Abstract
Background
Four practice guidelines incorporate the use of gene expression profiling (GEP) tests for early-stage, hormone-receptor positive, HER2 negative breast tumors. Few studies describe factors associated with GEP testing in US oncology practice. We assessed the relationship between clinical, demographic, and group-level socioeconomic variables and test use in women under age 65.
Patients and Methods
Data from five state cancer registries were linked with insurance claims data and GEP test results. We assessed rates of testing and variables associated with test use in an incident cohort of 9444 commercially-insured women under age 65, newly-diagnosed with Stage I or II hormone-receptor positive breast cancer from 2006–2012.
Results
Rates of testing for women with N0 disease increased from 20.4% in 2006 to 35.2% in 2011. Variables associated with higher rates of testing, beyond clinical factors such as nodal status (P < .001), included being diagnosed from 2008–2012 vs. 2006–2007 (adjusted odds ratio, 1.67; 95% CI, 1.47 to 1.90), having preexisting comorbidities (adjusted odds ratio, 1.35; 95% CI, 1.14 to 1.59), and higher out-of-pocket pharmacy costs (adjusted odds ratio, 1.66; 95% CI, 1.40 to 1.97). Women under age 50 were more likely to be tested if they had Stage I vs. Stage II disease (P < .0001).
Conclusions
In an insured population of women under age 65, GEP testing increased following its inclusion in guidelines and mounting evidence. Additional research is needed to better understand oncologists’ decision not to order GEP testing for their patients who are otherwise eligible.
Keywords: Breast cancer, gene expression profiling, genomic testing
Introduction
Four practice guidelines incorporate consideration of gene expression profiling (GEP) of early-stage, hormone-receptor positive, human epidermal growth factor receptor 2 (HER2) negative breast tumors with existing pathologic features to refine recurrence estimates and guide treatment recommendations.1–4 More limited evidence supports its use in patients with one to three positive nodes5 and testing is not included in clinical guidelines for these patients. The Oncotype DX® Breast Cancer Assay (Genomic Health, Redwood City, CA), the most commonly used GEP test in the US and the only GEP in NCCN guidelines,6–8 is a 21-gene assay that provides women and clinicians with a Recurrence Score® (RS) result that, combined with other prognostic variables, can be used to identify women at either low, intermediate, or high risk of recurrence and estimate the benefit of adjuvant chemotherapy.9,10–16
Studies of GEP test adoption, and use of Oncotype DX specifically, suggest that between 20–50% of eligible women were tested.17–20 This rate of testing is likely aided by the incorporation of Oncotype DX testing into guidelines,1, 2 and the eventual attainment of nearly universal reimbursement by health plans for guideline-informed testing.21, 22 These studies have found that while rates of testing have increased since 2007, the year testing was first incorporated into a clinical practice guideline, the likelihood of testing varies across practice settings and is related to several clinical and demographic variables. Overall, rates of testing were highest among women with less aggressive disease features and with estrogen- and/or progesterone-receptor (ER/PR) positive, HER2 negative disease that reflects clinical guidelines. Likewise, women with no or limited comorbidities were more likely to be tested, as were White vs. non-White women and those in their 50s (vs. younger or older patients).19, 20, 23
Previous studies of GEP test use are limited by small sample sizes,20, 23 lack of representativeness of community practice,19 or an inability to measure additional explanatory variables such as group-level economic variables or regional variation. Further, previous studies have focused on women of all ages. Given rates of testing are highest in women under age 65,19, 20, 23, 24 further attention is warranted to assess variables associated with testing in this population. The objectives of this study are to examine rates of GEP testing and to determine patient-level clinical, sociodemographic, and group-level socioeconomic variables associated with testing in an incident cohort of newly diagnosed women under age 65 with commercial health insurance. This study allows us to assess test use in a large sample of newly diagnosed women receiving care across five states.
Methods
Patient Selection and Study Cohort
Details of data linkages are presented in Appendix I. Briefly, our linked database consists of five state cancer registries containing clinical and pathological variables linked with claims data maintained by HealthCore Inc. (Wilmington, DE), an independent subsidiary to Anthem, Inc., an independent licensee of the Blue Cross and Blue Shield Association. We linked RS results through collaboration with Genomic Health, the patent holder of the Oncotype DX test. A total of 16064 women between ages 24–64 diagnosed with breast cancer (according to tumor registry data) and successfully linked with HealthCore claims were assessed for eligibility based on Anthem Inc. coverage policies for Oncotype DX testing. These coverage policies were consistent with NCCN guidelines during the study period. We selected only those women diagnosed with a first invasive breast cancer (n = 14710). Based on guidelines for GEP testing, we excluded women with in situ disease, or stage III or IV or with missing stage (n = 2728). We also excluded women with both ER and PR missing, both ER and PR negative or at least one negative/borderline and the other missing (n = 2538). These exclusions resulted in a final cohort of 9444 women who were diagnosed through April 30, 2012 who may have been considered for GEP testing. The participating registries, HealthCore, and Georgetown obtained all necessary IRB and HIPAA approvals for this linkage.
Study Measures
Receipt of GEP testing was identified by a linkage between HealthCore and Genomic Health test data for Anthem covered members with breast cancer whose GEP testing is performed by Genomic Health. From registry data we obtained age at diagnosis, race-ethnicity, marital status, year of diagnosis, and diagnosis of prior primary cancers other than breast cancer, including non-melanoma skin cancers. Staging was created using the American Joint Committee on Cancer Breast Cancer Staging (version 7) which is based on the Tumor, Node, Metastasis (TNM) system.25 We obtained ER, PR, HER2, nodal status and histological grade from registry data. We grouped ER and PR positive cases vs. women having either PR or ER positive tumors (but not both). We compared borderline HER2 status and those with “unknown” status to those with HER2 negative cases. HER2 status was derived using the SEER Collaborative Stage Site-Specific Factor 15 (positive/negative/borderline/unknown). We also compared those with N0 disease to those with N1mic and N1 disease. Well and moderately differentiated tumors were compared to poorly or undifferentiated tumors. From HealthCore’s Integrated Research Database (HIRDSM),26 we ascertained 31 individual health comorbid conditions diagnosed between 1 year prior to their breast cancer diagnosis up to and including the month of diagnosis based on the Elixhauser comorbidity index.27 For each condition, we used a commonly applied algorithm that required an inpatient diagnosis and/or at least 2 outpatient diagnosis codes at least 30 days apart to minimize false-positives. HIRD also contains information on copays, deductibles, and coinsurance for services provided that were used to create an out-of-pocket pharmacy payment burden variable (in quintiles) over the prior six months before the breast cancer diagnosis. Finally, members’ residential 5-digit zip codes were linked to derive sociodemographic data based on 2007–2011 American Community Survey of US Census, including median household income (in quintiles) and urban vs. rural location.
Statistical Analysis
We examined the bivariate relationship between the receipt of testing and each variable. We included all variables in a multivariable logistic regression model with GEP test receipt as a binary dependent variable and all other variables as main effects. Prior to running our final multivariable model, we tested several hypothesized interactions individually when added to the main effects model (age x year tested, stage, comorbidity; out of pocket pharmacy costs x year tested, stage, comorbidity; year tested x stage). We only report those interaction terms that met our criteria for statistical significance (Type I error of 0.05) in the final multivariable model. All tests were two-sided and we used a Type I error of 0.05. Due to the high number of cases with unknown HER2 status, we performed sensitivity analyses, including only those with known HER2 status (n = 4980) as well as among those with node negative (N0; n = 7054) vs. node positive (N1mic/N1; n = 2390) disease and among eligible patients for GEP testing according to practice guidelines (n = 6546). We report adjusted odds ratios and 95% confidence intervals (CI) produced by the logistic regressions. All calculations were done using SAS 9.3 (Cary, NC).
Results
In this cohort, 2371 women (25.1%) received GEP testing (Table 1). The 9444 women with early stage, hormone-receptor positive disease were evenly divided across years of diagnosis, were primarily White, previously unaffected with cancer, had no comorbidity, and resided in urban areas. While the majority of tested patients had breast cancers whose clinical features aligned with practice guidelines, with an overall 31.4% rate of testing across all years, 9.0% of tested women (n = 213) did not meet these guidelines.
Table 1.
Characteristics of Selected Cohort, Tested Cases and Relationship with Test Usea
| Selected Cohort |
Tested | ||
|---|---|---|---|
| No. | Row % | P | |
| Total | 9444 | 25.1 | |
| Year Diagnosed | <.0001 | ||
| 2006–2007 | 3199 | 18.7 | |
| 2008–2009 | 3067 | 28.1 | |
| 2010–2012 | 3178 | 28.7 | |
| Age at diagnosis | <.0001 | ||
| 24–39 | 660 | 17.4 | |
| 40–49 | 2907 | 26.3 | |
| 50–59 | 3841 | 25.9 | |
| 60–64 | 2036 | 24.4 | |
| Race - Ethnicity | <.0001 | ||
| NH White | 7942 | 26.2 | |
| NH Black | 400 | 17.0 | |
| Asian / Pacific Islander | 616 | 20.0 | |
| Hispanic | 386 | 20.2 | |
| Marital Status | .52 | ||
| Not Married | 2660 | 25.6 | |
| Married | 6614 | 24.9 | |
| Prior Cancer | .49 | ||
| No | 8948 | 25.0 | |
| Yes | 496 | 26.4 | |
| State | .28 | ||
| CA | 3937 | 25.1 | |
| GA | 1442 | 27.3 | |
| KY | 745 | 25.0 | |
| NY | 1579 | 24.1 | |
| OH | 1741 | 24.3 | |
| Area | .009 | ||
| Rural | 479 | 20.0 | |
| Urban | 8851 | 25.4 | |
| Median Household Income | .09 | ||
| 1 lowest | 1733 | 22.7 | |
| 2 | 1787 | 24.5 | |
| 3 | 1937 | 25.8 | |
| 4 | 1905 | 25.8 | |
| 5 highest | 1968 | 26.4 | |
| Out-of-Pocket Pharmacy Costs | <.0001 | ||
| 1 lowest | 1807 | 19.0 | |
| 2 | 1808 | 20.9 | |
| 3 | 1861 | 23.8 | |
| 4 | 1964 | 28.7 | |
| 5 highest | 2004 | 32.2 | |
| Stage | <.0001 | ||
| I | 5582 | 31.6 | |
| II | 3862 | 15.7 | |
| Nodal Involvement | <.0001 | ||
| N0 | 7054 | 30.2 | |
| N1mi | 609 | 19.0 | |
| N1 | 1781 | 7.1 | |
| HER2 Status | <.0001 | ||
| Positive | 688 | 11.5 | |
| Negative | 4175 | 31.7 | |
| Borderline/ Unknown | 4581 | 21.2 | |
| Hormone Receptor Status | <.0001 | ||
| Both ER and PR positive | 7990 | 26.4 | |
| At least one positive | 1454 | 18.0 | |
| Histological Grade | <.0001 | ||
| 1–2 | 6869 | 27.7 | |
| 3 | 2177 | 18.3 | |
| One Year Comorbidities | <.0001 | ||
| 0 (ref) | 8531 | 24.1 | |
| 1 or more | 913 | 34.3 | |
Note: missing values not included in table.
Selected cohort: missing or unknown grade (398), missing median household income (114), missing area (114), missing marital status (170), missing race (100)
Tested cohort: missing or unknown grade (70), missing median household income (29), missing area (29), missing marital status (43), missing race (23)
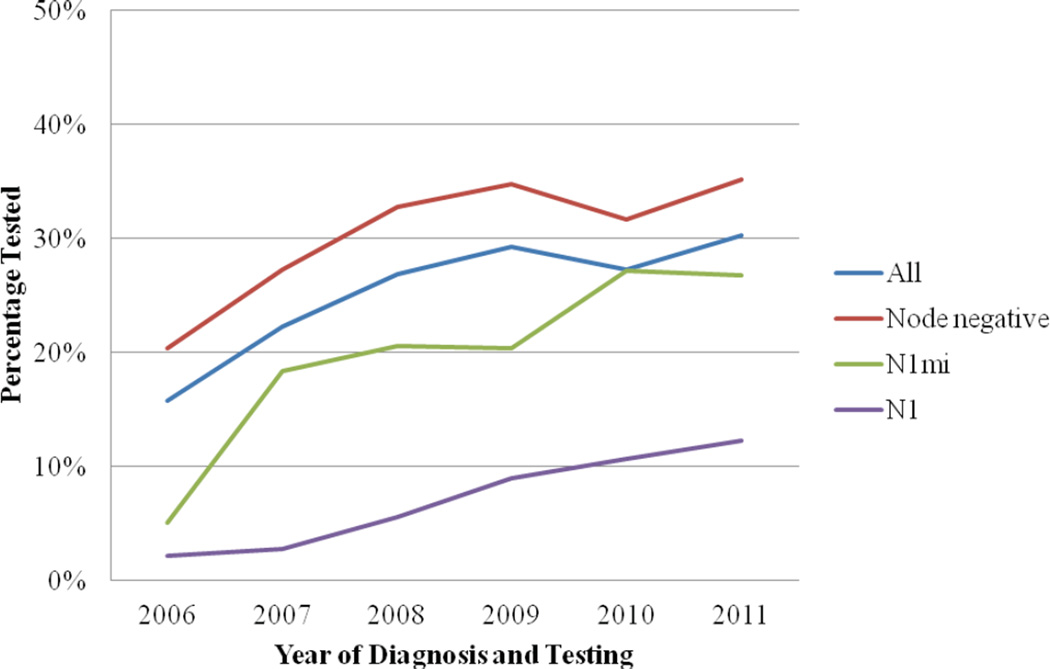
Testing increased significantly over time (P < .0001; Figure 1). Rates of testing among patients with N0 disease increased from 2006 (20.4%) to 2011 (35.2%). Rates of testing among patients with N1mic disease increased substantially from 2006 (5.1%) to 2007 (18.4%) and then again in 2010 (27.2%). Rates of testing in patients with N1 disease have steadily increased over time, though remain lower (12.3%) over all years than patients with N0 or N1mic disease.
Figure 1. Rates of testing by year (2006–2011).
A test of each linear trend was significant for the cohort and each subgroup (P<.0001). Patients diagnosed in 2012 were not included due to incomplete data for this year.
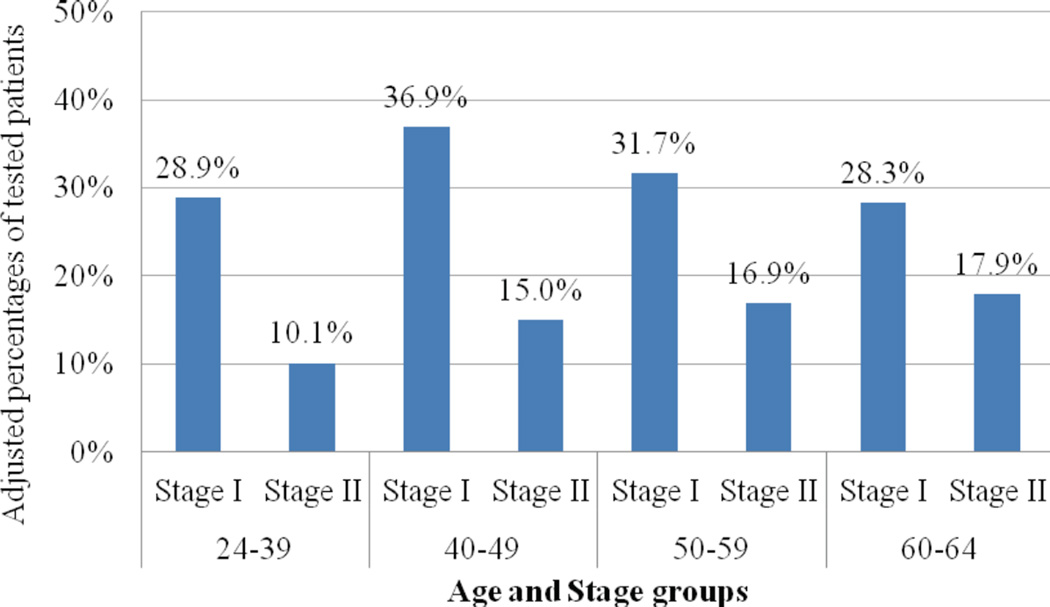
Several clinical variables were independently associated with testing after adjustment for all other variables (Table 2). Characteristics associated with lower likelihood of testing included either ER or PR positivity vs. ER and PR positivity (OR, 0.69 [95% CI, 0.59 to 0.81]; P < .0001), borderline/unknown vs. negative HER2 status (OR, 0.51 [95% CI, 0.44 to 0.59]; P < .0001), N1mic (OR, 0.67 [95% CI, 0.52 to 0.86]) or N1 (OR, 0.22 [95% CI, 0.17 to 0.27]) as compared to N0 disease (P < .0001) and poorly or undifferentiated tumor grade vs. well or moderately differentiated grade (OR, 0.81 [95% CI, 0.71 to 0.93]; P = .002). There was a significant interaction of age and stage (P < .0001). The adjusted odds of being tested was significantly higher for stage I vs. stage II disease among women diagnosed at ages 24 to 39 (OR, 1.94 [95% CI, 1.21 to 3.09]) and ages 40 to 49 (OR, 1.80 [95% CI, 1.41 to 2.18]), while there were no significant differences in test use by stage in the older age groups (Figure 2).
Table 2.
Multivariable Model of Variables Associated with GEP testing
| OR | (95% CI) | P | ||
|---|---|---|---|---|
| Year Diagnosed | <.0001 | |||
| 2006–2007 (ref) | ||||
| 2008–2009 | 1.67 | (1.47–1.90) | ||
| 2010–2012 | 1.54 | (1.34–1.76) | ||
| Race - Ethnicity | .001 | |||
| NH White (ref) | ||||
| NH Black | 0.61 | (0.45–0.82) | ||
| Asian and Pac. Islanders | 0.83 | (0.66–1.04) | ||
| Hispanic | 0.76 | (0.58–1.01) | ||
| Marital Status | .54 | |||
| Not Married | 1.04 | (0.92–1.16) | ||
| Married (ref) | ||||
| Prior Cancer | .42 | |||
| No (ref) | ||||
| Yes | 0.91 | (0.72–1.14) | ||
| State | <.0001 | |||
| CA | 0.63 | (0.53–0.76) | ||
| GA (ref) | ||||
| KY | 0.77 | (0.62–0.97) | ||
| NY | 0.83 | (0.69–1.01 | ||
| OH | 0.91 | (0.76–1.09) | ||
| Area | .02 | |||
| Rural | 0.74 | (0.57–0.95) | ||
| Urban (ref) | ||||
|
Median Household Income (area, Quintiles, Low to High) |
.60 | |||
| 1 lowest (ref) | ||||
| 2 | 1.08 | (0.91–1.28) | ||
| 3 | 1.13 | (0.95–1.34) | ||
| 4 | 1.14 | (0.95–1.37) | ||
| 5 highest | 1.14 | (0.95–1.37) | ||
|
Out-of-Pocket Pharmacy Costs (Quintiles, Low to High) |
<.0001 | |||
| 1 lowest (ref) | ||||
| 2 | 1.06 | (0.89–1.27) | ||
| 3 | 1.22 | (1.02–1.45) | ||
| 4 | 1.52 | (1.29–1.80) | ||
| 5 highest | 1.66 | (1.40–1.97) | ||
| Nodal Involvement | <.0001 | |||
| N0 (ref) | ||||
| N1mi | 0.67 | (0.52–0.86) | ||
| N1 | 0.22 | (0.17–0.27) | ||
| HER2 Status | <.0001 | |||
| Positive | 0.30 | (0.23–0.39) | ||
| Negative (ref) | ||||
| Borderline/ Unknown | 0.51 | (0.44–0.59) | ||
| Hormone Receptor Status | <.0001 | |||
| ER and PR both positive (ref) | ||||
| At least one positive | 0.69 | (0.59–0.81) | ||
| Histological Grade | .002 | |||
| 1–2: Well/moderately differentiated (ref) |
||||
| 3: Poorly or not differentiated | 0.81 | (0.71–0.93) | ||
| One year Comorbidities | .0006 | |||
| 0 (ref) | ||||
| 1 or more | 1.35 | (1.14–1.59) | ||
| Age at diagnosis |
Stage at Diagnosis |
.0001 | ||
| 24–39 | Stage I | 1.94 | (1.21–3.09) | |
| Stage II | ||||
| 40–49 | Stage I | 1.80 | (1.41–2.18) | |
| Stage II | ||||
| 50–59 | Stage I | 1.18 | (0.97–1.44) | |
| Stage II | ||||
| 60–64 | Stage I | 0.91 | (0.70–1.18) | |
| Stage II | ||||
Figure 2. Interaction between age and stage among tested women.
Adjusted odds of being tested was significantly higher for Stage I versus Stage II disease among women diagnosed under age 50. There were no significant differences in test use by Stage in women aged 50 and over.
The overall effect for race/ethnicity was significant (P < .0001) with Non-Hispanic Black women the only group to be significantly less likely to be tested than Non-Hispanic White women (OR, 0.61 [95% CI, 0.45 to 0.82]). Women with at least one comorbid condition prior to their cancer diagnosis were more likely to be tested than women with no comorbidities (OR, 1.35 [95% CI, 1.14 to 1.59]; P = 0.0006). There was significant regional variation with Georgia having the highest adjusted percentages of testing, and California (OR, 0.63 [95% CI, 0.53 to 0.76]) and Kentucky (OR, 0.77; 95% CI, 0.62 to 0.97) the lowest testing rates. Rural patients also were less likely to be tested than urban patients (OR, 0.74 [95% CI, 0.57 to 0.95]; P = 0.0206). Testing was higher among those in the top three quartiles of out-of-pocket pharmacy costs (OR, 1.22 [95% CI, 1.02 to 1.45], OR, 1.52 [95% CI, 1.29 to 1.80, and OR, 1.66 [95% CI, 1.40 to 1.97], respectively) as compared to those in the lowest quintile.
Results of sensitivity analyses were similar to our primary model, with a few minor exceptions, mostly resulting in a loss of statistical significance for variables due to loss of sample size in our sensitivity analyses (see Table A1).
Conclusions
To our knowledge, this is the largest and most representative study of US oncology practice among treated women under age 65 to investigate the use of Oncotype DX testing. We found that multiple clinical, demographic and group-level economic variables are associated with the likelihood of testing in women under age 65 with early-stage breast cancer.
Testing rates among women with node negative disease nearly doubled in the first few years after clinical guidelines incorporated GEP testing, reaching 35% among women with N0, hormone-receptor positive, HER2 negative disease. This is lower than marketing data publicly-reported by Genomic Health in 2015 which suggest that testing of guideline-eligible women of all ages has continued to increase after 2011.28 Rates of testing among women with node positive disease also increased as the evidence base for this practice began to develop, as well as with the opening of the RxPONDER trial (SWOG S1007, NCT01272037), which examines the effectiveness of chemohormonal therapy vs. hormonal therapy alone for women with 1 to 3 positive nodes.29, 30 Nonetheless, rates of testing remain lower for this group. Overall, time trends in test use appear to be influenced by factors such as the evidence for clinical utility, coverage by insurers, and incorporation into guidelines.31 Future changes in adoption are likely following publication of ongoing trials and other validation studies, as well as any increases in patient demand for testing. Qualitative studies with oncologists suggest they take a number of factors into consideration when ordering testing. These factors include not only clinical variables, but also patients’ pre-test preferences for chemotherapy32 and the degree of uncertainty regarding their recommendation for chemotherapy.33 Additional multi-method research is needed to further understand oncologists’ decision making processes about the use of GEP testing, and precision medicine more broadly, as well as how they involve patients in this process.
Likelihood of testing was strongly associated with clinical criteria that align with guidelines. A minority of tested patients had disease features that made them ineligible for testing according to guidelines that are consistent with Anthem’s policies for testing during our study period. However, some oncologists could have determined that testing of N1 women outside of the guidelines would add value based on the current literature5 pending the results of the RxPONDER clinical trial.29 It also is possible that these patients were discovered to have positive nodes subsequent to GEP testing.34 We also found that a small number of women with HER2 positive disease were also tested outside guidelines.
Our findings for age by stage suggest that oncologists are more likely to test younger women if they have Stage I vs. Stage II disease. While we found similar, non-significant trends for older patients, this pattern of care could reflect how oncologists use patient variables to determine the clinical utility of testing for individual patients, and therefore, whether they should order testing. For example, the presence of more aggressive Stage II disease in younger women as well as well as greater tolerance for chemotherapy-related side effects from chemotherapy in this group could lead oncologists to determine that chemotherapy is the optimal treatment for these patients, regardless of their Recurrence Score.35, 36 Additionally, in the studies that established the clinical utility of the Oncotype DX test and led to its incorporation in clinical guidelines, less than 10% of the women were under the age of 40.13, 37 Oncologists may therefore be generally less inclined to test younger patients under the age of 40 and those presenting with Stage II disease due to the potential benefit offered by chemotherapy regardless of test result. Conversely, they could be more open to omitting chemotherapy for Stage I disease.32
Our results also suggest that the likelihood of testing in our cohort was higher among patients with at least one preexisting comorbidity. These results differ from previous studies that have uniformly found rates of testing are inversely related to the presence of comorbidites.19, 24 This difference may be due to our younger cohort, as greater comorbidity may be associated with less benefit from chemotherapy and increases the probability of adverse toxicity events from chemotherapy.38 Whereas GEP testing and chemotherapy may be less likely to be considered for women over age 65, in our cohort, the positive association of testing with comorbidity may reflect the use of the test to help identify the subgroup of women at a higher risk of chemotherapy-induced toxicity and reductions in quality of life who might safely forgo chemotherapy.39
While our sample was large, we were limited in the inclusion of non-White and Hispanic participants. Despite this small subsample, our results replicated several studies that found lower rates of testing among non-Hispanic Black women.19, 20, 23 The consistency of this finding suggests that this may represent an emerging but unexplained disparity in the use of genomic medicine among women with breast cancer that requires further investigation. Although we did not have information on care settings, such as whether patients were treated in an academic or community setting, all patients in our sample were covered by commercial insurance and therefore, should have somewhat comparable access. We adjusted for out-of-pocket pharmaceutical payment burden to help mitigate possible variability in access within our covered population. Further, we believe that this is the first study to document regional variation in the ordering of GEP testing, as well as lower rates of testing among rural vs. urban patients. While these effects were diminished in sensitivity analyses among women with confirmed HER2 status, regional variations in other unrelated aspects of cancer care and outcomes are well-documented.40–42 Regional variation in GEP testing could reflect continued clinical uncertainty or professional disagreements among oncologists regarding the utility of GEP testing. Finally, we found that testing rates were higher for patients with greater out of pocket burden for pharmacy costs. While we are uncertain about the specific reasons for these differences, we speculate that this finding may be partly due to efforts on the part of oncologists to save patients with higher cost-sharing burden from the cost of chemotherapy if it is not going to significantly lower their risk for recurrence. Overall, our results suggest that oncologists consider not only disease characteristics, but additional non-clinical factors that appear to affect who receives GEP. There is room for an additional increase of testing among patients who are otherwise eligible per guidelines.
Our study is limited by the high proportion of patients with unknown HER2 status. We were unable to account for certain variables possibly related to test use, such as academic centers vs. community centers, the specialty of the oncologist ordering the GEP test and unmeasured patient-level variables. Finally, our study may have limited generalizability to all community practice. Our cohort includes women less than 65 with commercial health insurance in five US states. Our results reflect regional variation that might not reflect use in the US overall.
Conclusions
To our knowledge, this is the largest population-based study to assess use of GEP testing in US oncology practice. We found that the use of testing increased substantially following inclusion in clinical guidelines. Our results suggest that there are many eligible patients who are not tested. Although it appears that oncologists have incorporated testing for selected patients, rates of testing are associated with variables associated with the clinical guidelines for testing (hormonal status, nodal involvement) and the clinical utility of testing based on the potential that test results will inform treatment decision making (age, stage, comorbidities). Additional variables associated with rates of testing, including race and out of pocket pharmacy costs, as well as regional variation, suggest that testing might not be evenly disseminated to eligible patients. Ongoing trials will help to further establish the clinical utility of testing in these subgroups and likely stimulate greater dissemination of the test in practice.
Supplementary Material
Acknowledgments
Study funded by R01CA160671 and P30CA051008 from the National Cancer Institute. Manuscript preparation supported by MRSG 10-110-01-CPPB from the American Cancer Society.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Conflicts of Interest: Calvin Chao is an employee of Genomic Health, Inc. Chunfun Liu, Bola F. Ekezue PhD, and Nandini Selvam are employees of HealthCore, Inc.
We thank the following persons for facilitating the linkage of cancer registry data for this project: Drs. Tom Tucker and Ms. Jaclyn Nee, Kentucky Cancer Registry; Ms. Lynn Giljahn, Ohio Department of Health; Dr. Maria J. Schymura and Ms. Amy Kahn, NY State Tumor Registry; Drs. A. Rana Bayakly, Georgia Comprehensive Cancer Registry, and Dr. Kevin Ward, Georgia Center for Cancer Statistics; Drs. Rosemary Cress and Arti Parikh-Patel, California Cancer Registry.
References
- 1.Goldhirsch A, Winer EP, Coates AS, et al. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol. 2013;24(9):2206–2223. doi: 10.1093/annonc/mdt303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harris L, Fritsche H, Mennel R, et al. American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol. 2007;25(33):5287–5312. doi: 10.1200/JCO.2007.14.2364. [DOI] [PubMed] [Google Scholar]
- 3.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) [Accessed January 5, 2015];Breast Cancer. Version 3. 2014 Available at: http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. [Google Scholar]
- 4.Senkus E, Kyriakides S, Penault-Llorca F, et al. Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(Suppl 6):vi7–vi23. doi: 10.1093/annonc/mdt284. [DOI] [PubMed] [Google Scholar]
- 5.Dowsett M, Cuzick J, Wale C, et al. Prediction of risk of distant recurrence using the 21-gene recurrence score in node-negative and node-positive postmenopausal patients with breast cancer treated with anastrozole or tamoxifen: a TransATAC study. J Clin Oncol. 2010;28(11):1829–1834. doi: 10.1200/JCO.2009.24.4798. [DOI] [PubMed] [Google Scholar]
- 6.Carlson JJ, Roth JA. The impact of the Oncotype Dx breast cancer assay in clinical practice: a systematic review and meta-analysis. Breast Cancer Res Treat. 2013;141(1):13–22. doi: 10.1007/s10549-013-2666-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Evaluation of Genomic Applications in Practice and Prevention (EGAPP) Working Group. Recommendations from the EGAPP Working Group: can tumor gene expression profiling improve outcomes in patients with breast cancer? Genet Med. 2009;11(1):66–73. doi: 10.1097/GIM.0b013e3181928f56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Genomic Health Inc. Genomic Health announces third quarter 2014 financial results and reports continued growth in U.S. invasive breast cancer business. [Accessed January 5, 2015];2014 Nov 4; press release. Available at: http://investor.genomichealth.com/releaseDetail.cfm?releaseID=880398. [Google Scholar]
- 9.Mamounas EP, Tang G, Fisher B, et al. Association between the 21-gene recurrence score assay and risk of locoregional recurrence in node-negative, estrogen receptor-positive breast cancer: results from NSABP B-14 and NSABP B-20. J Clin Oncol. 2010;28(10):1677–1683. doi: 10.1200/JCO.2009.23.7610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldstein LJ, Gray R, Badve S, et al. Prognostic utility of the 21-gene assay in hormone receptor-positive operable breast cancer compared with classical clinicopathologic features. J Clin Oncol. 2008;26(25):4063–4071. doi: 10.1200/JCO.2007.14.4501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hornberger J, Alvarado MD, Rebecca C, Gutierrez HR, Yu TM, Gradishar WJ. Clinical validity/utility, change in practice patterns, and economic implications of risk stratifiers to predict outcomes for early-stage breast cancer: a systematic review. J Natl Cancer Inst. 2012;104(14):1068–1079. doi: 10.1093/jnci/djs261. [DOI] [PubMed] [Google Scholar]
- 12.Lyman GH, Cosler LE, Kuderer NM, Hornberger J. Impact of a 21-gene RT-PCR assay on treatment decisions in early-stage breast cancer: an economic analysis based on prognostic and predictive validation studies. Cancer. 2007;109(6):1011–1018. doi: 10.1002/cncr.22506. [DOI] [PubMed] [Google Scholar]
- 13.Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351(27):2817–2826. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 14.Sparano JA, Paik S. Development of the 21-gene assay and its application in clinical practice and clinical trials. J Clin Oncol. 2008;26(5):721–728. doi: 10.1200/JCO.2007.15.1068. [DOI] [PubMed] [Google Scholar]
- 15.Tang G, Shak S, Paik S, et al. Comparison of the prognostic and predictive utilities of the 21-gene Recurrence Score assay and Adjuvant! for women with node-negative, ER-positive breast cancer: results from NSABP B-14 and NSABP B-20. Breast Cancer Res Treat. 2011;127(1):133–142. doi: 10.1007/s10549-010-1331-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang G, Cuzick J, Costantino JP, et al. Risk of recurrence and chemotherapy benefit for patients with node-negative, estrogen receptor-positive breast cancer: recurrence score alone and integrated with pathologic and clinical factors. J Clin Oncol. 2011;29(33):4365–4372. doi: 10.1200/JCO.2011.35.3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeFrank JT, Salz T, Reeder-Hayes K, Brewer NT. Who gets genomic testing for breast cancer recurrence risk? Public Health Genomics. 2013;16(5):215–222. doi: 10.1159/000353518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Genomic Health Inc. MyBreastCancerTreatment.org. Learn about Oncotype DX. [Accessed January 5, 2015];2015 Available at: http://www.mybreastcancertreatment.org/en-US/LearnAboutOncotypeDX/FAQPage.aspx#.VIisvrctD9J. [Google Scholar]
- 19.Hassett MJ, Silver SM, Hughes ME, et al. Adoption of gene expression profile testing and association with use of chemotherapy among women with breast cancer. J Clin Oncol. 2012;30(18):2218–2226. doi: 10.1200/JCO.2011.38.5740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lund MJ, Mosunjac M, Davis KM, et al. 21-gene recurrence scores: racial differences in testing, scores, treatment, and outcome. Cancer. 2012;118(3):788–796. doi: 10.1002/cncr.26180. [DOI] [PubMed] [Google Scholar]
- 21.Anthem. Gene expression profiling for managing breast cancer treatment. [Accessed January 5, 2015];2015 Available at: http://www.anthem.com/medicalpolicies/policies/mp_pw_a049879.htm. [Google Scholar]
- 22.Genomic Health Inc. MyBreastCancerTreatment.org. Paying for the Oncotype DX test. [Accessed January 5, 2015]; Available at: http://www.mybreastcancertreatment.org/en-US/LearnAboutOncotypeDX/InsuranceAndOncotypeDX.aspx#.VKsQdLctD9J. [Google Scholar]
- 23.Guth AA, Fineberg S, Fei K, Franco R, Bickell NA. Utilization of Oncotype DX in an inner city population: race or place? Int J Breast Cancer. 2013;2013:653805. doi: 10.1155/2013/653805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen C, Dhanda R, Tseng WY, Forsyth M, Patt DA. Evaluating use characteristics for the oncotype dx 21-gene recurrence score and concordance with chemotherapy use in early-stage breast cancer. J Oncol Pract. 2013;9(4):182–187. doi: 10.1200/JOP.2012.000638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.American Joint Committee on Cancer. AJCC Cancer Staging Manual. 7th. New York: Springer; 2010. [Google Scholar]
- 26.HealthCore. HealthCore Research Integrated DatabaseSM. [Accessed January 5, 2015]; Available at: http://healthcore.com/home/research_enviro.php?page=Research. [Google Scholar]
- 27.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 28.Genomic Health Inc. Genomic Health to present at the 33rd Annual J.P. Morgan Healthcare Conference; [Accessed January 5, 2015]. January 5, 2015 press release. Available at: http://investor.genomichealth.com/releaseDetail.cfm?releaseID=889415. [Google Scholar]
- 29.Ramsey SD, Barlow WE, Gonzalez-Angulo AM, et al. Integrating comparative effectiveness design elements and endpoints into a phase III, randomized clinical trial (SWOG S1007) evaluating oncotypeDX-guided management for women with breast cancer involving lymph nodes. Contemp Clin Trials. 2013;34(1):1–9. doi: 10.1016/j.cct.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wong WB, Ramsey SD, Barlow WE, Garrison LP, Jr, Veenstra DL. The value of comparative effectiveness research: projected return on investment of the RxPONDER trial (SWOG S1007) Contemp Clin Trials. 2012;33(6):1117–1123. doi: 10.1016/j.cct.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dubois RW, Lauer M, Perfetto E. When is evidence sufficient for decision-making? A framework for understanding the pace of evidence adoption. J Comp Eff Res. 2013;2(4):383–391. doi: 10.2217/cer.13.39. [DOI] [PubMed] [Google Scholar]
- 32.Spellman E, Sulayman N, Eggly S, et al. Conveying genomic recurrence risk estimates to patients with early-stage breast cancer: oncologist perspectives. Psychooncology. 2013;22(9):2110–2116. doi: 10.1002/pon.3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bombard Y, Rozmovits L, Trudeau M, Leighl NB, Deal K, Marshall DA. The value of personalizing medicine: medical oncologists' views on gene expression profiling in breast cancer treatment. Oncologist. 2015;20(4):351–356. doi: 10.1634/theoncologist.2014-0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stemmer SM, Klang SH, Ben-Baruch N, et al. The impact of the 21-gene Recurrence Score assay on clinical decision-making in node-positive (up to 3 positive nodes) estrogen receptor-positive breast cancer patients. Breast Cancer Res Treat. 2013;140(1):83–92. doi: 10.1007/s10549-013-2603-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kimmick G. Adjuvant chemotherapy for breast cancer in older women: emerging evidence to aid in decision making. Curr Treat Options Oncol. 2011;12(3):286–301. doi: 10.1007/s11864-011-0159-z. [DOI] [PubMed] [Google Scholar]
- 36.Spano JP, Falandry C, Chaibi P, Freyer G. Current targeted therapies in breast cancer: clinical applications in the elderly woman. Oncologist. 2011;16(8):1144–1153. doi: 10.1634/theoncologist.2011-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Habel LA, Shak S, Jacobs MK, et al. A population-based study of tumor gene expression and risk of breast cancer death among lymph node-negative patients. Breast Cancer Res. 2006;8(3):R25. doi: 10.1186/bcr1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chia VM, Page JH, Rodriguez R, Yang SJ, Huynh J, Chao C. Chronic comorbid conditions associated with risk of febrile neutropenia in breast cancer patients treated with chemotherapy. Breast Cancer Res Treat. 2013;138(2):621–631. doi: 10.1007/s10549-013-2454-9. [DOI] [PubMed] [Google Scholar]
- 39.Hall PS, McCabe C, Stein RC, Cameron D. Economic evaluation of genomic test-directed chemotherapy for early-stage lymph node-positive breast cancer. J Natl Cancer Inst. 2012;104(1):56–66. doi: 10.1093/jnci/djr484. [DOI] [PubMed] [Google Scholar]
- 40.Hines R, Markossian T, Johnson A, Dong F, Bayakly R. Geographic residency status and census tract socioeconomic status as determinants of colorectal cancer outcomes. Am J Public Health. 2014;104(3):e63–e71. doi: 10.2105/AJPH.2013.301572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Monson JR, Probst CP, Wexner SD, et al. Failure of evidence-based cancer care in the United States: the association between rectal cancer treatment, cancer center volume, and geography. Ann Surg. 2014;260(4):625–631. doi: 10.1097/SLA.0000000000000928. [DOI] [PubMed] [Google Scholar]
- 42.Watanabe-Galloway S, Zhang W, Watkins K, et al. Quality of end-of-life care among rural Medicare beneficiaries with colorectal cancer. J Rural Health. 2014;30(4):397–405. doi: 10.1111/jrh.12074. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.