Abstract
Protein bodies of cereal seeds consist of ordered, largely insoluble heteropolymers formed by prolamin storage proteins within the endoplasmic reticulum (ER) of developing endosperm cells. Often these structures are permanently unable to traffic along the secretory pathway, thus representing a unique example for the use of the ER as a protein storage compartment. In recent years, marked progress has been made in understanding what is needed to make a protein body and in formulating hypotheses on how protein body formation might have evolved as an efficient mechanism to store large amounts of protein during seed development, as opposed to the much more common system of seed storage protein accumulation in vacuoles. The major key evolutionary events that have generated prolamins appear to have been insertions or deletions that have disrupted the conformation of the eight-cysteine motif, a protein folding motif common to many proteins with different functions and locations along the secretory pathway, and, alternatively, the fusion between the eight-cysteine motif and domains containing additional cysteine residues.
Keywords: cereals, evolution of subcellular compartments, prolamins, protein bodies, protein sorting, seeds
Introduction
Almost 50% of the global food protein supply comes from cereal seeds (faostat.fao.org). Most of these proteins accumulate in protein bodies (PB), large polymers formed in the endoplasmic reticulum (ER). First discovered in the endosperm cells of maize developing seeds (Duvick, 1961), PBs are the most striking example for an alternative, perhaps surprising, use of the ER.
The ER is the port of entry of the secretory pathway, which leads to the cell surface or the inner hydrolytic compartments using the Golgi apparatus and various endosomes as intermediate sorting stations. The ER takes care of promoting efficient, correct structural maturation of proteins that enter the secretory pathway and selects for disposal those with incurable structural defects (Anelli and Sitia, 2008). In this respect, it is therefore mainly a compartment of transit. Its use to temporarily store proteins is very uncommon and almost exclusive to plants (Herman and Schmidt, 2004). More strikingly, the permanent ER residence of proteins that do not play a role in typical ER functions seems a feature unique to the major storage proteins of Poaceae seeds, the most studied being those of cereals (Shewry and Halford, 2002).
ER-Located Protein Bodies Evolved with Grasses
The Poaceae family (commonly termed grasses) originated less than 80 million years ago (Gaut, 2002), a long time after the most common classes of seed storage proteins, the 2S albumins and the 7S/11S globulins universally present in seeds and even in fern spores, had appeared (Rödin and Rask, 1990; Shutov et al., 1998). 2S albumins and 7S/11S globulins accumulate in protein storage vacuoles (PSVs, Vitale and Hinz, 2005), which are therefore the first subcellular compartment that evolved to store seed proteins. PSV proteins start their life in the ER and reach their destination mainly by Golgi- and endosome-mediated traffic, even if direct traffic from the ER to PSVs also occurs (Vitale and Hinz, 2005). PSVs have higher luminal pH and lower hydrolytic activity than vegetative, lytic vacuoles and they are ready to receive hydrolytic enzymes during germination, when they are transformed into lytic vacuoles for the rapid degradation of storage proteins.
Prolamins evolved with grasses, where they are in most cases the major, or almost exclusive, storage proteins (Shewry and Halford, 2002). Unlike the soluble 2S albumins and 7S/11S globulins, most prolamins rapidly form very large polymers that become insoluble to various extents because of inter-chain disulfide bonds or hydrophobic interactions. These electron dense, round-shaped structures with diameters between 0.5 and 2 μm were originally termed protein granules (Duvick, 1961; Khoo and Wolf, 1970) and then renamed PB (Graham et al., 1962). PB often detach from the ER cisternae, but retain ribosomes on their cytosolic face and do not fuse with other subcellular structures (Burr and Burr, 1976; Yamagata and Tanaka, 1986). Rice, maize, and sorghum prolamins exclusively form PB, whereas other prolamins, such as those of wheat, can either form PB or traffic through the Golgi complex to PSVs, or undergo autophagy (Rubin et al., 1992). The destiny depends on the individual prolamin, but also on the seed developmental stage: in wheat, the ability to traffic is more prominent at early stages and the formation of PB in the ER lumen favored at later stages (Tosi et al., 2009).
A Wide Superfamily of Small Cys-Rich Proteins
Common features of many, but not all, prolamins (Table 1) are a repeated domain rich in Pro or Gln residues (hence the name) and a domain derived from the eight-cysteine motif (8CM, José-Estanyol et al., 2004), characterized by eight Cys residues arranged in a specific order, forming four intra-chain disulfide bridges necessary to maintain a scaffold of alpha-helical segments connected by variable loops. The 8CM motif is common to a wide group of proteins termed the prolamin superfamily, with different functions and localizations along the secretory pathway. Besides many prolamins, the superfamily includes cereal α-globulins, trypsin inhibitors and α-amylase inhibitors, as well as hybrid proline-rich proteins (HyPRPs), non-specific lipid transfer proteins (nsLTPs) and 2S albumins, the latter three classes being widespread in plants. HyPRPs are cell wall proteins. nsLTPs are also secreted, often remaining anchored to the plasma membrane via a GPI anchor. 2S albumins, cereal α-globulins, trypsin inhibitors and α-amylase inhibitors are seed proteins accumulated in PSV. In prolamins and in the PSV-located members of the group three conserved regions, termed A, B, and C, can be identified within the 8CM motif, which in this case is therefore also termed ABC domain (Kreis et al., 1985).
Table 1.
Summary of the main characteristics of the rice, maize, and wheat prolamins of group III and II (Xu and Messing, 2009) analyzed in this review.
| Plant | Prolamin group | Prolamin | Repeated domain | Extra cysteine residues | 8CM | Requiring reducing agents to be solubilized | PB biogenesis in heterologous systems |
|---|---|---|---|---|---|---|---|
| Rice | II | 10 kD oryzeins | NO | YES | Altered by deletions | n.d. | n.d. |
| II | 16 kD oryzeins | NO | YES | Altered by deletions | n.d. | n.d. | |
| II | 13 kD oryzeins | NO | NO | Altered by deletions | YES | YES (13a polypeptide) | |
| Maize | II | β-zein | NO | NO | Altered by deletions | YES | YES |
| II | 27 kD γ-zein | Medium | YES | Intact | YES | YES | |
| Wheat | II | γ-gliadin | Medium | NO | Intact | NO | Dubious |
| III | HMW glutenins | Long | NO | Altered by insertion of the long repetitive domain and deletions | YES | YES | |
n.d.: left determined.
Based on the presence or not of the 8CM/ABC domain and additional Cys residues available for inter-chain disulfide bonds, prolamins have been divided into high molecular weight (HMW), cysteine rich (S-rich), cysteine poor (S-poor), and α-prolamins, the latter being the only group that does not seem related to 8CM proteins (Shewry and Halford, 2002). Based on amino acid sequence similarity and phylogenetic analysis, prolamins have also been divided into three groups named I, II, and III (Xu and Messing, 2009). The two criteria indeed largely overlap: α–prolamins are in group I, S-rich and S-poor in group II and HMW in group III. Group II members are present in all cereals. Group III is present in Pooideae (wheat, barley, Brachypodium) but not in Panicoideae (maize, sorghum, millet) and rice, whereas group I is only present in Panicoideae and perhaps Oryzeae.
Group III is considered the most ancient, followed by II and then I. Sequence similarity indicates that an ancestral group III prolamin may have originated by duplication of the α-globulin gene before the divergence of the different Poaceae subfamilies (Xu and Messing, 2009; Gu et al., 2010). Sequence alignments of orthologous regions of chromosomes suggest that the absence of group III prolamins in non-Pooideae families is due to gene loss after duplication (Xu and Messing, 2009).
The Evolutionary Shift From PSV to the ER
Phylogenetic studies thus indicate that early prolamins may have evolved by insertions/mutations in a gene encoding a PSV protein, causing a tendency to shift accumulation from PSV to the ER itself. Retention in the port of entry of the secretory pathway is clearly more parsimonious than traffic to vacuoles and can therefore increase cellular fitness in tissues that spend considerable energy to accumulate storage material, but it raises two new requirements: (i) minimizing deleterious effects on ER functions, (ii) finding a new strategy for the degradation of storage proteins during germination. The rapid formation of large polymers seems to have satisfied the first requirement. For the second, a key event has probably been the change in the tissue accumulation from cotyledons to the endosperm: the latter undergoes programmed cell death at the end of maturation. The loss of membrane integrity allows access of hydrolytic enzymes synthesized and secreted by the surrounding aleurone during germination. Roles in the efficient formation of PBs are played by protein machineries specific of the endosperm (Vitale and Boston, 2008; Holding, 2014) and by elements in the coding mRNAs that target the transcripts to specific ER regions (Tian and Okita, 2014). However, a number of prolamins can efficiently form PBs also when expressed individually in vegetative tissues of transgenic plants (Table 1; Geli et al., 1994; Shani et al., 1994; Bagga et al., 1995; Bellucci et al., 2000; Saito et al., 2009), indicating that PB formation exploits the general folding machinery of the ER present in all tissues and that the key features to form a PB reside in prolamins themselves, as we discuss in the next two paragraphs.
Rice and Wheat
Rice prolamins, encoded by about twenty genes, are small polypeptides (10, 13, or 16 kD) assigned to group II prolamins (Xu and Messing, 2009) and lacking any Pro- or Gln- rich repeated domain, indicating that the repetitions found in many other prolamins are not strictly necessary to form PBs (Onda and Kawagoe, 2011; Saito et al., 2012). A rice α-globulin-GFP fusion that is correctly sorted to PSV instead accumulates into ER-located PBs when the formation of the disulfide bond between Cys79 in the B region and Cys171 in the C region is inhibited by mutagenesis (Kawagoe et al., 2005). The hypervariable loop between regions B and C is much shorter in rice prolamins than in α-globulin, whereas in wheat HMW prolamins it is extensively elongated by the insertion of the very large repetitive domain rich in Pro and Gln (Kawagoe et al., 2005; Onda and Kawagoe, 2011). The authors suggested that these changes in length inhibit the formation of a critical intra-chain disulfide bond of the 8CM motif, making two Cys residues available for inter-chain disulfide bonds that allow the formation of PBs (Kawagoe et al., 2005). These insertions/deletions in α-globulin may thus have been the oldest events leading to group III formation in Pooideae and group II formation in Pooideae and rice (Table 1). It should also be underlined that only two 13 kD rice prolamins have the complete set of Cys of the 8CM motif and no other Cys residue, the other rice polypeptides having from 0 to 13 Cys residues in total (Onda and Kawagoe, 2011). Moreover, the two HMW prolamins of wheat (x-type and y-type HMW glutenin) have four to seven Cys residues, depending on the alleles. When expressed individually in transgenic tobacco these wheat prolamins form large polymers that are soluble only when reduced (Shani et al., 1994). Therefore, the loss of key Cys residues of the 8CM motif or the acquisition of new Cys residues can also be crucial for PB formation. Furthermore, at least once the initial rice PB core is formed, the rice prolamins without Cys residues can join it through interactions that are most probably of hydrophobic nature (Onda and Kawagoe, 2011).
Rice 13a prolamin has four Cys residues and is located in mid regions of the mature PB. When expressed in yeast as a GFP fusion, it forms insoluble PBs, whereas rice α-globulin similarly tagged with GFP is delivered to the yeast vacuole (Masumura et al., 2015). Deletions studies made on GFP fusions indicated the 13a portions corresponding to the B or C, but not A, regions form structures that resemble PBs by fluorescence microscopy, consistently with the view that the Cys residues in the B and C domains are important for PB formation. Unlike the full-length protein, these deletion mutants are soluble also in the absence of reducing agent, suggesting that there is not a direct causal relationship between insolubility and PB assembly. The polymerization state was not investigated, but the authors concluded that hydrophobic interactions may be important for the assembly of 13a prolamin into PBs (Masumura et al., 2015).
Maize
Maize prolamins (zeins) are grouped into four classes (Holding, 2014). α-zeins, polypeptides between 19 and 22 kD encoded by about twenty genes, are the most abundant. Three genes encode the γ-zeins (16kD, 27 kD, which is another very abundant zein, and 50 kD), whereas β-zein (15 kD) and δ-zein (10 kD, 18 kD) are the products of one and two genes, respectively. During seed development, γ- and β- zeins, which belong to group II prolamins, are synthesized first. The 27 kD γ-zein and β-zein form PBs when expressed individually in transgenic vegetative tissues (Table 1; Geli et al., 1994; Bagga et al., 1995).
Treatments of isolated maize PBs with reducing agents solubilize the 27 and 50 kD γ-zeins but not the other zeins (Vitale et al., 1982). The 27 kD γ-zein is perhaps the best studied prolamin and plays a fundamental role in the correct formation of maize PBs. A natural duplication of its locus generates Quality Protein Maize (Wu et al., 2010; Liu et al., 2016), whereas a deletion encompassing the 27 and 50 kD γ-zein genes reduces PB number to 12% and alters PB morphology (Yuan et al., 2014). The hypothesis that the more recent group I prolamins exploited the PB forming ability of the more ancient ones is also supported by the finding that co-expression of 27 kD γ-zein enhances the accumulation of a 19 kD α-zein polypeptide expressed in transgenic tobacco (Coleman et al., 1996). 27 kD γ-zein is composed of an N-terminal domain characterized by eight repeats of the amphipathic sequence ValHisLeuProProPro and seven Cys residues, followed by a C-terminal 8CM/ABC domain. The N-terminal domain promotes PB formation when fused to other proteins that are otherwise available for intracellular traffic (Mainieri et al., 2004; Llop-Tous et al., 2010), although this dominant effect is not universal (de Virgilio et al., 2008; Ceresoli et al., 2016). Deletion of the N-terminal domain from γ-zein causes secretion of the remaining ABC domain, and the reciprocal deletion causes ER retention of the N-terminal domain, although in this case the typical round-shaped PBs are not formed (Geli et al., 1994). Zeolin, a fusion of the N-terminal domain with entire sequence of the 7S storage protein phaseolin (which does not contain Cys residues), forms PBs that, like those formed by the wild type γ-zein, are insoluble unless reduced, and it traffics along the secretory pathway when cells are treated in vivo with reducing agents (Mainieri et al., 2004; Pompa and Vitale, 2006). The importance of inter-chain disulfide bonds was confirmed by mutagenesis of the full-length γ-zein: when the seven Cys residues of the N-terminal domain were mutated to Ser, γ-zein was efficiently secreted (Mainieri et al., 2014). Progressive mutagenesis of these Cys residues gives rise to intermediate phenotypes, both in the full-length protein and a fusion between the N-terminal-domain and GFP: PBs become progressively smaller and ER retention is negatively affected (Llop-Tous et al., 2010; Mainieri et al., 2014). Deletion of the amphipathic repeats indicates that these are also important for PB formation (Llop-Tous et al., 2010). Altogether, these data indicate that the addition of an amphipathic sequence rich in Cys residues to an 8CM sequence has been crucial for this prolamin.
If six out of seven Cys residues of the N-terminal domain are mutated, the 27 kD γ-zein is in large part secreted, but a detectable proportion is sorted to the vacuole via a pathway that is sensitive to brefeldin A and wortmannin, two inhibitors of Golgi-mediated, vacuolar sorting (Mainieri et al., 2014). This indicates that limited multimerization avoids secretion and favors PSV sorting, whereas extensive polymerization promotes PB biogenesis. Protein sorting to PSVs requires sorting motifs recognized by specific receptors, but key recognition events could occur in the ER lumen and transient polymerization could contribute to sorting efficiency (Robinson et al., 2005; Robinson and Pimpl, 2014). Remarkably, the natural vacuolar sorting signal of phaseolin can be in part replaced by the insertion of a novel Cys residue that leads to polymerization of trimers (Pompa et al., 2010), underlining the evolutionary relationships between traffic to the vacuole and PB formation.
A Working Model For the Origin of Protein Bodies
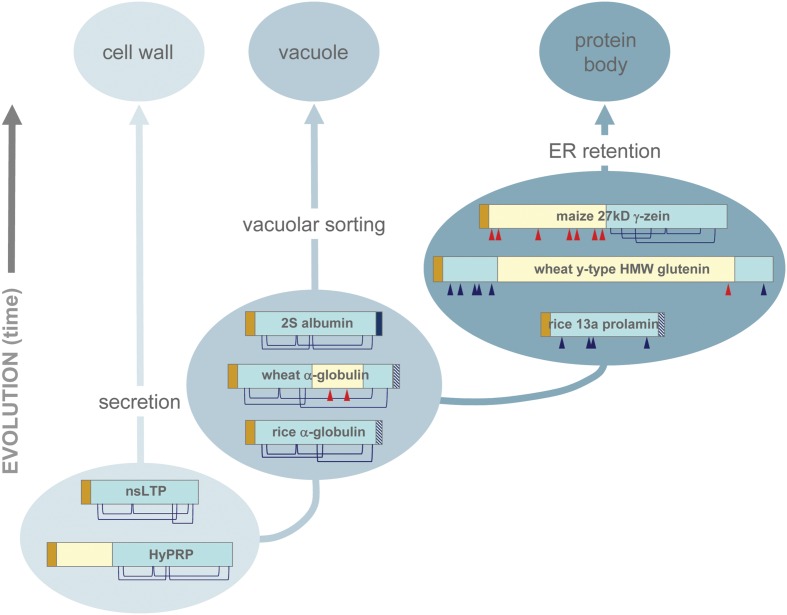
The use of the ER to store proteins has evolved only recently and constitutes an interesting example of a simpler solution for protein accumulation (accumulation in the ER itself) evolving from more complex ones (sorting to vacuoles or secretion). Figure 1 presents its possible evolutionary paths. All the evidence obtained studying prolamins that are self-sufficient to form PBs indicates that these structures have emerged by both exploiting and remodeling the 8CM scaffold. New availability of Cys residues involved in inter-chain disulfide bonds has been critical in this process and has resulted from either of two probably independent events: extensive insertions in the hypervariable region between the B and C domains characterize group III prolamins, whereas the addition of a new Cys-containing domain to an 8CM/ABC domain that can be virtually intact or mutated to variable extents characterizes many group II prolamins. The changes that have generated prolamins must thus have occurred more than once in evolution. Their success in being tolerated, without causing misfolding that would have led to degradation by ER quality control may be due to the rather simple folds of the small A, B, and C domains. The full explanation, however, must be more complex, since for example 7S globulins have a three-dimensional structure that does not tolerate great alterations (Hoffman et al., 1988; Pedrazzini et al., 1997; Lasserre et al., 2015), but zeolin is a very stable PB-forming protein fusion (Mainieri et al., 2014) and, conversely, a proportion of at least one rice 13 kD prolamin is detected in a ubiquitinated form in rice endosperm in which proteasome activity was inhibited, suggesting that normally this prolamin fails in part to fold properly and is degraded by ER quality control (Ohta and Taikawa, 2015).
FIGURE 1.
A model of the evolutionary events at the origin of protein bodies. The model describes the origin of prolamins that are self-sufficient for PB formation and probably have a dominant effect on those that evolved later. Proteins with 8CM motifs are secreted or sorted to the vacuole, depending on the presence or not of vacuolar sorting signals. Insertions that do not alter the disulphide bonds of the 8CM motif do not affect protein destiny. Extensive polymerization that leads to PB formation can result from insertions that inhibit the correct formation of the four intra-chain bonds, deletions that involve the loss of critical Cys residues, or additions of domains containing new Cys residues. The repetitive domains may also mediate interactions with the ER membrane. Vacuolar sorting signals may be present or absent in PB proteins, but PB formation has anyway a dominant effect on intracellular traffic. Phylogenetic analysis and the results of protein engineering and expression of individual genes indicate that the PB forming prolamins evolved from superfamily members that are sorted to the vacuole, not from secreted members. The schematic protein structures are based on the following GenBank accessions: EU968356.1 (maize nsLTP), EU964401.1 (maize HyPRP), CAA40015.1 (2S albumin), ABG68034.1 (wheat α-globulin), BAA09308.1 (rice α-globulin), 27 kD zein, ABG68035.1 (wheat y-type HMW glutenin), and BAA36697.1 (rice 13a prolamin). Brown: signal peptide; light blue: conserved 8CM motif, or portions of it; yellow: Pro-rich or Glu-rich domain; blue: vacuolar sorting sequence; striped blue: putative vacuolar sorting sequence; blue lines: intra-chain disulphide bonds of the 8CM motif; blue triangles: Cys residues originating from the 8CM motif; red triangle: Cys residues that do not originate from the 8CM motif.
It should be underlined that the formation of very large polymers is not per se sufficient to avoid traffic from the ER, at least in animal cells, a typical example being the secretion of procollagen fibrils (Bonfanti et al., 1998; Saito and Katada, 2015). Assembled PBs are heteropolymeric ordered structures in which certain polypeptides, such as γ- and β-zeins and rice 13–16 kD prolamins, are preferentially located at the PB surface (Lending and Larkins, 1989; Saito et al., 2012). Persistent interactions with chaperones and direct interactions with the luminal face of the ER membrane could thus play a major role in ER retention. Indeed, purified gliadins or a synthetic version of the Pro-rich repeat of 27 kD γ-zein directly interact in vitro with systems that mimic the inner face of the ER membrane (Kogan et al., 2004; Banc et al., 2009). It was suggested that also in gliadins the interactions could be mediated by the repeated region (Banc et al., 2009), even if the gliadin repeats are hydrophilic, unlike the amphipathic repeat of γ-zein, and the ability of γ-gliadin to form PB in the absence of other wheat prolamins is dubious (Napier et al., 1997; Tosi et al., 2009). Cell wall HyPRP proteins also have a hydrophilic, proline-rich repetitive N-terminal domain and an 8CM C-terminal domain (Dvorakova et al., 2007), but they are clearly unable to form PB. More detailed analysis and in vivo testing of the prolamin repetitive domains is therefore needed to determine their role in PB formation.
Author Contributions
EP and AV wrote the manuscript. DM and CM contributed to the critical selection of the works discussed in the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Aldo Ceriotti, Massimilano Lauria, Angelo Viotti for the useful suggestions.
Footnotes
Funding. Supported by “Filagro” project of CNR-Regione Lombardia.
References
- Anelli T., Sitia R. (2008). Protein quality control in the early secretory pathway. EMBO J. 27 315–327. 10.1038/sj.emboj.7601974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagga S., Hank Adams H., John D., Kemp J. D., Sengupta-Gopalan C. (1995). Accumulation of 15-kilodalton zein in nove1 protein bodies in transgenic tobacco. Plant Physiol. 107 13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banc A., Desbat B., Renard D., Popineau Y., Mangavel C., Navailles L. (2009). Exploring the interactions of gliadins with model membranes: effect of confined geometry and interfaces. Biopolymers 91 610–622. 10.1002/bip.21188 [DOI] [PubMed] [Google Scholar]
- Bellucci M., Alpini A., Paolocci F., Cong L., Arcioni S. (2000). Accumulation of maize γ-zein and γ-zein:KDEL to high levels in tobacco leaves and differential increase of BiP synthesis in transformants. Theor. Appl. Genet. 101 796–804. 10.1007/s001220051546 [DOI] [Google Scholar]
- Bonfanti L., Mironov A. A., Jr., Martinez-Menarguez J. A., Martella O., Fusella A., Baldassarre M., et al. (1998). Procollagen traverses the Golgi stack without leaving the lumen of cisternae: evidence for cisternal maturation. Cell 95 993–1003. 10.1016/S0092-8674(00)81723-7 [DOI] [PubMed] [Google Scholar]
- Burr B., Burr F. A. (1976). Zein synthesis in maize endosperm by polyribosomes attached to protein bodies. Proc. Natl. Acad. Sci. U.S.A. 73 515–519. 10.1073/pnas.73.2.515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceresoli V., Mainieri D., Del Fabbro M., Weinstein R., Pedrazzini E. (2016). A fusion between domains of the human bone morphogenetic protein-2 and maize 27 kD γ-Zein accumulates to high levels in the endoplasmic reticulum without forming protein bodies in transgenic tobacco. Front. Plant Sci. 7:358 10.3389/fpls.2016.00358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman C. E., Herman E. M., Takasaki K., Larkins B. A. (1996). The maize γ-zein sequesters α-zein and stabilizes its accumulation in protein bodies of transgenic tobacco endosperm. Plant Cell 8 2335–2345. 10.1105/tpc.8.12.2335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Virgilio M., De Marchis F., Bellucci M., Mainieri D., Rossi M., Benvenuto E., et al. (2008). The human immunodeficiency virus antigen Nef forms protein bodies in leaves of transgenic tobacco when fused to zeolin. J. Exp. Bot. 59 2815–2829. 10.1093/jxb/ern143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvick D. N. (1961). Protein granules of maize endosperm cells. Cereal Chem. 38 374–385. [Google Scholar]
- Dvorakova L., Cvrckova F., Fischer L. (2007). Analysis of the hybrid proline-rich protein families from seven plant species suggests rapid diversification of their sequences and expression patterns. BMC Genomics 8:412 10.1186/1471-2164-8-412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaut B. S. (2002). Evolutionary dynamics of grass genomes. New Phytol. 154 15–28. 10.1046/j.1469-8137.2002.00352.x [DOI] [Google Scholar]
- Geli M. I., Torrent M., Ludevid D. (1994). Two structural domains mediate two sequential events in γ-zein targeting: protein endoplasmic reticulum retention and protein body formation. Plant Cell 6 1911–1922. 10.1105/tpc.6.12.1911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham J. S. D., Jennings A. C., Morton R. K., Palk B. A., Raison J. K. (1962). Protein bodies and protein synthesis in developing wheat endosperm. Nature 196 967–969. 10.1038/196967a0 [DOI] [Google Scholar]
- Gu Y. Q., Wanjugi H., Coleman-Derr D., Kong X., Anderson O. A. (2010). Conserved globulin gene across eight grass genomes identify fundamental units of the loci encoding seed storage proteins. Funct. Integr. Genomics 10 111–122. 10.1007/s10142-009-0135-x [DOI] [PubMed] [Google Scholar]
- Herman E. M., Schmidt M. A. (2004). Endoplasmic reticulum to vacuole trafficking of endoplasmic reticulum bodies provides an alternate pathway for protein transfer to the vacuole. Plant Physiol. 136 3440–3446. 10.1104/pp.104.051722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman L. M., Donaldson D. D., Herman E. M. (1988). A modified storage protein is synthesized, processed, and degraded in the seeds of transgenic plants. Plant Mol. Biol. 11 717–729. 10.1007/BF00019513 [DOI] [PubMed] [Google Scholar]
- Holding D. R. (2014). Recent advances in the study of prolamin storage protein organization and function. Front. Plant Sci. 5:276 10.3389/fpls.2014.00276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- José-Estanyol M., Gomis-Rüth F., Puigdomènech P. (2004). The eight-cysteine motif, a versatile structure in plant proteins. Plant Physiol. Biochem. 42 355–365. 10.1016/j.plaphy.2004.03.009 [DOI] [PubMed] [Google Scholar]
- Kawagoe Y., Suzuki K., Tasaki M., Yasuda H., Akagi K., Katoh E., et al. (2005). The critical role of disulfide bond formation in protein sorting in the endosperm of rice. Plant Cell 17 1141–1153. 10.1105/tpc.105.030668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoo U., Wolf M. J. (1970). Origin and development of protein granules in maize endosperm. Am. J. Bot. 57 1042–1050. 10.2307/2441269 [DOI] [Google Scholar]
- Kogan M. J., Lopez O., Cocera M., Lopez-Iglesias C., De La Maza A., Giralt E. (2004). Exploring the interaction of the surfactant N-terminal domain of gamma-Zein with soybean phosphatidylcholine liposomes. Biopolymers 73 258–268. 10.1002/bip.10578 [DOI] [PubMed] [Google Scholar]
- Kreis M., Forde B. G., Rahman S., Miflin B. J., Shewry P. R. (1985). Molecular evolution of the seed storage proteins of barley, rye and wheat. J. Mol. Biol. 183 499–502. 10.1016/0022-2836(85)90017-8 [DOI] [PubMed] [Google Scholar]
- Lasserre E., Ko T., Dyer J., Murai N. (2015). Modified bean seed protein phaseolin did not accumulate stably in transgenic tobacco seeds after methionine enhancement mutations. Am. J. Plant Sci. 6 640–650. 10.4236/ajps.2015.65069 [DOI] [Google Scholar]
- Lending C. R., Larkins B. A. (1989). Changes in the zein composition of protein bodies during maize endosperm development. Plant Cell 1 1011–1023. 10.2307/3869002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Shi J., Sun C., Gong H., Fan X., Qiu F., et al. (2016). Gene duplication confers enhanced expression of 27-kDa γ-zein for endosperm modification in quality protein maize. Proc. Natl. Acad. Sci. U.S.A. 113 4964–4969. 10.1073/pnas.1601352113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llop-Tous I., Madurga S., Giralt E., Marzabal P., Torrent M., Ludevid M. D. (2010). Relevant elements of a maize γ-zein domain involved in protein body biogenesis. J. Biol. Chem. 285 35633–35644. 10.1074/jbc.M110.116285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainieri D., Morandini F., Maîtrejean M., Saccani A., Pedrazzini E., Vitale A. (2014). Protein body formation in the endoplasmic reticulum as an evolution of storage protein sorting to vacuoles: insights from maize γ-zein. Front. Plant Sci. 5:331 10.3389/fpls.2014.00331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainieri D., Rossi M., Archinti M., Bellucci M., De Marchis F., Vavassori S., et al. (2004). Zeolin: a new recombinant storage protein constructed using maize γ-zein and bean phaseolin. Plant Physiol. 136 3447–3456. 10.1104/pp.104.046409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masumura T., Shigemitsu T., Morita S., Satoh S. (2015). Identification of the region of rice 13 kDa prolamin essential for the formation of ER-derived protein bodies using a heterologous expression system. Biosci. Biotechnol. Biochem. 79 566–573. 10.1080/09168451.2014.991684 [DOI] [PubMed] [Google Scholar]
- Napier J. A., Richard G., Turner M. F. P., Shewry P. R. (1997). Trafficking of wheat gluten proteins in transgenic tobacco plants: γ-gliadin does not contain an endoplasmic reticulum retention signal. Planta 203 488–494. 10.1007/s004250050218 [DOI] [PubMed] [Google Scholar]
- Ohta M., Taikawa F. (2015). OsHrd3 is necessary for maintaining the quality of endoplasmic reticulum-derived protein bodies in rice endosperm. J. Exp. Bot. 66 4585–4593. 10.1093/jxb/erv229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onda Y., Kawagoe Y. (2011). Oxidative protein folding: selective pressure for prolamin evolution in rice. Plant Signal. Behav. 6 1966–1972. 10.4161/psb.6.12.17967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedrazzini E., Giovinazzo G., Bielli A., de Virgilio M., Frigerio L., Pesca M., et al. (1997). Protein quality control along the route to the plant vacuole. Plant Cell 9 1869–1880. 10.2307/3870531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pompa A., De Marchis F., Vitale A., Arcioni S., Bellucci M. (2010). An engineered C-terminal disulfide bond can partially replace the phaseolin vacuolar sorting signal. Plant J. 61 782–791. 10.1111/j.1365-313X.2009.04113.x [DOI] [PubMed] [Google Scholar]
- Pompa A., Vitale A. (2006). Retention of a bean phaseolin/maize γ-zein fusion in the endoplasmic reticulum depends on disulfide bond formation. Plant Cell 18 2608–2621. 10.1105/tpc.106.042226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson D. G., Oliviusson P., Hinz G. (2005). Protein sorting to the storage vacuoles of plants: a critical appraisal. Traffic 6 615–625. 10.1111/j.1600-0854.2005.00303.x [DOI] [PubMed] [Google Scholar]
- Robinson D. G., Pimpl P. (2014). Receptor-mediated transport of vacuolar proteins: a critical analysis and a new model. Protoplasma 251 247–264. 10.1007/s00709-013-0542-7 [DOI] [PubMed] [Google Scholar]
- Rödin J., Rask L. (1990). Characterization of matteuccin, the 2.2S storage protein of the ostrich fern. Evolutionary relationship to angiosperm seed storage proteins. FEBS J. 192 101–107. 10.1111/j.1432-1033.1990.tb19201.x [DOI] [PubMed] [Google Scholar]
- Rubin R., Levanony H., Galili G. (1992). Evidence for the presence of 2 different types of protein bodies in wheat endosperm. Plant Physiol. 99 718–724. 10.1104/pp.99.2.718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito K., Katada T. (2015). Mechanisms for exporting large-sized cargoes from the endoplasmic reticulum. Cell. Mol. Life Sci. 72 3709–3720. 10.1007/s00018-015-1952-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito Y., Kishida K., Takata K., Takahashi H., Shimada T., Tanaka K., et al. (2009). A green fluorescent protein fused to rice prolamin forms protein body-like structures in transgenic rice. J. Exp. Bot. 60 615–627. 10.1093/jxb/ern311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito Y., Shigemitsu T., Yamasaki R., Sasou A., Goto F., Kishida K., et al. (2012). Formation mechanism of the internal structure of type I protein bodies in rice endosperm: relationship between the localization of prolamin species and the expression of individual genes. Plant J. 70 1043–1055. 10.1111/j.1365-313X.2012.04947.x [DOI] [PubMed] [Google Scholar]
- Shani N., Rosenberg N., Kasarda D. D., Galili G. (1994). Mechanisms of assembly of wheat high molecular weight glutenins inferred from expression of wild-type and mutant subunits in transgenic tobacco. J. Biol. Chem. 269 8924–8930. [PubMed] [Google Scholar]
- Shewry P. R., Halford N. G. (2002). Cereal seed storage proteins: structures, properties and role in grain utilization. J. Exp. Bot. 53 947–958. 10.1093/jexbot/53.370.947 [DOI] [PubMed] [Google Scholar]
- Shutov A. D., Braun H., Chesnokov Y. V., Bäumlein H. (1998). A gene encoding a vicilin-like protein is specifically expressed in fern spores. Eur. J. Biochem. 252 79–89. 10.1046/j.1432-1327.1998.2520079.x [DOI] [PubMed] [Google Scholar]
- Tian L., Okita T. W. (2014). mRNA-based protein targeting to the endoplasmic reticulum and chloroplasts in plant cells. Curr. Opin. Plant Biol. 22 77–85. 10.1016/j.pbi.2014.09.007 [DOI] [PubMed] [Google Scholar]
- Tosi P., Parker M., Gritsch C. S., Carzaniga R., Martin B., Shewry P. R. (2009). Trafficking of storage proteins in developing grain of wheat. J. Exp. Bot. 60 979–991. 10.1093/jxb/ern346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale A., Boston R. S. (2008). Endoplasmic reticulum quality control and the unfolded protein response: insights from plants. Traffic 9 1581–1588. 10.1111/j.1600-0854.2008.00780.x [DOI] [PubMed] [Google Scholar]
- Vitale A., Hinz G. (2005). Sorting of proteins to storage vacuoles: how many mechanisms? Trends Plant Sci. 10 316–323. 10.1016/j.tplants.2005.05.001 [DOI] [PubMed] [Google Scholar]
- Vitale A., Smaniotto E., Longhi R., Galante E. (1982). Reduced soluble proteins associated with maize endosperm protein bodies. J. Exp. Bot. 33 439–448. 10.1093/jxb/33.3.439 [DOI] [Google Scholar]
- Wu Y., Holding D. R., Messing J. (2010). Gamma-zein is essential for endosperm modification in quality protein maize. Proc. Natl. Acad. Sci. U.S.A. 107 12810–12815. 10.1073/pnas.1004721107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J.-H., Messing J. (2009). Amplification of prolamin storage protein genes in different subfamilies of the Poaceae. Theor. Appl. Genet. 119 1397–1412. 10.1007/s00122-009-1143-x [DOI] [PubMed] [Google Scholar]
- Yamagata H., Tanaka K. (1986). The site of synthesis and accumulation of rice storage proteins. Plant Cell Physiol. 27 135–145. [Google Scholar]
- Yuan L., Dou Y., Kianian S. F., Zhang C., Holding D. R. (2014). Deletion mutagenesis identifies a haploinsufficient role for γ-zein in opaque2 endosperm modification. Plant Physiol. 164 119–130. 10.1104/pp.113.230961 [DOI] [PMC free article] [PubMed] [Google Scholar]