Abstract
Aim:
The aim of this study was to examine the activation of neuronal Kv7/KCNQ channels by a novel modified Kv7 opener QO58-lysine and to test the anti-nociceptive effects of QO58-lysine on inflammatory pain in rodent models.
Methods:
Assays including whole-cell patch clamp recordings, HPLC, and in vivo pain behavioral evaluations were employed.
Results:
QO58-lysine caused instant activation of Kv7.2/7.3 currents, and increasing the dose of QO58-lysine resulted in a dose-dependent activation of Kv7.2/Kv7.3 currents with an EC50 of 1.2±0.2 μmol/L. QO58-lysine caused a leftward shift of the voltage-dependent activation of Kv7.2/Kv7.3 to a hyperpolarized potential at V1/2=-54.4±2.5 mV from V1/2=-26.0±0.6 mV. The half-life in plasma (t1/2) was derived as 2.9, 2.7, and 3.0 h for doses of 12.5, 25, and 50 mg/kg, respectively. The absolute bioavailabilities for the three doses (12.5, 25, and 50 mg/kg) of QO58-lysine (po) were determined as 13.7%, 24.3%, and 39.3%, respectively. QO58-lysine caused a concentration-dependent reduction in the licking times during phase II pain induced by the injection of formalin into the mouse hindpaw. In the Complete Freund's adjuvant (CFA)-induced inflammatory pain model in rats, oral or intraperitoneal administration of QO58-lysine resulted in a dose-dependent increase in the paw withdrawal threshold, and the anti-nociceptive effect on mechanical allodynia could be reversed by the channel-specific blocker XE991 (3 mg/kg).
Conclusion:
Taken together, our findings show that a modified QO58 compound (QO58-lysine) can specifically activate Kv7.2/7.3/M-channels. Oral or intraperitoneal administration of QO58-lysine, which has improved bioavailability and a half-life of approximately 3 h in plasma, can reverse inflammatory pain in rodent animal models.
Keywords: QO58-lysine, Kv7/KCNQ, M-channel, pharmacokinetics, XE991, retigabine, inflammatory pain
Introduction
Neuronal Kv7/KCNQ/M-type potassium channels, activated by membrane depolarization, mediate low-threshold, slowly activating and non-deactivating outward potassium (K+) currents that hyperpolarize cell membranes and exert inhibitory control over neuronal hyperexcitability1,2. Neuronal hyperexcitability defines the fundamental mechanism of many neurological diseases such as epilepsy and pain2,3,4,5. Loss-of-function mutations in KCNQ2/3 or M-channels can cause myokymia and benign familial neonatal convulsions, a neonatal form of epilepsy3. Therefore, the pharmacological activation of KCNQ2/3 channels by specific channel openers can provide therapeutic benefits for the treatment of epilepsy, pain and possibly other neuropsychiatric disorders2,6.
Chemical agents designed to activate KCNQ/M-channels have been previously identified and developed, including the first KCNQ2/3 opener retigabine (Potiga), which was approved by the FDA in 2011 for the treatment of partial epilepsy7,8,9. Because of the broad action of retigabine on all neuronal Kv7 channels, retigabine has also been tested for the treatment of anxiety, pain, ophthalmic diseases and tinnitus in animal models6,10,11,12,13,14,15,16,17. However, because retigabine acts on all neuronal Kv7 channels and has potential side effects, efforts have been devoted to searching for more specific KCNQ2/3 channel openers, such as zinc pyrithione, which can rescue the channel mutants18, and PF-05020182, which suppresses convulsions in animals19.
We recently designed and synthesized a novel series of pyrazolo[1, 5-a]pyrimidin-7(4H)-ones (PPOs) that can selectively activate KCNQ/M-channels20. The lead compound 5-(2,6-dichloro-5-fluoropyridin-3-yl)-3-phenyl-2-(trifluoromethyl) pyrazolo[1,5-a]pyrimidin-7(4H)-one (QO-58) of the PPO series shows anti-seizure and anti-nociceptive effects in a model of sciatic nerve chronic constriction injury (CCI)21. However, the compound QO-58 series has issues with low lipophilicity and hydrophilicity, which results in low bioavailability and irregular absorption after oral administration22. To increase the solubility of QO-58, we synthesized the salt form of the compound, QO58-lysine, based on QO-58 (Figure 1A). We also developed a simple, reliable and efficient assay to analyze the bioavailability of intravenous QO58-lysine in rat plasma using high-performance liquid chromatography with UV detection23.
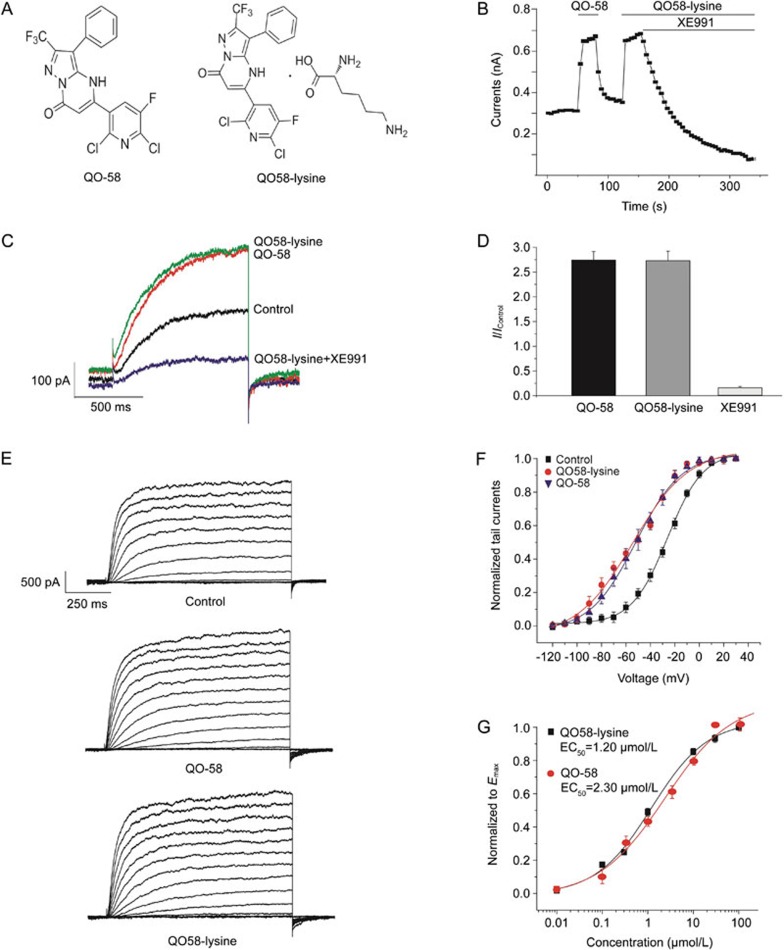
Figure 1.
Dose- and voltage-dependent activation of Kv7.2/Kv7.3 channels by QO58-lysine. (A) Chemical structure of QO58-lysine. (B) Whole-cell patch clamp recordings of Kv7.2/Kv7.3 channel activation by either QO58-lysine (10 μmol/L) or QO-58 compound (10 μmol/L); the current was blocked by XE991 (0.3 μmol/L). HEK293 cells were held at −80 mV for 50 s and stepped to −40 mV (100 s). (C) Representative outward currents elicited by step depolarization to −40 mV from a holding potential of −80 mV in the presence of QO-58 (10 μmol/L), QO58-lysine (10 μmol/L) and XE991 (0.3 μmol/L). (D) Summary of the data from B. (E) Comparison of the voltage-dependent activation of Kv7.2/Kv7.3 channels by QO-58 or QO58-lysine. The typical outward currents were elicited by depolarizing voltage steps from −120 to +30 mV in 10 mV increments and a holding potential of −100 mV in the presence of QO-58 (10 μmol/L) or QO58-lysine (10 μmol/L). (F) Voltage-dependent activation curves for Kv7.2/Kv7.3 currents were generated from tail currents (at −100 mV) and were fitted with a Boltzmann function. QO58-lysine and QO-58 caused the leftward shift of activation curves to −54.4±2.5 mV (V1/2) and −51.9±1.7 mV (V1/2) from −26.0±0.6 mV (V1/2), respectively (n=5, P<0.05). (G) Comparison of the dose-dependent activation of Kv7.2/Kv7.3 currents by QO-58 and QO58-lysine in HEK293 cells held at −80 mV and depolarized at −40 mV. The dose-response curves were fitted with a logistic function. The EC50 for QO-58 was 2.3±0.8 μmol/L, and the slope factor was 0.6±0.1 (n=6). The EC50 for QO58-lysine was 1.2±0.2 μmol/L, and the slope factor was 0.7±0.1 (n=5–8).
In this study, we took advantage of the validated HPLC assay and determined the oral bioavailability of QO58-lysine in rats as well as tested the pharmacological effects of QO58-lysine on inflammatory pain induced by formalin or Complete Freund's adjuvant (CFA) in rodent models. Our findings show that the elimination half-time of the enteric compound QO58-lysine was approximately 3 h. Oral or peritoneal administration of QO58-lysine resulted in anti-nociceptive effects that could be reversed by the specific channel blocker XE991.
Materials and methods
Chemicals
QO58-lysine (purity at 98.5%) and retigabine (purity >98% by HPLC-DAD) were synthesized at the Department of New Drugs Development, School of Pharmacy, Hebei Medical University, and their purity was verified using MS and NMR analysis20. XE991 was purchased from Sigma Aldrich (St Louis, MO, USA). Nitrendipine (purity at 99.0%), used as an internal standard (IS), was purchased from the National Institutes for Food and Drug Control (Beijing, China). Tween-80 (chemical purity) was purchased from Sinopharm Chemical Reagent Co, Ltd (Shanghai, China). Heparin sodium (titer≥175 USP units/mg) was purchased from Beijing Solarbio Science & Technology Co, Ltd (Beijing, China). HPLC-grade acetonitrile, methanol and analytical-grade ammonium acetate were purchased from Dikma Technologies Inc (Beijing, China). CFA and chloral hydrate were purchased from Sigma-Aldrich. Ultrapure water (18.2 MΩ) was obtained from a Milli-Q plus PF system (Merck Millipore, Shanghai, China).
Electrophysiology
Stable HEK293 cells expressing Kv7.2/7.3 channels were grown in DMEM supplemented with 10% fetal calf serum, 1× non-essential amino acids, 600 mg/mL G418 and 600 mg/mL hygromycin B. Plasmids encoding human Kv7.2 and rat Kv7.3 (GenBank accession numbers: AF110020 and AF091247, respectively) were kindly provided by Diomedes E Logothetis (Virginia Commonwealth University, Richmond, VA, USA).
For measurements of current in the HEK293 cells, recordings were performed using the perforated (amphotericin B, 250 mg/mL, Sigma, St Louis, MO, USA) patch-clamp technique at room temperature. The signals were amplified using an HEAK EPC10 patch-clamp amplifier. The acquisition rate was 10 kHz, and signals were filtered at 2.5 kHz. Patch electrodes were pulled with a micropipette puller (Sutter Instruments, Novato, CA, USA) and fire polished to a final resistance of 1–2 MΩ. Series resistances were compensated by 60%–80%. The internal and external solution for the cell recording was as follows (in mmol/L): 150 KCl, 5 MgCl2, 10 HEPES, and pH 7.4 adjusted with KOH and 160 NaCl, 2.5 KCl, 1 MgCl2, 2 CaCl2, 10 glucose, 20 HEPES and pH 7.4 adjusted with NaOH, respectively21.
Pharmacokinetic analysis
Sprague-Dawley (SD) rats (200–220 g) were fasted for 12 h with water ad libitum. Then, QO58-lysine (12.5, 25, and 50 mg/kg) was orally administered. Blood samples were collected from the posterior orbital venous plexus into heparinized tubes at time points of 0.5, 1, 2, 3, 4, 6, 8, 10, 12, 18, and 24 h after administration. All samples were immediately centrifuged at 4000 rounds per minute for 15 min, and the plasma was separated and stored at −20 °C.
Rat plasma was thawed at room temperature prior to the HPLC assay. Then, 50 μL of IS (Nitrendipine, 50 μg/mL) was added to 100 μL of plasma and vortexed for 15 s. Subsequently 400 μL of acetonitrile was added to the sample, and the solution was vortexed for 1 min. Following centrifugation at 13 000 rounds per minute for 10 min at room temperature, the supernatant was transferred to another tube and gently desiccated by nitrogen at 45 °C. The sample was reconstituted with H2O-acetonitrile premix and was vortexed for 30 s.
All chromatography measurements were conducted using a Waters Alliance HPLC system consisting of a 2694 solvent management system, 2998 photodiode array detector and Empower chromatography workstation. The chromatographic column used in this study was a reversed-phase DiamonsilTM C18 column (150 mm×4.6 mm, 5 μm) equipped with EasyGuard II C18 Replacement Cartridges (Dikma Technologies Inc).
The HPLC column was preheated to 35 °C, and the operation conditions were as follows: the mobile phase was 0.2 mol/L ammonium acetate in H2O-acetonitrile (40:60, v/v), the flow rate was 1 mL/min, and the volume of one injection was 10 μL. The ultraviolet wavelength was set to 285 nm for detection.
Rodent models of pain
Adult male SD rats (200–220 g) and the ICR mice (18–22 g) were provided by the Department of Laboratory Animal Sciences, Peking University Health Science Center. All experimental animal procedures used in this study conformed to the Guidelines of the Committee of Research and Ethical Issues of International Association and were approved by the Animal Care and Use Committee of Peking University Health Science Center. Animals were housed under a 12-h alternating light/dark cycle with food and water available ad libitum. All behavioral experiments were conducted by an investigator that was blinded to the drug treatments.
Formalin-induced pain in mice
Formalin (5% methanol solution, 10 μL) was carefully injected into the plantar surface of the hindpaw using a microsyringe. After injection, licking and biting behaviors were measured. According to the nature of formalin induced inflammation, the nociceptive response, expressed by the sum of licking and biting duration, was defined as two distinct phases: phase I (0–5 min) and phase II (15–40 min). Mice in the control group were administered 10% Tween-80-Saline (0.1 mL/10 g body weight). As positive controls, morphine (3.5 mg/kg, sc) or retigabine (25 mg/kg, po) were administered 15 min or 1 h before formalin injection, respectively. In the experimental groups, doses of 12.5, 25, and 50 mg/kg QO58-lysine were orally administered for 2, 4, 6, or 8 h before the formalin was injected into the plantar surface of the hindpaw, and licking and biting behaviors were immediately observed.
CFA-induced pain in rats
Fifty percent CFA was freshly diluted in saline. SD rats were anaesthetized with 10% chloral hydrate. For the experimental groups, 100 μL of CFA was injected into the planter surface of the left hindpaw. For the control groups, 100 μL of saline was injected into the same spot. The mechanical and thermal tests were performed each day for 9 d, and the results of the pilot study showed that the most prominent changes in nociceptive behavior were obtained at 24 h.
Twenty-four hours after CFA injection, retigabine (25 mg/kg) or QO58-lysine (12.5, 25, and 50 mg/kg) was orally administered, and rats in the CFA model group were administered 10% Tween-80-Saline (0.5 mL/100 g body weight, po). The mechanical, thermal and postural tests were performed at time points 0, 0.5, 1, 2, 3, 4, 6, 8, 12, and 18 h, which are the same time points used for the pharmacokinetic research.
Before all behavioral tests, the rats were allowed to adapt to the environment for at least 15 min. In the mechanical test, an electronic von Frey hair (IITC Inc Life Science, USA) apparatus was applied to the inflamed hindpaw of the experimental rats. Mechanical pain sensitivity, measured as the paw withdrawal threshold, was recorded when rats withdrew their paws in response to mechanical pressure applied to the paw. In the thermal test, the irritated hindpaw of the rat was illuminated/heated by a fully automatic plantar analgesia meter (Institute of Biomedical Engineering, CAMS, China), and the hindpaw withdraw latency, the index for thermal hyper-analgesia, was recorded. In the postural test, irritated rats were mounted on an incapacitance analgesia meter (Institute of Biomedical Engineering, CAMS, China), and the weight bearing difference between the affected limb and its counterpart was automatically recorded. The measurement was performed for 20 s and is expressed as the weight difference summation (g). All three tests were separately performed with different animals.
Antagonistic effect of XE991 on QO58-lysine induced anti-nociception
To confirm the contribution of the KCNQ channel to the action of QO58-lysine, a specific KCNQ channel blocker, XE991, was used. All compounds were intraperitoneally administered at 24 h after CFA injection, and rats in the CFA group were administered 10% Tween-80-Saline (0.2 mL/100 g body weight, ip). The mechanical test was performed at time points of 0, 0.5, 1, 1.5, 2, 2.5, and 3 h following intraperitoneal administration.
Locomotor activity
Locomotor activity was measured in four identical chambers (25 cm×25 cm×45 cm, without ceiling) that were soundproofed in closed cabinets using Digbehv spontaneous activity monitors (DigBehv-LG, Shanghai Jiliang Software Technology Co Ltd, China). Horizontal locomotor activity was recorded with a video camera placed above the chamber and analyzed with Digbehv software (version 2.0, Shanghai Jiliang Software Technology Co Ltd, China)13,24.
Mice were individually placed in a cage for 10 min to allow for adaptation to the new environment. QO58-lysine (5, 10, and 20 mg/kg), retigabine (5 mg/kg) or vehicle (10% Tween-80 in 0.9% NaCl) was intraperitoneally administered immediately before testing. The test duration was 60 min.
Statistical analysis
The data are presented as the mean±SEM and were analyzed using Student's t-test or analysis of variance (ANOVA). DAS 2.1.1 was used to analyze the HPLC data, and Graph Pad Prism 5 software was used for behavioral data analysis. Statistical significance was considered as P<0.05.
Results
Dose-dependent activation of Kv7.2/7.3 channels by the compound QO58-lysine
The compound QO58-lysine, a salt form of the original QO-58 series, was synthesized by reacting QO-58 with an equal molar amount of L-lysine (Figure 1A). Evaluation of the QO58-lysine compound was conducted using whole-cell patch clamp recordings of Kv7.2/Kv7.3 channels stably expressed in HEK293 cells (Figure 1B). Both QO-58 and the QO58-lysine (10 μmol/L) caused instant activation of Kv7.2/7.3 currents, depolarized at −40 mV, upon extracellular administration; the effect could be washed out before further addition of QO-58 (10 μmol/L), and the QO58-lysine activated current could be blocked using the specific blocker XE991 (Figure 1B–1D). Similar to the original QO-58 compound (10 μmol/L), QO58-lysine (10 μmol/L) caused a leftward shift in the voltage-dependent activation of Kv7.2/Kv7.3 to a hyperpolarized potential at −54.4±2.5 mV (V1/2) from −26.0±0.6 mV (V1/2), and a saturation at approximately 0 mV (Figure 1E and 1F). Increasing the amount of QO58-lysine or QO-58 resulted in a dose-dependent activation of Kv7.2/Kv7.3 currents, with an EC50 at 1.2±0.2 μmol/L and a slope factor of 0.7±0.1 or an EC50 at 2.3±0.8 μmol/L and a slope factor of 0.6±0.1, respectively (Figure 1G). These results confirm that the modified QO58-lysine can directly activate Kv7.2/Kv7.3 channels in the same way as the original QO-58 compound.
Pharmacokinetic profile of QO58-lysine compound in rat plasma
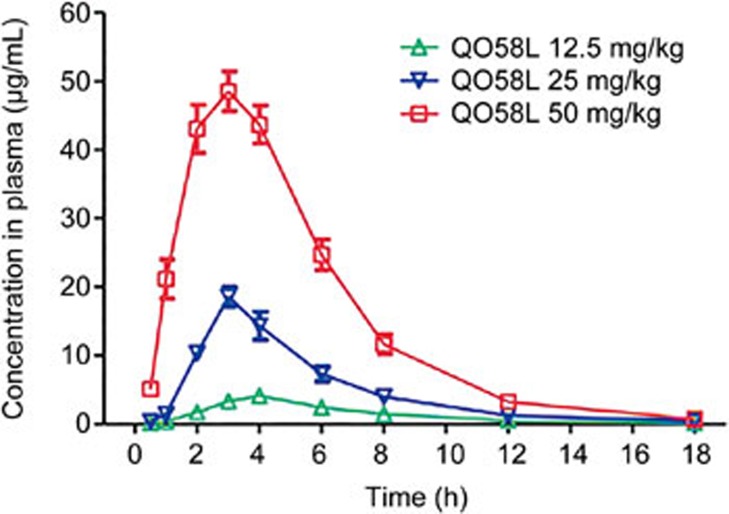
To determine the pharmacokinetic parameters for QO58-lysine, we utilized the HPLC assay that was previously validated in the pharmacokinetic study of intravenous QO58-lysine in rat with the elimination half-life of 2.2 h23. In this study, we determined the concentration-time parameter of oral QO58-lysine at different doses (12.5, 25, or 50 mg/kg). As shown in Figure 2, the time at which the peak concentration (Tmax) was reached at 3.8 h for 12.5 mg/kg, 3.0 h for 25 mg/kg and 3.2 h for 50 mg/kg. The peak concentration (Cmax) of QO58-lysine in plasma was obtained as 4.3 μg/mL for a dose of 12.5 mg/kg, 18.6 μg/mL for 25 mg/kg and 49.8 μg/mL for 50 mg/kg. As summarized in Table 1, the pharmacokinetic parameters were assessed using the non-compartment model, and the half-life in plasma (t1/2) was derived as 2.9, 2.7, and 3.0 h for doses of 12.5, 25, and 50 mg/kg, respectively. We also calculated the bioavailability based on oral QO58-lysine. The absolute bioavailabilities for the three doses (12.5, 25, and 50 mg/kg) of QO58-lysine (po) were determined as 13.7%, 24.3% and 39.3%, respectively, which were higher than 26.5% for a dose of 50 mg/kg of the QO-58 compound22. These results indicate that the modified salt form QO58-lysine is more easily absorbed than the original QO-58.
Figure 2.
Bioavailability after the oral administration of a single dose of QO58-lysine in rats. QO58-lysine compound was orally administered at different concentrations (12.5, 25, and 50 mg/kg) in SD rats. Blood was collected from the posterior orbital venous plexus during a period of 18 h after oral QO58-lysine. The QO58-lysine concentration in plasma was determined by HPLC. The data are expressed as the mean±SEM (n=5).
Table 1. Pharmacokinetic parameters of QO58-lysine after oral administration of different doses in rats. Data are expressed as mean±SD (n=5).
| Parameters | 12.5 mg/kg | 25 mg/kg | 50 mg/kg |
|---|---|---|---|
| t1/2 (h) | 2.89±0.53 | 2.72±0.97 | 2.98±0.52 |
| Tmax (h) | 3.83±0.41 | 3.00±0.00 | 3.20±0.45 |
| Cmax (mg/L) | 4.34±0.13 | 18.57±3.00 | 49.78±6.12 |
| Vz/F (L/kg) | 2.12±0.36 | 1.18±0.56 | 0.77±0.15 |
| CLz/F (L-h−1-kg−1) | 0.51±0.04 | 0.29±0.04 | 0.18±0.02 |
| AUC(0–t) (mg/L-h) | 24.03±1.99 | 85.84±14.32 | 281.01±30.25 |
| AUC(0–∞) (mg/L-h) | 24.68±2.11 | 87.30±13.19 | 281.85±30.29 |
Anti-nociceptive effects of QO58-lysine on formalin-induced pain in mice
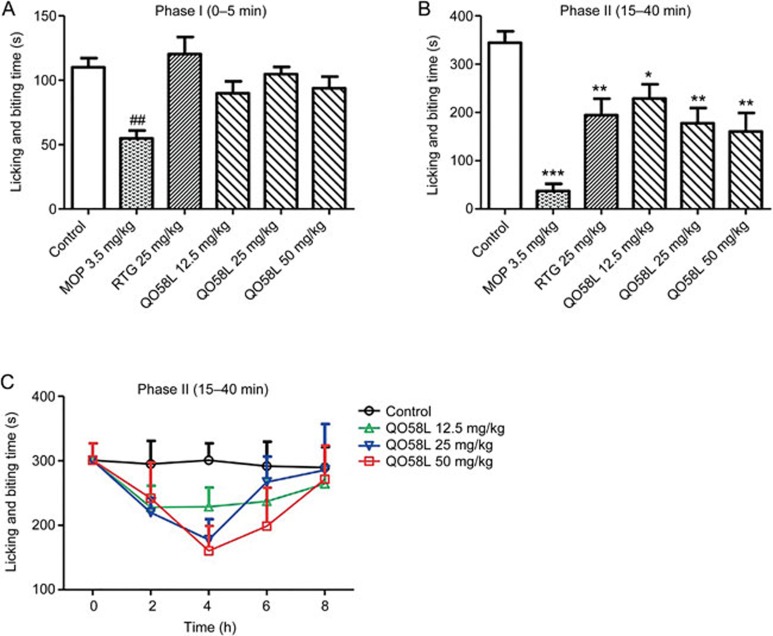
To test the anti-nociceptive effect of QO58-lysine, we utilized a model of inflammatory pain that is induced by intraplantar injection of 5% formalin into the mouse hindpaw. As a positive control, subcutaneous injection of morphine (3.5 mg/kg) 15 min before the formalin injection produced analgesic effects during the phase I (Figure 3A) and phase II responses (Figure 3B). As another positive control, pre-administration of retigabine (25 mg/kg) significantly reversed the licking and biting behavior induced by formalin in the phase II response (Figure 3B) but not in the phase I response (Figure 3A). Similarly, pre-administration of QO58-lysine caused a concentration-dependent inhibition of formalin-induced licking and biting behavior during phase II (P<0.05, n=10), compared to the vehicle control, but not in phase I (Figure 3A and 3B).
Figure 3.
Anti-nociceptive effect of oral administration of QO58-lysine on inflammatory pain induced by formalin in mice. (A) Effect of QO58-lysine on licking and biting behavior in phase I induced by intraplantar injection of formalin (5% methanol solution of 10 μL) in mice. Phase I was observed for a period of 0–5 min after formalin injection. Morphine (MOP, 3.5 mg/kg, sc) or retigabine (RTG, 25 mg/kg, po) was pre-administered 15 min and 1 h, respectively, before intraplantar injection of formalin into the hindpaw. Oral QO58-lysine (QO58L) was pre-administered at doses of 12.5, 25, and 50 mg/kg 4 h before formalin injection. Statistical significance is indicated as ##P<0.01, compared to the control group. (B) Effect of QO58-lysine on licking and biting behavior in phase II induced by intraplantar injection of formalin in mice. Phase II was observed for a period of 15–40 min after formalin injection. QO58-lysine at different concentrations inhibited licking and biting behavior during the phase II pain. Statistical significance is indicated as *P<0.05, **P<0.01, compared to the control group. (C) Time-dependent effect of QO58-lysine on the licking and biting behavior in phase II. Oral QO58-lysine was administered at doses of 12.5, 25, and 50 mg/kg, and the pain response was observed for 8 h after formalin injection. The data are expressed as the mean±SEM (n=9–10).
We also determined the time-dependent effect of QO58-lysine administered at different doses on licking and biting in phase II. As shown in Figure 3C, pre-treatment of QO58-lysine 4 h before formalin injection resulted in the maximum effect on pain related behaviors in phase II, followed by the slow reduction of the effect, which was consistent with the time-concentration pattern of plasma QO58-lysine after oral administration (Tmax=3.8 h, Figure 2). These results show that QO58-lysine exerted dose- and time-dependent anti-nociceptive effects on inflammatory pain induced by formalin.
Anti-nociceptive effect of QO58-lysine on CFA-induced inflammatory pain in rats
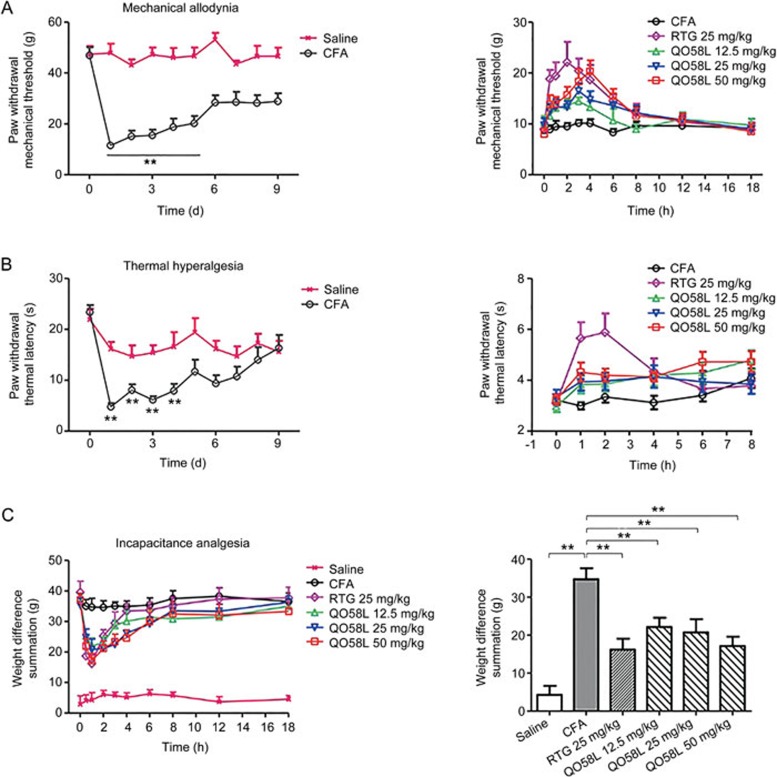
To test the anti-nociceptive effect of QO58-lysine on inflammatory pain induced by CFA in rats, we generated a pain model by using an intraplantar injection of 100 μL of CFA into the left hindpaw. After the CFA injection, rats developed both mechanical allodynia and thermal hyperalgesia within 24 h, which were maintained for 5 d before a slow recovery to baseline. The lowest withdrawal threshold measured by mechanical von Frey hair (Figure 4A, left panel) or heat radiation (Figure 4B, left panel) was obtained at approximately 24 h, compared to 24 h in the control group (Figure 4A left panel and 4B left panel). Therefore, the time point of 24 h after CFA injection was selected to evaluate the effects of compounds on inflammatory pain.
Figure 4.
Anti-nociceptive effects of oral QO58-lysine on inflammatory pain induced by CFA in rats. (A) Development of mechanical allodynia in CFA-induced rats (n=8). Paw withdrawal mechanical threshold was measured using an electric von Frey anesthesiometer over a 9-d period for the saline control and CFA groups. CFA was injected at time 0 and the withdrawal threshold was measured once a day for 9 d. The lowest withdrawal threshold occurred 1 d after CFA injection and was maintained for approximately 5 d. Student's t-test was used to indicate statistical significance between the saline group and the CFA group as **P<0.01. The right panel shows the effect of oral QO58-lysine on mechanical allodynia in CFA-induced rats (n=10) 24 h after intraplantar injection of CFA, retigabine (RTG, 25 mg/kg) or QO58-lysine (QO58L, 12.5, 25, and 50 mg/kg) was administered, and rats in the CFA group received 10% Tween-80-Saline. Paw withdrawal mechanical threshold was measured at 0, 0.5, 1, 2, 3, 4, 6, 8, 12, and 18 h after oral administration. (B) Development of thermal hyperalgesia in CFA-induced rats (n=8). The paw withdrawal latency of thermal hyperalgesia was measured using an automatic plantar analgesia tester over a 9 d period for the saline control group and the CFA group. CFA was injected at time 0, and the withdrawal thermal latency was measured once a day for 9 d. The lowest withdrawal latency occurred 1 d after CFA injection and was maintained for approximately 4 d. Student's t-test was used to indicate statistical significance between the saline group and the CFA group as **P<0.01. In the right panel, the effect of oral QO58-lysine on thermal hyperalgesia in CFA-induced rats is shown (n=10). Twenty-four hours after the intraplantar injection of CFA, retigabine (RTG, 25 mg/kg) or QO58-lysine (QO58L, 12.5, 25, and 50 mg/kg) was administered. Rats in the CFA group received 10% Tween-80-Saline. The paw withdraw latency of thermal hyperalgesia was measured at 0, 1, 2, 4, 6, and 8 h after oral administration. (C) Anti-nociceptive effect of oral QO58-lysine on CFA-induced pain in rats (n=10) using the incapacitance analgesia assay that measures the difference in weight bearing between the ipsilateral affected limb and the contralateral control limb. Measurements were obtained for 20 s, and they are represented as the weight difference summation (g). Twenty-four hours after the intraplantar injection of CFA, retigabine (RTG, 25 mg/kg, po) and QO58-lysine (QO58L, 12.5, 25, and 50 mg/kg, po) were administered. Rats in the CFA group were orally administered 10% Tween-80-Saline. The weight difference summation was measured at 0, 0.5, 1, 2, 3, 4, 6, 8, 12, and 18 h after oral administration. In the right panel, the weight difference summation was measured at 1 h. The data are expressed as the mean±SEM.
Oral administration of QO58-lysine at different doses (12.5, 25, and 50 mg/kg) resulted in a dose-dependent alleviation of mechanical allodynia induced by CFA, compared with the CFA model group (Figure 4A, right panel). The paw withdrawal threshold was increased, and the maximum effect was reached at 3–4 h after oral QO58-lysine administration; the effect lasted approximately 6 h before returning to baseline (Figure 4A, right panel). As a positive control, retigabine (25 mg/kg, po) increased the paw withdrawal threshold of mechanical allodynia (Figure 4A, right panel) and also increased the withdrawal latency of thermal hyperalgesia with maximum effect seen at 2 h (Figure 4B, right panel). However, oral administration of QO58-lysine at different doses (12.5, 25, and 50 mg/kg) only showed a moderate elevation of withdrawal latency of thermal hyperalgesia induced by CFA, but there was no statistical significance on withdrawal latency among the different dosing groups (Figure 4B, right panel).
To further confirm the anti-nociceptive effect of QO58-lysine, we also utilized an incapacitance meter assay that assesses the changes in hindpaw weight distribution between the left (intraplantar injection of CFA) and right (contralateral control) limbs. When challenged by CFA under chronic conditions, rats can transfer their bodyweight from the irritated paw to the healthy counterpart25,26. Therefore, the weight difference between the paw pair reflects the severity of pain. As shown in the right panel of Figure 4C, oral administration of QO58-lysine or retigabine attenuated CFA-induced chronic pain, and the maximal effect was reached in approximately 1 h. The anti-nociceptive effect of retigabine was gradually reduced and diminished by approximately 4 h. In contrast, the anti-nociceptive effect of QO58-lysine lasted approximately 6–8 h (Figure 4C, left panel). We also observed the effect of repeated administration of QO58-lysine for 3 d on incapacitance, and the results showed that multiple doses of QO58-lysine could attenuate CFA-induced incapacitance analgesia in a dose-dependent manner, which is consistent with the effect obtained from the administration of a single dose (data not shown).
Reversal of anti-nociceptive effects of intraperitoneal QO58-lysine by the specific blocker, XE991
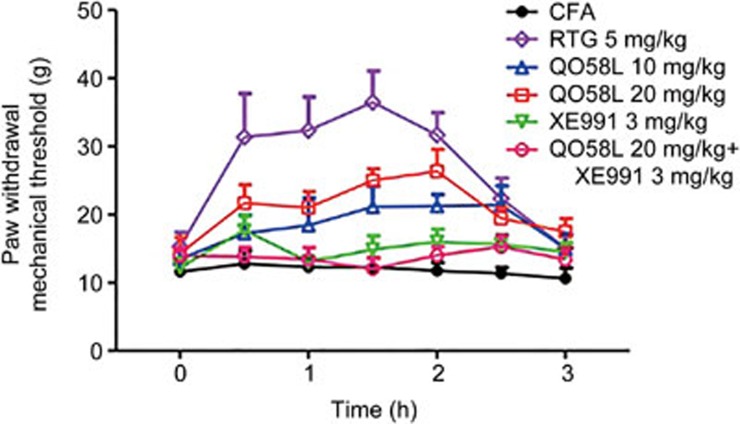
To confirm the specific action of QO58-lysine on inflammatory pain through the activation of KCNQ/Kv7 channel function, we used the specific KCNQ/Kv7 channel blocker, XE991, which selectively inhibits channel function12,27. Intraperitoneal administration of QO58-lysine (10 or 20 mg/kg) resulted in a time-dependent increase of the paw withdrawal threshold, and the anti-nociceptive effect of QO58-lysine was observed 0.5 h after injection and lasted approximately 2 h before returning to baseline (Figure 5). In contrast, the anti-nociceptive effect of QO58-lysine was antagonized by the co-administration of the blocker XE991 (3 mg/kg) with QO58-lysine (20 mg/kg), whereas XE991 (3 mg/kg) alone had no obvious effect (Figure 5). These results suggest that the anti-nociceptive effect of QO58-lysine is mediated by the activation of Kv7.2/7.3 channels.
Figure 5.
Anti-nociceptive effect of intraperitoneal QO58-lysine on mechanical allodynia in CFA-induced rats and its reversal by the specific blocker XE991. Twenty-four hours after the intraplantar injection of CFA, intraperitoneal administration of retigabine (RTG, 5 mg/kg) or XE991 (3 mg/kg) or QO58-lysine (QO58L, 10 and 20 mg/kg) was carried out. The CFA group of rats received an intraperitoneal administration of 10% Tween-80-Saline. The paw withdrawal mechanical threshold was measured at time points 0, 0.5, 1, 1.5, 2, 2.5, and 3 h after the intraperitoneal administration of the compounds. The data are expressed as the mean±SEM (n=10).
Effect of QO58-lysine on locomotor activity in mice
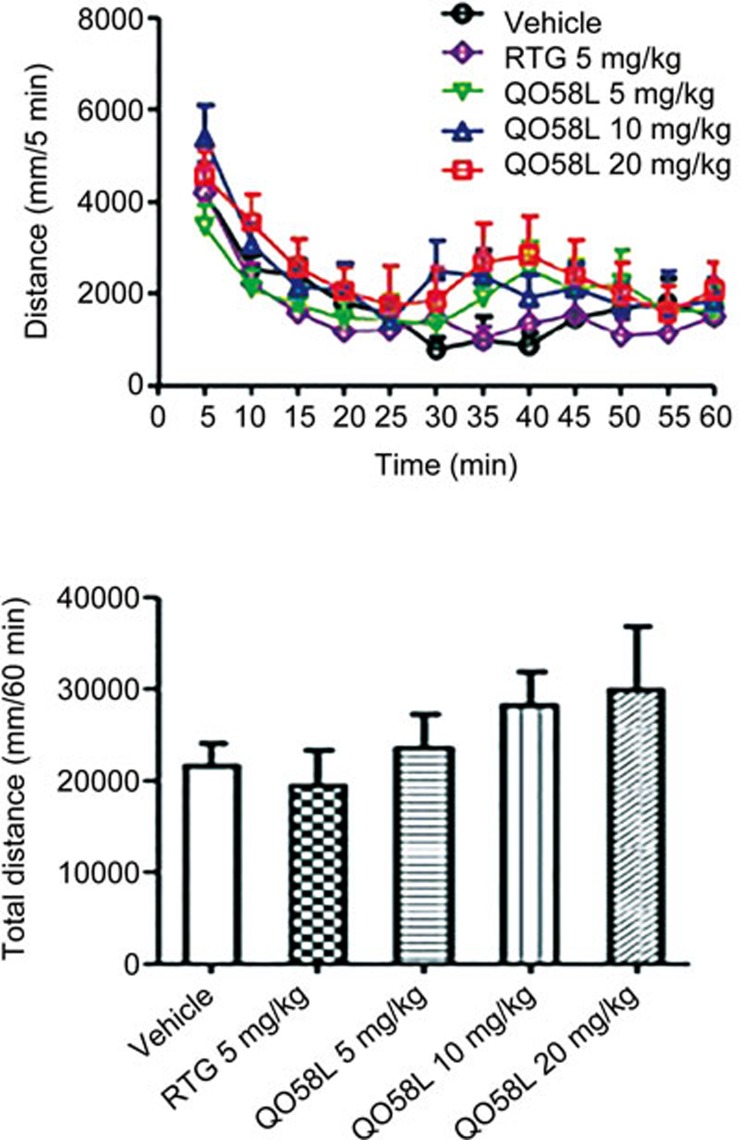
To evaluate the effect of QO58-lysine on locomotion, we also quantitatively determined the locomotor activity (total distance traveled) in mice. Intraperitoneal administration of different concentrations of QO58-lysine (5, 10, and 20 mg/kg, ip) had only a slight increase in the locomotor activity, which had no statistical significance compared to the vehicle or 5 mg/kg of retigabine (Figure 6). This result indicates that mouse locomotion behavior is not affected by the QO58-lysine compound.
Figure 6.
Effect of QO58-lysine on locomotor activity in mice. Mice were intraperitoneally administered different doses of QO58-lysine (5, 10, and 20 mg/kg, ip), retigabine (5 mg/kg, ip) or vehicle (10% Tween-80 in 0.9% NaCl) (top panel). Locomotion was observed for 60 min. Column graphs depicting the cumulative distance of locomotion within the 60 min period (bottom panel), and the data were analyzed by one-way ANOVA. The data are expressed as the mean±SEM (n=10).
Discussion
The goal of this study was to determine the bioavailability of the salt form (QO58-lysine) of a newly synthesized and modified compound that specifically activates the KCNQ/Kv7 channel and to investigate whether the oral QO58-lysine compound was effective in alleviating inflammatory pain in rodent models.
Before testing QO58-lysine for in vivo pharmacology, we investigated the pharmacokinetics of oral QO58-lysine in rat plasma using a previously validated HPLC assay23. The absolute bioavailability of QO58-lysine indicates that the modified salt form is more easily absorbed than the original QO5822. Based on the pharmacokinetic profile of QO58-lysine, we investigated the anti-nociceptive effect of QO58-lysine on inflammatory pain that was induced by formalin or CFA in mouse and rat models. QO58-lysine exhibits an anti-nociceptive effect during the second phase of pain induced by formalin. This anti-nociceptive effect increases gradually after intragastric administration of the compound and reached its peak at 4 h after administration. The result is consistent with the pharmacokinetic data; the time to reach the peak concentration (Tmax) is approximately 3 h after administration. The lack of effect of QO58-lysine against the first phase of pain behavior is consistent with the observations obtained using retigabine12.
We also tested the anti-nociceptive effect of QO58-lysine on chronic inflammatory pain induced by CFA. QO58-lysine increases the withdrawal mechanical threshold in rats in both a time- and dose-dependent manner. The fact that the anti-nociceptive effect of QO58-lysine can be antagonized by the KCNQ channel blocker XE991 indicates that the specific activation of KCNQ channels mediates the pharmacological effects of QO58-lysine on pain.
In previous studies, some anticonvulsant drugs, including retigabine, can cause a short period of motor disturbance. In our study, we used a relatively lower concentration of retigabine (oral 25 mg/kg and intraperitoneal 5 mg/kg) to avoid such impaired motor performance. During the behavior tests, we observed no significant performance impairment in all tested animals. We also tested the effect of QO58-lysine and XE991 on normal rats, and no abnormal pain sensation or any abnormality was observed. Taken together, our findings show that a novel specific Kv7/KCNQ channel opener, QO58-lysine, reverses inflammatory pain in rodent models without exhibiting obvious toxic effects.
Acknowledgments
This work was supported by research grants to KeWei WANG from the Ministry of Science and Technology of China (No 2013CB531302, 2013ZX09103001-015 and 2014ZX09507003-006-004) and to Jin-long QI from the Hebei Province Education Department (No YQ2013033).
References
- Wang HS, Pan Z, Shi W, Brown BS, Wymore RS, Cohen IS, et al. KCNQ2 and KCNQ3 potassium channel subunits: molecular correlates of the M-channel. Science 1998; 282: 1890–3. [DOI] [PubMed] [Google Scholar]
- Xiong Q, Gao Z, Wang W, Li M. Activation of Kv7 (KCNQ) voltage-gated potassium channels by synthetic compounds. Trends Pharmacol Sci 2008; 29: 99–107. [DOI] [PubMed] [Google Scholar]
- Cooper EC, Aldape KD, Abosch A, Barbaro NM, Berger MS, Peacock WS, et al. Colocalization and coassembly of two human brain M-type potassium channel subunits that are mutated in epilepsy. Proc Nat Acad Sci U S A 2000; 97: 4914–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubisch C, Schroeder BC, Friedrich T, Lütjohann B, El-Amraoui A, Marlin S, et al. KCNQ4, a novel potassium channel expressed in sensory outer hair cells, is mutated in dominant deafness. Cell 1999; 96: 437–46. [DOI] [PubMed] [Google Scholar]
- Charlier C, Singh NA, Ryan SG, Lewis TB, Reus BE, Leach RJ, et al. A pore mutation in a novel KQT-like potassium channel gene in an idiopathic epilepsy family. Nat Genet 1998; 18: 53–5. [DOI] [PubMed] [Google Scholar]
- Blackburn-Munro G, Dalby-Brown W, Mirza NR, Mikkelsen JD, Blackburn-Munro RE. Retigabine: chemical synthesis to clinical application. CNS Drug Rev 2005; 11: 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeks ED. Retigabine (ezogabine): in partial-onset seizures in adults with epilepsy. CNS Drugs 2011; 25: 887–900. [DOI] [PubMed] [Google Scholar]
- French JA, Abou-Khalil BW, Leroy RF, Yacubian EM, Shin P, Hall S, et al. Randomized, double-blind, placebo-controlled trial of ezogabine (retigabine) in partial epilepsy. Neurology 2011; 76: 1555–63. [DOI] [PubMed] [Google Scholar]
- Harris JA, Murphy JA. Retigabine (ezogabine) as add-on therapy for partial-onset seizures: an update for clinicians. Ther Adv Chronic Dis 2011; 2: 371–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korsgaard MP, Hartz BP, Brown WD, Ahring PK, Strobaek D, Mirza NR. Anxiolytic effects of Maxipost (BMS-204352) and retigabine via activation of neuronal Kv7 channels. J Pharmacol Exp Ther 2005; 314: 282–92. [DOI] [PubMed] [Google Scholar]
- Slomko AM, Naseer Z, Ali SS, Wongvravit JP, Friedman LK. Retigabine calms seizure-induced behavior following status epilepticus. Epilepsy Behav 2014; 37: 123–32. [DOI] [PubMed] [Google Scholar]
- Blackburn-Munro G, Jensen BS. The anticonvulsant retigabine attenuates nociceptive behaviours in rat models of persistent and neuropathic pain. Eur J Pharmacol 2003; 460: 109–16. [DOI] [PubMed] [Google Scholar]
- Hirano K, Kuratani K, Fujiyoshi M, Tashiro N, Hayashi E, Kinoshita M. Kv7.2–7.5 voltage-gated potassium channel (KCNQ2-5) opener, retigabine, reduces capsaicin-induced visceral pain in mice. Neurosci Lett 2007; 413: 159–62. [DOI] [PubMed] [Google Scholar]
- Li H, Wang F, Wang X, Sun R, Chen J, Chen B, et al. Antinociceptive efficacy of retigabine in the monosodium lodoacetate rat model for osteoarthritis pain. Pharmacology 2015; 95: 251–7. [DOI] [PubMed] [Google Scholar]
- Pottabathini R, Kumar A, Bhatnagar A, Garg S. Possible involvement of nitric oxide modulatory mechanism in the protective effect of retigabine against spinal nerve ligation-induced neuropathic pain. Cell Mol Neurobiol 2015; 35: 137–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passmore GM, Selyanko AA, Mistry M, Al-Qatari M, Marsh SJ, Matthews EA, et al. KCNQ/M currents in sensory neurons: significance for pain therapy. J Neurosci 2003; 23: 7227–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalappa BI, Soh H, Duignan KM, Furuya T, Edwards S, Tzingounis AV, et al. Potent KCNQ2/3-specific channel activator suppresses in vivo epileptic activity and prevents the development of tinnitus. J Neurosci 2015; 35: 8829–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Q, Sun H, Li M. Zinc pyrithione-mediated activation of voltage-gated KCNQ potassium channels rescues epileptogenic mutants. Nat Chem Biol 2007; 3: 287–96. [DOI] [PubMed] [Google Scholar]
- Davoren JE, Claffey MM, Snow SL, Reese MR, Arora G, Butler CR, et al. Discovery of a novel Kv7 channel opener as a treatment for epilepsy. Bioorg Med Chem Lett 2015; 25: 4941–4. [DOI] [PubMed] [Google Scholar]
- Qi J, Zhang F, Mi Y, Fu Y, Xu W, Zhang D, et al. Design, synthesis and biological activity of pyrazolo[1,5-a]pyrimidin-7(4H)-ones as novel Kv7/KCNQ potassium channel activators. Eur J Med Chem 2011; 46: 934–43. [DOI] [PubMed] [Google Scholar]
- Zhang F, Mi Y, Qi JL, Li JW, Si M, Guan BC, et al. Modulation of Kv7 potassium channels by a novel opener pyrazolo[1,5-a]pyrimidin-7(4H)-one compound QO-58. Br J Pharmacol 2013; 168: 1030–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CF, Qi JL, Zhang HL, Jia QZ. Pharmacokinetic study of QO-58: a new potassium channel opener. Chin Pharmacol Bull 2014; 30: 574–7. [Google Scholar]
- Ma T, Wang K. Development of high-performance liquid chromatography assay for pharmacokinetic analysis of KCNQ/M-channel opener QO58-lysin in rat plasma. J Chin Pharm Sci 2014; 23: 153–8. [Google Scholar]
- Qin WJ, Wang YT, Zhang M, Wen RT, Liu Q, Li YL, et al. Molecular chaperone heat shock protein 70 participates in the labile phase of the development of behavioural sensitization induced by a single morphine exposure in mice. Int J Neuropsychopharmacol 2013; 16: 647–59. [DOI] [PubMed] [Google Scholar]
- Patchornik S, Ram E, Ben Shalom N, Nevo Z, Robinson D. Chitosan-hyaluronate hybrid gel intraarticular injection delays osteoarthritis progression and reduces pain in a rat meniscectomy model as compared to saline and hyaluronate treatment. Adv Orthop 2012; 2012: 979152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen KD, Mata BA, Gabr MA, Huebner JL, Adams SB Jr, Kraus VB, et al. Kinematic and dynamic gait compensations resulting from knee instability in a rat model of osteoarthritis. Arthritis Res Ther 2012; 14: R78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Wu Y, Bi Y, Tan L, Gan Y, Wang K. Activation of voltage-gated KCNQ/Kv7 channels by anticonvulsant retigabine attenuates mechanical allodynia of inflammatory temporomandibular joint in rats. Mol Pain 2010; 6: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]