Abstract
Background and objectives:
Evidence suggests associations between vitamin D deficiency and cardiovascular disease (CVD) risk factors, including hypertension and excessive cortisol levels. Also, vitamin D levels may impact exercise performance. Thus, we aimed to investigate the effects of vitamin D intake on cardiovascular risk factors, free urinary cortisol and exercise performance.
Methods:
A randomized placebo-controlled single-blinded parallel trial was conducted in healthy participants (n = 15). They received 2000 IU (50 µg) vitamin D3 per day (n = 9) or placebo (lactose) (n = 6) for 14 days. Body composition, systolic blood pressure (SBP), diastolic blood pressure (DBP) and arterial elasticity (as measured by pulse wave velocity, PWV) were recorded at baseline, day 7 and day 14 of intervention. A total of two 24-hour urine samples were collected to estimate free cortisol and cortisone levels. Exercise performance was assessed at the baseline and day 14 of the intervention using a bike ergometer in which BP and PWV were measured before and after exercise. The distance cycled in 20 minutes and the Borg Scale rate of perceived exertion (RPE) were recorded.
Results:
In the intervention arm, at day 14, vitamin D supplementation significantly reduced SBP and DBP from 115.8 ± 17.1 and 75.4 ± 10.3 at baseline to 106.3 ± 10.9 (p = 0.022) and 68.5 ± 10.1 mmHg (p = 0.012) respectively. Also arterial stiffness was markedly reduced in the vitamin D group (from 7.45 ± 1.55 to 6.11 ± 1.89, p = 0.049). Urinary free cortisol levels and cortisol/cortisone ratio were significantly reduced from 162.65 ± 58.9 nmol/day and 2.22 ± 0.7 to 96.4 ± 37.2 (p = 0.029) and 1.04 ± 0.4 (p = 0.017) respectively. Exercise-induced SBP and DBP were significantly reduced post vitamin D intake from 130.7 ± 12.2 to 116.1 ± 8.1 (p = 0.012) and from 76.2 ± 8.4 to 70.5 ± 7.7 mmHg (p = 0.042) respectively. The distance cycled in 20 minutes significantly increased from 4.98 ± 2.65 to 6.51 ± 2.28km (p = 0.020), while the Borg Scale RPE reduced from 5.13 ± 1.36 to 4.25 ± 0.71 RPE (p = 0.021). In the placebo arm, no significant effects on CVD risk factors and exercise performance were observed.
Conclusion:
These results suggest that daily vitamin D supplementation may ameliorate CVD risk factors including a decrease in 11β-HSD1 activity, as evidenced by the decrease in the cortisol/cortisone ratio, and improve exercise performance in healthy individuals. However, large scale studies are required to verify our findings.
Keywords: blood pressure, cardiovascular disease, exercise performance, oxidative stress, pulse wave velocity, vitamin D
Introduction
There is an interesting body of evidence for the multiplicity of roles of vitamin D in the human body exerted through vitamin D receptors (VDRs), which is present in many tissues including endothelial cells [Martins et al. 2014]. VDRs have a role in the conversion of 25(OH)D3 (25-hydroxyvitamin D3) to its active form 1,25(di-OH)D (1,25-dihydroxyvitamin D3). This has many functions including antiproliferative effects on vascular smooth muscle, immune modulation, stimulating release of inflammatory cytokines and modulates renin–angiotensin–aldosterone system (RAS) [Giallauria et al. 2012]. The discovery of vitamin D receptors in many tissues has provided new insights into the broad classical and nonclassical functions of vitamin D and the adverse effects of its deficiency [Kendrick et al. 2009]. Vitamin D deficiency is highly prevalent worldwide. Low levels of serum vitamin D are present in as many as 30–50% of otherwise healthy adults [Wang L et al. 2008]. Limited synthesis due to inadequate sun exposure, low intake of vitamin D-rich food, pigmented skin, indoor lifestyle and use of sunscreen creams are the main causes of low serum vitamin D levels, while poor dietary habits and intake of vitamin D in food or supplements also contribute to the risk of deficiency. Hypovitaminosis D is very common in winter months in the UK; synthesis of vitamin D3 is almost impossible and the majority of the UK population might be vitamin D deficient [Close et al. 2013]. Hypovitaminosis D is defined as a serum vitamin D level of <40 nmol/l [Hyppönen and Power, 2007], though some researchers recommend a level of >50 nmol/l to be adequate [Zitterman et al. 2009]. The Endocrine Society Clinical Practice Guidelines (2011) recommended levels for optimal vitamin D of >75 nmol/l.
Cardiovascular diseases (CVDs) such as heart attack, congenital heart disease and stroke are a major cause of morbidity and mortality. An estimated £19 billion is spent on CVD-related treatment [Kendrick et al. 2009; British Heart Foundation, 2015]. Recent literature has implicated vitamin D deficiency as a risk factor for CVD and its deficiency has developed to be a key biological predictor of increased rates of CVD [Mheid et al. 2011; Gotsman et al. 2012]. Vitamin D inadequacy has also been associated with hypertension, obesity, atherosclerosis, diabetes mellitus type 2 and oxidative stress [Anderson et al. 2010; Giallauria et al. 2012; Gotsman et al. 2012; Antoniades et al. 2009]. For instance, the Framingham Offspring Study showed that low levels of vitamin D are independently related to CVD incidence [Wang T et al. 2008]. In addition, low vitamin D levels have been linked with reduced exercise performance. Vitamin D deficiency has been associated with reduced exercise performance in athletes [Fitzgerald et al. 2015]. Researchers have investigated whether vitamin D may benefit exercise performance [Koundourakis et al. 2014], and reported that in trained athletes, vitamin D intake improved their performance. However, the understanding of the effect of vitamin D supplementation on both CVD risk factors and exercise performance has been compromised by limited prospective studies, the suboptimal dosing of vitamin D and the lack of understanding of the mechanism by which vitamin D mediates benefit to CVD risk factors and exercise performance [Giallauria et al. 2012].
In humans, vitamin D, known as the ‘sunshine vitamin’, is a fat-soluble unique vitamin because it can be synthesized by the body in the skin from sun exposure, specifically ultraviolet-B (UVB) radiation. The sun contributes to 80–90% of vitamin D supply [Zitterman et al. 2005], while the diet is a poor source of vitamin D accounting for only 20% of vitamin D supply [Zitterman et al. 2009]. Food sources which do contain some vitamin D include oily fish, egg, fortified spreads and breakfast cereals [Wang L et al. 2008]. The term ‘vitamin D’ is an umbrella term for the two forms of the vitamin: vitamin D3, which is also known as cholecalciferol, and vitamin D2, which is also known as ergocalciferol. Both of these forms of the vitamin are biologically inactive and so must undergo metabolism to their active form before they can exert their beneficial effects on the body. The aims of this study are to investigate the effects of vitamin D supplementation on cardiovascular risk factors, urinary free glucocorticoids and exercise performance in healthy volunteers.
Materials and methods
Participant recruitment
Participant recruitment was held at Queen Margaret University (QMU), Scotland and following the approval of the Research Ethics Committee, a recruitment email was sent via the university’s internal email system. Eligibility criteria were assessed using a health status questionnaire. The inclusion criteria included both healthy males and females aged 19–53 years. The exclusion criteria included people suffering from CVD, hyperglycaemia, primary hyperthyroidism, renal failure, diabetes mellitus and vitamin D intolerance. In addition, individuals who were already taking vitamin D supplements and pregnant and breastfeeding women were excluded. Information sheets were presented to the participants and once participants decided to participate they were asked to sign the consent form, and all data collected from the volunteers were kept anonymous. All measurements took place in QMU between 15 February 2015 and 1 May 2015.
Study design
A parallel, single-blinded, randomized, placebo-controlled trial was conducted with a duration of 2 weeks (Table 1). Participants were advised to avoid vitamin supplementation and not to change their dietary habits. In order to decrease intra-patient variability, and thus increase statistical power, the cardiovascular risk factors, BP and PVW, were measured at the beginning, middle and at the end of the intervention [Vickers, 2003]. All parameters were recorded three times at each visit and the average reading was taken. Participants taking vitamin D were given 2000 IU (50 μg in tablet form containing also 100 mg lactose) daily for two weeks, and placebo tablets (containing 100 mg lactose) which resembled vitamin D tablets were given to the placebo group. A total of 15 participants were recruited; 9 people selected to take vitamin D and 6 for the placebo group.
Table 1.
Outline of the study design.
| Day | Scheduled activities |
|---|---|
| Day –5 | 5 day washout period 2 day diet diary to be completed Health status questionnaire |
| Day –1 | 24 hour urine collection |
| Day 0 | Baseline BP, PWV, height, weight, BMI all recorded Baseline distance for 20 minutes cycling |
| Vitamin D supplementation or placebo starts | |
| Day 8–9 | BP, PWV recorded |
| Day 13 | 24 hour urine collection 2 day diet diary to be completed Health status questionnaire |
| Day 14–15 | Final BP, PWV, height, weight, BMI measured Distance for 20 minutes cycling recorded |
BMI, body mass index; BP, blood pressure; PWV, pulse wave velocity.
Blood pressure, pulse wave velocity and BMI measurements
A digital sphygmomanometer (Omron Healthcare Europe BVWegalaan 57NL-2132 JD Hoofddorp M5-I) was used to measure BP while the participants laid down comfortably in a 30-degree position and were allowed to relax for 10 minutes in a quiet room to avoid ‘white coat’ hypertension [Mheid et al. 2011]. PWV was measured to assess arterial stiffness using a noninvasive devise, VicorderTM (Skidmore Medical, Bristol, UK). Participants were rested in a supine position in a quiet room and the PWV was measured by simultaneously inflating both cuffs to 60 mmHg. PWV is calculated by measuring pulse transient time and distance between the two sites. The distance (cm) between sites was measured manually using a tape measure [Weber et al. 2009]. Height and weight were measured using standard techniques and BMI was calculated. Samples of urine were collected as 24-hour urine collections to assess urinary cortisol and cortisone levels using specific enzyme-linked immunosorbent assay (ELISA) methods.
Exercise performance
Exercise performance was investigated using a bike ergometer [Ronge, 1952]. BP and PWV were measured before and after exercise. The intensity of the bike ergometer was set to 100 watts to reduce inter- and intra-individual variability. The distance was recorded after 20 minutes of exercise. The same bike was used at both sessions to minimize variance between different bike ergometers. To investigate the strain of exercise the Borg Scale rate of perceived exertion (RPE) was used [Borg, 1982]. Diet diaries and health status questionnaires were measured before and after intervention to ensure any changes in CVD risk factors and exercise performance were due to vitamin D supplementation.
Biochemical markers
24-hour urine collections were obtained at baseline and at day 14 to assess oxidative stress, cortisol and cortisone levels. All samples were weighed and stored at −20°C to avoid bacterial and fungal growth and to reduce polyphenol oxidation.
A thiobarbituric acid reactive substances (TBARS) assay was performed to measure lipid peroxidation (oxidative stress) [Yagi, 1984]. The TBARS method is used for screening and monitoring lipid peroxidation, which is a major indicator of cellular injury and thus considered to be an oxidative stress marker [Amstrong and Browne, 1994]). At present, the TBARS assay is the most widely employed method for assessing plasma lipid peroxidation that allows comparison with other published studies. In brief, 0.1 ml of urine sample, 0.2 ml of Tris buffer and 0.1 ml of ascorbic acid was added to the incubation tube. After 15 minutes of incubation at 37oC, 0.4 ml of Trichloroacetic acid (TCA) and 0.8 ml of 2-thiobarbituric acid (TBA) solution were added into the tubes. Tubes were then incubated at 99oC for 15 minutes and centrifuged at 4000 rpm for 10 minutes. The absorbance of the samples was read at 532 nm. The data were calculated from a standard curve constructed simultaneously. The within-individual run coefficient of variation was 1.8–3.3%, which is fairly specific and acceptable [Amstrong and Browne, 1994].
Urinary free cortisol and cortisone levels were measured using highly specific and sensitive ELISA methods previously described by Al-Dujaili and colleagues [Al-Dujaili et al. 2012]. The urine samples were extracted by taking 100 μl of urine samples and 1.5 ml of ether and the tubes were vortex mixed for 10 minutes. Tubes were then placed in a −80oC freezer for 10 minutes and the upper layer was dried in glass tubes and reconstituted in 1 ml assay buffer. Briefly, the ELISA plate was coated with the steroid-BSA conjugate and left in the fridge overnight. The plate was washed with wash buffer and blocked using 200 μl of blocking buffer. The plate was then incubated at 37oC for 1 hour. After emptying the buffer, 50 μl of sample or standard was added into the assigned wells, followed by the addition of 100 μl of antibody in assay buffer. The plate was incubated at room temperature for 2 hours and washed. Enzyme coupled to a double antibody solution was added followed by a 1 hour incubation at room temperature. The plate was then washed and 100 μl of substrate was added and incubated for 15 minutes, 50 μl of stop solution was then added and the plate was read at 450 nm in the MRX ELISA reader (Dynex, USA).
Statistical analysis
All statistics were performed using the Statistical Package for the Social Sciences (SPSS, version 19.0) and numerical variables were first tested for normal distribution. Differences in baseline characteristics were examined using independent Student’s t-tests for scale data and Chi-square test for categorical variables. A one-way analysis of variance (ANOVA) was performed to assess changes between baseline, day 7 and day 14 in BP and PWV. The models included the main effects of treatment and post-hoc Bonferroni adjustment was used to account for multiple testing. Paired Student’s t-tests were used to establish changes between baseline and day14 in exercise performance, oxidative stress and cortisol/cortisone levels. Values were presented as the mean ± SD or SEM and p ⩽ 0.05 [Field, 2005].
Results
Participants characteristics
Overall, 15 healthy nonsmokers aged 19–53 were recruited; the participants were randomly allocated either to receive vitamin D supplementation (n = 9) or placebo (n = 6) (Table 2). A total of 6 out of all the 15 participants were regular exercisers with mean ± SD of 2.77 ± 3.59 hours/week and no participants were on contraceptive drugs. The baseline characteristics of the participants are presented in Table 2.
Table 2.
Baseline characteristics of the participants in the study.
| Baseline characteristics |
p-value | ||
|---|---|---|---|
| Vitamin D |
Placebo |
||
| Mean ± SD | Mean ± SD | ||
| Age | 27.75 ± 11.3 | 25.20 ± 3.1 | 0.61 |
| BMI (kg/m2) | 22.39 ± 4.2 | 22.76 ± 4.3 | 0.41 |
| Physical activity (hours/week) | 4.2 ± 3.9 | 2.80 ± 2.8 | 0.21 |
| Alcohol (units/week) | 3.75 ± 11.7 | 3.2 ± 2.9 | 0.071 |
| n (%) | n (%) | ||
| Gender | 9 (100) | 6 (100) | 0.52 |
| Male | 2 (22.2) | 2 (33.3) | |
| Female | 7 (77.7) | 4 (66.6) | |
| Ethnicity | 9 (100) | 6 (100) | 0.72 |
| White | 4 (44.4) | 3 (50.0) | |
| Chinese | 2 (22.2) | 0 (0) | |
| Pakistani | 3 (33.3) | 3 (50.0) | |
| Caffeine | 9 (100) | 6 (100) | 0.072 |
| Never/1–3 times per week | 6 (42.4) | 4 (66.6) | |
| 1–2 cups/day | 1 (7.3) | 1 (16.6) | |
| 2–3 cups/day | 1 (7.3) | 1 (16.6) | |
| 4 cups/day | 1 (7.3) | 0 (0) | |
Independent Student’s t-test; 2 Chi-square test. The percentages were calculated out of the total number of volunteers in the study.
BMI, body mass index; SD, standard deviation.
Physiological markers
In the intervention arm, vitamin D supplementation significantly reduced both SBP and DBP from 115.8 ± 17.1 and 75.4 ± 10.3 to 109.1 ± 11.3 (p = 0.022) and 70.3 ± 11.6 mmHg (p = 0.014) respectively at day 7. PWV was only reduced slightly from 7.45 ± 1.55 to 6.79 ± 1.28 (p = 0.08). At day 14 of intervention SBP and DBP were further reduced to 106.3 ± 10.9 (p = 0.02) and 68.5 ± 10.1 mmHg (p = 0.01) respectively. Arterial stiffness, measured as PWV, was markedly reduced from 7.45 ± 1.55 to 6.11 ± 1.89 (p = 0.04) (Figure 1).
Figure 1.
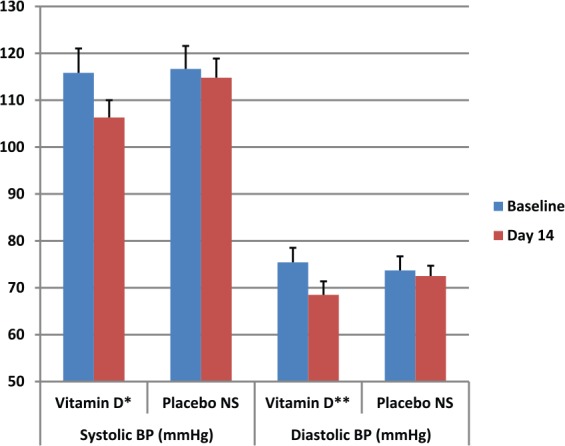
Effects of vitamin D supplementation on basal Systolic & Diastolic BP (mean ± SEM). *p = 0.02, 95% CI (0.4–11.6); **p = 0.01, 95% CI (0.3–7.9).
BP, blood pressure; CI, confidence interval; NS, Not significant; SEM, standard error of the mean.
In the placebo arm, baseline SBP and DBP were slightly reduced at day 7 but this was not significant; from 116.7 ± 9.3 and 73.7 ± 7.9 mmHg to 114.4 ± 8.1 (p = 0.306) and 72.4 ± 5.8 (p = 0.689) respectively, and again no significant reduction was noted between baseline and day 14 (114.8 ± 7.6; p = 0.3 and 72.9 ± 8.3; p = 0.26) (Figure 1). A one-way ANOVA test was not significant for SBP, DBP baseline between the vitamin D and placebo groups. The Δ changes in SBP, DBP and PWV in vitamin D participants versus the placebo group are shown in Figure 2.
Figure 2.
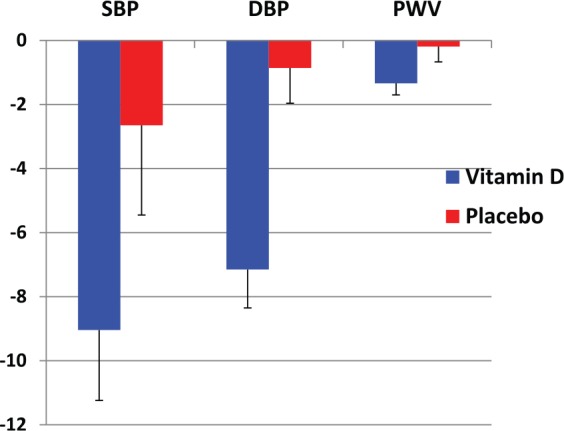
The Δ changes in SBP, DBP and PWV following vitamin D and placebo intervention at day 14.
DBP, diastolic blood pressure; PWV, pulse wave velocity; SBP, systolic blood pressure.
Biochemical markers
At baseline the biochemical markers were calculated by analyzing 24-hour urine collections to measure oxidative stress using TBARS (3.82 ± 1.12 μM). An ELISA was used to measure stress levels through cortisol (162.6 ± 58.9 nmol/day) and cortisone (74.2 ± 76.9 nmol/day). Following intervention, cortisol levels were significantly reduced to 96.4 ± 37.42 nmol/day (p = 0.029). However, no significant change in TBARS (3.38 ± 1.30 μM), or cortisone levels (92.6 ± 52.6 nmol/day) were found. Cortisol:cortisone ratio was significantly reduced from 2.22 ± 0.7 to 1.04 ± 0.4 (p = 0.017). In the placebo group, no significant changes were evident (Table 3).
Table 3.
Changes in TBARS, cortisol, cortisone and cortisol:cortisone ratio between baseline and intervention at day 14 (mean ± SD) following vitamin D supplementation.
| Marker | Baseline (mean ± SD) | Intervention (mean ± SD) | p-value | |
|---|---|---|---|---|
| Vitamin D | TBARS | 3.82 ± 1.12 | 3.38 ± 1.30 | 0.190 |
| Cortisol | 162.6 ± 58.9 | 96.4 ± 37.2 | 0.029 | |
| Cortisone | 74.2 ± 76.9 | 92.6 ± 52.58 | 0.210 | |
| Cortisol:cortisone ratio | 2.22 ± 0.70 | 1.04 ± 0.42 | 0.017 | |
| Placebo | TBARS | 3.24 ± 0.56 | 4.74 ± 0.32 | 0.250 |
| Cortisol | 238.8 ± 105.3 | 187.6 ± 125.7 | 0.494 | |
| Cortisone | 75.4 ± 39.67 | 65.1 ± 14.50 | 0.339 | |
| Cortisol:cortisone ratio | 3.16 ± 1.24 | 2.89 ± 2.37 | 0.788 |
p-value calculated using paired Student’s t-test.
SD, standard deviation; TBARS, thiobarbituric acid reactive substances.
Effect on exercise performance
The results of this study showed a significant reduction in pre- to post-exercise SBP and DBP following 2 weeks of vitamin D intervention. At baseline, the BP measurements after exercise were: SBP (130.7 ± 12.6 mmHg) and DBP (76.2 ± 8.4 mmHg) in participants receiving vitamin D supplementation and SBP (128.7 ± 8.7 mmHg), DBP (74.1 ± 7.5 mmHg) in the placebo group. After two weeks of vitamin D supplementation the exercise-induced rise in SBP was reduced to 116.1 ± 8.2 mmHg (p = 0.012) and DBP reduced to 70.5 ± 7.7 mmHg (p = 0.04) after 20 minutes exercise (Figure 3). However, there was no significant changes in the placebo group as SBP was 127.2 ± 11.1 mmHg (p = 0.2) and DBP 73.7 ± 5.9 mmHg (p = 0.827).
Figure 3.
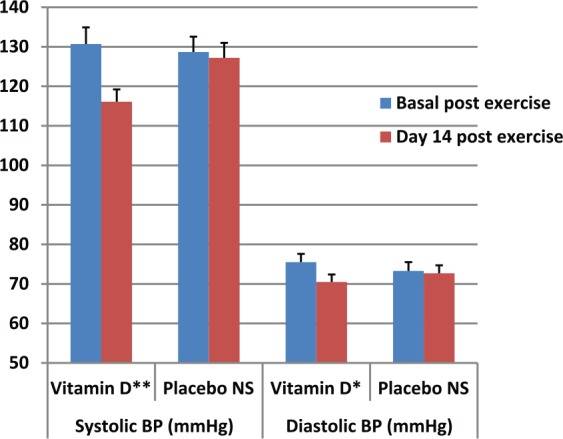
Effects of vitamin D supplementation on exercise-induced systolic and diastolic BP (mean ± SEM). *p = 0.042; 95% CI (0.3–21.3); **p = 0.012; 95% CI (0.1–10.1).
BP, blood pressure; CI, confidence interval; NS, Not significant; SEM, standard error of the mean.
The vitamin D group showed a statistically significant increase in the distance cycled from 4.98 ± 2.65 km to 6.51 ± 2.28 km (p = 0.02) and a significant reduction in the Borg Scale RPE from 5.13 ± 0.80 RPE to 4.25 ± 0.70 RPE (p = 0.02) following 2 weeks of intervention. No significant changes were seen in the placebo group for either the distance cycled (4.14 ± 2.06km to 4.83 ± 1.17km, p = 0.24) or the Borg Scale values (5.8 ± 1.89 RPE to 5.80 ± 1.79 RPE, p = 0.98) (Figures 4A and 4B).
Figure 4.
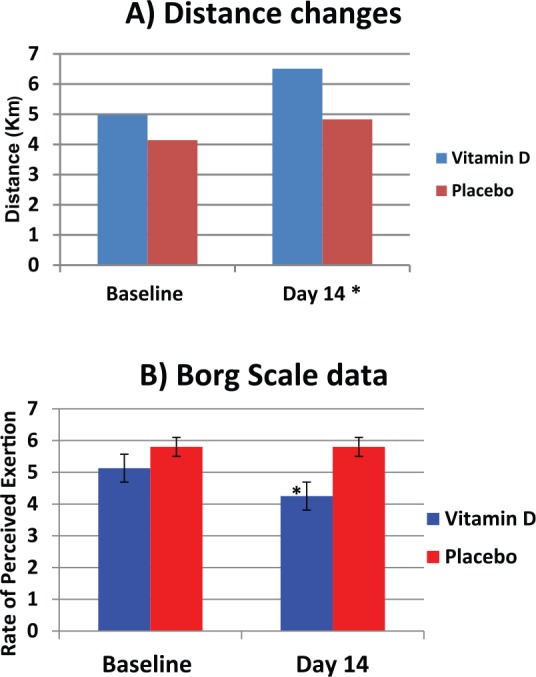
Effects of vitamin D supplementation on exercise performance measured as (A) distance, and (B) Borg Scale rate of perceived exertion
Results are expressed as mean ± SEM; (A): Participants distance cycled at baseline and day 14 of intervention, *p = 0.02; 95% CI [(-2.74) to (-0.320)]; (B): Participants rate of exhaustion after exercise calculated using Borg Scale, p = 0.02; 95% CI [(-2.74) to (-0.32)].
CI, confidence interval; SEM, standard error of the mean.
Diet
Results from the food diaries collected from all participants showed no significant changes in the macronutrients and energy intake for the vitamin D group at baseline and during intervention. BMI was also calculated and no significant difference was noted between the baseline and intervention phase (Table 4). In addition, analysis of the health status questionnaires showed no significant difference in the amount of exercise per week, total alcohol consumption and coffee consumption.
Table 4.
Dietary intake of participants at baseline and intervention phase (mean ± SD).
| Dietary intake | |||
|---|---|---|---|
| Baseline (mean ± SD) | Intervention (mean ± SD) | p-value | |
| Fat (g) | 59.65 ± 16.66 | 55.05 ± 12.78 | 0.383 |
| CHO (g) | 167.65 ± 42.91 | 138.98 ± 49.02 | 0.141 |
| Protein (g) | 79.95 ± 46.52 | 74.16 ± 40.59 | 0.877 |
| Total energy (kcal) | 1413.96 ± 334.51 | 1281.46 ± 240.60 | 0.156 |
| Vitamin D (µg) | 0.87 ± 0.41 | 0.88 ± 0.27 | 0.937 |
| BMI | 22.54 ± 4.08 | 22.65 ± 4.01 | 0.358 |
p-value calculated using paired Student’s t-test.
BMI, body mass index; CHO, carbohydrates; SD, standard deviation
Discussion
This pilot study found that healthy adults supplemented with vitamin D had both lower blood pressure and lower levels of the stress hormone cortisol in their urine compared with those given a placebo. Furthermore, the vitamin D group increased the distance cycled by 1.5 km (30%) in 20 minutes and showed lower signs of physical exertion than the placebo group following 2 weeks of intervention.
Around 10 million people in the UK may have low vitamin D levels. On average, 1 in 10 adults has low levels of vitamin D in the summer, compared with 2 in 5 in winter. Because people with darker skin are less efficient at using sunlight to make vitamin D, up to three out of four adults with dark skin are deficient in winter. Our pilot study suggests that taking vitamin D supplements can improve fitness levels and lower CVD risk factors such as BP and PWV. Our study adds to the body of evidence showing the importance of tackling this widespread problem.
A significant reduction was noted in SBP and DBP between baseline and after vitamin D intervention. A similar improvement in BP was seen in a prospective double-blind study in 283 black individuals with 1000, 2000 and 4000 IU of vitamin D supplementation [Forman et al. 2013]. Vitamin D exerts antihypertensive effects through the inhibition of the renin–angiotensin–aldosterone system (RAS) and the association between vitamin D levels and renin activity was first established in 1986 [Resnick et al. 1986; Wu et al. 2010]. RAS is a vital regulator of BP through renin activity, in which renin cleaves angiotensin I to angiotensin II and once bound to the receptor, it exerts regulatory effects on BP. Inappropriate stimulation of RAS proceeds to hypertension suggesting that the inhibition of RAS by vitamin D may reduce BP [Li et al. 2002]. Conversely, a number of studies have reported no reduction in BP with vitamin D. One study [Zittermann et al. 2009] reported that 83 μg vitamin D daily gave no reduction in BP in 200 participants. However, the study showed marked variations and was confined as the BP was measured only on two occasions limiting the detection of small but significant changes in BP. More recently, 100,000 IU vitamin D supplements every 2 months failed to reduce BP in 75 participants, however the dose is too large to be given at once and might disappear in the adipose tissue, and the daily available vitamin D is reported to be insufficient to exert the biological effects [Heaney, 2005; Witham et al. 2013]. In addition, concomitant medication has prevented vitamin D from exerting beneficial effects, explaining no effect on BP in many studies [Witham et al. 2012].
Arterial stiffness, the reduced ability of an artery to respond to blood pressure changes, has been associated with vitamin D deficiency [Giallauria et al. 2012; Pirro et al. 2012]. Vitamin D deficiency causes arterial stiffness through the stimulation of vascular smooth muscle proliferation, macrophage invasion and calcification [Mheid et al. 2011]. In the current study, vitamin D supplementation induced a small but statically reduction in PWV, which is the gold standard index for measuring arterial stiffness [Pirro et al. 2012]. Similarly, 2500 IU vitamin D daily exerted no effect on arterial stiffness in 114 participants, although average circulating levels of 25(OH)D were increased [Gepner et al. 2012]. More recently, arterial stiffness in 199 participants was nonsignificantly affected by 50,000 IU and 100,000 IU single intramuscular vitamin D [McGreevy et al. 2015]. In contrast, two other studies supplemented with 2500 IU vitamin D daily for 4 months and 100,000 IU vitamin D for 3 months significantly reduced PWV in African individuals [Dong et al. 2010; Martins et al. 2014]. However, data from both studies cannot be extrapolated on the general population as both studies included black individuals living in high latitudes and skin colour Africans have a higher prevalence of vitamin D deficiency. Vasoprotective functions of vitamin D are believed to be exerted by direct and indirect effects on vascular cells and suppression of RAS [Dong et al. 2010]. The reduction in PWV might also impact favourably the development of CVD later in adulthood [Dong et al. 2010]. In addition, this study did not have sufficient power to detect a moderate but significant change in PWV. The method of assessing PWV may have introduced possible errors in the study as PWV is advised to be measured in a dark, temperature controlled environment [Mheid et al. 2011]. Moreover, the intervention period was insufficient as improvement in calcification may take longer than 3 months to the point where arterial compliance may improve and make structural or functional changes to the arteries [Witham et al. 2012; Ryu et al. 2014]. Therefore, longer trials investigating the potential of vitamin D in stimulating favourable alterations in cardiovascular function are warranted.
Vitamin D has been associated with CVD due to its effects on oxidative stress. Tarcin and colleagues demonstrated that vitamin D deficient individuals presented higher plasma TBARS [Tarcin et al. 2009]. Free radical oxidation of cellular components in CVD has been widely recognized and oxidative modification of low density lipoprotein and cellular constituents subsidises mechanism of atherogenesis [Canale et al. 2014]. In addition, reactive oxygen species promote oxidative stress, since they induce specific post-translational modifications which alter the function of vital cellular proteins and signalling pathways to the heart [Ho et al. 2013]. Biomarkers of oxidation, specifically malondialdehyde (MDA), often measured using TBARS, are elevated in relationship with CVD risk factors. Since the 1990s the association between vitamin D supplementation and TBARS has been thoroughly investigated [Wiseman, 1993; Kuzmenko et al. 1997]. In this particular study, no change in TBARS was observed in samples taken before exercise, suggesting oxidative stress was unaffected by vitamin D supplements or post-urine samples should have been collected to test the effect following the challenge of exercise. A study supplemented with 300,000 IU vitamin D monthly, for 3 months, effectively reduced TBARS in 23 asymptomatic participants, possibly due to an improvement of endothelial function with vitamin D supplementation, whereas measurements of flow-mediated dilation were significantly lower in 25(OH)D-deficient participants [Tarcin et al. 2009]. Activated T-cells induce oxidative stress and studies have found that the effect of vitamin D on RAS inhibits the release of inflammatory cytokines from activated T-cells, thus, reducing oxidative stress [Judd et al. 2010]. In addition, Wiseman suggested that the hydrophobic sections of 1,25(OH)D3 have the capability to impair the viscosity of the membrane, thus, protecting the cell membrane from lipid peroxidation and the harmful effects of free radicals, suggesting a role for vitamin D as a potential antioxidant [Wiseman, 1993].
Vitamin D supplementation significantly reduced cortisol levels and cortisol:cortisone ratio but had a nonsignificant effect on cortisone. Cortisol is vital for the body’s recovery from stress; however excessive levels of cortisol have direct effects on cardiac output and has been associated with hypertension [Vogelzangs et al. 2010]. Cortisol release is stimulated by the activation of the hypothalamic-pituitary-adrenal (HPA) axis which promotes the release of corticotrophin releasing hormone and adrenocorticotropic hormone. Also the enzyme 11β-HSD1 exerts an important role in modulating the levels of active cortisol. The reduction in the ratio of urinary cortisol:cortisone indicated an inhibition of 11β-HSD1activity, explaining the decrease in cortisol levels. Hyperactivity of the HPA axis results in an excessive production of cortisol, and once cortisol is bound to the mineralocorticoid receptor, proinflammatory effects and vascular cell calcification are promoted, causing damaging effects in CVD [Bhathena et al. 1991; Vogelzangs et al. 2010: Kumari et al. 2011]. Cortisone and the cortisol:cortisone ratio have been linked with increased CVD events [Quinkler and Stewart, 2003]. To date, no study has investigated the potential effect of vitamin D supplementation on cortisol levels, although a limited number of studies have investigated the effects of vitamin E and C on cortisol levels [Peters et al. 2001]. For instance, vitamin C supplementation significantly reduced cortisol levels after ultramarathon racing in 45 participants [Peters et al. 2001]. The current study indicates that vitamin D has the potential to reduce cortisol levels and the cortisol:cortisone ratio.
Suboptimal levels of vitamin D have been associated with impaired exercise performance, as it reduces muscle action and skeletal mineralisation [Wyon et al. 2014; Fitzgerald et al. 2015]. Many studies have argued that vitamin D status is linked with muscle strength, aerobic capacity and speed, however, the specific mechanism by which vitamin D exerts its effects on performance is debated [Wyon et al. 2014; Koundourakis et al. 2014]. One proposed mechanism is that vitamin D supplementation increases adenosine triphosphate (ATP) content in muscle, while others argue that vitamin D improves muscle function through the presence of VDRs in muscle fibres by controlling serum calcium concentration [Wyon et al. 2014]. In addition, vitamin D influences VO2 max via effects on erythropoiesis, thus modifying the oxygen supply to exercising muscles, consequently improving aerobic exercise performance [Koundourakis et al. 2014]. In the current study, vitamin D supplementation significantly reduced SBP and DBP, and led to a slight reduction in PWV, thus, improving performance and tolerance as a high BP induces fatigue, dyspnea, mild exertion and excessive ventilator response to any load of exercise [D’Alonzo et al. 1987]. This was first suggested when acute intravenous verapamil reduced systolic and arterial stiffness, thus, enhancing aerobic exercise performance [Chen et al. 1999]. It may be possible to detect a greater reduction in PWV if the duration of vitamin D intervention was increased, as intervention may take 3 months before a marked improvement in arterial stiffness is detected [Witham et al. 2013]. In the current study, vitamin D supplementation significantly improved chosen measures of physical performance including distance cycled on a bike ergometer and the Borg Scale as an increase in distance and a reduction in Borg Scale RPE were noted. The Borg Scale is considered to be the best indicator of degree of physical strain and a reduction indicated improved performance [Borg, 1982]. Despite the small sample size, 2000 IU vitamin D significantly improved some markers of performance, suggesting the potential of vitamin D to reduce fatigue and improve endurance and aerobic capacity, thus a role of vitamin D as an ergogenic aid might be considered. Vitamin D deficiency was associated with an increased rate of perceived exertion assessed by the Borg Scale in heart failure patients [Boxer et al. 2011]. Similarly, Wyon and colleagues reported beneficial effects of 2000 IU vitamin D supplementation for 4 months on performance parameters including muscle strength and vertical jump height in 24 ballet dancers, but lacked proper randomization of participants into groups and consideration of confounding variables [Wyon et al. 2014]. Confounding variables including caffeine and alcohol, which are consumed by some athletes as ergogenic aids to reduce fatigue before exercise, were all taken into consideration [Mohr et al. 2013; Koundourakis et al. 2014]. No significant change between these variable was found proving beneficial effects on exercise performance were due to vitamin D supplementation. However, larger scale studies investigating effects of different doses of vitamin D and the mechanisms by which vitamin D exerts its effects on exercise performance are warranted.
Limitations and strengths of this study
This study was limited by the reduced sample size, short intervention period and the single-blinded approach. In addition, recommended dietary intake of vitamin D is 10–20 μg/day and the participant’s dietary intake was 1.87 ± 0.41; since plasma levels of 25(OH)D were not measured, it can be assumed that participants were vitamin D-deficient [Hollis, 2005]. Thus, the vitamin D supplementation may have only restored the body’s vitamin D levels but full biological effects were not observed [Heaney, 2005]. More females than males were recruited in this study and sex could be a confounding factor as BP is associated with gender [Caro et al. 2012].
Furthermore, diet diaries were used as an indicator of dietary vitamin D intake and self-reporting can lead to errors such as under-reporting of food consumption [Hughes et al. 2012]. Of note, food frequency questionnaires focusing on food and supplements rich in vitamin D and sun exposure questionnaires to quantify UVB exposure have been reported to give an accurate indication of total vitamin D intake [Caro et al. 2012]. Despite the limitations this study has strengths including the analysis of confounding variables such as exercise, alcohol and caffeine to ensure beneficial effects were due to vitamin D supplementation, and the fact that the study was performed during the winter and early spring months and no patients had been on holidays 3 months prior to the study. In addition, the current study gives merit to the fact that healthy participants who were not on medication were included, thus there was no interruption in the biological actions of vitamin D. Also, measurement of BP on three separate occasions, taking the average of three readings on each occasion, allowed the detection of small significant changes in BP after vitamin D supplementation.
Conclusion
We have shown that vitamin D supplementation improved some CVD risk factors in healthy volunteers. Blood pressure reduction suggests that vitamin D has antihypertensive effects possibly due to its inhibitory effects on the RAS and 11β-HSD1 activity. Arterial stiffness was slightly reduced during the short duration of the study suggesting that long term vitamin D intake may improve arterial stiffness. Urinary cortisol and the cortisol:cortisone ratio reduction suggests a decrease in the stress hormone levels, that may be due to the reduction of 11β-HSD1 activity (the enzyme responsible for the activation of cortisone to its active form, cortisol). Exercise performance was markedly improved due to vitamin D supplementation. Our pilot study showed a reduction in the exercise-induced rise in BP which may improve tolerance and reduce fatigue thus improving exercise performance. It is worth mentioning that vitamin D deficiency could be dormant and difficult to detect, as vitamin D can affect muscle work in various ways and there may be no visible symptoms of the deficiency [Ward et al. 2009]. Future studies should assess plasma baseline and post-intervention 25(OH)D levels, include a larger sample and perform a double-blinded clinical trial to verify these findings.
Acknowledgments
The authors would like to thank all volunteers who responded positively and have completed the study. Emad Al-Dujaili was responsible for planning, supervising, executing, and writing the manuscript of the study. Raquel Revuelta Iniesta was responsible for co-supervising the research work and analyzing the data. Nimrah Munir recruited the volunteers and conducted the analyses.
Footnotes
Author’s Note: The corresponding author is currently affiliated to: Professor of Clinical & Medical Biochemistry Faculty of Pharmacy, Middle East University Amman, Jordan Email: ealdujaili@meu.edu.jo
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Emad A. S. Al-Dujaili, Queen Margaret University, Queen Margaret University Drive, Musselburgh, Edinburgh, East Lothian EH21 6UU, UK.
Nimrah Munir, Dietetics, Nutrition and Biological Sciences, Queen Margaret University, Edinburgh, UK.
Raquel Revuelta Iniesta, Dietetics, Nutrition and Biological Sciences, Queen Margaret University, Edinburgh, UK.
References
- Al-Dujaili E., Baghdadi H., Howie F., Mason J. (2012) Validation and application of a highly specific and sensitive ELISA for the estimation of cortisone in saliva, urine and in vitro cell-culture media by using a novel antibody. Steroids 77: 703–709. [DOI] [PubMed] [Google Scholar]
- Anderson J., May H., Horne B., Bair T., et al. (2010) Relation of vitamin D deficiency to cardiovascular risk factors, disease status, and incident events in a general healthcare population. Am J Cardiol 106: 963–968. [DOI] [PubMed] [Google Scholar]
- Antoniades C., Shirodaria C., Leeson P., Antonopoulos A., et al. (2009) Association of plasma asymmetrical dimethylarginine (ADMA) with elevated vascular superoxide production and endothelial nitric oxide synthase uncoupling: implications for endothelial function in human atherosclerosis. Eur Heart J 30: 1142–1150. [DOI] [PubMed] [Google Scholar]
- Armstrong D., Browne R. (1994) The analysis of free radicals, lipid peroxides, antioxidant enzymes and compounds related to oxidative stress as applied to the clinical chemistry laboratory. Adv Exp Med Biol 366: 43–58. [DOI] [PubMed] [Google Scholar]
- Bhathena S., Berlin E., Judd J., Kim Y., Law J., et al. (1991) Effects of omega 3 fatty acids and vitamin E on hormones involved in carbohydrate and lipid metabolism in men. Am J Clin Nutrition 54: 684–688. [DOI] [PubMed] [Google Scholar]
- British Heart Foundation (2015) Cardiovascular disease statistics UK factsheet. Heart statistics research. British Heart Foundation, UK. Available at: https://www.bhf.org.uk/research/heart-statistics (accessed November 2015).
- Borg G. (1982) Psychophysical bases of perceived exertion. Med Sci Sports Exercise 14: 377–381. [PubMed] [Google Scholar]
- Boxer R., Kenny A., Cheruvu V., Vest M., et al. (2010) Serum 25-hydroxyvitamin D concentration is associated with functional capacity in older adults with heart failure. Amer Heart J 160: 893–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canale M., Noce A., Capria A., et al. (2015) Coronary artery calcifications predict long term cardiovascular events in nondiabetic Caucasian hemodialysis patients. Aging 7: 269–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caro Y., Negron V., Palacios C. (2012) Association between vitamin D levels and blood pressure in a group of Puerto Ricans. Puerto Rico Health Sci J 31: 123–129. [PMC free article] [PubMed] [Google Scholar]
- Chen C., Nakayama M., Talbot M., Nevo E., Fetics B., et al. (1999) Verapamil acutely reduces ventricular-vascular stiffening and improves aerobic exercise performance in elderly individuals. J Amer College Cardiol 33: 1602–1609. [DOI] [PubMed] [Google Scholar]
- Close G., Russell J., Cobley J., et al. (2013) Assessment of vitamin D concentration in professional athletes and healthy adults during the winter months in the UK: implications for skeletal muscle function. J Sports Sci 31: 344–353. [DOI] [PubMed] [Google Scholar]
- D’Alonzo G., Gianotti L., Pohil R., Reagle R., Duree S., Fuentes F., et al. (1987) Comparison of progressive exercise performance of normal subjects and patients with primary pulmonary hypertension. Chest 92: 57–62. [DOI] [PubMed] [Google Scholar]
- Dong Y., Stallmann-Jorgensen I., Pollock N., Harris R., et al. (2010) A 16-week randomized clinical trial of 2000 international units daily vitamin D3 supplementation in Black youth: 25-hydroxyvitamin d, adiposity, and arterial stiffness. J Clin Endocrinol Metab 95: 4584–4591. [DOI] [PubMed] [Google Scholar]
- Field A. (2005) Discovering Statistics Using SPSS. 2nd edition London: Sage. [Google Scholar]
- Fitzgerald J., Peterson B., Warpeha J., Johnson S., Ingraham S. (2015) Association between vitamin D status and maximal-intensity exercise performance in junior and collegiate hockey players. J Strength Condition Res 29: 2513–2521. [DOI] [PubMed] [Google Scholar]
- Forman J., Scott J., Drake B., Suarez E., Hayden D., Bennett G., et al. (2013) Effect of vitamin D supplementation on blood pressure in Blacks. Hypertension 61: 779–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gepner A., Ramamurthy R., Krueger D., Korcarz C., Binkley N., Stein J. (2012) A prospective randomized controlled trial of the effects of vitamin D supplementation on cardiovascular disease risk. PLoS One 7: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giallauria F., Milaneschi Y., Tanaka T., Maggio M., Canepa M., et al. (2012) Arterial stiffness and vitamin D levels: the Baltimore longitudinal study of ageing. J Clin Endocrine Metab 97: 3717–3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotsman I., Shauer A., Zwas D., Hellman Y., Keren A., Lotan C., et al. (2012) Vitamin D deficiency is a predictor of reduced survival in patients with heart failure; vitamin supplementation improves outcome. Eur J Heart Failure 14:357–366. [DOI] [PubMed] [Google Scholar]
- Heaney R. (2005) The vitamin D requirement in health and disease. J Steroid Biochem Mol Biol 97: 13–19. [DOI] [PubMed] [Google Scholar]
- Ho E., Galoughi K., Liu C., Bhindi R., Figtree G. (2013) Biological markers of oxidative stress: applications to cardiovascular research and practice. Redox Biol 8: 483–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollis B. (2005) Circulating 25-hydroxyvitamin D levels indicative of vitamin D sufficiency: implications for establishing a new effective dietary intake recommendation for vitamin D. Amer Soc Nutritional Sci 135: 317–322. [DOI] [PubMed] [Google Scholar]
- Hughes C., Woodside J., McGartland C., Roberts M., Nichollis D., McKeown P. (2012) Nutritional intake and oxidative stress in chronic heart failure. Nutrition Metabol Cardiovasc Dis 22: 376–382. [DOI] [PubMed] [Google Scholar]
- Hyppönen E., Power C. (2007) Hypovitaminosis D in British adults at age 45 y: nationwide cohort study of dietary and lifestyle predictors. Am J Clin Nutr 85: 860–868. [DOI] [PubMed] [Google Scholar]
- Judd S., Raiser S., Kumari M., Tangpricha V. (2010) 1,25-dihydroxyvitamin D3 reduces systolic blood pressure in hypertensive adults: a pilot feasibility study. J Steroid Biochem Mol Biol 121: 445–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendrick J., Targher G., Smits G., Chonchol M. (2009) 25-hydroxyvitamin D deficiency is independently associated with cardiovascular disease in the Third National Health and Nutrition Examination Survey. Atherosclerosis 205: 255–260. [DOI] [PubMed] [Google Scholar]
- Koundourakis N., Androulakis N., Malliaraki N., Margioris A. (2014) Vitamin D and exercise performance in professional soccer players. PLoS One 9: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari M., Shipley M., Stafford M., Kivimaki M. (2011) Association of diurnal patterns in salivary cortisol with all-cause and cardiovascular mortality: findings from the Whitehall II Study. J Clin Endocrinol Metab 95: 1478–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzmenko A., Morozova R., Nikolenko I., Korniets G., Kholodova Y. (1997) Effects of vitamin D3 and ecdysterone on free-radical lipid peroxidation. Biochem 62: 609–612. [PubMed] [Google Scholar]
- Li Y., Kong J., Wei M., Chen Z., Liu S., Cao L. (2002) 1,25-dihydroxyvitamin D3 is a negative endocrine regulator of the renin–angiotensin system. J Clin Invest 110: 229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins D., Meng Y., Tareen N., Artaza J., Lee J., Farodolu C., et al. (2014) The effect of short term vitamin D supplementation on the inflammatory and oxidative mediators of arterial stiffness. Health 6: 1503–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGreevy C., Barry M., Davenport C., Byrne B., Donaghy C., et al. (2015) The effect of vitamin D supplementation on arterial stiffness in an elderly community–based population. J Amer Soc Hypertension 9: 176–183. [DOI] [PubMed] [Google Scholar]
- Mheid I., Patel R., Murrow J., Morris A., Rahman A., Fike L., et al. (2011) Vitamin D Status is associated with arterial stiffness and vascular dysfunction in healthy humans. J Amer College Cardiol 58: 186–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr M., Nielsen J., Bangsbo J. (2011) Caffeine intake improves intense intermittent exercise performance and reduces muscle interstitial potassium accumulation. J Appl Physiol 111: 1372–1379. [DOI] [PubMed] [Google Scholar]
- Peters E., Anderson R., Nieman D., Fickl H., Jogessar V. (2001) Vitamin C supplementation attenuates the increases in circulating cortisol, adrenaline, and anti-inflammatory polypeptides following ultramarathon running. Nutrition 22: 537–543. [DOI] [PubMed] [Google Scholar]
- Pirro M., Manfredelli M., Helou R., Scarponi A., Schillaci G., et al. (2012) Association of parathyroid hormone and 25-OH-vitamin D levels with arterial stiffness in postmenopausal women with vitamin D insufficiency. J Atheroscler Thromb 19: 924–931. [DOI] [PubMed] [Google Scholar]
- Quinkler M., Stewart P. (2003) Hypertension and the cortisol–cortisone shuttle. J Clin Endocrinol Metab 88: 2384–2392. [DOI] [PubMed] [Google Scholar]
- Resnick L., Muller F., Laragh J. (1986) Calcium-regulating hormones in essential hypertension, relation to plasma renin activity and sodium metabolism. Ann Intern Med 105: 649–654. [DOI] [PubMed] [Google Scholar]
- Ronge H. (1952) Increase of physical effectiveness by systematic ultraviolet irradiation. Strahlentherapie 88: 563–566. [PubMed] [Google Scholar]
- Ryu O., Chung W., Lee S., Hong K., Choi M., Yoo H. (2014) The effect of high-dose vitamin D supplementation on insulin resistance and arterial stiffness in patients with type 2 diabetes. Korean Assoc Intern Med 29: 620–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarcin O., Yavuz D., Ozben B., Telli A., Ogunc A., et al. (2009) Effect of vitamin D deficiency and replacement on endothelial function in asymptomatic subjects. Endocrine Res 94: 4023–4030. [DOI] [PubMed] [Google Scholar]
- Vickers A. (2003) How many repeated measures in repeated measures designs? Statistical issues for comparative trials. BMC Med Res Methodol 3: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelzangs N., Beekman A., Milaneschi Y., Bandinelli S., Ferrucci L., Penninx W. (2010) Urinary cortisol and six-year risk of all-cause and cardiovascular mortality. J Clin Endocrinol Metab 95: 4959–4964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Manson J., Buring E., Lee I., Sesso H. (2008) Dietary intake of dairy products, calcium, and vitamin D and the risk of hypertension in middle-aged and older women. Hypertension 51: 1073–1079. [DOI] [PubMed] [Google Scholar]
- Wang T., Pencina M., Booth S., Jacques P., Ingelsson E., et al. (2008) Vitamin D deficiency and risk of cardiovascular disease. Circulation 117: 503–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward K., Das G., Berry J., Roberts S., Rawer R., Adams J., et al. (2009) Vitamin D status and muscle function in post-menarchal adolescent girls. J Clin Endocrinol Metab 94: 559–563. [DOI] [PubMed] [Google Scholar]
- Weber T., Ammer M., Rammer M., et al. (2009) Noninvasive determination of carotid–femoral pulse wave velocity depends critically on assessment of travel distance: a comparison with invasive measurement. J Hypertension 27: 1624–1630. [DOI] [PubMed] [Google Scholar]
- Wiseman T. (1993) Vitamin D is a membrane antioxidant. Ability to inhibit iron-dependent lipid peroxidation in liposomes compared to cholesterol, ergosterol and tamoxifen and relevance to anticancer action. FEBS Lett 326: 285–288. [DOI] [PubMed] [Google Scholar]
- Witham M., Dove F., Khan F., Lang C., Belch J., Struthers A. (2013) Effects of vitamin D supplementation on markers of vascular function after myocardial infarction: a randomized controlled trial. Int J Cardiol 167: 745–749. [DOI] [PubMed] [Google Scholar]
- Witham M., Dove F., Sugden J., Doney A., Struthers A. (2012) The effect of vitamin D replacement on markers of vascular health in stroke patents: a randomized controlled trial. Nutrition Metab Cardiovasc Dis 22: 864–870. [DOI] [PubMed] [Google Scholar]
- Wu S., Ho S., Zhong L. (2010) Effects of vitamin D supplementation on blood pressure. Southern Med J 103: 729–737. [DOI] [PubMed] [Google Scholar]
- Wyon M., Koutedakis Y., Wolman R., Nevill A., Allen N. (2014) The influence of winter vitamin D supplementation on muscle function and injury occurrence in elite ballet dancers: A controlled study. J Sci Med Sport 17: 8–12. [DOI] [PubMed] [Google Scholar]
- Yagi K. (1984) Assay for blood plasma or serum. Meth Enzymol 105: 328–331. [DOI] [PubMed] [Google Scholar]
- Zittermann A., Frisch S., Berthold H., Gotting C., Kuhn J., Kleesiek K., et al. (2009) Vitamin D supplementation enhances the beneficial effects of weight loss on cardiovascular disease risk markers. Amer J Clin Nutrition 89: 1321–1327. [DOI] [PubMed] [Google Scholar]
- Zittermann A., Schleithoff S., Koerfer R. (2005) Putting cardiovascular disease and vitamin D insufficiency into perspective. Br J Nutr 94: 483–492. [DOI] [PubMed] [Google Scholar]


