Abstract
Aim:
Increasing evidence suggests that probucol, a lipid-lowering agent with anti-oxidant activities, may be useful for the treatment of ischemic stroke with hyperlipidemia via reduction in cholesterol and neuroinflammation. In this study we examined whether probucol could protect against brain ischemic injury via anti-neuroinflammatory action in normal and hyperlipidemic mice.
Methods:
Primary mouse microglia and murine BV2 microglia were exposed to lipopolysaccharide (LPS) for 3 h, and the release NO, PGE2, IL-1β and IL-6, as well as the changes in NF-κB, MAPK and AP-1 signaling pathways were assessed. ApoE KO mice were fed a high-fat diet containing 0.004%, 0.02%, 0.1% (wt/wt) probucol for 10 weeks, whereas normal C57BL/6J mice received probucol (3, 10, 30 mg·kg-1·d-1, po) for 4 d. Then all the mice were subjected to focal cerebral ischemia through middle cerebral artery occlusion (MCAO). The neurological deficits were scored 24 h after the surgery, and then brains were removed for measuring the cerebral infarct size and the production of pro-inflammatory mediators.
Results:
In LPS-treated BV2 cells and primary microglial cells, pretreatment with probucol (1, 5, 10 μmol/L) dose-dependently inhibited the release of NO, PGE2, IL-1β and IL-6, which occurred at the transcription levels. Furthermore, the inhibitory actions of probucol were associated with the downregulation of the NF-κB, MAPK and AP-1 signaling pathways. In the normal mice with MCAO, pre-administration of probucol dose-dependently decreased the infarct volume and improved neurological function. These effects were accompanied by the decreased production of pro-inflammatory mediators (iNOS, COX-2, IL-1, IL-6). In ApoE KO mice fed a high-fat diet, pre-administration of 0.1% probucol significantly reduced the infarct volume, improved the neurological deficits following MCAO, and decreased the total- and LDL-cholesterol levels.
Conclusion:
Probucol inhibits LPS-induced microglia activation and ameliorates cerebral ischemic injury in normal and hyperlipidemic mice via its anti-neuroinflammatory actions, suggesting that probucol has potential for the treatment of patients with or at risk for ischemic stroke and hyperlipidemia.
Keywords: probucol, LPS, microglia, ischemic stroke, focal cerebral ischemia, hyperlipidemia, inflammatory cytokines, neuroinflammation
Introduction
Neuroinflammation mediated by the activation of microglia is an important factor that contributes to neuronal death and magnifies damage in ischemic brain injury1. Microglia is the resident macrophage of the brain and plays a central role in neuroinflammation. Microglia participates in host defense in the brain and act as phagocytes that engulf tissue debris and dead cells. Microglia excessively produce pro-inflammatory factors, including nitric oxide (NO), prostaglandin E2 (PGE2), TNF-α, IL-1β and IL-6, in response to ischemic brain injury, and these factors subsequently aggravate the neuroinflammation, which further increases the severity of brain damage1,2. Thus, the identification of novel agents to regulate neuroinflammation is regarded as a promising approach for the treatment of ischemic brain injury.
Hyperlipidemia with an accompanying increase in peripheral inflammation is a risk factor for stroke3. Although hyperlipidemia is not a direct predictor of stroke, half of stroke patients have hyperlipidemia4. Hyperlipidemia induces inflammation in the brain5,6,7 and exacerbates ischemic brain damage8,9. Therefore, decreasing cholesterol and inflammation may be an effective therapeutic strategy for reducing the influences of both hyperlipidemia and ischemic stroke.
Probucol is a bisphenol compound that was synthesized as an anti-oxidant and found to have the potential to reduce cholesterol10,11. Probucol is an anti-oxidant and lipid-reducing drug that has been in clinical use during the past few decades for the prevention and treatment of cardiovascular diseases. In experimental and clinical studies, probucol has been reported to reduce intimal proliferation following balloon injury in animals12,13 and to inhibit restenosis after coronary angioplasty with14 and without15 stent in humans. Probucol also dramatically retards atherosclerosis in hyperlipidemic animals16,17,18. Furthermore, probucol has been demonstrated to posse the capacity to attenuate inflammation in animal models of aging19,20, focal cerebral ischemia8,9 and ischemic myocardial injury21,22, as well as in diabetic23 and atherosclerotic rabbits24. Based on these reports, probucol may be useful for the treatment of ischemic stroke with hyperlipidemia via reductions in cholesterol and neuroinflammation.
In the present study, we investigated whether probucol protects the brain against ischemic injury via an anti-neuroinflammatory effect. To accomplish this, we explored the anti-neuroinflammatory effects of probucol and the mechanisms of the production of pro-inflammatory cytokines in microglial cells stimulated with LPS in vitro and the suppression of ischemic brain injury in normal and hyperlipidemic mice by probucol. To investigate the molecular effects of probucol on neuroinflammation, BV2 microglial cells and primary microglial cells were used to test the effects of probucol on LPS-induced iNOS, COX-2 and proinflammatory cytokine expression. Because both the NF-κB and MAPK pathways participate in the regulation of neuroinflammation25, both pathways were examined as possible underlying molecular mechanisms. We then determined the potential efficacy of probucol against ischemic stroke in both normal and hyperlipidemic mice and examined the effects of probucol on inflammatory markers in ischemic brains. The current study provides evidence for the potential use of probucol for the treatment of patients with or at risk for ischemic stroke and hyperlipidemia.
Materials and methods
Cell culture, palmitic acid-BSA treatment and hypoxia conditions
The murine BV2 cell line and 293-T cells were maintained in DMEM (Gibco, Grand Island, NY, USA) supplemented with 10% FBS (Gibco, Waltham, MA, USA), penicillin (Gibco, Waltham, MA, USA, 100 units/mL) and streptomycin (Gibco, Waltham, MA, USA, 100 μg/mL) at 37 °C in a humidified incubator under 5% CO2. The probucol [4,4'-(isopropylidenedithio)bis(2,6-di-t-butylophenol)] was kindly donated by Otsuka Pharmaceutical (Tokushima, Japan) and was dissolved in DMSO. Dilutions were then made in DMEM. The final concentration of DMSO in the medium was less than 0.01% (v/v), and this concentration was found to have no influence on cell growth. In all experiments, the cells were treated with the indicated concentrations of probucol for 3 h prior to the addition of LPS (1 μg/mL for the BV2 cells and 10 ng/mL for the primary microglia; Sigma-Aldrich, St Louis, MO, USA). To mimic hyperlipidemic mice in vitro, we used palmitic acid (PA) with BSA26. PA complexed with fatty acid-free BSA (Sigma-Aldrich, St Louis, MO, USA) was prepared as previously described26. PA (Sigma-Aldrich, St Louis, MO, USA) was dissolved in 100% EtOH to a concentration of 195 mmol/L. Fatty acid-free BSA dissolved in DMEM and prepared at 10%. PA (195 mmol/L) was mixed with 10% BSA, and the final molar ratio of PA to BSA was 6:1. For the hypoxic conditions, cells were incubated in a hypoxic incubator (Astec, Kasuya, Fukuoka, Japan) that maintained a low oxygen tension (5% CO2, 5% O2 balanced with N2).
Isolation of neonatal mouse microglia cells (primary microglia culture)
Microglial cells were isolated from brains of 2 or 3-d-old C57BL/6J mice as previously described with some modification27. In brief, mixed glial cultures were prepared from the cerebral cortex, and mechanical and chemical dissociations (0.5% trypsin-EDTA: Gibco, Waltham, MA, USA) were performed. Cortical cells were seeded in DMEM with 10% FBS and cultured until confluence. Microglial cultures were prepared via the mild trypsinization method. The cell medium was removed, and the cells were incubated at 37 °C with mild trypsin solution (trypsin-EDTA:DMEM=1:2, 1 mmol/L CaCl2). After 20–30 min, the astroglial layer was detached. When the astroglial layer was fully detached, it was discarded via aspiration, and DMEM/15% FBS medium was added. Twenty-four hours were allowed for the stabilization of the microglial cells, and the experiments were then performed.
Nitrite determination
The production of NO in the culture supernatants was measured with a colorimetric assay using the Griess reaction (Enzo Life Sciences, Farmingdale, NY, USA). Briefly, BV2 cells (4×104 cells/mL) were seeded in 24-well plates and incubated with probucol for 3 h prior to incubation with LPS (1 μg/mL). Following LPS stimulation for 6 h, 50 μL of the conditioned culture medium was mixed with the same volume of Griess reagent [1% sulfanilamide and 0.1% N-(1-naphthyl)-ethylenediamine dihydrichloride in 5% H3PO4]. The NO concentration was calculated using a standard solution of sodium nitrite prepared in the medium. The absorbance was determined at 540 nm using a SpectraMax190 microplate reader (Molecular Devices, Sunnyvale, CA, USA).
Enzyme-linked immunosorbent assay (ELISA)
The levels of PGE2 were measured with ELISA kits according to the manufacturer's instructions (Enzo Life Sciences, Farmingdale, NY, USA). The absorbance was determined at 450 nm using a SpectraMax190 microplate reader (Molecular Devices, Sunnyvale, CA, USA).
Western blot analysis
The proteins from the BV2 cells and brain tissues were isolated according to standard techniques, separated by 8%–12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and transferred onto a nitrocellulose membrane (Amersham Biosciences, Piscataway, NJ, USA). Next, the immunoblot analysis was performed with the following primary antibodies and secondary antibodies conjugated with horseradish peroxidase: iNOS (BD Biosciences, San Jose, CA, USA); COX-2 and NF-κB p65 (Santa Cruz Biotechnology, Dallas, TX, USA); p38, p-p38, JNK, p-JNK, ERK, p-ERK, and c-Jun (Cell Signaling, Danvers, MA, USA); and IL-1β (Santa Cruz Biotechnology, Dallas, TX, USA). The chemiluminescence intensity was measured using an ImageQuant LAS 4000 apparatus (GE Healthcare Life Sciences, Uppsala, Sweden). The membrane was then reprobed with anti-β-actin (Sigma-Aldrich, St Louis, MO, USA) and anti-lamin B (Santa Cruz Biotechnology, Dallas, TX, USA) antibodies as an internal control.
Reverse transcription-polymerase chain reaction (RT-PCR) and real-time PCR
Total RNAs were isolated from the cells and mouse brains using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's recommendations. The RNA was then reverse-transcribed for one hour at 42 °C with Moloney Murine Leukemia Virus reverse transcriptase (Promega, Madison, WI, USA) to produce cDNA. For the RT-PCR, RT-generated cDNA encoding the iNOS, COX-2, and GAPDH genes was amplified using the primers provided in Table 1. The PCR thermal cycling conditions consisted of 95 °C for 3 min, followed by 25 cycles of 95 °C for 5 s, 60 °C for 30 s and 72 °C for 20 s. Real-time PCR was conducted using a Rotor-Gene Q real-time PCR system (Qiagen, Düsseldorf, Hilden, Germany) with SYBR Green PCR Master Mix (Qiagen, Düsseldorf, Hilden, Germany), and the results were normalized to the GAPDH gene expression. All experiments were performed in triplicate and repeated at least three times using the primers provided in Table 1. The threshold cycles (Ct) were used to quantify the mRNA expression of the target genes.
Table 1. Sequences of primers used in RT-PCR and real time PCR analysis.
| Gene | Primer | Length (bp) | Sequence (5′–3′) |
|---|---|---|---|
| RT-PCR | |||
| Mouse iNOS | sense primer | 461 | CACTTGGATCAGGAACCTGAAG |
| antisense primer | CCAGCTTCTTCAATGTGGTAGC | ||
| Mouse COX-2 | sense primer | 271 | TTCAACACACTCTATCAC |
| antisense primer | AGAAGCGTTTGCGGTACT | ||
| Mouse GAPDH | sense primer | 486 | ATGACCACAGTCCATGCCATCA |
| antisense primer | TTACTCCTTGGAGGCCATGTAG | ||
| Real-time PCR | |||
| Mouse iNOS | sense primer | 93 | TCCTGGACATTACGACCCCT |
| antisense primer | AGGCCTCCAATCTCTGCCTA | ||
| Mouse COX-2 | sense primer | 178 | CATCCCCTTCCTGCGAAGTT |
| antisense primer | CATGGGAGTTGGGCAGTCAT | ||
| Mouse IL-1β | sense primer | 196 | GGATGAGGACATGAGCACCT |
| antisense primer | TCCATTGAGGTGGAGAGCTT | ||
| Mouse IL-6 | sense primer | 191 | AGTTGCCTTCTTGGGACTGA |
| antisense primer | CAGAATTGCCATTGCACAAC | ||
| Mouse GAPDH | sense primer | 83 | CCTGGCCAAGGTCATCCAT |
| antisense primer | TCTTCTGGGTGGCAGTGATG |
Luciferase activity
Luciferase reporter plasmids were transfected into 293-T cells using Lipofectamine 2000 transfection reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's protocol. The cell lysates were then prepared with passive lysis buffer from a Promega assay system and used to measure the luciferase activity according to the manufacturer's instructions for the dual luciferase reporter assay system (Promega, Fitchburg, WI, USA). A luciferase assay was performed out using an Infinite M200 multifunctional detector system (Tecan Austria GmbH, Austria).
Confocal laser scanning microscopy study
NF-κB p65 nuclear localization was evaluated with indirect immunofluorescence assays using confocal microscopy. Briefly, BV2 cells were cultured directly on glass coverslips in 6-well plates for 24 h and incubated with 5 μmol/L probucol for 3 h before incubation with LPS (1 μg/mL). Following LPS stimulation for 6 h, the cells were fixed with 4% paraformaldehyde in PBS and then permeabilized with 0.2% Triton X-100 in PBS. The cells were subsequently blocked with 1.5% FBS serum (Invitrogen, Carlsbad, CA, USA), and polyclonal antibody against NF-κB p65 (Santa Cruz Biotechnology, Dallas, TX, USA) was then applied for 3 h followed by 1 h of incubation with Texas Red anti-rabbit IgG (Vector Laboratories, Burlingame, CA, USA). After washing with PBS three times, the coverslips were mounted in permanent mounting media (Vector Laboratories, Burlingame, CA, USA), and the fluorescence was visualized using a Zeiss LSM 700 laser scanning confocal device (Carl Zeiss, Oberkochen, Germany).
General surgical preparation
Male C57BL/6J (20–25 g) and ApoE KO mice (Japan SLC, Hamamatsu, Japan) on a C57BL/6J genetic background were housed under diurnal light conditions and allowed food and tap water ad libitum. The animal protocol used in this study was reviewed by the Pusan National University-Institutional Animal Care and Use Committee (PNU-IACUC) in term of ethical procedures and scientific care, and the protocol was approved (Approval Number PNU-2011-000419). The C57BL/6J mice received probucol (3, 10, or 30 mg/kg in 1% DMSO, orally) once per day for 4 d prior to the ischemic event. Four-week-old ApoE KO mice were fed Western-type high-fat diets (HFD; 42% of the total calories were from fat; 0.15% cholesterol; Research Diet, New Brunswick, NJ, USA) containing 0.004%, 0.02%, or 0.1% (wt/wt) probucol for 10 weeks. Anesthesia was achieved with isoflurane (2% induction and 1.5% maintenance in 80% N2O and 20% O2) administered via a facemask. The femoral artery was catheterized for the measurement of the mean arterial blood pressure using a model MLT844 physiological pressure transducer (AD Instruments, Medford, MA, USA). The data were continuously recorded using a Power Lab data acquisition and analysis system (AD Instruments, Medford, MA, USA) and stored in a computer. A suitable depth of anesthesia was ensured based on the absence of cardiovascular changes in response to tail pinches. The rectal temperature was maintained at 36.5–37.5 °C using a PanlabTM thermostatically controlled heating mat (Harvard Apparatus, Holliston, MA, USA). The arterial blood gases and pH were measured before ischemia using an i-Stat System (Abbott, Abbott Park, IL, USA).
Focal cerebral ischemia
Focal cerebral ischemia was induced via middle cerebral artery occlusion (MCAO) using a previously described intraluminal filament technique28. A fiber-optic probe was affixed to the skull over the MCA for the measurement of the regional CBF (rCBF) using a PeriFlux Laser Doppler System 5000 (Perimed, Stockholm, Sweden). The baseline values were measured before internal carotid artery ligation (these values were taken as 100% flow). MCA occlusion was induced with a silicon-coated 7–0 monofilament in the internal carotid artery, and the monofilament was advanced to occlude the MCA. In all animals, the rCBF was measured to confirm the achievement of consistent and similar levels of ischemic induction. The filament was withdrawn 1 h or 45 min after occlusion, and reperfusion was confirmed using laser Doppler imaging. The surgical wound was sutured, and the mice were allowed to recover from the anesthesia. The brains were removed 24 h after MCA occlusion. The cerebral infarct size was determined on 2,3,5-triphenyltetrazolium chloride (TTC)-stained, 2-mm-thick brain sections. The infarction areas were quantified using the iSolution full image analysis software (Image & Microscope Technology, Vancouver, Canada). To account for and eliminate the effects of swelling/edema, the infarction volume was calculated via an indirect measure produced by summing the volumes of each section according to the following formula: contralateral hemisphere (mm3) - undamaged ipsilateral hemisphere (mm3)29.
Neurological score
The neurological deficits on each mouse were scored 24 h after ischemic insult in a blinded fashion according to the following graded scoring system: 0=no deficit, 1=forelimb weakness and torso turning to the ipsilateral side when held by the tail, 2=circling to the affected side, 3=unable to bear weight on the affected side, and 4=no spontaneous locomotor activity or barrel rolling30.
Statistical analysis
All data are expressed as the mean±the standard error of the mean (SEM). In the in vitro study, the non-LPS stimulated cells were compared to the cells that were treated with LPS in the absence of probucol with unpaired t-tests, and the cells treated with LPS in the absence of probucol were compared to those treated with each concentration of probucol with a one-way ANOVA followed by Dunnett's tests. In the in vivo study, the vehicle group was compared with the groups that were treated with each concentration of probucol with one-way ANOVA followed by Dunnett's tests. Differences were considered statistically significant when the P values were <0.05. All statistical analyses were performed using SigmaPlot 11.2 (Systat Software Inc, San Jose, CA, USA).
Results
Probucol inhibits NO and PGE2 production in LPS-stimulated BV2 cells without cell toxicity
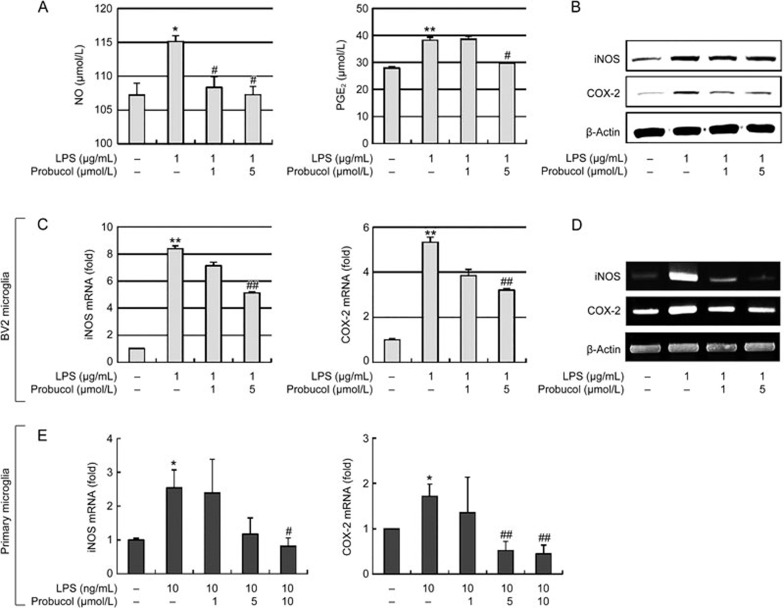
We first investigated the effects of probucol on the production of NO and PGE2, which are key inflammatory mediators in LPS-stimulated BV2 microglia (Figure 1A). LPS induced significant increases in the production of NO and PGE2; however, probucol pretreatment significantly and dose-dependently inhibited the LPS-stimulated NO and PGE2 production in the BV2 cells. To exclude the possibility that the inhibitions of NO and PGE2 production were due to cytotoxicity of probucol, MTT assays were performed with BV2 cells that were treated with probucol alone and in combination with LPS. The concentration (1–5 μmol/L) used to inhibit NO and PGE2 production did not affect cell viability (data not shown), which suggests that the inhibitions of NO and PGE2 production in LPS-stimulated BV2 cells were not due to cytotoxic effects of probucol.
Figure 1.
Effects of probucol on NO and PGE2 production, iNOS mRNA and COX-2 mRNA expression in LPS-stimulated microglial cells. BV2 microglia were treated with the indicated concentrations of probucol (1 or 5 μmol/L) for 3 h prior to LPS (1 μg/mL) treatment. (A) The levels of NO and PGE2 in the media were measured using Griess reagent and ELISA, respectively, 6 h after LPS treatment (n=4). (B) The iNOS and COX-2 protein levels were determined 6 h after LPS stimulation. Actin was used as an internal control for the Western blotting analyses. (C and D) iNOS and COX-2 mRNA levels were determined 6 h after LPS stimulation. GAPDH was used as an internal control for the (C) real-time PCR and (D) RT-PCR assays (n=4). The images are representative of those obtained from four independent experiments. (E) Primary microglia isolated from the neonatal mouse brains were treated with the indicated concentrations of probucol (1, 5, or 10 μmol/L) for 3 h prior to LPS (10 ng/mL) treatment. The iNOS and COX-2 mRNA levels were determined (n=5). The results are expressed as the mean±SEM. *P<0.05, **P<0.01 vs cells without LPS; #P<0.05 and ##P<0.01 vs cells treated with LPS in the absence of probucol.
Probucol inhibits iNOS and COX-2 expression in LPS-stimulated microglial cells
To further investigate whether the suppressions of NO and PGE2 production by probucol occurred in parallel with the expression of their corresponding synthases, ie, iNOS and COX-2, we evaluated the effects of probucol on LPS-stimulated iNOS and COX-2 gene expression in BV2 cells with Western blot analyses, real-time PCR and RT-PCR. The Western blot analyses revealed that the iNOS and COX-2 proteins were markedly upregulated at 6 h after LPS (1 μg/mL) treatment, whereas the probucol attenuated iNOS and COX-2 protein expression in the LPS-stimulated BV2 microglia (Figure 1B). The effects of probucol on the iNOS and COX-2 mRNA levels were also evaluated 6 h after LPS treatment. Real-time PCR analyses revealed that the reductions in the iNOS and COX-2 mRNAs were correlated with the reductions in the corresponding protein levels (Figure 1C), and these data were confirmed by RT-PCR (Figure 1D). Thus, probucol significantly reduced iNOS and COX-2 in LPS-stimulated BV2 cells. Primary microglial cells isolated from neonatal mouse brains were treated with probucol (1, 5, or 10 μmol/L). The iNOS and COX-2 mRNA levels were significantly decreased in the LPS-stimulated primary microglial cells (Figure 1E). Taken together, these results suggest that the inhibitions of NO and PGE2 production by probucol were due to reduced expression of iNOS and COX-2.
Probucol inhibits expression of pro-inflammatory cytokines in LPS-stimulated BV2 cells
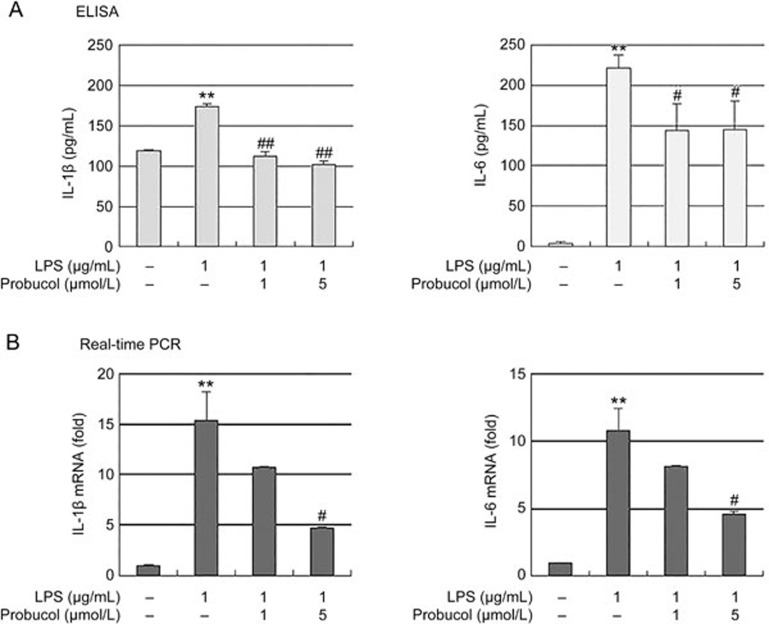
We next investigated whether probucol suppresses pro-inflammatory cytokines, such as IL-1β and IL-6, in LPS-stimulated BV2 cells. BV2 cells were pretreated with probucol for 3 h before treatment with LPS (1 μg/mL) for 6 h, and the cytokine levels in the culture media were subsequently evaluated with ELISA (Figure 2A). LPS induced significant increases in IL-1β and IL-6 production, but these increases were significantly blocked by treatment with probucol. In a parallel experiment, real-time PCR was used to determine whether probucol regulated the expression of these cytokines at the transcription level (Figure 2B). Probucol also reduced the LPS-induced IL-1β and IL-6 mRNA levels in dose-dependent manners. These results suggest that probucol effectively suppressed of IL-1β and IL-6 production via the alteration of the transcription levels in the activated microglia.
Figure 2.
Effect of probucol on IL-1β and IL-6 production in LPS-stimulated BV2 cells. The cells were pretreated with the indicated doses of probucol (1 and 5 μmol/L) for 3 h prior to LPS (1 μg/mL) treatment, and the total RNA and supernatants were isolated 6 h after LPS treatment. (A) The extracellular levels of IL-1β and IL-6 were measured in the culture media using commercial ELISA kits. (B) The levels of IL-1β and IL-6 mRNA were determined by real-time PCR. GAPDH was used as an internal control. The results are expressed as the mean±SEM (n=4). **P<0.01 vs cells without LPS; #P<0.05 and ##P<0.01 vs cells treated with LPS in the absence of probucol.
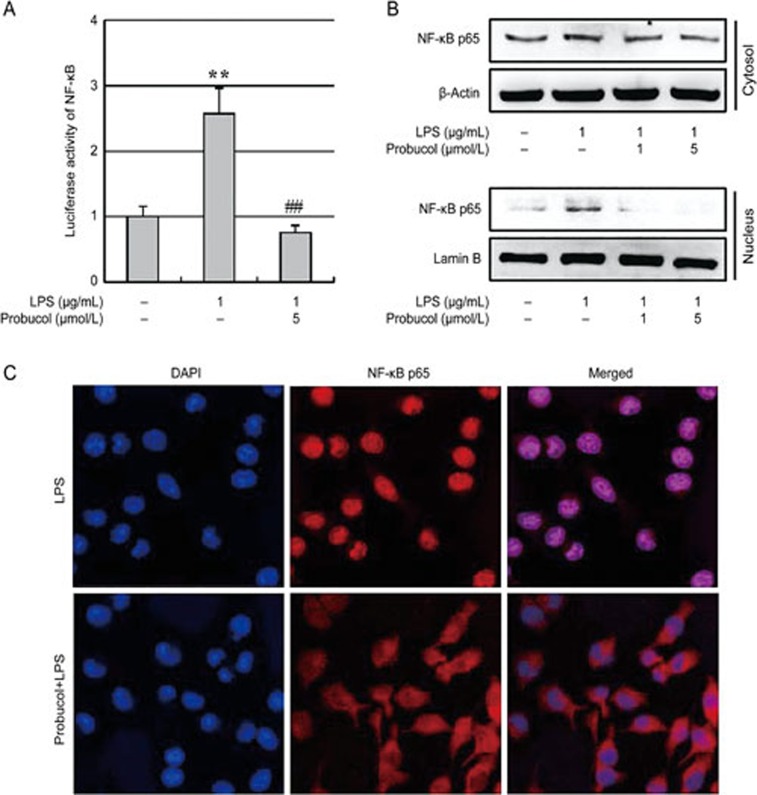
Probucol inhibits the activation of NF-κB in LPS-stimulated BV2 cells
NF-κB is well known as an important transcription factor that regulates the gene expression of pro-inflammatory mediators; therefore, we analyzed the transcriptional activity and nuclear translocation of NF-κB using a luciferase assay, Western blot and immunofluorescence analysis (Figure 3). The LPS-enhanced NF-κB activity was significantly inhibited following 3 h of pretreatment with 5 μmol/L probucol (Figure 3A). To confirm the effects of probucol on NF-κB activity, we investigated the effects of probucol on LPS-induced NF-κB p65 nuclear translocation because NF-κB activated by LPS enters the nucleus and induces gene expression. Immunoblotting revealed that probucol pretreatment significantly attenuated the translocation of the NF-κB p65 protein subunit to the nucleus (Figure 3B), and similar results were found using immunofluorescence microscopy (Figure 3C). These results indicate that the inhibition of NF-κB activation by probucol may be responsible for the suppressions of NO, PGE2, and pro-inflammatory cytokines in BV2 cells.
Figure 3.
Effect of probucol on NF-κB activity. (A) Transfected 293-T cells were pretreated with 5 μmol/L probucol for 3 h and then stimulated with LPS (1 μg/mL) for 6 h. NF-κB activity is expressed as the relative luciferase activity. The results are expressed as the mean±SEM (n=3). **P<0.01 vs cells without LPS; ##P<0.01 vs cells treated with LPS in the absence of probucol. (B) The total cytosolic and nuclear proteins were subjected to Western blotting using anti-NF-κB p65. Actin and lamin B were used as internal controls. (C) The localization of NF-κB p65 was visualized by confocal microscopy following immunofluorescence staining with anti-NF-κB p65 (red). The cells were stained with DAPI to visualize the nuclei (blue). The results are representative of those obtained from three independent experiments.
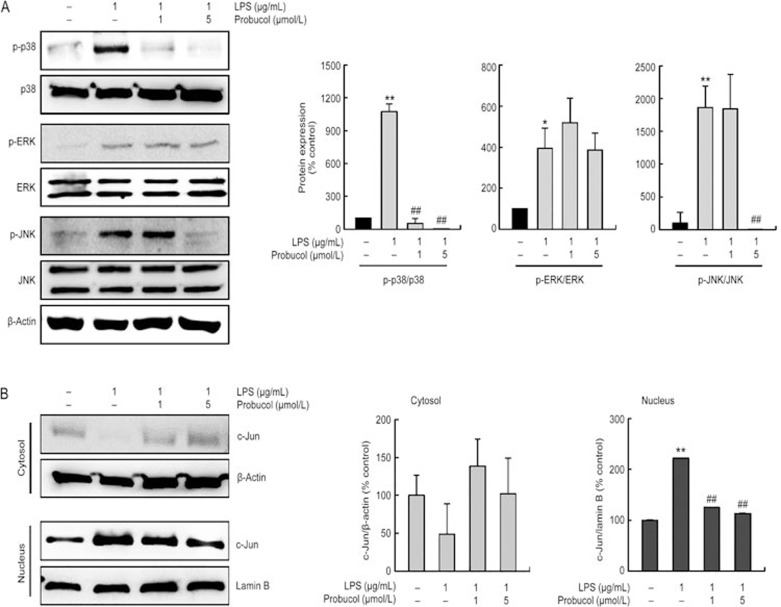
Probucol decreases LPS-induced MAPK activations in BV2 microglia cells
Mitogen-activated protein kinases (MAPKs) are among the most important signaling molecules that are involved in inflammatory responses31. Therefore, we investigated the effects of probucol on p38, ERK-1/2 and JNK activations 6 h after LPS stimulation of the BV2 cells. The phosphorylation of p38, ERK and JNK was markedly upregulated in response to LPS stimulation, whereas the probucol treatment significantly decreased the p38 and JNK MAPK activations (Figure 4A). However, the phosphorylation of ERK-1/2 was not affected. The total amounts of p38, ERK-1/2 and JNK were unaffected by the LPS and probucol treatments. These findings suggest that probucol is capable of disrupting the p38 and JNK pathways that are activated by LPS in BV2 microglia. We next investigated the translocation of c-Jun, which is the major component of the AP-1 family, from cytosol to the nucleus using Western blot analysis to determine whether AP-1 was involved in the inhibition of inflammatory mediators by probucol (Figure 4B). LPS stimulation caused c-Jun to translocated from the cytosol to the nucleus, whereas probucol inhibited this translocation.
Figure 4.
Probucol inhibited the activations of MAPKs in the LPS-stimulated BV2 cells. BV2 cells were treated with the indicated dose of probucol (1 or 5 μmol/L) 3 h before LPS treatment (1 μg/mL) for 6 h. (A) Probucol treatment significantly decreased the LPS-induced p38 and JNK MAPK activations. Total protein (50 μg) was subjected to 10% SDS-PAGE followed by Western blotting using anti-p-p38, anti-p38, anti-p-ERK-1/2, anti-ERK-1/2, anti-p-JNK, and anti-JNK. Quantification graphs (n=3). (B) The total cytosolic and nuclear proteins were subjected to Western blotting using anti-c-Jun. Actin and lamin B were used as internal controls. Quantification graphs (n=3).
Probucol prevents ischemic brain injury in mice via the attenuation of pro-inflammatory cytokine production in the ischemic brain
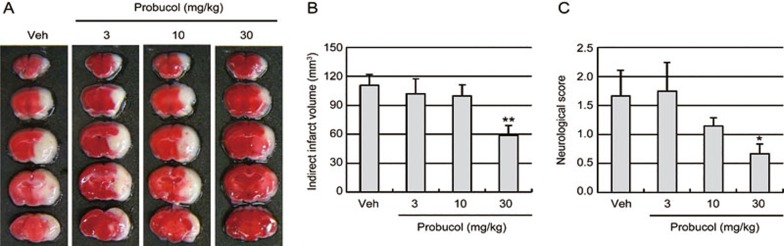
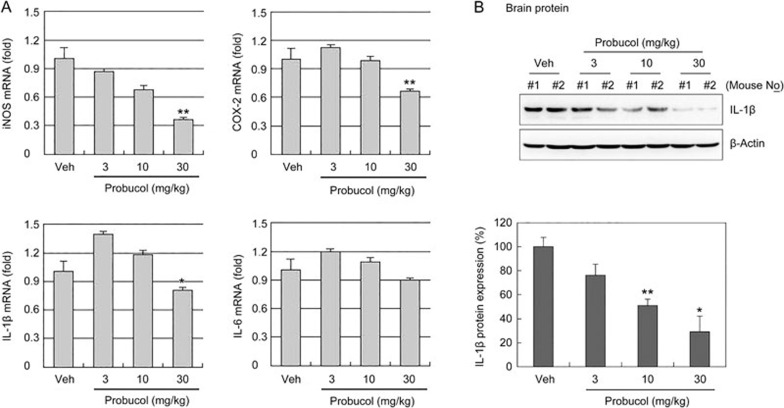
To determine whether probucol improved the tissue and functional outcomes following focal cerebral ischemia in healthy mice, the infarct sizes and neurological score were measured 24 h after 1 h of MCAO in C57BL/6J mice (Figure 5). The mice received probucol (3, 10, or 30 mg/kg in 1% DMSO, orally) once per day for 4 d prior to the ischemic event. TTC staining revealed that probucol (30 mg/kg) significantly decreased the cerebral infarct volume relative to the volumes observed in the vehicle mice (Figure 5A, 5B). Consistent with the smaller infarct sizes, probucol led to prominent improvements in neurological function 24 h after ischemic injury (Figure 5C). These findings suggested that pretreatment with probucol significantly attenuated ischemic brain injury. We next assessed the iNOS, COX-2, IL-1β, and IL-6 mRNA levels in the brain tissues 24 h after ischemic brain injury to examine the anti-neuroinflammatory effects of probucol on focal cerebral ischemia (Figure 6). Quantitative PCR data revealed that the iNOS, COX-2 and IL-1β mRNA levels in the ischemic brain were significantly decreased by treatment with 30 mg/kg probucol (Figure 6A). Probucol treatment did not affect the expression of these genes in normal brains (Supplementary Figure S1). Western blot analysis revealed that IL-1β protein expression in the ischemic brain was also decreased by treatment with probucol (Figure 6B).
Figure 5.
Effect of probucol on infarct volumes and neurological deficits following focal cerebral ischemia in normal mice. (A) Representative photographs of coronal brain sections from vehicle- (Veh) and probucol-treated mice stained with 2,3,5-triphenyltetrazolium chloride. C57BL/6J mice received probucol (3, 10, and 30 mg/kg in 1% DMSO, orally) once per day for 4 d prior to the ischemic insults. White indicates the infarct area. (B) Quantification of the infarct volumes 24 h after 1 h of middle cerebral artery occlusion (n=6). The infarct volumes were calculated by integrating the infarct areas in 2 mm-thick coronal slices. (C) Neurological deficits were assessed in each mouse 24 h after the ischemic insults in a blinded fashion (n=6). The values are presented as the mean±SEM. *P<0.05 and **P<0.01 vs vehicle group (Veh).
Figure 6.
Effect of probucol on iNOS, COX-2, IL-1β and IL-6 expression in the ischemic brain. (A) Bar graph showing the mRNA levels of iNOS, COX-2, IL-1β, and IL-6 in the ischemic brain tissues 24 h after focal cerebral ischemia as assessed with real-time PCR. These gene expression levels were normalized against GAPDH. The results are expressed as the mean±SEM (n=4). *P<0.05 and **P<0.01 vs vehicle group (Veh). (B) Western blotting for IL-1β using ischemic brain lysate (n=4). *P<0.05 and **P<0.01 vs vehicle group (Veh).
Probucol attenuates ischemic brain injury in hyperlipidemic mice
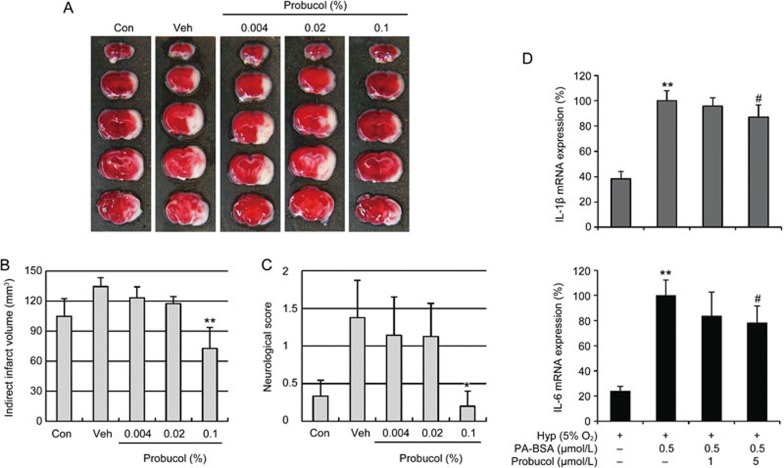
Finally, we investigated whether probucol attenuates the hyperlipidemia-induced exacerbation of ischemic brain injury by inducing MCAO in ApoE KO mice that had previously been fed a HFD for 10 weeks (Figure 7). The body weights of the ApoE KO mice fed a HFD were slightly greater than those of the normal diet-fed mice (control), and the treatment with 0.004%, 0.02%, and 0.1% probucol did not affect the body weights or blood pressures of the HFD-fed mice (Table 2). After 10 weeks of HFD, large increases in plasma total cholesterol and low-density lipoprotein (LDL) cholesterol levels were observed in the ApoE KO mice (Table 2, P<0.01 vs control); however, these increased were significantly mitigated by the 0.1% probucol treatment (P<0.01 vs vehicle). To determine whether probucol improved the tissue outcomes following cerebral ischemia in hyperlipidemic mice, the infarct sizes and neurological scores were measured 24 h after 45-min transient MCA occlusions. The treatment with 0.1% probucol significantly reduced the infarct volumes (Figure 7A, 7B) and improved the neurological functions (Figure 7C) of the ApoE KO that were fed the HFD for 10 weeks. The BV2 microglial cells were exposed to in vitro hyperlipidemic conditions (0.5 μmol/L palmitic acid-BSA) under hypoxia (5% O2), and we observed IL-1β and IL-6 mRNA expression (Figure 7D). In the hypoxic conditions, palmitic acid (PA) treatment induced significant increases in both IL-1β and IL-6, and these increases were mitigated by probucol. These data are consistent with the findings from the in vivo hyperlipidemic mice. Based on our results, we suggest that probucol attenuates ischemic brain damage in both young healthy and hyperlipidemic mice.
Figure 7.
Effects of probucol on the infarct volumes and functional outcomes following focal cerebral ischemia in hyperlipidemic mice. (A) Representative topical TTC-stained brains from ApoE KO mice that were fed HFD with or without 0.004%, 0.02%, or 0.1% probucol for 10 weeks. The mice were subjected to 45 min of MCA occlusion followed by 24 h of reperfusion. White indicates the infarct area. (B) Quantification of the infarct volumes 24 h after ischemia (n=7–8). The infarct volumes were calculated by integrating the infarct areas in 2 mm-thick coronal slices. (C) The neurological deficits of each mouse were assessed 24 h after ischemic insult in a blinded fashion (n=7–8). The values are presented as the mean±SEM. *P<0.05, **P<0.01 vs age- and diet-matched ApoE KO mice (Veh, vehicle). (D) BV2 microglial cells were exposed to in vitro hyperlipidemic conditions (0.5 μmol/L palmitic acid-BSA) with hypoxia (5% O2). Palmitic acid (PA)-BSA treated cells were incubated for 3 h, moved to hypoxic conditions and further incubated for 16 h. We observed the IL-1β and IL-6 mRNA expression with real-time PCR. The PA-BSA-induced upregulations of IL-1β and IL-6 were decreased by probucol (n=6). **P<0.01 vs hypoxia group (Hyp); #P<0.05 vs PA-BSA with hyp group (Veh).
Table 2. Physiological parameters.
| Control | Vehicle | 0.004% Probucol | 0.02% Probucol | 0.1% Probucol | |
|---|---|---|---|---|---|
| Body weight (g) | 32.64±1.24 | 35.38±0.68 | 37.67±0.74 | 36.44±0.97 | 33.92±1.65 |
| MABP (mmHg) | 110.33±2.04 | 112.32±2.52* | 110.84±2.43 | 113.98±1.64 | 107.10±3.51 |
| pH | 7.40±0.01 | 7.43±0.01 | 7.43±0.02 | 7.40±0.01 | 7.39±0.02 |
| pO2 (mmHg) | 96.40±2.16 | 96.50±1.38 | 96.43±2.19 | 95.88±1.38 | 96.81±0.86 |
| pCO2 (mmHg) | 35.07±1.71 | 32.29±1.29 | 32.33±1.89 | 33.68±1.00 | 36.30±1.67 |
| Total cholesterol | 108.17±9.64 | 240.38±21.39** | 292.14±20.26 | 264.75±37.69 | 114.00±32.24# |
| LDL-cholesterol | 27.83±2.41 | 153.25±16.81** | 167.86±9.77 | 157.00±32.74 | 59.00±14.67# |
Values are the mean±SEM. Body weight is expressed in grams. MABP (mean arterial blood pressure), pO2, and pCO2 are expressed in mmHg.
*P<0.05,
**P<0.01 vs age-matched ApoE KO without HFD (Control);
#P<0.05 vs age- and diet-matched ApoE KO (Vehicle).
Discussion
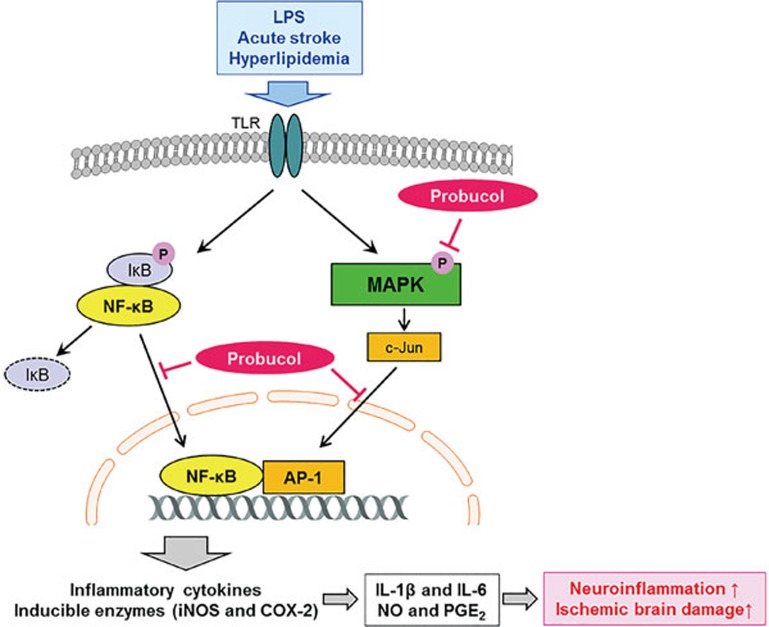
The present study was conducted to elucidate the anti-neuroinflammatory effects of probucol and the mechanisms of the influences of probucol on the production of pro-inflammatory mediators in LPS-stimulated microglial cells and ischemic brain damage in normal and hyperlipidemic mice. Probucol significantly and dose dependently inhibited the pro-inflammatory mediators at the level of transcription in the LPS-stimulated BV2 cells and primary microglia. This study also revealed that the pharmacological actions of probucol were associated with the downregulations of the NF-κB, MAPK, and AP-1 signaling pathways. In the in vivo study, treatment with probucol resulted in significantly reduced infarct volumes and improved neurological functions 24 h after ischemic brain injury, and these effects were possibly due to the inhibition of the production of pro-inflammatory mediators. Treatment with 0.1% probucol for 10 weeks also reduced the infarct volumes and improved the neurological deficits in addition to decreasing the total- and LDL-cholesterol in the ApoE KO mice that had been fed a high-fat diet. These findings suggest that probucol modulated LPS-induced neuroinflammatory responses and is a potential therapeutic candidate for the treatment of ischemic brain injury in both young healthy and hyperlipidemic mice (Figure 8).
Figure 8.
Schematic model of the anti-neuroinflammatory effects of probucol in ischemic brain injury.
Microglia is the resident macrophages of the brain and plays a key role in brain inflammation. Increasing evidence implicates microglial overactivation following acute ischemic stroke in the excess production of inflammatory mediators, including NO, PGE2 and pro-inflammatory cytokines, which then contribute to secondary brain injury and exacerbate neuronal injury1,2. Therefore, the identification of novel agents that regulate neuroinflammation by inhibiting microglial activation is regarded as a significant strategy for the treatment of ischemic stroke. Several animal studies have demonstrated that probucol exerts beneficial effects via anti-inflammation mechanisms in aging mice19,20, focal cerebral ischemic mice8,9, heart failure rats21,22, diabetic rabbits23 and atherosclerotic rabbits24. Probucol has also been demonstrated to prevent atherogenesis via the induction of the anti-inflammatory enzyme heme oxygenase-1 independently of lipid oxidation and cholesterol reduction in models of atherosclerosis, restenosis and type 2 diabetes32. Most recently, we found that probucol and probucol plus cilostazol decrease microglial activation in the ischemic cortices of hyperlipidemic mice8,9. Therefore, we investigated the effects of probucol on the expression of inflammation-related genes in LPS-stimulated microglia.
NO production from iNOS can lead to inflammatory disorders, such as ischemic and neurodegenerative diseases33. Similarly, PGE2 is a well-known inflammatory mediator that is derived from arachidonic acid via the action of COX-2. COX-2 emerged as a major player in brain inflammation, and increased COX-2 expression is believed to contribute to brain injury34. In the present study, probucol concentration-dependently inhibited both NO and PGE2 production via the down-regulations of iNOS and COX-2 expression at the mRNA and protein levels in the LPS-stimulated BV2 cells and primary microglia (Figure 1). Moreover, the in vivo investigation revealed that 30 mg/kg probucol significantly suppressed iNOS and COX-2 gene expression in the ischemic brain tissues. Similar results have been reported by Takechi et al. who found that long-term probucol therapy completely abolishes the saturated fat-induced COX-2 increase in a diet-induced model of cerebral capillary dysfunction20. These data suggest that the anti-inflammatory activity of probucol is involved in the modulation of iNOS and COX-2 expression.
Microglia is the main source of excess cytokines that elicit inflammatory responses, including IL-1β and IL-6. These pro-inflammatory cytokines are thought to be responsible for some of the harmful effects of ischemic brain injuries35. IL-1β is a potent pro-inflammatory cytokine that acts through the IL-1 receptors found on numerous cell types, including neurons and microglia. Moreover, IL-1β has been proposed to be an important mediator of neuroimmune interactions that directly participate in stroke36. IL-6 is a crucial inflammatory factor as demonstrated by the finding of a significant increase in IL-6 in stroke patients shortly following the ischemic event. Moreover, IL-6 plays a vital role as a messenger molecule between leucocytes, the vascular endothelium, and the resident parenchyma cells37. Therefore, the inhibition of inflammatory cytokine production or function serves as a key mechanism in the control of brain damage and neuroinflammation.
Probucol has exhibited favorable results in the modulation of inflammatory cytokine production. Specifically, probucol has been found to have the capacity to inhibit the expression of oxidation-sensitive inflammatory factors, such as VCAM-138, MCP-139, and IL-140. Additionally, the serum concentrations of inflammatory cytokines (CRP, IL-6, IL-18, and TNF-α) are markedly lower in probucol-treated atherosclerotic rabbits24. The expression of TNF-α, TGF-β, and HSP70 is also significantly downregulated by probucol treatment in alloxan-induced diabetic rabbits23. Consistent with these reports, our findings provide evidence that treatment with probucol significantly reduced IL-1β and IL-6 production in addition to IL-1β and IL-6 gene expression in LPS-activated microglial BV2 cells (Figure 2). These data are in agreement with in vivo results that demonstrated that the increased IL-1β mRNA and IL-1β protein levels in the ischemic brains were reduced in response to treatment with probucol (Figure 6). Based on these findings, probucol exerts anti-inflammatory properties via the inhibition of pro-inflammatory cytokines.
NF-κB is activated in cerebral ischemia and promotes ischemic brain injury through the regulation of the expression of various genes that are involved in the production of many pro-inflammatory cytokines and enzymes that are related to the inflammatory process41,42. The phosphorylation of NF-κB p65 (Ser536) is required for the activation and nuclear translocation of NF-κB, which results in transcriptional induction of inflammation-associated genes42. We demonstrated that probucol prevents LPS-induced NF-κB activity and nuclear translocation (Figure 3), which suggests that the attenuation of the NF-κB signaling pathways in microglia by probucol might result in the down-regulation of inflammatory mediators and cytokines and thus result in an anti-inflammatory effect.
Aside from NF-κB, MAPKs have been implicated as critical mediators of inflammatory responses in three MAPK cascades, ie, the p38, ERK-1/2, and JNK subgroups31. The MAPKs p38, ERK and JNK are known to be activated by LPS, and several studies have demonstrated the significance of MAPKs in the transcriptional regulation of the LPS-induced production of inflammatory mediators via the activation of transcription factor AP-143. In the present study, we demonstrated that treatment with probucol significantly inhibited LPS-stimulated phosphorylation of p38 and JNK (but not Erk1/2) and the activity of c-Jun, which is a major component of the AP-1 family that is responsible for the over-reactive inflammatory responses of BV2 cells. These results indicate that probucol also suppresses inflammatory mediators through the inhibition of MAPK signaling pathways and the attenuation of the activity of transcription factor AP-1. Taken together, our data demonstrated that the inhibitions of COX-2, iNOS, IL-1β, and IL-6 by probucol in LPS-stimulated BV2 microglia might be due to its inhibitory effects on the NF-κB, MAPK, and AP-1 signaling pathways.
Stroke is characterized by massive inflammation in areas surrounding the ischemic injury that magnify damage to the brain1. Therefore, the finding of novel agents to regulate neuroinflammation could be an efficient approach to protecting the brain against ischemic injury. The anti-neuroinflammatory effects of probucol are further supported by an in vivo focal cerebral ischemia mouse model. Probucol (30 mg/kg) improved the tissue and functional outcomes 24 h after 1 h of MCA occlusion (Figure 5), and the iNOS, COX-2, and IL-1β levels in the ischemic brains were significantly decreased by treatment with probucol (Figure 6), and the IL-6 mRNA levels exhibited a trend toward a decrease. These findings suggest that the inhibitory effects of probucol on inflammatory mediator expression in the ischemic brain enabled the identification of one of the mechanisms responsible for its beneficial effects against focal cerebral ischemia. In addition to its anti-neuroinflammatory effects, probucol has been demonstrated to protect forebrain ischemia-induced hippocampal neuronal loss, energy depletion, and oxidative stress in the hippocampal CA1 region44; these findings suggest that probucol may be an agent of therapeutic value for individuals with ischemic stroke.
Hyperlipidemia is a major risk factor associated with ischemic stroke, and half of all stroke patients have hyperlipidemia4. It is well known that hyperlipidemia induces inflammation in the brain5,6,7 and that hyperlipidemia exacerbates the ischemic brain damage that is linked to neuroinflammation8,9. There is also considerable evidence that hyperlipidemia contributes to the disruption of cerebrovascular reflexes and the breakdown of the blood-brain barrier45,46. Therefore, the modulation of hyperlipidemia and neuroinflammation via probucol may be an effective therapeutic strategy for reducing the effects of both hyperlipidemia and ischemic stroke. A promising model of hyperlipidemia is the spontaneously hyperlipidemic ApoE KO mouse in which HFD induces inflammation and exacerbates the development of severe lesions in the arteries47. Via the use of an experimental stroke model in hyperlipidemic mice, the present study investigated whether probucol attenuates the hyperlipidemia-induced exacerbation of ischemic brain injury. Cerebral ischemia was induced by 45 min of MCA occlusion and 24 h reperfusion in hyperlipidemic mice and by 1 h of MCA occlusion and 24 h reperfusion in normal mice. This approach was selected because the cerebral infarctions and neurological deficits were greater in the hyperlipidemic mice than in the normal mice. Our data revealed that 0.1% probucol significantly decreased the total and LDL cholesterol levels and reduced the larger infarct volumes and neurological deficits that were observed in the ApoE KO that were fed HFD for 10 weeks compared with the ApoE KO that were fed a regular diet. The pharmacological inhibition of the cholesterol level and neuroinflammation by probucol are important targets for potential treatments of ischemic stroke in the presence of hyperlipidemia.
In conclusion, probucol exhibits anti-inflammatory effects in LPS-stimulated microglia via the down-regulation of pro-inflammatory mediators corresponding to the suppression of NF-κB and MAPK activation. Additionally, probucol prevents cerebral ischemic damage in normal and hyperlipidemic mice via anti-neuroinflammatory action, which suggests that probucol could have potential for the treatment of patients with or at risk of ischemic stroke with hyperlipidemia. These findings suggest that probucol may be used as a potential neurotherapeutic to treat the inflammatory components of acute brain injury.
Author contribution
Yeon Suk JUNG, Byung Tae CHOI, Sae-Won LEE, and Hwa Kyoung SHIN participated in the research design; Yeon Suk JUNG, Jung Hwa PARK, Hyunha KIM, Ji Young HWANG, and So Young KIM conducted the experiments; Ki Whan HONG and Hwa Kyoung SHIN contributed new reagents or analytic tools; Yeon Suk JUNG, Sae-Won LEE, Jung Hwa PARK, Hyunha KIM, and So Young KIM performed the data analysis; Yeon Suk JUNG, Sun Sik BAE, Byung Tae CHOI, Sae-Won LEE, and Hwa Kyoung SHIN wrote or contributed to the writing of the manuscript.
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (2014R1A5A2009936).
Footnotes
Supplementary Figure S1 is available at the Acta Pharmacologica Sinica's website.
Supplementary Information
References
- Wang Q, Tang XN, Yenari MA. The inflammatory response in stroke. J Neuroimmunol 2007; 184: 53–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z, Yenari MA. Post-ischemic inflammation: molecular mechanisms and therapeutic implications. Neurol Res 2004; 26: 884–92. [DOI] [PubMed] [Google Scholar]
- Engstrom G, Lind P, Hedblad B, Stavenow L, Janzon L, Lindgarde F. Effects of cholesterol and inflammation-sensitive plasma proteins on incidence of myocardial infarction and stroke in men. Circulation 2002; 105: 2632–7. [DOI] [PubMed] [Google Scholar]
- Rother J, Alberts MJ, Touze E, Mas JL, Hill MD, Michel P, et al. Risk factor profile and management of cerebrovascular patients in the REACH Registry. Cerebrovasc Dis 2008; 25: 366–74. [DOI] [PubMed] [Google Scholar]
- Rahman SM, Van Dam AM, Schultzberg M, Crisby M. High cholesterol diet results in increased expression of interleukin-6 and caspase-1 in the brain of apolipoprotein E knockout and wild type mice. J Neuroimmunol 2005; 169: 59–67. [DOI] [PubMed] [Google Scholar]
- Thirumangalakudi L, Prakasam A, Zhang R, Bimonte-Nelson H, Sambamurti K, Kindy MS, et al. High cholesterol-induced neuroinflammation and amyloid precursor protein processing correlate with loss of working memory in mice. J Neurochem 2008; 106: 475–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue QS, Sparks DL, Streit WJ. Microglial activation in the hippocampus of hypercholesterolemic rabbits occurs independent of increased amyloid production. J Neuroinflammation 2007; 4: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Park SH, Bae SS, Hong KW, Kim YD, Park KP, et al. Combinatorial effect of probucol and cilostazol in focal ischemic mice with hypercholesterolemia. J Pharmacol Exp Ther 2011; 338: 451–7. [DOI] [PubMed] [Google Scholar]
- Kim JH, Hong KW, Bae SS, Shin YI, Choi BT, Shin HK. Probucol plus cilostazol attenuate hypercholesterolemiainduced exacerbation in ischemic brain injury via anti-inflammatory effects. Int J Mol Med 2014; 34: 687–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnhart JW, Sefranka JA, McIntosh DD. Hypocholesterolemic effect of 4,4'-(isopropylidenedithio)-bis(2,6-di-t-butylphenol) (probucol). Am J Clin Nutr 1970; 23: 1229–33. [DOI] [PubMed] [Google Scholar]
- Yamashita S, Matsuzawa Y. Where are we with probucol: a new life for an old drug? Atherosclerosis 2009; 207: 16–23. [DOI] [PubMed] [Google Scholar]
- Schneider JE, Berk BC, Gravanis MB, Santoian EC, Cipolla GD, Tarazona N, et al. Probucol decreases neointimal formation in a swine model of coronary artery balloon injury. A possible role for antioxidants in restenosis. Circulation 1993; 88: 628–37. [DOI] [PubMed] [Google Scholar]
- Lau AK, Leichtweis SB, Hume P, Mashima R, Hou JY, Chaufour X, et al. Probucol promotes functional reendothelialization in balloon-injured rabbit aortas. Circulation 2003; 107: 2031–6. [DOI] [PubMed] [Google Scholar]
- Tardif JC, Gregoire J, Schwartz L, Title L, Laramee L, Reeves F, et al. Effects of AGI-1067 and probucol after percutaneous coronary interventions. Circulation 2003; 107: 552–8. [DOI] [PubMed] [Google Scholar]
- Tardif JC, Cote G, Lesperance J, Bourassa M, Lambert J, Doucet S, et al. Probucol and multivitamins in the prevention of restenosis after coronary angioplasty. Multivitamins and Probucol Study Group. N Engl J Med 1997; 337: 365–72. [DOI] [PubMed] [Google Scholar]
- Sasahara M, Raines EW, Chait A, Carew TE, Steinberg D, Wahl PW, et al. Inhibition of hypercholesterolemia-induced atherosclerosis in the nonhuman primate by probucol. I. Is the extent of atherosclerosis related to resistance of LDL to oxidation? J Clin Invest 1994; 94: 155–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasen JH, Koenig K, Bach H, Kontush A, Heinle H, Witting PK, et al. Comparison of the effects of alpha-tocopherol, ubiquinone-10 and probucol at therapeutic doses on atherosclerosis in WHHL rabbits. Atherosclerosis 2002; 163: 249–59. [DOI] [PubMed] [Google Scholar]
- Braun A, Zhang S, Miettinen HE, Ebrahim S, Holm TM, Vasile E, et al. Probucol prevents early coronary heart disease and death in the high-density lipoprotein receptor SR-BI/apolipoprotein E double knockout mouse. Proc Natl Acad Sci U S A 2003; 100: 7283–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takechi R, Galloway S, Pallebage-Gamarallage MM, Lam V, Dhaliwal SS, Mamo JC. Probucol prevents blood-brain barrier dysfunction in wild-type mice induced by saturated fat or cholesterol feeding. Clin Exp Pharmacol Physiol 2013; 40: 45–52. [DOI] [PubMed] [Google Scholar]
- Takechi R, Pallebage-Gamarallage MM, Lam V, Giles C, Mamo JC. Long-term probucol therapy continues to suppress markers of neurovascular inflammation in a dietary induced model of cerebral capillary dysfunction. Lipids Health Dis 2014; 13: 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sia YT, Lapointe N, Parker TG, Tsoporis JN, Deschepper CF, Calderone A, et al. Beneficial effects of long-term use of the antioxidant probucol in heart failure in the rat. Circulation 2002; 105: 2549–55. [DOI] [PubMed] [Google Scholar]
- Sia YT, Parker TG, Liu P, Tsoporis JN, Adam A, Rouleau JL. Improved post-myocardial infarction survival with probucol in rats: effects on left ventricular function, morphology, cardiac oxidative stress and cytokine expression. J Am Coll Cardiol 2002; 39: 148–56. [DOI] [PubMed] [Google Scholar]
- Fu H, Li G, Liu C, Li J, Wang X, Cheng L, et al. Probucol prevents atrial remodeling by inhibiting oxidative stress and TNF-alpha/NF-kappaB/TGF-beta signal transduction pathway in alloxan-induced diabetic rabbits. J Cardiovasc Electrophysiol 2015; 26: 211–22. [DOI] [PubMed] [Google Scholar]
- Li T, Chen W, An F, Tian H, Zhang J, Peng J, et al. Probucol attenuates inflammation and increases stability of vulnerable atherosclerotic plaques in rabbits. Tohoku J Exp Med 2011; 225: 23–34. [DOI] [PubMed] [Google Scholar]
- Dai ZK, Lin TC, Liou JC, Cheng KI, Chen JY, Chu LW, et al. Xanthine derivative KMUP-1 reduces inflammation and hyperalgesia in a bilateral chronic constriction injury model by suppressing MAPK and NFkappaB activation. Mol Pharm 2014; 11: 1621–31. [DOI] [PubMed] [Google Scholar]
- Leroy C, Tricot S, Lacour B, Grynberg A. Protective effect of eicosapentaenoic acid on palmitate-induced apoptosis in neonatal cardiomyocytes. Biochim Biophys Acta 2008; 1781: 685–93. [DOI] [PubMed] [Google Scholar]
- Saura J, Tusell JM, Serratosa J. High-yield isolation of murine microglia by mild trypsinization. Glia 2003; 44: 183–9. [DOI] [PubMed] [Google Scholar]
- Huang Z, Huang PL, Panahian N, Dalkara T, Fishman MC, Moskowitz MA. Effects of cerebral ischemia in mice deficient in neuronal nitric oxide synthase. Science 1994; 265: 1883–5. [DOI] [PubMed] [Google Scholar]
- Lin TN, He YY, Wu G, Khan M, Hsu CY. Effect of brain edema on infarct volume in a focal cerebral ischemia model in rats. Stroke 1993; 24: 117–21. [DOI] [PubMed] [Google Scholar]
- Li X, Blizzard KK, Zeng Z, DeVries AC, Hurn PD, McCullough LD. Chronic behavioral testing after focal ischemia in the mouse: functional recovery and the effects of gender. Exp Neurol 2004; 187: 94–104. [DOI] [PubMed] [Google Scholar]
- Kim SH, Smith CJ, Van Eldik LJ. Importance of MAPK pathways for microglial pro-inflammatory cytokine IL-1 beta production. Neurobiol Aging 2004; 25: 431–9. [DOI] [PubMed] [Google Scholar]
- Wu BJ, Kathir K, Witting PK, Beck K, Choy K, Li C, et al. Antioxidants protect from atherosclerosis by a heme oxygenase-1 pathway that is independent of free radical scavenging. J Exp Med 2006; 203: 1117–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GC, Bal-Price A. Inflammatory neurodegeneration mediated by nitric oxide, glutamate, and mitochondria. Mol Neurobiol 2003; 27: 325–55. [DOI] [PubMed] [Google Scholar]
- Kawano T, Anrather J, Zhou P, Park L, Wang G, Frys KA, et al. Prostaglandin E2 EP1 receptors: downstream effectors of COX-2 neurotoxicity. Nat Med 2006; 12: 225–9. [DOI] [PubMed] [Google Scholar]
- Lambertsen KL, Biber K, Finsen B. Inflammatory cytokines in experimental and human stroke. J Cereb Blood Flow Metab 2012; 32: 1677–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell N, Allan S, Toulmond S. The role of interleukin 1 in acute neurodegeneration and stroke: pathophysiological and therapeutic implications. J Clin Invest 1997; 100: 2648–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenhar-Tsarfaty S, Ben Assayag E, Bova I, Shopin L, Fried M, Berliner S, et al. Interleukin-6 as an early predictor for one-year survival following an ischaemic stroke/transient ischaemic attack. Int J Stroke 2010; 5: 16–20. [DOI] [PubMed] [Google Scholar]
- Wu BJ, Di Girolamo N, Beck K, Hanratty CG, Choy K, Hou JY, et al. Probucol [4,4'-[(1-methylethylidene)bis(thio)]bis-[2,6-bis(1,1-dimethylethyl)phenol]] inhibits compensatory remodeling and promotes lumen loss associated with atherosclerosis in apolipoprotein E-deficient mice. J Pharmacol Exp Ther 2007; 321: 477–84. [DOI] [PubMed] [Google Scholar]
- Chang MY, Sasahara M, Chait A, Raines EW, Ross R. Inhibition of hypercholesterolemia-induced atherosclerosis in the nonhuman primate by probucol. II. Cellular composition and proliferation. Arterioscler Thromb Vasc Biol 1995; 15: 1631–40. [DOI] [PubMed] [Google Scholar]
- Ku G, Doherty NS, Schmidt LF, Jackson RL, Dinerstein RJ. Ex vivo lipopolysaccharide-induced interleukin-1 secretion from murine peritoneal macrophages inhibited by probucol, a hypocholesterolemic agent with antioxidant properties. FASEB J 1990; 4: 1645–53. [DOI] [PubMed] [Google Scholar]
- Schneider A, Martin-Villalba A, Weih F, Vogel J, Wirth T, Schwaninger M. NF-kappaB is activated and promotes cell death in focal cerebral ischemia. Nat Med 1999; 5: 554–9. [DOI] [PubMed] [Google Scholar]
- Ridder DA, Schwaninger M. NF-kappaB signaling in cerebral ischemia. Neuroscience 2009; 158: 995–1006. [DOI] [PubMed] [Google Scholar]
- Moriyama N, Taniguchi M, Miyano K, Miyoshi M, Watanabe T. ANP inhibits LPS-induced stimulation of rat microglial cells by suppressing NF-kappaB and AP-1 activations. Biochem Biophys Res Commun 2006; 350: 322–8. [DOI] [PubMed] [Google Scholar]
- Al-Majed AA. Probucol attenuates oxidative stress, energy starvation, and nitric acid production following transient forebrain ischemia in the rat hippocampus. Oxid Med Cell Longev 2011; 2011: 471590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayata C, Shin HK, Dilekoz E, Atochin DN, Kashiwagi S, Eikermann-Haerter K, et al. Hyperlipidemia disrupts cerebrovascular reflexes and worsens ischemic perfusion defect. J Cereb Blood Flow Metab 2013; 33: 954–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalayci R, Kaya M, Uzun H, Bilgic B, Ahishali B, Arican N, et al. Influence of hypercholesterolemia and hypertension on the integrity of the blood-brain barrier in rats. Int J Neurosci 2009; 119: 1881–904. [DOI] [PubMed] [Google Scholar]
- Nakashima Y, Plump AS, Raines EW, Breslow JL, Ross R. ApoE-deficient mice develop lesions of all phases of atherosclerosis throughout the arterial tree. Arterioscler Thromb 1994; 14: 133–40. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.