Abstract
Objectives:
The influence of type of insulin treatment - insulin analogs versus human insulin - on the development of diabetes related vascular complications has been sparsely investigated. We examine here possible differences regarding kidney function and hemoglobin levels.
Methods:
Multiple linear regression was used to investigate the relationship between the following characteristics measured in 509 type 1 diabetic patients who were recruited in an outpatient practice: current clinical status and treatment modalities, type of injected insulin and the routine laboratory parameters hemoglobin, HbA1c, serum creatinine, eGFR, hs CRP and urinary albumin/creatinine ratio.
Results:
Compared with human insulin, multiple regression analysis taking into account possible confounders revealed that treatment with insulin analogs was associated with increased eGFR (+7.1 ml/min; P=0.0002), lower urinary albumin/creatinine ratio (ratio logarithm -0.4; P=0.003) and higher hemoglobin concentration (+0.31 g/dl; P=0.04). Stratification by type of insulin showed the best renal status for treatment with insulins Glargine and Lispro. Differences were consistent both for patients with normal (eGFR → 90 ml/min) and with an impaired (eGFR ← 90 ml/min) kidney function.
Conclusions:
Present results suggest that treatment of type 1 diabetic patients with normal and impaired renal function with insulin analogs, especially Glargine and Lispro, is associated with better kidney function, lower urinary albumin/creatinine ratio and lower hemoglobin concentration compared to therapy with human insulin. If confirmed by other studies, treatment with insulin analogs may be a further possibility in delaying progression of nephropathy and in preventing early hemoglobin decline.
Keywords: albumin excretion, aspart, detemir, diabetic nephropathy, glargine, hemoglobin, insulin analog, kidney function, lispro, type 1 diabetes
Background
Insulin analogs (IAs) are widely used in the treatment of insulin-dependent diabetic patients. Compared with human insulin (HI), short-acting IAs decrease postprandial blood glucose peaks by their faster onset of action. Long-acting IAs decrease the risk of hypoglycemia by their balanced pharmacokinetics. Owing to these effects, IAs do not just improve glycemic control but may also lower the risk of vascular complications. Despite its clinical relevance, the relationship between the type of insulin therapy and the onset of diabetic complications has been poorly investigated. In a previous study, we reported IA to be associated with a better kidney function and smaller decrease in hemoglobin concentrations with declining kidney function than HI [Hasslacher et al. 2010]. Due to the small number of patients, differentiation considering types of IAs was not possible in this early publication. Present analyses rely on data from approximately 500 patients, thus allowing stratification by the type of IA and/or insulin combinations. The primary investigated endpoints are kidney function and Hb level with declining kidney function in type 1 diabetic patients.
Methods
A total of 509 patients with type 1 diabetes who attended the outpatient clinic of St. Josefs hospital Heidelberg between 2006 and 2012 and agreed to participate in the study were included if they fulfilled the following inclusion criteria: age 20–85 years, duration of diabetes at least 3 years, and no change in the insulin therapy during the previous 12 months. Patients affected by severe illnesses including malignoma, liver cirrhosis, congestive heart failure, renal insufficiency [estimated glomerular filtration rate (eGFR) < 15 ml/min], acute infection, bronchial asthma (steroid dependent), rheumatoid arthritis requiring any type of therapy, any organ transplantation, congenital cardiac or renal anomalies, pregnant patients and patients on erythropoietin (EPO) therapy were excluded from the study.
Medical history and demographic data as well as concomitant therapy were recorded at the first patient visit. The following laboratory parameters were measured: HbA1c, full blood count, creatinine, lipids, high-sensitivity C-reactive protein (hsCRP), urine status and urinary albumin/creatinine ratio. eGFR was calculated according to the Cockcroft–Gault formula. All measurements were performed by the MVZ Lab Dr. Limbach u. Kollegen in Heidelberg, Germany. HbA1c was measured by high-pressure liquid chromatography, urine albumin by a turbidimetric immunoassay in a sample of first morning urine after exclusion of any urinary tract infection and menstruation. Other laboratory measurements were conducted using routine methods by an autoanalyser (Dimension, Siemens, Germany) using serum or lithium-heparinized plasma.
Diabetic patients were grouped according to the applied long-acting insulin (human insulin, insulin detemir, insulin glargine) and short-acting insulin (human insulin, insulin aspart, insulin glulisine, insulin lispro). In general, treatment with IA was initiated by patients’ GP/diabetologist and the reasons for change from HI to IA were mostly hypoglycemic events during night, high postprandial glucose levels or insufficient glycemic control. The time of treatment with the specified type of IA was recorded according to patients’ statements and/or information from the GP/diabetologists’ practice.
Relative frequencies were used to describe ordinal scaled factors, the location and spread of ratio scaled variables were represented by medians with 5th and 95th percentiles. Differences between groups of patients treated with different insulin types were assessed by uncorrected chi-squared and Wilcoxon tests as appropriate. Univariate linear regression was used to identify patient characteristics related to hemoglobin levels and characteristics related to laboratory parameters reflecting renal function (eGFR and the urinary albumin/creatinine ratio, which was log-transformed to obtain a nearly normally distributed variable). The identified related patient characteristics were taken into account in multiple linear regression models which were applied to quantify differences among types of insulin in hemoglobin levels and renal function. Locally weighted scatter plot smoothing (LoWeSS) regression was used to visually depict the relationship between eGFR and hemoglobin levels in the investigated patient collective. Statistical analyses were conducted using the SAS system version 9.3 and the R language and environment for statistical computing.
The study was approved by the local ethics committee and written informed consent was obtained from all patients.
Results
The baseline demographic and laboratory characteristics of the investigated patients, with their treatment modalities and concomitant diseases are summarized in Table 1. Most patients used an intensified insulin therapy (91%), 7.3% of them with an insulin pump. Exclusive treatment with an IA received 46.8% of patients, 31.4 % received exclusively HI and 21.8 % received a combination of HI and IA. Average treatment times with specific IA types were 4.9 (lispro), 4.6 (aspart), 5.1 (glargine) and 4.5 years (detemir). The majority of patients (78%) had been treated for more than 3 years before study measurements with the respective IA.
Table 1.
Main patient characteristics (median values with 5th and 95th percentiles) and probability values for no difference with patients treated with human insulin from Wilcoxon and chi-squared tests.
|
Type of insulin
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Human (n = 160) | Analog (n = 238) |
Human + Analog (n = 111) |
|||||||
| Characteristic | Unit | Med | 5th to 95th | Med | 5th to 95th | p | Med | 5th to 95th | p |
| Age | Years | 51 | 28 to 71 | 51 | 25 to 72 | 0.63 | 48 | 24 to 71 | 0.01 |
| Female gender | % | 53 | 46 | 0.18 | 41 | 0.04 | |||
| Diabetes duration | Years | 19 | 3 to 44 | 20 | 5 to 45 | 0.25 | 18 | 3 to 44 | 0.44 |
| BMI | kg/m² | 24 | 20 to 30 | 25 | 20 to 33 | 0.16 | 25 | 20 to 31 | 0.53 |
| HbA1c | mmol/mol | 59.6 | 40.9 to 82.5 | 58.5 | 38.8 to 86.9 | 0.42 | 58.5 | 42.1 to 95.6 | 0.42 |
| hsCRP | mg/l | 1.7 | 0.0 to 8.0 | 1.4 | 0.2 to 9.9 | 0.71 | 1.0 | 0.2 to 8.3 | 0.26 |
| Triglycerides | mg/dl | 87 | 43 to 308 | 80 | 40 to 244 | 0.01 | 78 | 37 to 182 | 0.05 |
| Cholesterol | mg/dl | 198 | 136 to 263 | 192 | 139 to 262 | 0.04 | 188 | 127 to 241 | 0.05 |
| HDL | mg/dl | 58 | 34 to 110 | 63 | 39 to 95 | 0.33 | 62 | 38 to 95 | 0.47 |
| LDL | mg/dl | 115 | 65 to 169 | 107 | 63 to 170 | 0.18 | 103 | 64 to 160 | 0.14 |
| eGFR | ml/min | 83 | 34 to 128 | 94 | 45 to 134 | 0.01 | 101 | 40 to 137 | 0.01 |
| U-albumin/creatinine | mg/g | 7 | 2 to 205 | 6 | 1 to 383 | 0.01 | 6 | 1 to 233 | 0.10 |
| Hemoglobin | g/dl | 13.9 | 11.6 to 16.2 | 13.7 | 11.3 to 15.8 | 0.02 | 14.1 | 11.8 to 16.3 | 0.24 |
| Ferritin | µl/l | 90 | 7 to 148 | 108 | 10 to 360 | 0.11 | 92 | 43 to 514 | 0.45 |
| Iron | µl/l | 837 | 102 to 1320 | 820 | 470 to 1214 | 0.78 | 790 | 164 to 1484 | 0.83 |
| Prevalence of retinopathy | % | 39 | 31 | 0.15 | 35 | 0.45 | |||
| Prevalence of hypertension | % | 58 | 61 | 0.64 | 50 | 0.16 | |||
| Systolic blood pressure | mmHg | 134 | 104 to 180 | 132 | 110 to 169 | 0.95 | 130 | 108 to 163 | 0.12 |
| Diastolic blood pressure | mmHg | 78 | 60 to 93 | 78 | 61 to 95 | 0.47 | 76 | 63 to 92 | 0.63 |
| Prevalence of treatment with RAS inhibitors* | % | 43 | 47 | 0.37 | 39 | 0.56 | |||
Bold type denotes statistical significance at the 5% confidence level.
Including ACE inhibitors and AT1 blockers.
ACE, angiotensin-converting enzyme; BMI, body mass index; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; hsCRP, high-sensitivity C-reactive protein; LDL, low-density lipoprotein; RAS, renin–angiotensin system.
Age, gender, diabetes duration, body mass index (BMI), HbA1c, hsCRP, high-density lipoprotein (HDL) and low-density lipoprotein (LDL) cholesterol differences between patients treated with HI and IA did not reach statistical significance. Total cholesterol (p = 0.04) and triglyceride levels (p = 0.01) were slightly higher in the HI group. Prevalence of hypertension, antihypertensive therapy with angiotensin-converting enzyme (ACE)/AT1 blockers and the quality of blood pressure control were similar in the HI and IA groups.
The median unadjusted Hb concentration was 13.7 g/dl in the IA group, somewhat lower than in the HI group (13.9 g/dl; p = 0.02). The kidney function (eGFR) was within the normal range (eGFR > 90 ml/min) for 49.3% of the patients. Among diabetes patients with decreased eGFR (<90 ml/min), 96.9% showed a renal function in chronic kidney disease (CKD) stages II and III (eGFR 30–89 ml/min), 3.1% in CKD stage IV (eGFR = 15–29 ml/min).
The kidney function in patients treated with IA was on average 13.3% higher (p = 0.01), the urinary albumin/creatinine ratio (ACR) approximately 19% lower (p = 0.01) than in the HI group. Patients treated with a combination of HI and IA presented with the highest eGFR in the three groups. However, ACR and hemoglobin concentrations did not significantly differ from HI-treated patients (Table 1).
Table 2 shows the results of univariate linear regression models examining the possible relationship between patient characteristics, and eGFR, ACR and Hb. For example, a 1-year increase in the patient age translated into an eGFR decrease of 1.2 (p < 0.0001). In the subsequent multiple regression analyses, differences in eGFR, ACR and hemoglobin by type of insulin treatment were investigated taking into account the identified confounders in univariate analyses.
Table 2.
Univariate analysis of possible associations with eGFR, urinary ACR and hemoglobin in the investigated collective.
| Characteristic |
eGFR
|
Log(ACR)
|
Hemoglobin
|
|||
|---|---|---|---|---|---|---|
| Parameter estimate | p | Parameter estimate | p | Parameter estimate | p | |
| Age | −1.1671 | <.0001 | 0.0087 | 0.0111 | −0.0149 | 0.001 |
| Female gender | −11.5398 | <.0001 | −0.2505 | 0.0083 | −1.1563 | <.0001 |
| Diabetes duration | −0.7145 | <.0001 | 0.0026 | 0.50 | −0.0122 | 0.02 |
| BMI | 1.6321 | <.0001 | 0.0189 | 0.16 | −0.0178 | 0.32 |
| HbA1c | −1.1798 | 0.23 | 0.1212 | 0.0004 | −0.0628 | 0.17 |
| hsCRP | −0.7160 | 0.18 | 0.0154 | 0.40 | −0.0633 | 0.01 |
| Hypertension | −23.0817 | <.0001 | 0.4491 | <.0001 | −0.1783 | 0.18 |
| ACE-inhibitors/AT1-blockers | −20.9838 | <.0001 | 0.4192 | <.0001 | −0.3890 | 0.003 |
| Systolic blood pressure | −0.2842 | <.0001 | 0.0104 | <.0001 | 0.0028 | 0.41 |
| eGFR | – | – | −0.0082 | <.0001 | 0.0173 | <.0001 |
Bold type denotes statistical significance at the 5% confidence level.
ACE, angiotensin-converting enzyme; ACR, albumin/creatine ratio; BMI, body mass index; eGFR, estimated glomerular filtration rate; hsCRP, high-sensitivity C-reactive protein.
Differences in kidney function and urinary albumin excretion by type of insulin treatment
Table 3 and Figure 1(a) show the mean differences in eGFR between patients treated with IA or HI and IA versus HI-treated patients as the reference group adjusted for age, sex, diabetes duration, BMI, prevalent hypertension, concomitant therapy with ACE inhibitors or AT1 blockers, and systolic blood pressure. Multiple regression analysis revealed that the average eGFR of patients in the IA group was approximately 7.1 ml/min higher (p = 0.0002) and in the HI and IA group approximately 4.7 ml/min higher (p = 0.06) than in patients treated with human insulin. The goodness of the fitted multiple regression model was R2 = 0.43.
Table 3.
Results from multiple linear regression analyses of the effect of insulin type on eGFR for all patients and those with eGFR lower than 90 ml/min. Results are provided as mean differences between named IA- and HI-treated patients as reference. Estimates are adjusted for identified confounders.
| Patients | Insulin type | Subtype | Patients (n) | Mean difference | 95% CI | p | |
|---|---|---|---|---|---|---|---|
| Any | HI | 160 | Reference | ||||
| IA | 238 | 7.1369 | 3.3351 | 10.9387 | 0.0002 | ||
| HI and IA | 111 | 4.6973 | −0.0172 | 9.4117 | 0.06 | ||
| IA | Lispro | 34 | 9.3943 | 1.5514 | 17.2371 | 0.02 | |
| Lispro glargine | 76 | 5.7618 | 0.6467 | 10.8768 | 0.03 | ||
| Lispro detemir | 23 | 6.0301 | −2.4029 | 14.4631 | 0.16 | ||
| Aspart glargine | 47 | 8.8780 | 2.5101 | 15.2459 | 0.006 | ||
| Aspart detemir | 41 | 5.9424 | −1.3433 | 13.2282 | 0.10 | ||
| Glargine based | 123 | 6.6890 | 2.3417 | 11.0363 | 0.003 | ||
| Detemir based | 64 | 5.8006 | 0.0272 | 11.5740 | 0.05 | ||
| eGFR <90ml/min | IA | Lispro | 12 | 4.4565 | −5.8492 | 14.7621 | 0.40 |
| Lispro glargine | 34 | 3.5690 | −2.9863 | 10.1243 | 0.29 | ||
| Lispro detemir | 9 | 1.5344 | −9.4584 | 12.5272 | 0.78 | ||
| Aspart glargine | 21 | 7.9266 | 0.7134 | 15.1397 | 0.03 | ||
| Aspart detemir | 19 | −2.6071 | −11.9392 | 6.7249 | 0.58 | ||
| Glargine based | 55 | 5.4526 | 0.2646 | 10.6406 | 0.04 | ||
| Detemir based | 28 | −0.4824 | −7.9455 | 6.9806 | 0.90 | ||
Bold type denotes statistical significance at the 5% confidence level.
CI, confidence interval; eGFR, estimated glomerular filtration rate; HI, human insulin; AI, insulin analog.
Figure 1.
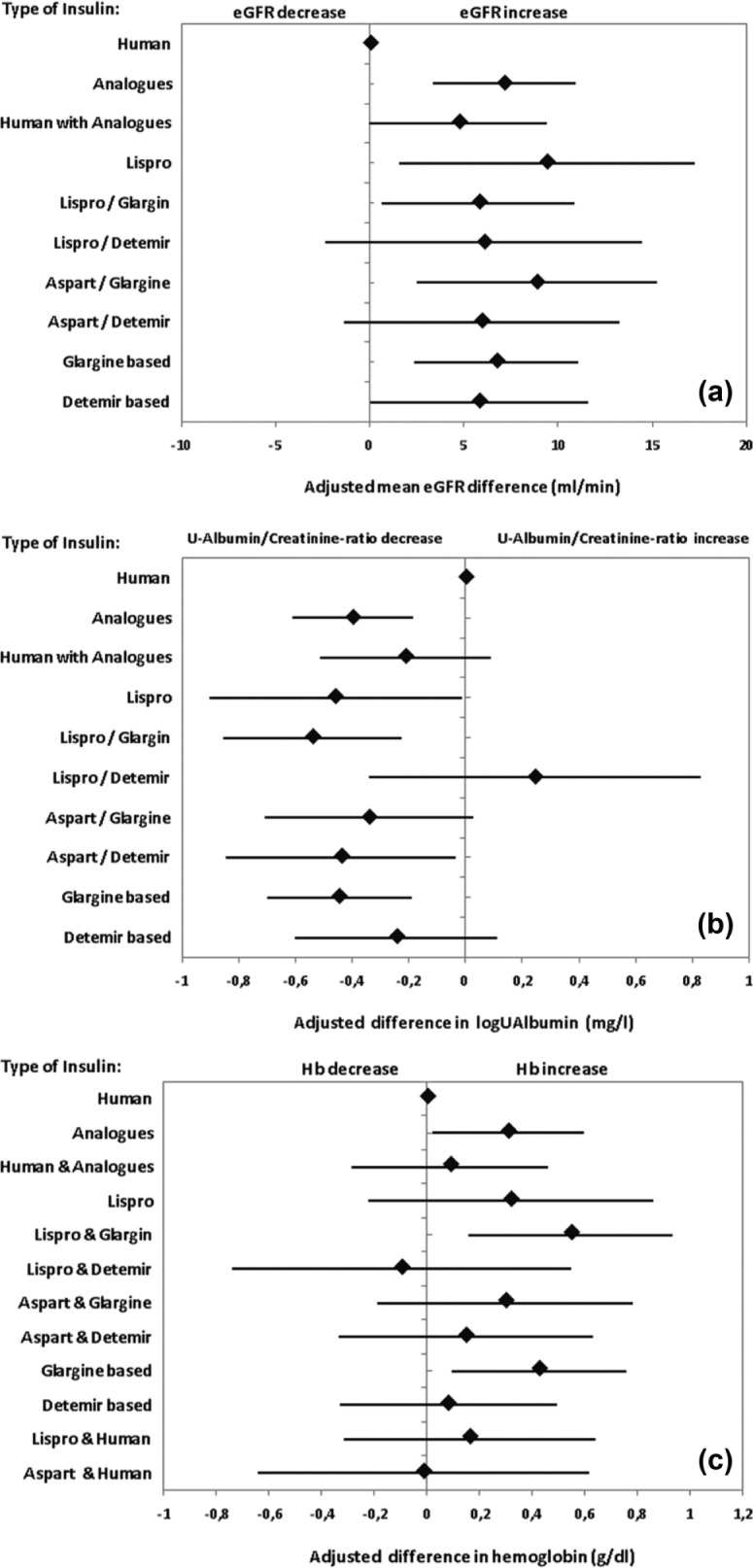
Results from a multiple linear regression model of the effect of insulin type on: (a) estimated glomerular filtration rate (eGFR) summarized by mean eGFR differences between specified insulin analog (IA)- and human insulin (HI)-treated patients as the reference, (b) on the logarithm of the urinary albumin/creatinine ratio summarized by mean log(ACR) differences between specified IA- and HI-treated patients and (c) on hemoglobin concentration differences between specified IA- and HI-treated patients.
Stratified analyses by IA type showed the largest mean difference eGFR for the ‘lispro’ (p = 0.02) and the ‘aspart/glargine’ (p = 0.006) groups. However, differences among particular IA types did not reach statistical significance (overlapping confidence intervals). In the ‘glargine-based’ therapy cluster, the eGFR was about 6.7 ml/min higher (p = 0.003) than in the HI group. For the cluster ‘detemir-based’, it was about 5.8 ml/min higher with borderline significance (p = 0.05). Seventeen patients treated with insulin glulisine (glulisine/glargine n = 11; glulisine/detemir n = 6) were not considered in this and further analyses due to the low number of observations.
In order to examine whether these differences are present both in patients with normal (eGFR >90 ml/min) and reduced (eGFR <90 ml/min) kidney function, statistical analyses were accordingly stratified. For the patient group with normal kidney function, differences between insulin types were not statistically significant (data not shown). Multiple regression results for patients with an eGFR <90 ml/min are shown in Table 3. Patients treated with aspart/glargine and ‘glargine-based’ presented with a higher eGFR than HI-treated patients [by 7.9 ml/min (p = 0.03) and 5.5 ml/min (p = 0.04), respectively].
Table 4 and Figure 1(b) show mean differences in the logarithm of the ACR between patients treated with IA or HI and IA compared with HI-treated patients as the reference group adjusted for the identified confounders, i.e. age, sex, HbA1c, prevalent hypertension, concomitant therapy with ACE inhibitors or AT1 blockers, and systolic blood pressure (Table 2). Based on multiple linear regression analyses, the ACR was significantly lower under IA treatment than with HI therapy (p = 0.0003). No statistical difference was found in patients treated with a combination of HI and IA. The goodness of fit of the multiple regression model was poor (R2 = 0.07). Stratification of patients according to particular IA type revealed that treatment with insulin lispro, lispro/glargine and aspart/detemir as well as the cluster ‘glargine-based’ insulin therapy were associated with a significantly lower ACR.
Table 4.
Results from multiple linear regression analyses of the effect of insulin type on the logarithm of the urinary albumin/creatinine ratio. Results are provided as mean differences between named IA and HI treated patients as reference. Estimates are adjusted for identified confounders.
| Patients | Insulin type | Subtype | Patients (n) | Mean difference | 95% CI | p | |
|---|---|---|---|---|---|---|---|
| Any | HI | 160 | Reference | ||||
| IA | 238 | −0.3971 | −0.6098 | −0.1845 | 0.0003 | ||
| HI and IA | 111 | −0.2131 | −0.5150 | 0.0889 | 0.17 | ||
| IA | Lispro | 34 | −0.4603 | −0.9061 | −0.0145 | 0.04 | |
| Lispro glargine | 76 | −0.5406 | −0.8555 | −0.2256 | 0.0008 | ||
| Lispro detemir | 23 | 0.2437 | −0.3423 | 0.8296 | 0.42 | ||
| Aspart glargine | 47 | −0.3416 | −0.7087 | 0.0256 | 0.07 | ||
| Aspart detemir | 41 | −0.4388 | −0.8445 | −0.0331 | 0.03 | ||
| Glargine based | 123 | −0.4457 | −0.7011 | −0.1904 | 0.0006 | ||
| Detemir based | 64 | −0.2446 | −0.6020 | 0.1128 | 0.18 | ||
| eGFR <90ml/min | IA | Lispro | 12 | −0.5161 | −1.4235 | 0.3914 | 0.27 |
| Lispro glargine | 34 | −0.7756 | −1.3444 | −0.2068 | 0.008 | ||
| Lispro detemir | 9 | 0.5154 | −0.4889 | 1.5196 | 0.32 | ||
| Aspart glargine | 21 | −0.4874 | −1.1524 | 0.1776 | 0.15 | ||
| Aspart detemir | 19 | −0.2834 | −1.0429 | 0.4760 | 0.47 | ||
| Glargine based | 55 | −0.6660 | −1.1185 | −0.2134 | 0.004 | ||
| Detemir based | 28 | 0.0288 | −0.6116 | 0.6691 | 0.93 | ||
| eGFR > 90ml/min | IA | Lispro | 22 | −0.3282 | −0.7970 | 0.1405 | 0.17 |
| Lispro glargine | 42 | −0.3442 | −0.6813 | −0.0071 | 0.04 | ||
| Lispro detemir | 14 | −0.0873 | −0.7626 | 0.5881 | 0.80 | ||
| Aspart glargine | 26 | −0.1530 | −0.5508 | 0.2448 | 0.45 | ||
| Aspart detemir | 22 | −0.4040 | −0.8252 | 0.0172 | 0.06 | ||
| Glargine based | 68 | −0.2548 | −0.5524 | 0.0428 | 0.09 | ||
| Detemir based | 36 | −0.3270 | −0.7256 | 0.0716 | 0.10 | ||
Bold type denotes statistical significance at the 5% confidence level.
CI, confidence interval; eGFR, estimated glomerular filtration rate; HI, human insulin; IA, insulin analog.
After patient stratification according to kidney function, both patients groups with an eGFR higher and lower than 90 ml/min showed a lower ACR under IA treatment (p = 0.03 and p = 0.04, respectively). The group treated with HI and IA did not show a significant difference in comparison to the reference group (Table 4).
After stratification according to the particular type of IAs, the ACR in the group with normal kidney function was only lower for the combination lispro/glargine than in the reference group (p = 0.04). Patients with reduced kidney function under lispro/glargine administration and ‘glargine-based’ insulin therapy presented with a decreased ACR than the reference group (p = 0.008 and p = 0.004, respectively; Table 4).
Effect of the type of insulin on the hemoglobin level
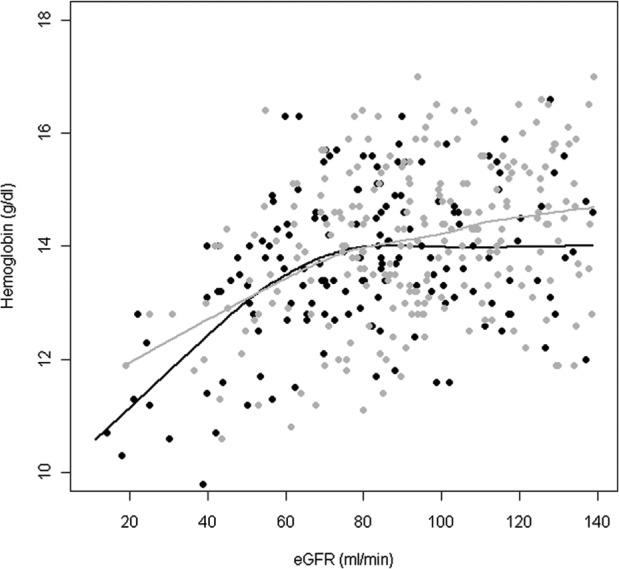
Figure 2 represents the relationship between hemoglobin level and kidney function under treatment with HI and IA. The hemoglobin level was higher for IA- than for HI-treated patients for any eGFR value, with particularly large differences in the hemoglobin level for eGFR values in the upper and lower ranges.
Figure 2.
Relationship between hemoglobin concentration and estimated glomerular filtration rate (eGFR) without consideration of additional covariates. Individual data and fitted locally weighted scatter plot smoothing (LoWeSS) curves are plotted in gray for analog insulin and in black for human insulin treated patients.
Table 5 and Figure 1 (c) shows the mean differences in hemoglobin levels between patients treated with IA and patients treated with a combination of HI and IA compared with HI-treated patients as a reference group. Multiple linear regression taking into account possible confounders (age, sex, diabetes duration, hsCRP, concomitant therapy with ACE inhibitors and AT1 blockers) indicated that the hemoglobin level of patients treated with IA was 0.31 g/dl higher (p = 0.04) than in HI-treated patients. The difference between patients treated with HI and IA and HI-treated patients did not reach statistical significance. The goodness of the fitted multiple regression model was in this case R2 = 0.18. After stratification according to particular type of IA, increased hemoglobin levels were only found for the combination lispro/glargine (0.54 g/dl, p = 0.006) and for the cluster ‘glargine-based’ insulin therapy (0.42 g/dl, p = 0.01).
Table 5.
Results from multiple linear regression analyses of the effect of hemoglobin levels. Results are provided as mean differences between named IA and HI treated patients as reference. Estimates are adjusted for identified confounder.
| Patients | Insulin type | Subtype | Patients (n) | Mean difference | 95% CI | p | |
|---|---|---|---|---|---|---|---|
| Any | HI | 160 | Reference | ||||
| IA | 238 | 0.3081 | 0.0212 | 0.5949 | 0.04 | ||
| HI and IA | 111 | 0.0872 | −0.2853 | 0.4597 | 0.65 | ||
| IA | Lispro | 34 | 0.3185 | −0.2229 | 0.8599 | 0.25 | |
| Lispro glargine | 76 | 0.5458 | 0.1571 | 0.9346 | 0.006 | ||
| Lispro detemir | 23 | −0.0972 | −0.7429 | 0.5484 | 0.77 | ||
| Aspart glargine | 47 | 0.2969 | −0.1882 | 0.7820 | 0.23 | ||
| Aspart detemir | 41 | 0.1454 | −0.3378 | 0.6286 | 0.56 | ||
| Glargine based | 123 | 0.4251 | 0.0919 | 0.7583 | 0.01 | ||
| Detemir based | 64 | 0.0808 | −0.3322 | 0.4937 | 0.70 | ||
| eGFR | IA | Lispro | 12 | 0.0124 | −1.0505 | 1.0752 | 0.98 |
| <90 | Lispro glargine | 34 | 0.6847 | 0.0592 | 1.3102 | 0.03 | |
| ml/min | Lispro detemir | 9 | 0.5486 | −0.4815 | 1.5787 | 0.30 | |
| Aspart glargine | 21 | −0.1202 | −0.8832 | 0.6428 | 0.76 | ||
| Aspart detemir | 19 | −0.1703 | −0.9697 | 0.6292 | 0.68 | ||
| Glargine based | 55 | 0.3811 | −0.1632 | 0.9254 | 0.17 | ||
| Detemir based | 28 | 0.1268 | −0.5386 | 0.7923 | 0.71 | ||
| eGFR | IA | Lispro | 22 | 0.1785 | −0.3081 | 0.6652 | 0.47 |
| >90 | Lispro glargine | 42 | 0.4199 | 0.0033 | 0.8365 | 0.05 | |
| ml/min | Lispro detemir | 14 | −0.3877 | −1.0871 | 0.3117 | 0.28 | |
| Aspart glargine | 26 | 0.2961 | −0.2945 | 0.8867 | 0.33 | ||
| Aspart detemir | 22 | 0.5220 | −0.0631 | 1.1070 | 0.08 | ||
| Glargine based | 68 | 0.3551 | −0.0371 | 0.7473 | 0.08 | ||
| Detemir based | 36 | 0.2155 | −0.3202 | 0.7512 | 0.43 | ||
Bold type denotes statistical significance at the 5% confidence level.
CI, confidence interval; eGFR, estimated glomerular filtration rate; HI, human insulin; IA, insulin analog.
After patient stratification according to normal and reduced kidney function (eGFR ⩾ 90 ml/min and < 90 ml/min, respectively), Hb levels were significantly higher in patients treated with lispro/glargine than in HI-treated patients in both groups (eGFR > 90 ml/min: +0.42 g/dl, p = 0.05; eGFR < 90 ml/min: +0.68 g/dl, p = 0.03). Differences for other types of insulin treatment did not reach statistical significance.
Discussion
The study shows for the first time that treatment of type 1 diabetes patients with IA, especially with insulin glargine and lispro, is associated with a better kidney function and higher hemoglobin levels in early CKD stages when compared with HI therapy.
IA are widely used in the treatment of insulin-requiring diabetic patients, especially in cases with transient glucose peaks or risk of hypoglycemia. IA cannot just improve the glycemic control of diabetes but may also lower the risk of cardiovascular events, as reported in several studies in Type 2 diabetes patients [Rhoads et al. 2009; Scholz and Dippel, 2012; Rathmann and Kostev, 2013]. Their effects on development of diabetic nephropathy, a further diabetes- related complication with poor renal and cardiovascular prognosis, are unknown as of yet. In our study on a high number of Type 1 diabetic patients with normal or impaired kidney function we found that patients treated with IA showed on average a 7.2 ml/min higher eGFR and lower urinary albumin excretion than patients treated with HI. After stratification according to kidney function, higher eGFR values were only detected in IA treated patients with an eGFR < 90 ml/min, a lower urinary albumin excretion in both groups, i.e. eGFR > 90 ml/min and < 90 ml/min. Stratified analyses of particular IA types revealed that the above-described effects were consistently found under administration of insulin glargine and/or lispro, rarely under administration of aspart or detemir. For all comparisons, potential confounders were taken into account.
The causes of the described results are not clear. The patients treated with HI and IA did not differ according to essential factors that could affect kidney function such as age, duration of diabetes, gender distribution, control of diabetes and blood pressure, treatment with renin–angiotensin (RAS) inhibitors. Transient glucose peaks and hypoglycemia, which are observed more rarely under IA therapy than under HI administration, are not known to be typical risk factors for the occurrence of a nephropathy. Kulozik and Hasslacher [Kulozik and Hasslacher, 2015] recently showed that low 1,5-AG levels as indicator of transient hyperglycemia are not associated with an increased prevalence of nephropathy. Ruggenenti and colleagues [Ruggenenti et al. 2003] found in a study on the renal hemodynamics that postprandial hyperperfusion of the glomeruli, which is detectable under HI therapy, does not occur under treatment with insulin lispro. It is not known to what extent this effect on glomerular hemodynamics can also be found with other IA, and whether this effect has a protective influence on the course of nephropathy.
Impairment of oxygen metabolism in kidney tissue has been discussed as a possible partial cause for the development of nephropathy [Nangaku and Eckardt, 2007; Miyata van Ypersele de Strihou, 2010]. Damage to the arterial and/or glomerular blood flow or lower hemoglobin levels may decrease the oxygen supply especially to the tubular structures and may lead to various reactions with formation of fibrosis in the renal medulla. Several studies on diabetic patients with earlier CKD stages have shown an association between hemoglobin concentration and loss of kidney function [Breyer et al. 1996; Babazono et al. 2006; Oh et al. 2012]. In the present study, we could confirm that the hemoglobin level already starts to be lower in the early stages of nephropathy (Figure 2) [Bosmann et al. 2002; El-Achkar et al. 2005]. However, under administration of insulin glargine and lispro or the combination of both, the hemoglobin levels of patients with an eGFR < 90 ml/min were 0.69 g/dl higher than under treatment with HI, even when numerous confounders were considered. Significant differences could not be detected with administration of insulin detemir or aspart or combined administration of IA and HI when compared to human insulin. Patients with normal kidney function also showed slightly higher hemoglobin values with glargine/lispro administration than with HI, and the difference bordered on statistical significance. These results confirm overall the findings of our earlier study in a smaller patient collective and extend them by the identification of two IAs that are essentially responsible for the hemoglobin-stabilizing effect [Hasslacher et al. 2010].
As a cause of the observed effects on hemoglobin concentration, primarily potential differences in the mitogenic potency between HI and the individual IA are to be discussed, which are mediated by insulin and IGF-1 receptors. Insulin receptors primarily of the subtype A were detected in the cells of the hematopoietic system, which mediate mitogenic effects in addition to metabolic effects [Dainiak and Kreczko, 1985; Benecke et al. 1992; Belfiore et al. 2009]. The cells of the bone marrow further have IGF-1 as well as hybrid receptors, which mediate both IGF-1 and insulin effects [Miyagawa et al. 2000; Bailyes et al. 1997; Federici et al. 1997]. Various in vitro studies have already shown that insulin and IGF-1 stimulate proliferation and growth of late erythroid progenitors [Aoki et al. 1994; Sawada et al. 1989; Shih et al. 1999].
The affinity of HI and IA to the insulin and/or IGF-1 receptors differs [Kurtzhals et al. 2000]. Thus, when compared with HI, insulin glargine was shown to have a several times higher affinity for the IGF-1 receptor and stronger mitogenic effects in different cell systems with high IGF-1 receptor density [Kurtzhals et al. 2000; Mayer et al. 2008; Sommerfeld et al. 2010; Varewijck et al. 2013]. Insulin glargine also stimulated the IR/IGF1 hybrid receptor in vitro significantly more strongly than HI [Pierre-Eugene et al. 2012]. Insulin lispro also has an increased affinity for the IGF-1 receptor, the hybrid receptor IR/IGF1R is slightly more stimulated than with HI as well [Kurtzhals et al. 2000; Pierre-Eugene et al. 2012]. Insulin aspart showed in a tumor cell line a slightly increased mitogenic potency that, however, was not confirmed in other studies [Pierre-Eugene et al. 2012; Gammeltoft et al. 1999; Eckhardt et al. 2007]. Analyses regarding binding and dissociation of aspart regarding the IGF1-1 or the hybrid receptor did not show any differences in comparison with HI [Kurtzhals et al. 2000; Pierre-Eugene et al. 2012]. Insulin detemir shows substantially weaker binding to the IGF-1 receptor and a higher dissociation velocity than HI [Kurtzhals et al. 2000], corresponding results were also found for the hybrid receptor [Pierre-Eugene et al. 2012]. The mitogenic potency of detemir is lower when compared with HI, or is not different [Kurtzhals et al. 2000; Mayer et al. 2008; Sommerfeld et al. 2010; Shukla et al. 2009]. These results obtained in vitro on the mitogenic potency of the different IA are in concordance with our clinical findings of higher hemoglobin levels during treatment with the IA glargine and lispro. It should be emphasized that the administered insulin doses were within the usual clinical range, as Kulozik and Hasslacher [Kuzolik and Hasslacher, 2013] could recently show in a subgroup analysis of this patient collective. Glargine and lispro doses were not higher in patients with normal kidney function than HI doses and slightly lower in patients with reduced kidney function.
The main limitation of our study is its cross-sectional design. Therefore, a ‘selection bias’ cannot be excluded with certainty. This disadvantage should be ameliorated by the large number of patients examined, performance of the study at a center according to fixed criteria and requests to the pretreating family physicians for the confirmation of information given by patients. Another limitation is that the time of exposure to specific types of IA could not be precisely determined in all patients. The majority of patients (78%) had been treated for more than 3 years before the time of the study with the respective IA. This time span may be long enough for the detection of effects on kidney function and hemoglobin concentration. A further limitation may be that other factors that may influence kidney function or hemoglobin levels such as nonsteroidal anti-inflammatory drug (NSAID) use, smoking habits, EPO levels could not be considered due to information unavailability or large proportions of missing values in our dataset. Further studies with repeated longitudinal follow up of diabetic patients or with intervention should be conducted to validate our findings.
In summary, our results suggest that treatment of type 1 diabetic patients with IAs, especially insulin glargine and lispro, associates with a better renal status and a reduced decrease in hemoglobin levels with impaired kidney function. If confirmed in future prospective studies, this may present an opportunity in delaying progression of diabetic nephropathy and the occurrence of anemia, with the well-known unfavorable effects on the renal and cardiovascular prognosis.
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was kindly supported by an unrestricted grant of Manfred Lautenschläger Stiftung Diabetes, Wiesloch, Germany.
Conflict of interest statement: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Contributor Information
Christoph Hasslacher, Diabetesinstitut Heidelberg and Department of Clinical Studies, St. Josefskrankenhaus Heidelberg GmbH, c/o St. Josefskrankenhaus, Landhausstr. 25, 69115 Heidelberg, Germany.
Felix Kulozik, Diabetesinstitut Heidelberg and Department of Clinical Studies, St. Josefskrankenhaus Heidelberg GmbH, Germany.
Justo Lorenzo Bermejo, Institute of Medical Biometry and Informatics, University of Heidelberg, Germany.
References
- Aoki I., Taniyama M., Toyama K., Homori M., Ishikawa K. (1994) Stimulatory effect of human insulin on erythroid progenitors (CFU-E and BFU-E) in human CD34+ separated bone marrow cells and the relationship between insulin and erythropoietin. Stem cells 12: 329–338. [DOI] [PubMed] [Google Scholar]
- Babazono T., Hanai K., Suzuki K., Kiuchi Y., Inoue A., Tanaka M., et al. (2006) Lower haemoglobin level and subsequent decline in kidney function in type 2 diabetic adults without clinical albuminuria. Diabetologia 49: 1387–1393. [DOI] [PubMed] [Google Scholar]
- Bailyes E., Navé B., Soos M., Orr S., Hayward A., Siddle K. (1997) Insulin receptor/IGF-I receptor hybrids are widely distributed in mammalian tissues: quantification of individual receptor species by selective immunoprecipitation and immunoblotting. Biochem J 327: 209–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belfiore A., Frasca F., Pandini G., Sciacca L., Vigneri R. (2009) Insulin receptor isoforms and insulin receptor/insulin-like growth factor receptor hybrids in physiology and disease. Endocr Rev 30: 586–623. [DOI] [PubMed] [Google Scholar]
- Benecke H., Flier J., Moller D. (1992) Alternatively spliced variants of the insulin receptor protein. Expression in normal and diabetic human tissues. J Clin Invest 89: 2066–2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosmann D., Winkler A., Marsden J., Macdougall I., Watkins P. (2002) Anaemia with erythropoietin deficiency occurs early in diabetic nephropathy. Diab Care 224: 495–499. [DOI] [PubMed] [Google Scholar]
- Breyer J., Bain R., Evans J., Nahman N., Lewis E., Cooper M., et al. (1996) Predictors of the progression of renal insufficiency in patients with insulin-dependent diabetes and overt diabetic nephropathy. The Collaborative Study Group. Kidney Int 50: 1651–1658. [DOI] [PubMed] [Google Scholar]
- Dainiak N., Kreczko S. (1985) Interactions of insulin, insulinlike growth factor II, and platelet-derived growth factor in erythropoietic culture. J Clin Invest 76: 1237–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhardt K., May C., Koenen M., Eckel J. (2007) IGF-1 receptor signalling determines the mitogenic potency of insulin analogues in human smooth muscle cells and fibroblasts. Diabetologia 50: 2534–2543. [DOI] [PubMed] [Google Scholar]
- El-Achkar T., Ohmit S., McCullough P., Crook E., Brown W., Grimm R., et al. (2005) Higher prevalence of anemia with diabetes mellitus in moderate kidney insufficiency: The Kidney Early Evaluation Program. Kidney Int 67: 1483–1488. [DOI] [PubMed] [Google Scholar]
- Federici M., Porzio O., Zucaro L., Fusco A., Borboni P., Lauro D., et al. (1997) Distribution of insulin/insulin-like growth factor-I hybrid receptors in human tissues. Mol Cell Endocrinol 129: 121–126. [DOI] [PubMed] [Google Scholar]
- Gammeltoft S., Hansen B., Dideriksen L., Lindholm A., Schäffer L., Trüb T., et al. (1999) Insulin aspart: a novel rapid-acting human insulin analogue. Expert Opin Investig Drugs 8: 1431–1442. [DOI] [PubMed] [Google Scholar]
- Hasslacher C., Collenberg E., Möcks J. (2010) Effect of insulin analogs on the decline of hemoglobin in diabetic patients with nephropathy. Exp Clin Endocrinol Diabetes 118: 341–345. [DOI] [PubMed] [Google Scholar]
- Kulozik F., Hasslacher C. (2013) Insulin requirements in patients with diabetes and declining kidney function: differences between insulin analogues and human insulin? Ther Adv Endocrinol Metab 4: 113–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulozik F., Hasslacher C. (2015) 1,5-Anhydroglucitol as marker of short-term hyperglycemic excursions in well-controlled type 2 diabetes mellitus: associations with the prevalence of vascular complications. Diabetes Res Treat Open Access 2: 120. [Google Scholar]
- Kurtzhals P., Schäffer L., Sørensen A., Kristensen C., Jonassen I. (2000) Correlations of receptor binding and metabolic and mitogenic potencies of insulin analogs designed for clinical use. Diabetes 49: 999–1005. [DOI] [PubMed] [Google Scholar]
- Mayer D., Shukla A., Enzmann H. (2008) Proliferative effects of insulin analogues on mammary epithelial cells. Arch Physiol Biochem 114: 38–44. [DOI] [PubMed] [Google Scholar]
- Miyagawa S., Kobayashi M., Konishi N., Sato T., Ueda K. (2000) Insulin and insulin-like growth factor I support the proliferation of erythroid progenitor cells in bone marrow through the sharing of receptors. Brit. J Haemat 109: 555–562. [DOI] [PubMed] [Google Scholar]
- Miyata T., van Ypersele de Strihou C. (2010) Diabetic nephropathy: a disorder of oxygen metabolism? Nat Rev Nephrol 6: 83–95. [DOI] [PubMed] [Google Scholar]
- Nangaku M., Eckardt K. (2007) Hypoxia and the HIF system in kidney disease. J Mol Med Berl Ger 85: 1325–1330. [DOI] [PubMed] [Google Scholar]
- Oh S., Baek S., Kim Y., Goo H., Chin H., Na K., et al. (2012) Higher hemoglobin level is associated with subtle declines in renal function and presence of cardiorenal risk factors in early CKD stages. Nephrol Dial Transplant 27: 267–275. [DOI] [PubMed] [Google Scholar]
- Pierre-Eugene C., Pagesy P., Nguyen T., Neuillé M., Tschank G., Tennagels N., et al. (2012) Effect of insulin analogues on insulin/IGF1 hybrid receptors: increased activation by glargine but not by its metabolites M1 and M2. PloS One 7: e41992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathmann W., Kostev K. (2013) Lower incidence of recorded cardiovascular outcomes in patients with type 2 diabetes using insulin aspart vs. those on human regular insulin: observational evidence from general practices. Diabetes Obes Metab 15: 358–363. [DOI] [PubMed] [Google Scholar]
- Rhoads G., Kosiborod M., Nesto R., Fonseca V., Lu S., Zhang Q., et al. (2009) Comparison of incidence of acute myocardial infarction in patients with type 2 diabetes mellitus following initiation of neutral protamine Hagedorn insulin versus insulin glargine. Am J Cardiol 104: 910–916. [DOI] [PubMed] [Google Scholar]
- Ruggenenti P., Flores C., Aros C., Ene-Iordache B., Trevisan R., Ottomano C., et al. (2003) Renal and metabolic effects of insulin lispro in type 2 diabetic subjects with overt nephropathy. Diabetes Care 26: 502–509. [DOI] [PubMed] [Google Scholar]
- Sawada K., Krantz S., Dessypris E., Koury S., Sawada S. (1989) Human colony-forming units erythroid do not require accessory cells but do require direct interaction with insulin like growth factor 1 and/or insulin for erythroid development. J Clin Invest 83: 1701–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz B., Dippel F. (2012) incidence of cardiovascular outcomes in type 2 diabetic patients treated with insulin glulisine or other rapid acting insulin analogs under real-life conditions: a retrospective database analysis. Diabetol Stoffwechsel 7: 302–307. [Google Scholar]
- Shih L., Huang J., Lee C. (1999) Insulin-like growth factor I plays a role in regulating erythropoiesis in patients with end-stage renal disease and erythrocytosis. J Am Soc Nephrol 10: 315–322. [DOI] [PubMed] [Google Scholar]
- Shukla A., Grisouard J., Ehemann V., Hermani A., Enzmann H., Mayer D. (2009) Analysis of signaling pathways related to cell proliferation stimulated by insulin analogs in human mammary epithelial cell lines. Endocr Relat Cancer 16: 429–441. [DOI] [PubMed] [Google Scholar]
- Sommerfeld M., Müller G., Tschank G., Seipke G., Habermann P., Kurrle R., et al. (2010) In vitro metabolic and mitogenic signaling of insulin glargine and its metabolites. PloS One 5: e9540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varewijck A., Yki-Järvinen H., Schmidt R., Tennagels N., Janssen J. (2013) Concentrations of insulin glargine and its metabolites during long-term insulin therapy in type 2 diabetic patients and comparison of effects of insulin glargine, its metabolites, IGF-I, and human insulin on insulin and IGF-I receptor signaling. Diabetes 62: 2539–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]