Abstract
Attention deficit hyperactivity disorder (ADHD) is associated with increased risk of tobacco dependence. Nicotine, the main psychoactive component of tobacco, appears to be implicated in ADHD-related tobacco dependence. However, the behavioral responsiveness to nicotine of the prevalent animal model of ADHD, the spontaneously hypertensive rat (SHR), is currently underinvestigated. The present study examined the activational effects of acute and chronic nicotine on the behavior of adult male SHRs, relative to Wistar Kyoto (WKY) controls. Experiment 1 verified baseline strain differences in open-field locomotor activity. Experiment 2 tested for baseline strain differences in rotational behavior using a Rotorat apparatus. Adult SHR and WKY rats were then exposed to a 7-day regimen of 0.6 mg/kg/d s.c. nicotine, or saline, prior to each assessment. A separate group of SHRs underwent similar training, but was pre-treated with mecamylamine, a cholinergic antagonist. Nicotine sensitization, context conditioning, and mecamylamine effects were then tested. Baseline strain differences were observed in open-field performance and in the number of full rotations in the Rotorat apparatus, but not in the number of 90° rotations or direction changes. In these latter measures, SHRs displayed weaker nicotine-induced rotational suppression than WKYs. Both strains expressed nicotine-induced sensitization of rotational activity, but evidence for strain differences in sensitization was ambiguous; context conditioning was not observed. Mecamylamine reversed the effects of nicotine on SHR performance. These findings are consistent with the hypothesis that a reduced aversion to nicotine (expressed in rats as robust locomotion) may facilitate smoking among adults with ADHD.
Keywords: Nicotine, Sensitization, Suppression, Spontaneously Hypertensive Rat, Wistar Kyoto, ADHD
1. Introduction
Attention deficit-hyperactivity disorder (ADHD) is a childhood neurodevelopmental disorder characterized by symptoms of inattention, hyperactivity, and impulsivity [1] that often persist into adulthood [2-4]. ADHD is a risk factor for smoking and tobacco dependence [5-7]. Adolescents with ADHD begin experimenting with tobacco and progress to regular use at a younger age than their non-diagnosed peers, and are more likely to continue smoking as adults [8-10]. Prevalence of smoking among adolescents and adults with ADHD is double that of the general population [8,10]. In addition, smokers with ADHD consume more cigarettes per day and are measurably more dependent on tobacco than smokers without ADHD [11,12]. Once dependent, smokers with ADHD report more frequent but less successful quit attempts than smokers without ADHD, and exhibit more severe withdrawal symptoms [6,13-15].
Smokers with ADHD report that smoking is reinforcing, induces wakefulness, enhances cognition, decreases irritability, and improves mood [16,17]. Although smokers without ADHD also report these effects [18], smokers with ADHD report stronger effects [16,17] and report that cigarette puffs are more satisfying and better “liked” [17]. Nicotine, the primary psychoactive component of tobacco [19], appears to mediate the impact of smoking on cognition and affect [18,20-23]. Collectively, these findings suggest a strong, potentially nicotine-mediated impact of tobacco use on the behavior and cognition of smokers with ADHD.
The physiological mechanisms underlying the heightened responsiveness to tobacco in individuals with ADHD are yet unknown. To test candidate mechanisms, including those involving nicotinic-receptor function, animal models may be employed. Behavioral responsiveness to nicotine is expressed in adult rat models as locomotor suppression (initially) and sensitization (after repeated exposures). Tolerance to the suppression of locomotion induced by acute nicotine administration develops rapidly over a few exposures [24-27]. Nicotine-induced locomotor suppression appears to reflect the aversive properties of acute nicotine in adult rats. For instance, nicotine pre-exposure reduces nicotine-induced locomotor suppression [28] and taste aversion [29], but facilitates nicotine-induced place preference [30]. These effects suggest that tolerance to the aversive effects of nicotine develops during pre-exposure, and may be expressed as attenuated nicotine-induced locomotor suppression.
Repeated nicotine exposure leads to an escalation in nicotine-induced locomotion [31,32]. Behavioral sensitization induced by nicotine and other drugs reflects neural adaptations in the dopaminergic mesolimbic pathway that are associated with drug dependence [33-35]. Therefore, nicotine-induced behavioral sensitization is a potential measure of vulnerability to nicotine dependence; it complements other measures that involve more complex learning mechanisms, such as nicotine self-administration [36].
This study investigated whether the most widely used animal model of ADHD, the spontaneously hypertensive rat (SHR) [37,38], displays the pattern of nicotine-induced locomotor suppression and sensitization that may reflect the heightened vulnerability to tobacco dependence in humans with ADHD. Prior studies suggest that, relative to its progenitor and most commonly used control strain, the inbred Wistar Kyoto rat (WKY) [39-43], SHR is more responsive to nicotine. In particular, young SHR self-administers intravenous nicotine at a higher rate [44], and appears to display more robust nicotine-induced conditioned place preference [45] than WKY. These effects, however, may reflect differential learning deficits and interference by non-learning factors across strains [46,47]. Watterson and colleagues [45] also reported heightened dose-dependent nicotine-induced locomotion in adolescent SHR relative to WKY, but did not explicitly test for sensitization or at other ages.
One disadvantage of the SHR model—for the purpose of this study—is that it exhibits elevated baseline levels of locomotor activity relative to WKY [39,42,43,69]. Preexisting strain differences confound potential differences in nicotine-induced locomotor effects between strains. To circumvent this obstacle, the present study sought to assess strain differences in nicotine-induced sensitization of a behavior that (a) does not differ between strains at baseline, (b) involves mesolimbic dopamine activity, and thus (c) displays a pattern of suppression and sensitization over repeated exposure that is potentially indicative of vulnerability to dependence. This study tested various rotational behavior measures as potential target behaviors [48,49].
Experiment 1 verified differences in baseline levels of locomotor activity between strains in the open field arena. Experiment 2 evaluated differences in baseline levels of various rotational behaviors in the Rotorat apparatus. Measures of rotational behavior that did not differ significantly between strains served as dependent measures to evaluate nicotine-induced behavioral suppression and sensitization. Experiment 2 tested the hypothesis that, to the extent that the SHR strain models ADHD-related responsiveness to nicotine, SHR rats will display reduced nicotine-induced suppression or enhanced nicotine-induced sensitization of baseline-equated rotational behavior, relative to WKY rats.
2. Material and Methods
2.1 Animals
Adult male rats of two inbred strains, spontaneously hypertensive rats (SHR/NCrl; Charles River Laboratories) and Wistar Kyoto (WKY/NHsd; Harlan Laboratories) served as subjects. Strains and breeders were selected to model the ADHD combined subtype [41]. All rats had previous experimental history with a variable-interval (VI) schedule of food reinforcement, procured by lever pressing, and during which access to food was restricted to 1 h/d. Training and food regimens were identical for both strains. Food restriction was discontinued for at least 3 days prior to the present experiments; food and water were provided ad libitum in the home cage throughout experimentation. All subjects were pair-housed in a colony room with a 12:12-h light-dark cycle, with lights on at 1900 h and experiments conducted during the dark phase. All experimental protocols were conducted in accordance with the guidelines provided by the National Institute of Health and approved by the Arizona State University Institutional Animal Care and Use Committee.
2.2 Apparatus
Horizontal locomotion and rotational behavior were assessed using an open field arena (experiment 1) and a Rotorat apparatus (experiment 2), respectively. The open field arena consisted of a black plastic box with an open top measuring 90 × 90 × 40 cm, located in a dimly lit room. A single shielded white light bulb was suspended 90 cm above the center of the arena to illuminate it. The arena was divided into two zones of interest: the center zone, defined as a 54 × 54 cm square in the center of the arena, and the perimeter, defined as the remaining area of the arena; no stimulus demarcated the boundary between center and perimeter. Video tracking software (EthoVision XT 8.1, Noldus Information Technology, Wageningen, Netherlands) recorded the position of the animal and horizontal distance traveled, sampling at 5 Hz.
The Rotorat apparatus consisted of a stainless steel bowl (40.6 cm diameter × 25.4 cm height; model ENV-500, Med Associates, St. Albans, VT) surrounded by clear Plexiglas walls. A spring tether was secured to the top of the apparatus by a rotational sensor that recorded rotational activity. A zip-tie collar was placed loosely around the neck of the rat and connected to the spring tether via a stainless steel alligator clip.
2.3 Drugs
Nicotine hydrochloride tartrate (NIC; Sigma, St. Louis, MO, USA) and mecamylamine hydrochloride (MEC; Sigma, St. Louis, MO, USA) were dissolved in saline (0.9% NaCl). MEC is a non-selective nicotinic receptor antagonist; it served as a pre-treatment to verify the cholinergic dependency of NIC-induced rotational behavior in SHR [50,51]. NIC solutions were adjusted to a pH of 7.2. Both drugs and saline (SAL) were administered subcutaneously (s.c.) in a volume of 1 ml/kg only in experiment 2. NIC doses were calculated as the freebase weight.
2.4 Experiment 1: Horizontal Locomotion in the Open Field Arena
Behavioral testing was conducted using 12 rats of each strain, beginning on post-natal day (PND) 123. In three consecutive daily 10-min sessions, each rat was permitted to freely move about the open field arena individually. Deionized water was used to thoroughly clean the arena between sessions. Two standard measures of open-field activity, the total time spent in the center of the open field arena and the total distance traveled (e.g., [52]), were tracked daily for each rat.
2.5 Experiment 2: Rotational Behavior in the Rotorat Apparatus
Behavioral testing was conducted using 23 SHR and 16 WKY rats, beginning on PND 99. Experimental events and group assignment are outlined in Table 1. All daily sessions were 30-min in duration. On the first day of acclimation, rats were placed in the Rotorat apparatus but were not connected to the spring tether. On the second day of acclimation, rats were injected with SAL (s.c.) immediately prior to testing and were connected to the spring tether. Baseline locomotor activity was assessed on the first day following acclimation. During the 7-day chronic nicotine administration phase, rats received either a SAL or MEC (1.0 mg/kg, s.c.) pre-treatment injection; 20 min later they received either SAL or NIC (0.6 mg/kg, s.c.) and were immediately placed in the Rotorat apparatus. Rats were randomly assigned to drug treatment groups. No testing was conducted and no injections given for two days following the last day of chronic nicotine administration. All rats were then given a sensitization test, in which they were injected with 0.3 mg/kg NIC, s.c., immediately prior to testing (the testing dose was lower than the training dose to avoid ceiling effects that would undermine meaningful comparisons between strains). Two days later, all rats were injected with 1.0 mg/kg MEC, s.c., 20 min prior to a SAL injection, followed immediately by a test (MEC/Context Test). Full (360°) rotations, 90° rotations, and direction changes were recorded.
Table 1. Group assignment and dosing schedule in experiment 2.
| Group | Acclimation (PND 99) | Acclimation (PND 100) | Baseline (PND 101) | Chronic NIC (PND 102-108) | Sensitization Test (PND 111) | MEC/Context Test (PND 113) |
|---|---|---|---|---|---|---|
| WKY-SAL n = 8 |
No Injection | SAL | SAL | SAL:SAL | SAL:NIC | MEC:SAL |
| WKY-NIC n = 8 |
No Injection | SAL | SAL | SAL:NIC | SAL:NIC | MEC:SAL |
| SHR-SAL n = 7 |
No Injection | SAL | SAL | SAL:SAL | SAL:NIC | MEC:SAL |
| SHR-NIC n = 8 |
No Injection | SAL | SAL | SAL:NIC | SAL:NIC | MEC:SAL |
| SHR-MEC n = 8 |
No Injection | SAL | SAL | MEC:NIC | SAL:NIC | MEC:SAL |
Note. Cells within each column indicate the drug administered to rats in each phase. Each column is labeled with the corresponding phase and age of the rats during that phase. Cells with two drugs separated with a colon refer to the pretreatment drug administered 20 min prior to testing (to the left of the colon) and the drug administered immediately prior to testing (to the right of the colon). During the Chronic NIC phase, the nicotine dose administered to WKY-NIC, SHR-NIC, and SHR-MEC was 0.6 mg/kg, s.c. On Sensitization Test day all rats received a nicotine dose of 0.3 mg/kg, s.c.
2.6 Data Analysis
Strain differences in horizontal locomotion (experiment 1) were established using 2 (Strain) × 3 (Day) mixed ANOVAs that separately compared center times and distances traveled between strains and among test days. Baseline strain differences in rotational behavior (experiment 2) were established using three independent t-tests that separately compared Baseline full rotations, 90° rotations, and direction changes between SHR and WKY rats assigned to SAL and NIC groups. Significant Baseline strain differences on a dependent measure precluded further analysis of that measure. Dependent measures that did not vary significantly in Baseline were further analyzed, each separately. Specifically, Baseline data were subtracted from Day 1, Day 7, and Sensitization Test data, resulting in difference scores for each phase. Separate 2 (Strain: SHR vs. WKY) × 2 (Drug: NIC vs. SAL) ANOVAs were conducted on difference scores in each phase to examine the effect of acute (Day 1) and chronic (Day 7) NIC administration, the sensitization effects of NIC (Sensitization Test) and MEC, and the effects of conditioning context to NIC (MEC/Context Test). Difference scores were employed to focus the analysis on Strain and Drug effects in each Day, while excluding Day effects that are uninformative of the differential suppression/sensitization hypothesis under consideration (when scaled by baseline, comparisons across levels of Drug, not Day, may reveal suppression/sensitization effects).
Experiment 2 included an SHR-MEC group to determine the cholinergic dependency of rotational behavior in SHR induced by acute, chronic, and sensitized NIC. Because of this narrow objective, a WKY-MEC group was not included. Instead, to assess whether MEC blocked the effects NIC on the rotational behavior of SHR, the difference scores of SHR-MEC were compared to those of SHR-SAL and SHR-NIC, separately in each phase, using a one-way ANOVA.
In experiment 2, differential sensitization effects across strains were inferred from significant Strain × Drug interaction effects observed during the sensitization test. The MEC/Context test was included to assess a potential sensitization effect of MEC alone, and NIC-induced conditioning of rotational behavior to the training context. Comparing SHR-MEC and SHR-SAL performance in the MEC/Context test assessed the first effect; main and simple effects of Drug on MEC/Context performance assessed the second effect.
Tukey's HSD post hoc tests were conducted, where appropriate, following significant ANOVA results (p < .05). When sphericity was violated, a Huynh-Feldt correction was implemented. With the exception of Baseline comparisons of rotational behavior (experiment 2), only significant effects are reported.
3. Results
3.1 Experiment 1: Horizontal Locomotion in the Open Field Arena
Fig. 1 shows the mean (+/-SEM) distance traveled in the open field by each strain in each test day. ANOVA revealed a significant main effect of Day (F(1.42, 29.79) = 29.86, p < 0.001). Distance traveled was lowest on Day 1, increased significantly on Day 2 (p < 0.001) and then decreased significantly on Day 3 (p = 0.005). ANOVA also revealed that SHRs traveled significantly greater distances than WKYs (F(1, 21) = 52.70, p < 0.001).
Fig 1.
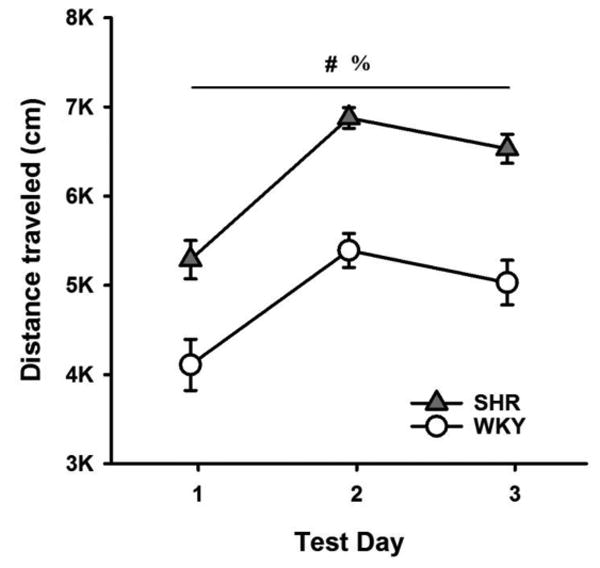
Mean (+/-SEM) total distance travelled by SHRs (filled triangles) and WKYs (open circles) in an open field arena, across test days. Significant main effect of Day. Significant main effect of Strain.
Fig. 2 shows the mean (+/-SEM) time spent in the center of the open field by each strain in each test day. ANOVA revealed a significant Strain × Day interaction effect (F(2, 42) = 4.99, p = 0.011). SHRs spent significantly more time in the center than WKYs on all three days (all p-values < 0.001); this difference appears to be particularly pronounced on the second and third test days.
Fig 2.
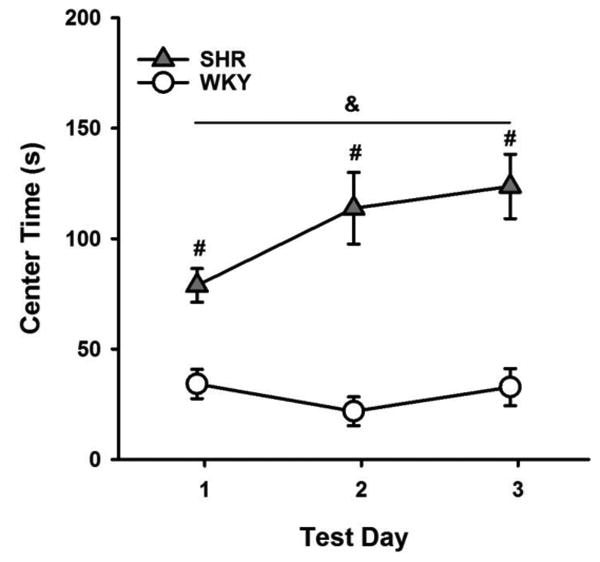
Mean (+/-SEM) time spent in the center of the open field by SHRs (filled triangles) and WKYs (open circles) across test days. &Significant Day × Strain interaction effect. #Significant simple effect of Strain.
3.2 Experiment 2: Rotational Behavior in the Rotorat Apparatus
Mean (± SEM) number of full rotations, 90° rotations, and direction changes are reported for Baseline, Day 1, Day 7, Sensitization Test, and MEC/Context Test in Table 2. Fig. 3 depicts these scores, grouped by strain (excluding SHR-MEC rats), at baseline. Overall, rats produced very few full rotations relative to other behaviors; SHRs produced significantly fewer full rotations than WKYs, (t(30) = 6.13, p < .001); this variable was, therefore, not further analyzed. No significant strain differences in 90° rotations and direction changes were observed at Baseline (respectively: t(30) = 0.88, p = 0.517; t(30) = 0.99, p = 0.328). These two measures were further analyzed separately for nicotinic effects.
Table 2. Mean (± SEM) rotational behavior scores in experiment 2.
| Score / Group | Baseline | Day 1 | Day 7 | Sensitization Test | MEC/Context Test |
|---|---|---|---|---|---|
| Full Rotations | |||||
| WKY-SAL | 8.63 (1.84) | 10.13 (2.21) | 17.00 (2.90) | 16.50 (4.22) | 11.00 (1.45) |
| WKY-NIC | 7.63 (1.67) | 6.63 (2.80) | 25.00 (3.85) | 18.50 (4.03) | 7.75 (2.57) |
| SHR-SAL | 0.75 (0.36) | 1.00 (0.37) | 6.75 (1.74) | 4.13 (0.83) | 3.75 (0.90) |
| SHR-NIC | 0.50 (0.19) | 12.38 (2.02) | 19.38 (5.80) | 3.00 (1.07) | 3.63 (0.49) |
| SHR-MEC | 4.28 (2.37) | 2.28 (0.94) | 3.42 (1.00) | 4.29 (0.89) | 6.57 (1.19) |
| 90° Rotations | |||||
| WKY-SAL | 280.00 (38.29) | 390.75 (71.04) | 493.88 (41.26) | 322.38 (31.03) | 580.25 (59.67) |
| WKY-NIC | 340.25 (55.28) | 82.00 (18.64) | 289.00 (36.89) | 570.38 (73.93) | 462.13 (50.72) |
| SHR-SAL | 363.00 (34.95) | 389.88 (15.23) | 520.25 (63.81) | 501.00 (84.69) | 639.00 (85.76) |
| SHR-NIC | 323.00 (68.15) | 248.75 (29.97) | 498.25 (42.52) | 843.63 (131.68) | 709.75 (70.84) |
| SHR-MEC | 394.42 (70.69) | 425.57 (27.17) | 482.57 (45.90) | 506.14 (69.99) | 635.71 (79.27) |
| Direction Changes | |||||
| WKY-SAL | 582.00 (62.68) | 762.50 (109.25) | 915.63 (53.78) | 523.50 (61.01) | 1312.75 (141.69) |
| WKY-NIC | 655.38 (115.39) | 101.88 (16.84) | 272.25 (22.14) | 1017.25 (168.44) | 1099.13 (115.82) |
| SHR-SAL | 773.50 (86.45) | 906.38 (44.68) | 1289.75 (161.29) | 1153.63 (176.04) | 1245.38 (247.47) |
| SHR-NIC | 653.25 (112.51) | 392.00 (36.19) | 864.50 (105.37) | 1705.13 (236.24) | 1481.50 (130.82) |
| SHR-MEC | 804.71 (105.11) | 872.57 (55.86) | 1082.86 (101.27) | 1069.71 (90.84) | 1348.86 (143.39) |
Fig 3.
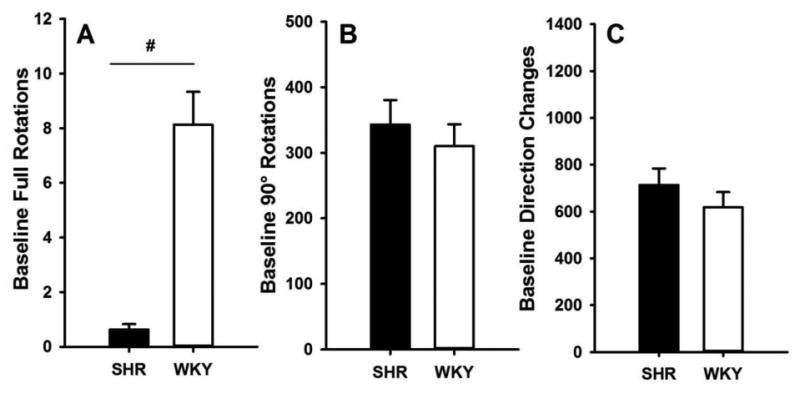
Mean (+/-SEM) Baseline full rotations (A), 90° rotations (B) and direction changes (C) for SHR (filled bars) and WKY (open bars). Significant main effect of strain.
3.2.1 Ninety-degree rotations
Difference scores for 90° rotations are shown in Fig. 4. A significant Strain × Drug interaction effect on these scores was observed on Day 1 (F(1, 28) = 7.07, p = 0.013) and on Day 7 (F(1, 28) = 6.07, p = 0.020). On both days, NIC reduced 90° rotations in WKY relative to SAL-treated WKY (all p-values ≤ 0.001) and NIC-treated SHR (all p-values ≤ 0.044). These findings suggest a stronger suppression of rotational behavior induced by acute NIC in WKYs than in SHRs, maintained over 7 days. During the Sensitization Test, NIC induced larger increases in 90° rotations in SHRs than WKYs (F(1, 28) = 6.49, p = 0.017) and in NIC-treated than in SAL-treated rats (F(1, 28) = 14.16, p = 0.001). The latter effect indicates that NIC sensitized 90° rotations. The absence of significant Strain and Drug effects on MEC/Context performance (all p > 0.104) suggest that context conditioning may not explain NIC-induced sensitization of 90° rotations.
Fig 4.
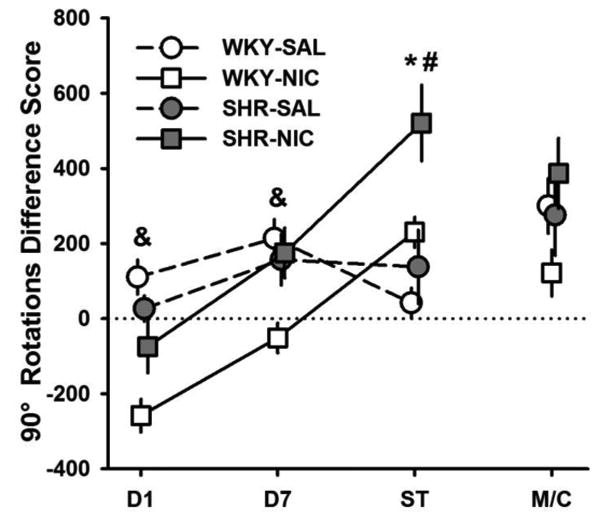
Mean (+/-SEM) difference in 90° rotations between Baseline and Day 1, Day 7, Sensitization Test (ST), and MEC/Context Test (M/C) for SHR (filled symbols) and WKY (open symbols) under nicotine (squares) and saline (circles) treatments. ST = Sensitization Test, M/C = MEC/Context Test. *Significant main effect of drug. #Significant main effect of strain. &Significant Strain x Drug interaction effect.
3.2.2 Direction changes
Difference scores for direction changes are shown in Fig. 5. NIC treatment reduced the number of direction changes on Day 1 (F(1, 28) = 34.42, p < 0.001) and Day 7 (F(1, 28) = 20.02, p < 0.001). SHRs produced more direction changes than WKYs in Day 7 (F(1, 28) = 11.58, p = 0.002) and during the Sensitization Test (F(1, 28) = 14.59, p = .001). During the Sensitization Test, NIC induced more direction changes in NIC-treated than in SAL-treated rats (F(1, 28) = 13.66, p = 0.001), indicating that nicotine sensitized direction-changing behavior. The absence of significant Strain and Drug effects on MEC/Context performance (all p > 0.191) suggest that context conditioning may not explain NIC-induced sensitization of direction changes.
Fig 5.
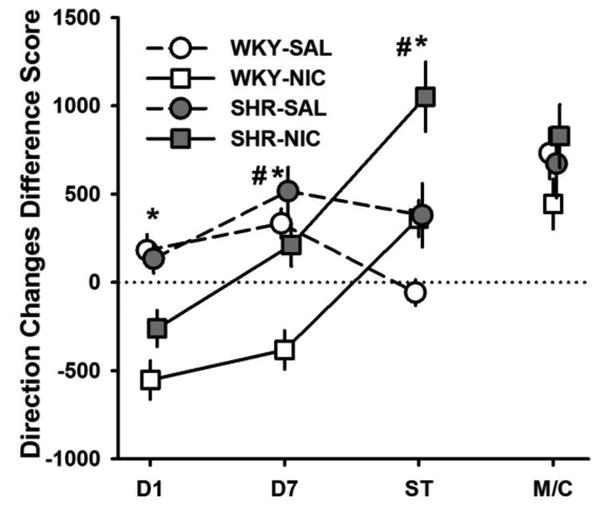
Mean (+/-SEM) difference in direction changes between Baseline and Day 1, Day 7, Sensitization Test (ST), and MEC/Context Test (M/C) for SHR (filled symbols) and WKY (open symbols) under nicotine (squares) and saline (circles) treatments. ST = Sensitization Test, M/C = MEC/Context Test. *Significant main effect of drug. #Significant main effect of strain.
3.2.3 Mecamylamine
Difference scores for 90° rotations in SHR-NIC, SHR-SAL, and SHR-MEC are shown in Fig. 6. A significant effect of Drug was detected on Sensitization-Test performance (F(2, 20) = 5.70, p = .011). The test dose of NIC induced a larger increase in 90° rotations in NIC-treated SHR rats than in SAL-treated (p = .010) and MEC-pretreated rats (p = .008). Difference scores for direction changes in these groups are shown in Fig. 7. A significant effect of Drug was detected during Day 1 (F (2,20) = 3.74, p = .041) and Sensitization Test (F (2,20) = 5.59, p = .012) on direction changes. On Day 1, NIC treatment reduced the number of direction changes in SHRs relative to SAL treatment (p = .018). During the Sensitization Test, NIC treatment increased the number of direction changes in SHRs relative to SAL treatment (p = .014) and MEC pretreatment (p = .006) groups. No significant effect of training Drug was observed on MEC/Context performance on 90° rotations or direction changes, suggesting that responsiveness to MEC was not sensitized. Furthermore, both measures of SHR-SAL performance in MEC/Context Test (when MEC was administered alone) relative to Day 7 (when no drug was administered) suggest that MEC alone did not suppress behavior. Taken together, these results suggest that MEC effectively blocked the sensitizing effect of nicotine on the number of 90° rotations and direction changes produced by SHRs.
Fig 6.
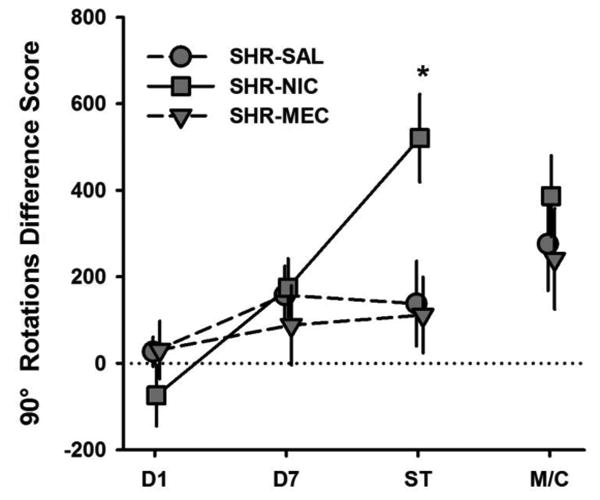
Mean (+/- SEM) differences in 90° rotations between Baseline and Day 1, Day 7, Sensitization Test (ST), and MEC/Context Test (M/C) for SHRs that were treated with saline (SHR-SAL; circles), nicotine (SHR-NIC; squares), and pretreated with mecamylamine prior to nicotine (SHR-MEC; downward triangles). ST = Sensitization Test, M/C = MEC/Context Test. *Significant main effect of Drug.
Fig 7.
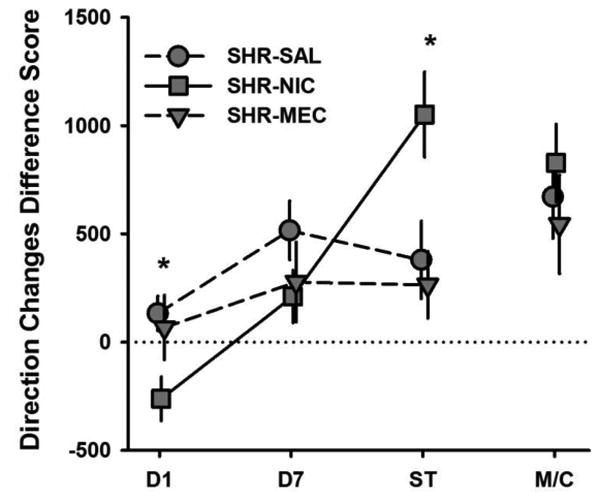
Mean (+/- SEM) differences in direction changes between Baseline and Day 1, Day 7, Sensitization Test (ST), and MEC/Context Test (M/C) for SHRs that were treated with saline (SHR-SAL; circles), nicotine (SHR-NIC; squares), and pretreated with mecamylamine prior to nicotine (SHR-MEC; downward triangles). ST = Sensitization Test, M/C = MEC/Context Test. *Significant main effect of Drug.
4. Discussion
Experiment 1 replicated previous reports showing that SHRs travel greater distances and spend more time in the center of the open field arena than WKYs [39,42,43,69]. In contrast, in experiment 2, baseline levels of 90° rotations and direction changes in the Rotorat apparatus were similar across strains. This difference in performance across tests indicates that motor differences between SHRs and WKYs depend on the apparatus used and the type of behavior it engenders. To characterize this difference across tests, it is important to consider two critical differences between them. First, the link between specific motivation-related neural mechanisms and performance is better characterized in open-field locomotion [33] than in rotational behavior, despite the well-established involvement of mesolimbic dopamine activity in the latter [49]. Second, although open-field hyperlocomotion has been demonstrated in SHR of a wide range of ages (e.g., [53,54]), this study only demonstrated cross-strain similarities in the rotational behavior of 101-day old rats. These limitations notwithstanding, rotational behavior appears to provide an alternative approach to study behavioral-activational effects in the SHR model, that are obscured by baseline differences in performance relative to WKY.
The source of differences in locomotion between strains is not well characterized. Elevated locomotion in SHR may emerge, for instance, from exploratory behavior elicited by the open field, or from reduced neophobia [70]. Moreover, open-field hyperlocomotion in SHR declines more slowly than in WKY [71,72,73]. Whatever process is responsible for strain effects on open-field performance, these do not appear to influence 90° rotations and direction changes, as these dependent measures were similar between strains (Fig. 3), and remained so in the absence of nicotine (Figs. 4 and 5).
Results from experiment 2 show that SHR is less sensitive than WKY to the suppressive effects of nicotine on rotational behavior. Nicotine initially suppressed 90° rotations and direction changes in both strains, but to a larger extent in WKY. The immediacy of the nicotine-induced behavioral suppression suggests that this effect is unconditioned, not mediated by a nicotine-associated context [28]. Although motor suppression induced by acute nicotine administration is common in adult rats, tolerance to this effect typically develops following a few additional administrations [24,25]. The results from experiment 2 are not consistent with this pattern of nicotine effects. After 7 daily administrations, the initial suppressive effects of nicotine were still visible in two rotational behaviors in WKY and in the direction changes of SHR. It is unclear whether the observed persistence of nicotine-induced motor suppression is due to the strains of rat tested, their pre-experimental history of instrumental training and food restriction, or because suppression was tested on rotational behavior. Either way, the present study shows that the suppressive effects of nicotine on locomotion are weaker in SHR than in WKY.
A chronic regimen of nicotine sensitized rotational behavior regardless of strain, as shown by the elevated response of nicotine-treated rats to nicotine in the sensitization test. Although nicotine-treated SHR produced more rotational behavior than nicotine-treated WKY during the sensitization test, the absence of a significant Strain x Drug interaction effect on sensitization performance precludes interpreting this difference as reflecting a heightened sensitization in SHR. It is possible, for instance, that the strain difference in sensitization performance was carried over from the last day of chronic administration, even though these assessments were separated by 72 h. More generally, it is possible that strain differences in the intensity and persistence of nicotine-induced behavioral suppression obscures potential differences in behavioral sensitization. Future tests that dissociate sensitizing from suppressive processes may be required to further investigate nicotine-dependence vulnerability in the SHR model.
Differences in test performance between rats exposed to nicotine vs. saline during training may arise from nicotine-induced context conditioning. That is, by virtue of its pairing with nicotine, the test context might have acquired the capacity to elicit high levels of rotational behavior. If such were the case, rats exposed to nicotine during training would display higher levels of activity than rats exposed to saline during training, even after nicotine is discontinued (i.e., during the MEC/Context test). Because such difference in performance was not observed, it is unlikely that context conditioning contributed significantly to performance during the sensitization test. Although it is possible that mecamylamine blocked the expression of conditioned activity during the MEC/Context test, it would be inconsistent with prior findings that mecamylamine does not block the expression of nicotine-induced conditioning [55].
Mecamylamine pretreatment appears to block the nicotine-induced suppression and sensitization of rotational behavior in SHR, suggesting that these nicotinic effects are mediated by central nAChRs. This finding also suggests that strain differences in cholinergic function mediate the strain differences in nicotine-induced behavioral suppression. In this regard, a phenotypic variation of particular interest is the globally reduced number of the α4β2 nAChR subtype in the young-adult SHR [56]. This variation is particularly noticeable in brain regions involved in nicotine-induced locomotion [57-58]. In particular, the α4 subunit appears to be necessary for nicotine-induced behavioral suppression in mice [59]. Polymorphisms of the α4 subunit gene CHRNA4 are associated with ADHD [60,61] and with initial aversive responses to tobacco smoking [62]. The present study thus raises the possibility that the adult SHR models a α4-mediated blunted response to the aversive properties of nicotine, which facilitates smoking in adults with ADHD.
Some characteristics of the subjects in the present study may represent substantial limitations. For instance, various criticisms have been raised on the SHR as a model of ADHD (e.g., [63]) and on the WKY as a normoactive control strain (e.g., [43,64]). Although it is unlikely for a single genetic model to capture the complexity and heterogeneity of ADHD symptoms, it is possible for a model to capture a subset of functionally related symptoms. To this end, the selection of appropriate breeders and substrains of SHR and WKY has been progressively fine-tuned [41]. The breeder selection in this study reflects such fine-tuning; the results obtained appear to validate such selection.
It is also possible that the pre-experimental history with instrumental conditioning and food restriction of rats in both experiments may have influenced their performance. It is important to note, however, that such history was very similar across strains, and therefore it may not explain the differential suppressive effect of nicotine observed in SHR and WKY. Moreover, the general pattern of effects of nicotine on performance (suppression followed by sensitization) replicates findings from other laboratories using different methods [24,25], suggesting that the main effect of nicotine on performance is robust across large variations in experimental procedures and, presumably, pre-experimental histories.
5. Conclusions
The present study suggests that adult SHRs are less sensitive than adult WKYs to the suppressive effect of nicotine on behavior, and that this effect is mediated by central nAChRs. To the extent that nicotine-induced behavioral suppression reflects the aversive properties of nicotine [28], these results suggest that the adult SHR model of ADHD is less responsive to these aversive properties. This finding is consistent with differences in the nicotine-dependent rewarding and mood-altering effects of smoking between individuals with and without ADHD [17,20,23]. It thus appears that the SHR is a useful model to investigate the neurobiological basis of such differential effects, constituting a much-needed preclinical tool for the study of individual differences in responsiveness to nicotine [65]. Nonetheless, future research may provide converging evidence of the utility of the SHR model using more direct measures of nicotine aversion, such as conditioned taste and place aversion [45,66,67] and self-administration [44]. Furthermore, it is important to emphasize that transient nicotine-induced behavioral suppression only models one of potentially many mechanisms contributing to tobacco dependence in adults with ADHD, which may include stronger cognitive-enhancing and withdrawal effects of nicotine among individuals with ADHD [16,68].
Highlights.
Baseline rotational activity is similar in SHR (ADHD model) and WKY (control) rats
SHR displays weaker nicotine-induced suppression of rotational activity than WKY
Nicotine sensitizes rotational activity in both strains
Nicotine effects on SHR performance are dependent of cholinergic receptors
SHR may model an ADHD-related reduction in nicotine aversion that promotes smoking
Acknowledgments
This study was supported by seed funding from the College of Liberal Arts and Sciences, Arizona State University, and by two grants from the National Institutes of Health (DA032632, MH094562). We thank Heather Bimonte-Nelson for lending her equipment for part of this project, and Sheri Hiroi and Bryan Camp for their support in data collection. We also thank Janet Neisewander and Laurie Chassin, who reviewed early drafts of this paper.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Psychiatric Association. (DSM-5), APA. 5th. Washington DC: 2013. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- 2.Döpfner M, Hautmann C, Görtz-Dorten A, Klasenm F, Ravens-Sieberer U BELLA Study Group. Long-term course of ADHD symptoms from childhood to early adulthood in a community sample. Eur Child Adolesc Psychiatry. 2015;24:665–673. doi: 10.1007/s00787-014-0634-8. [DOI] [PubMed] [Google Scholar]
- 3.Faraone SV, Perlis RH, Doyle AE, Smoller JW, Goralnick JJ, Holmgren MA, Sklar P. Molecular genetics of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1313–1323. doi: 10.1016/j.biopsych.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 4.Kessler RC, Adler L, Barkley R, Biederman J, Conners CK, Demler O, Faraone SV, Greenhill LL, Howes MJ, Secnik K, Spencer T, Ustun TB, Walters EE, Zaslavsky AM. The prevalence and correlates of adult ADHD in the United States: results from the national comorbidity survey replication. Am J Psychiatry. 2006;163:716–723. doi: 10.1176/appi.ajp.163.4.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glass K, Flory K. Why does ADHD confer risk for cigarette smoking? A review of psychosocial mechanisms. Clin Child Fam Psychol Rev. 2010;13:291–313. doi: 10.1007/s10567-010-0070-3. [DOI] [PubMed] [Google Scholar]
- 6.Kollins SH, McClernon FJ, Fuemmeler BF. Association between smoking and attention-deficit/hyperactivity disorder symptoms in a population-based sample of young adults. Arch Gen Psychiatry. 2005;62:1142–1147. doi: 10.1001/archpsyc.62.10.1142. [DOI] [PubMed] [Google Scholar]
- 7.Matthies S, Holzner S, Feige B, Scheel C, Perlov E, Ebert D, Tebart van Elst L, Philipsen A. ADHD as a serious risk factor for early smoking and nicotine dependence in adulthood. J Atten Disord. 2013;17:176–86. doi: 10.1177/1087054711428739. [DOI] [PubMed] [Google Scholar]
- 8.Breyer JL, Lee S, Winters KC, August GJ, Realmuto GM. A longitudinal study of childhood ADHD and substance dependence disorders in early adulthood. Psychol Addict Behav. 2014;28:238–46. doi: 10.1037/a0035664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang Z, Lichtenstein P, Larsson H. The effects of childhood ADHD symptoms on early-onset substance use: A Swedish twin study. J Abnorm Child Psychol. 2012;40:425–435. doi: 10.1007/s10802-011-9575-6. [DOI] [PubMed] [Google Scholar]
- 10.Lambert NM, Hartsough CS. Prospective study of tobacco smoking and substance dependencies among samples of ADHD and non-ADHD participants. J Learn Disabil. 1998;31:533–544. doi: 10.1177/002221949803100603. [DOI] [PubMed] [Google Scholar]
- 11.Ohlmeier MD, Peters K, Kordon A, Seifert J, Te Wildt B, Wiese B, Ziegenbein M, Emrich HM, Schneider U. Nicotine and alcohol dependence in patients with comorbid attention-deficit/hyperactivity disorder (ADHD) Alcohol Alcohol. 2007;42:539–543. doi: 10.1093/alcalc/agm069. [DOI] [PubMed] [Google Scholar]
- 12.Wilens TE, Vitulano M, Upadhyaya H, Adamson J, Sawtelle R, Ultzinger L, Biederman J. Cigarette smoking associated with attention deficit hyperactivity disorder. J Pediatr. 2008;153:414–419. doi: 10.1016/j.jpeds.2008.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Humfleet GL, Prochaska JJ, Mengis M, Cullen J, Muñoz R, Reus V, Hall SM. Preliminary evidence of the association between the history of childhood attention-deficit/hyperactivity disorder and smoking treatment failure. Nicotine Tob Res. 2005;7:453–60. doi: 10.1080/14622200500125310. [DOI] [PubMed] [Google Scholar]
- 14.Lasser K. Smoking and mental illness: A population-based prevalence study. JAMA J Am Med Assoc. 2000;284:2606–2610. doi: 10.1001/jama.284.20.2606. [DOI] [PubMed] [Google Scholar]
- 15.Pomerleau OF, Downey KK, Stelson FW, Pomerleau CS. Cigarette smoking in adult patients diagnosed with attention deficit hyperactivity disorder. J Subst Abuse. 1995;7:373–378. doi: 10.1016/0899-3289(95)90030-6. [DOI] [PubMed] [Google Scholar]
- 16.Mitchell JT, McIntyre EM, McClernon FJ, Kollins SH. Smoking motivation in adults with attention-deficit/hyperactivity disorder using the Wisconsin inventory of smoking dependence motives. Nicotine Tob Res. 2014;16:120–125. doi: 10.1093/ntr/ntt144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Voorhees E, McClernon FJ, Fuemmeler B, English J, Holdaway A, Hallyburton M, Dew R, Kollins S. An examination of differences in variables maintaining smoking behavior in adult smokers with and without attention-deficit/hyperactivity disorder. Addict Res Theory. 2012;20:72–81. [Google Scholar]
- 18.Harrell PT, Juliano LM. A direct test of the influence of nicotine response expectancies on the subjective and cognitive effects of smoking. Exp Clin Psychopharmacol. 2012;20:278–286. doi: 10.1037/a0028652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wonnacott S, Sidhpura N, Balfour DJK DJK. Nicotine: From molecular mechanism to behavior. Curr Opin Pharmacol. 2005;5:53–59. doi: 10.1016/j.coph.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 20.Darredeau C, Stewart SH, Barrett SP. The effects of nicotine content information on subjective and behavioural responses to nicotine-containing and denicotinized cigarettes. Behav Pharmacol. 2013;24:291–297. doi: 10.1097/FBP.0b013e3283635fd9. [DOI] [PubMed] [Google Scholar]
- 21.Juliano LM, Fucito LM, Harrell PT. The influence of nicotine dose and nicotine dose expectancy on the cognitive and subjective effects of cigarette smoking. Exp Clin Psychopharmacol. 2011;19:105–115. doi: 10.1037/a0022937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kassel JD, Evatt DP, Greenstein JE, Wardle MC, Yates MC, Veilleux JC. The acute effects of nicotine on positive and negative affect in adolescent smokers. J Abnorm Psychol. 2007;116:543–553. doi: 10.1037/0021-843X.116.3.543. [DOI] [PubMed] [Google Scholar]
- 23.Perkins KA, Doyle T, Ciccocioppo M, Conklin C, Sayette M, Caggiula A. Sex differences in the influence of nicotine dose instructions on the reinforcing and self-reported rewarding effects of smoking. Psychopharmacology. 2006;184:600–607. doi: 10.1007/s00213-005-0103-7. [DOI] [PubMed] [Google Scholar]
- 24.Clarke PB, Kumar R. The effects of nicotine on locomotor activity in non-tolerant and tolerant rats. Br J Pharmacol. 1983;78:329–337. doi: 10.1111/j.1476-5381.1983.tb09398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DiFranza JR, Wellman RJ. Sensitization to nicotine: how the animal literature might inform future human research. Nicotine Tob Res. 2007;9:9–20. doi: 10.1080/14622200601078277. [DOI] [PubMed] [Google Scholar]
- 26.Ksir C. Acute and chronic nicotine effects on measures of activity in rats: A multivariate analysis. Psychopharmacology (Berl) 1994;115:105–109. doi: 10.1007/BF02244758. [DOI] [PubMed] [Google Scholar]
- 27.Morrison CF, Stephenson JA. The occurrence of tolerance to a central depressant effect of nicotine. Br J Pharmacol. 1972;46:151–156. doi: 10.1111/j.1476-5381.1972.tb06857.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bevins RA, Palmatier MI. Nicotine-conditioned locomotor sensitization in rats: Assessment of the US-preexposure effect. Behav Brain Res. 2003;143:65–74. doi: 10.1016/s0166-4328(03)00009-3. [DOI] [PubMed] [Google Scholar]
- 29.Iwamoto ET, Williamson EC. Nicotine-induced taste aversion: Characterization and preexposure effects in rats. Pharmacol Biochem Behav. 1984;21:527–532. doi: 10.1016/s0091-3057(84)80034-9. [DOI] [PubMed] [Google Scholar]
- 30.Shoaib M, Stolerman IP, Kumar RC. Nicotine-induced place preferences following prior nicotine exposure in rats. Psychopharmacology. 1994;113:445–452. doi: 10.1007/BF02245221. [DOI] [PubMed] [Google Scholar]
- 31.Hamilton KR, Starosciak AK, Chwa A, Grunberg NE. Nicotine behavioral sensitization in Lewis and Fischer male rats. Exp Clin Psychopharmacol. 2012;20:345–51. doi: 10.1037/a0029088. [DOI] [PubMed] [Google Scholar]
- 32.Samaha AN, Yau WYW, Yang P, Robinson TE. Rapid delivery of nicotine promotes behavioral sensitization and alters its neurobiological impact. Biol Psychiatry. 2005;57:351–360. doi: 10.1016/j.biopsych.2004.11.040. [DOI] [PubMed] [Google Scholar]
- 33.Mao D, McGehee DS. Nicotine and behavioral sensitization. J Mol Neurosci. 2010;40:154–163. doi: 10.1007/s12031-009-9230-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morud J, Adermark L, Perez-Alcazar M, Ericson M, Söderpalm B. Nicotine produces chronic behavioral sensitization with changes in accumbal neurotransmission and increased sensitivity to re-exposure. Addict Biol. 2016;21:397–406. doi: 10.1111/adb.12219. [DOI] [PubMed] [Google Scholar]
- 35.Vezina P, McGehee D, Green W. Exposure to nicotine and sensitization of nicotine-induced behaviors, Progr. Neuro-Psychopharmacol. Biol Psychiatry. 2007;31:1625–1638. doi: 10.1016/j.pnpbp.2007.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Corrigall WA. Nicotine self-administration in animals as a dependence model. Nicotine Tob Res. 1999;1:11–20. doi: 10.1080/14622299050011121. [DOI] [PubMed] [Google Scholar]
- 37.Russell VA, Sagvolden T, Johansen EB. Animal models of attention-deficit hyperactivity disorder. Behav Brain Funct. 2005;1:9. doi: 10.1186/1744-9081-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sagvolden T, Johansen EB. Rat models of ADHD. Curr Top Behav Neurosci. 2012;9:301–315. doi: 10.1007/7854_2011_126. [DOI] [PubMed] [Google Scholar]
- 39.Hsieh YL, Yang CC. Age-series characteristics of locomotor activities in spontaneously hypertensive rats: A comparison with the Wistar-Kyoto strain. Physiol Behav. 2008;93:777–82. doi: 10.1016/j.physbeh.2007.11.032. [DOI] [PubMed] [Google Scholar]
- 40.Russell VA. Overview of animal models of attention deficit hyperactivity disorder (ADHD) Curr Protoc Neurosci. 2011;9:9.35. doi: 10.1002/0471142301.ns0935s54. [DOI] [PubMed] [Google Scholar]
- 41.Sagvolden T, Johansen EB, Wøien G G, Walaas SI, Storm-Mathisen J, Bergersen LH, Hvalby O, Jensen V, Aase H, Russell VA, Killeen PR, Dasbanerjee T, Middleton FA, Faraone SV. The spontaneously hypertensive rat model of ADHD—The importance of selecting the appropriate reference strain. Neuropharmacology. 2009;57:619–626. doi: 10.1016/j.neuropharm.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sagvolden T, Metzger M, Schiørbeck HK, Rugland AL, Spinnangr I, Sagvolden G. The spontaneously hypertensive rat (SHR) as an animal model of childhood hyperactivity (ADHD): Changed reactivity to reinforcers and to psychomotor stimulants. Behav Neural Biol. 1992;58:103–112. doi: 10.1016/0163-1047(92)90315-u. [DOI] [PubMed] [Google Scholar]
- 43.van den Bergh FS, Bloemarts E, Chan JSW, Groenink L, Olivier B, Oosting RS. Spontaneously hypertensive rats do not predict symptoms of attention-deficit hyperactivity disorder. Pharmacol Biochem Behav. 2006;83:380–390. doi: 10.1016/j.pbb.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 44.Chen H, Hiler KA, Toley EA, Matta SG, Sharp BM. Genetic factors control nicotine self-administration in isogenic adolescent rat strains. PLoS ONE. 2012;7:e44234. doi: 10.1371/journal.pone.0044234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Watterson E, Daniels CW, Watterson LR, Mazur GJ, Brackney RJ, Olive MF, Sanabria F. Nicotine-induced place conditioning and locomotor activity in an adolescent animal model of attention deficit/hyperactivity disorder (ADHD) Behav Brain Res. 2015;291:184–188. doi: 10.1016/j.bbr.2015.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rauhut AS, Zentner IJ, Mardekian SK, Tanenbaum JB. Wistar Kyoto and Wistar rats differ in the affective and locomotor effects of nicotine. Physiol Behav. 2008;93:177–188. doi: 10.1016/j.physbeh.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 47.Hill JC, Herbst K, Sanabria F. Characterizing operant hyperactivity in the spontaneously hypertensive rat. Behav Brain Funct. 2012;8:5. doi: 10.1186/1744-9081-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Janhunen S, Ahtee L. Differential nicotinic regulation of the nigrostriatal and mesolimbic dopaminergic pathways: Implications for drug development. Neurosci Biobehav Rev. 2007;31:287–314. doi: 10.1016/j.neubiorev.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 49.Koshikawa N. Role of the nucleus accumbens and the striatum in the production of turning behaviour in intact rats. Rev Neurosci. 1994;5:331–346. doi: 10.1515/revneuro.1994.5.4.331. [DOI] [PubMed] [Google Scholar]
- 50.Lapin EP, Maker HS, Sershen H, Hurd Y, Lajtha A. Dopamine-like action of nicotine: Lack of tolerance and reverse tolerance. Brain Res. 1987;407:351–363. doi: 10.1016/0006-8993(87)91114-0. [DOI] [PubMed] [Google Scholar]
- 51.Reavill C, Stolerman I. Interaction of nicotine with dopaminergic mechanisms assessed through drug discrimination and rotational behaviour in rats. J Psychopharmacol. 1987;1:264–273. doi: 10.1177/026988118700100408. [DOI] [PubMed] [Google Scholar]
- 52.Stöhr T, Wermeling DS, Weiner I, Feldon J. Rat strain differences in open-field behavior and the locomotor stimulating and rewarding effects of amphetamine. Pharmacol Biochem Behav. 1998;59:813–818. doi: 10.1016/s0091-3057(97)00542-x. [DOI] [PubMed] [Google Scholar]
- 53.Cierpial MA, Konarska M, McCarty R. Maternal effects on the development of spontaneous hypertension. Health Psychol. 1988;7:125–135. doi: 10.1037//0278-6133.7.2.125. [DOI] [PubMed] [Google Scholar]
- 54.Cierpial MA, Shasby DE, Murphy CA, Borom AH, Stewart RE, Swithers SE, McCarty R. Open-field behavior of spontaneously hypertensive and Wistar-Kyoto normotensive rats: Effects of reciprocal cross-fostering. Behav Neural Biol. 1989;51:203–210. doi: 10.1016/s0163-1047(89)90827-3. [DOI] [PubMed] [Google Scholar]
- 55.Sahraei H, Falahi M, Zarrindast MR, Sabetkasaei M, Ghoshooni H, Khalili M. The effects of nitric oxide on the acquisition and expression of nicotine-induced conditioned place preference in mice. Eur J Pharmacol. 2004;503:81–87. doi: 10.1016/j.ejphar.2004.08.054. [DOI] [PubMed] [Google Scholar]
- 56.Hohnadel EJ, Hernandez CM, Gearhart DA, Terry AV. Effect of repeated nicotine exposure on high-affinity nicotinic acetylcholine receptor density in spontaneously hypertensive rats. Neurosci Lett. 2005;382:158–163. doi: 10.1016/j.neulet.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 57.Gattu M, Pauly JR, Boss KL, Summers JB, Buccafusco JJ. Cognitive impairment in spontaneously hypertensive rats: Role of central nicotinic receptors. I, Brain Res. 1997;771:89–103. doi: 10.1016/s0006-8993(97)00793-2. [DOI] [PubMed] [Google Scholar]
- 58.Terry AV, Hernandez CM, Buccafusco JJ, Gattu M. Deficits in spatial learning and nicotinic-acetylcholine receptors in older, spontaneously hypertensive rats. Neuroscience. 2000;101:357–368. doi: 10.1016/s0306-4522(00)00377-8. [DOI] [PubMed] [Google Scholar]
- 59.Cahir E, Pillidge K, Drago J, Lawrence AJ. The necessity of α4* nicotinic receptors in nicotine-driven behaviors: Dissociation between reinforcing and motor effects of nicotine. Neuropsychopharmacology. 2011;36:1505–1517. doi: 10.1038/npp.2011.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kent L, Middle F, Hawi Z, Fitzgerald M, Gill M, Feehan C, Craddock N. Nicotinic acetylcholine receptor α4 subunit gene polymorphism and attention deficit hyperactivity disorder. Psychiatr Genet. 2001;11:37–40. doi: 10.1097/00041444-200103000-00007. [DOI] [PubMed] [Google Scholar]
- 61.Wallis D, Arcos-Burgos M, Jain M, Castellanos FX, Palacio JD, Pineda D, Lopera F, Stanescu H, Pineda D, Berg K. Polymorphisms in the neural nicotinic acetylcholine receptor α4 subunit (CHRNA4) are associated with ADHD in a genetic isolate. Attent Deficit Hyperactivity Disord. 2009;1:19–24. doi: 10.1007/s12402-009-0003-5. [DOI] [PubMed] [Google Scholar]
- 62.Pedneault M, Labbe A, Roy-Gagnon MH, Low NC, Dugas E, Engert JC, O'Loughlin J. The association between CHRN genetic variants and dizziness at first inhalation of cigarette smoke. Addict Behav. 2014;39:316–320. doi: 10.1016/j.addbeh.2013.08.033. [DOI] [PubMed] [Google Scholar]
- 63.Alsop B. Problems with spontaneously hypertensive rats (SHR) as a model of attention-deficit/hyperactivity disorder (AD/HD) J Neurosci Methods. 2007;162:42–48. doi: 10.1016/j.jneumeth.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 64.Drolet G, Proulx K, Pearson D, Rochford J, Deschepper CF. Comparisons of behavioral and neurochemical characteristics between WKY, WKHA, and Wistar rat strains. Neuropsychopharmacology. 2002;27:400–409. doi: 10.1016/S0893-133X(02)00303-2. [DOI] [PubMed] [Google Scholar]
- 65.Falco AM, Bevins RA. Individual differences in the behavioral effects of nicotine: A review of the preclinical animal literature. Pharmacol Biochem Behav. 2015;138:89–90. doi: 10.1016/j.pbb.2015.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Le Foll B, Goldberg SR. Nicotine induces conditioned place preferences over a large range of doses in rats. Psychopharmacology. 2005;178:481–492. doi: 10.1007/s00213-004-2021-5. [DOI] [PubMed] [Google Scholar]
- 67.Wilmouth CE, Spear LP. Adolescent and adult rats' aversion to flavors previously paired with nicotine. Ann N Y Acad Sci. 2004;1021:462–464. doi: 10.1196/annals.1308.065. [DOI] [PubMed] [Google Scholar]
- 68.McClernon FJ, Van Voorhees EE, English J, Hallyburton M, Holdaway A, Kollins SH. Smoking withdrawal symptoms are more severe among smokers with ADHD and independent of ADHD symptom change: Results from a 12-Day contingency-managed abstinence trial. Nicotine Tob Res. 2011;13:784–792. doi: 10.1093/ntr/ntr073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bayless DW, Perez MC, Daniel JM. Comparison of the validity of the use of the spontaneously hypertensive rat as a model of attention deficit hyperactivity disorder in males and females. Behav Brain Res. 2015;286:85–92. doi: 10.1016/j.bbr.2015.02.029. [DOI] [PubMed] [Google Scholar]
- 70.Delini-Stula A, Hunn C. Neophobia in spontaneous hypertensive (SHR) and normotensive control (WKY) rats. Behav Neural Biol. 1985;43:206–211. doi: 10.1016/s0163-1047(85)91377-9. [DOI] [PubMed] [Google Scholar]
- 71.Leaton RN, Cassella JV. Locomotor activity, auditory startle and shock thresholds in spontaneously hypertensive rats. Physiol Behav. 1983;31:103–109. doi: 10.1016/0031-9384(83)90103-8. [DOI] [PubMed] [Google Scholar]
- 72.Hendley ED, Wessel DJ, Atwater DG, Gellis J, Whitehorn D, Low WC. Age, sex and strain differences in activity and habituation in SHR and WKY rats. Physiol Behav. 1985;34:379–383. doi: 10.1016/0031-9384(85)90199-4. [DOI] [PubMed] [Google Scholar]
- 73.van den Buuse M, de Jong W. Open-field behaviour and blood pressure in spontaneously hypertensive rats. Clin Exp Hypertens A. 1988;10:667–684. doi: 10.3109/10641968809033917. [DOI] [PubMed] [Google Scholar]


