative stress in neurodegenerative diseases. J Alzheimer's Dis 2014; 42 Suppl 3: S125–52. [DOI] [PubMed] [Google Scholar]
- Pan TH, Kondo S, Le WD, Jankovic J. The role of autophagy-lysosome pathway in neurodegeneration associated with Parkinson's disease. Brain 2008; 131: 1969–78. [DOI] [PubMed] [Google Scholar]
- Dorsey ER, George BP, Leff B, Willis AW. The coming crisis: obtaining care for the growing burden of neurodegenerative conditions. Neurology 2013; 80: 1989–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tayeb HO, Yang HD, Price BH, Tarazi FI. Pharmacotherapies for Alzheimer's disease: beyond cholinesterase inhibitors. Pharmacol Ther 2012; 134: 8–25. [DOI] [PubMed] [Google Scholar]
- Kim HG, Oh MS. Herbal medicines for the prevention and treatment of Alzheimer's disease. Curr Pharm Des 2012; 18: 57–75. [PubMed] [Google Scholar]
- Peng WD. Hypotensive effect of tenuifolic saponin and its mechanism. Acta Pharmacol Sin 1999; 20: 639–42. [PubMed] [Google Scholar]
- Cheong MH, Lee SR, Yoo HS, Jeong JW, Kim GY, Kim WJ, et al. Anti-inflammatory effects of Polygalae Radix through inhibition of NF-κB activation in lipopolysaccharide-induced BV2 microglial cells. J Ethnopharmacol 2011; 137: 1402–8. [DOI] [PubMed] [Google Scholar]
- Shin IJ, Son SU, Park H, Kim Y, Park SH, Swanberg K, et al. Preclinical evidence of rapid-onset antidepressant-like effect in Radix Polygalae extract. PLoS One 2014; 9: e 88617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YJ, Huang XB, Li ZX, Yin LL, Chen WQ, Li L. Tenuigenin protects cultured hippocampal neurons against methylglyoxal-induced neurotoxicity. Eur J Pharmacol 2010; 645: 1–8. [DOI] [PubMed] [Google Scholar]
- Ikeya Y, Takeda S, Tunakawa M, Karakida H, Toda K, Yamaguchi T, et al. Cognitive improving and cerebral protective effects of acylated oligosaccharides in Polygala tenuifolia. Biol Pharm Bull 2004; 27: 1081–5. [DOI] [PubMed] [Google Scholar]
- Park CH, Choi SH, Koo JW, Seo JH, Kim HS, Jeong SJ, et al. Novel cognitive improving and neuroprotective activities of Polygala tenuifolia Willdenow extract, BT-11. J Neurosci Res 2002; 70: 484–92. [DOI] [PubMed] [Google Scholar]
- Lee JY, Kim KY, Shin KY, Won BY, Jung HY, Suh YH. Effects of BT-11 on memory in healthy humans. Neurosci Lett 2009; 454: 111–4. [DOI] [PubMed] [Google Scholar]
- Shin KY, Lee JY, Won BY, Jung HY, Chang KA, Koppula S, et al. BT-11 is effective for enhancing cognitive functions in the elderly humans. Neurosci Lett 2009; 465: 157–9. [DOI] [PubMed] [Google Scholar]
- Li CJ, Yang JZ, Yu SS, Chen NH, Xue W, Hu JF, et al. Triterpenoid saponins with neuroprotective effects from the roots of Polygala tenuifolia. Planta Med 2008; 74: 133–41. [DOI] [PubMed] [Google Scholar]
- Zhang D, Miyase T, Kuroyanagi M, Umehara K, Ueno A. Five new triterpene saponins, polygalasaponins XXVIII-XXXII from the root of Polygala japonica Houtt. Chem Pharm Bull (Tokyo) 1996; 44: 810–5. [DOI] [PubMed] [Google Scholar]
- Song XY, Hu JF, Sun MN, Li ZP, Wu DH, Ji HJ, et al. IMM-H004, a novel coumarin derivative compound, protects against amyloid beta-induced neurotoxicity through a mitochondrial-dependent pathway. Neuroscience 2013; 242: 28–38. [DOI] [PubMed] [Google Scholar]
- Lin HB, Yang XM, Li TJ, Cheng YF, Zhang HT, Xu JP. Memory deficits and neurochemical changes induced by C-reactive protein in rats: implication in Alzheimer's disease. Psychopharmacology 2009; 204: 705–14. [DOI] [PubMed] [Google Scholar]
- Xi S, Sun W, Wang F, Jin Y, Sun G. Transplacental and early life exposure to inorganic arsenic affected development and behavior in offspring rats. Arch Toxicol 2009; 83: 549–56. [DOI] [PubMed] [Google Scholar]
- Wang ZZ, Yang WX, Zhang Y, Zhao N, Zhang YZ, Liu YQ, et al. Phosphodiesterase-4D knock-down in the prefrontal cortex alleviates chronic unpredictable stress-induced depressive-like behaviors and memory deficits in mice. Sci Rep 2015; 5: 11332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun F, Sun JD, Han N, Li CJ, Yuan YH, Zhang DM, et al. Polygalasaponin F induces long-term potentiation in adult rat hippocampus via NMDA receptor activation. Acta Pharmacol Sin 2012; 33: 431–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JW, Ning N, Ma YZ, Zhang R, Tan F, Chen NH. Claulansine F suppresses apoptosis induced by sodium nitroprusside in PC12 cells. Free Radical Res 2013; 47: 488–97. [DOI] [PubMed] [Google Scholar]
- Hu JF, Chu SF, Ning N, Yuan YH, Xue W, Chen NH, et al. Protective effect of (–)clausenamide against Abeta-induced neurotoxicity in differentiated PC12 cells. Neurosci Lett 2010; 483: 78–82. [DOI] [PubMed] [Google Scholar]
- Choi JG, Kim HG, Kim MC, Yang WM, Huh Y, Kim SY, et al. Polygalae radix inhibits toxin-induced neuronal death in the Parkinson's disease models. J Ethnopharmacol 2011; 134: 414–21. [DOI] [PubMed] [Google Scholar]
- Naito R, Tohda C. Characterization of anti-neurodegenerative effects of Polygala tenuifolia in Abeta(25–35)-treated cortical neurons. Biol Pharm Bull 2006; 29: 1892–6. [DOI] [PubMed] [Google Scholar]
- Khan ZU, Martin-Montanez E, Navarro-Lobato I, Muly EC. Memory deficits in aging and neurological diseases. Prog Mol Biol Transl Sci 2014; 122: 1–29. [DOI] [PubMed] [Google Scholar]
- Ebert U, Kirch W. Scopolamine model of dementia: electroencephalogram findings and cognitive performance. Eur J Clin Invest 1998; 28: 944–9. [DOI] [PubMed] [Google Scholar]
- Wang R, Yan H, Tang XC. Progress in studies of huperzine A, a natural cholinesterase inhibitor from Chinese herbal medicine. Acta Pharmacol Sin 2006; 27: 1–26. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Ban JY, Koh SB, Seong NS, Song KS, Bae KW, et al. Polygalae radix extract protects cultured rat granule cells against damage induced by NMDA. Am J Chin Med 2004; 32: 599–610. [DOI] [PubMed] [Google Scholar]
- Hu Y, Liu P, Guo DH, Rahman K, Wang DX, Xie TT. Antidepressant effects of the extract YZ-50 from Polygala tenuifolia in chronic mild stress treated rats and its possible mechanisms. Pharm Biol 2010; 48: 794–800. [DOI] [PubMed] [Google Scholar]
- Korte M, Carroll P, Wolf E, Brem G, Thoenen H, Bonhoeffer T. Hippocampal long-term potentiation is impaired in mice lacking brain-derived neurotrophic factor. Proc Natl Acad Sci U S A 1995; 92: 8856–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luikart BW, Zhang W, Wayman GA, Kwon CH, Westbrook GL, Parada LF. Neurotrophin-dependent dendritic filopodial motility: a convergence on PI3K signaling. J Neurosci 2008; 28: 7006–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uluç K, Kendigelen P, Fidan E, Zhang L, Chanana V, Kintner D, et al. TrkB receptor agonist 7, 8 dihydroxyflavone triggers profound gender-dependent neuroprotection in mice after perinatal hypoxia and ischemia. CNS Neurol Disorders-Drug 2013; 12: 360–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue W, Hu JF, Yuan YH, Sun JD, Li BY, Zhang DM, et al. Polygalasaponin XXXII from Polygalae Radix improves hippocampal-dependent learning and memory. Acta Pharmacol Sin 2009; 30: 1211–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Luo X, Liu X, Liu D, Wang X, Guo Z, et al. Intraperitoneal administration of a novel TAT-BDNF peptide ameliorates cognitive impairments via modulating multiple pathways in two Alzheimer's rodent models. Sci Rep 2015; 5: 15032–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler WJ, Alonso M, Bramham CR, Pozzo-Miller LD. From acquisition to consolidation: on the role of brain-derived neurotrophic factor signaling in hippocampal-dependent learning. Learn Memory 2002; 9: 224–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature 1993; 361: 31–9. [DOI] [PubMed] [Google Scholar]