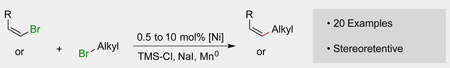
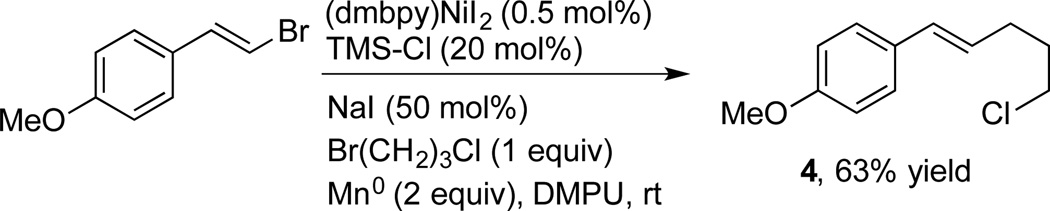Abstract
An improved method for the reductive coupling of aryl and vinyl bromides with alkyl halides is presented that achieves high yields for a variety of substrates at rt with a low (2.5 to 0.5 mol%) catalyst loading. Under the optimized conditions, difficult substrates, such as unhindered alkenyl bromides, can be coupled to furnish the desired olefins with minimal diene formation and good stereoretention. These improved conditions also work well for aryl bromides. For example, a gram-scale reaction is demonstrated with 0.5 mol% catalyst loading, while reactions at 10 mol% catalyst loading completed in as little as 20 min. Finally, a low-cost single-component pre-catalyst, (bpy)NiI2, is introduced that is both air- and moisture-stable over a period of months.
Keywords: C-C coupling, alkenes, reductive coupling, vinyl bromides
Graphical abstract
Win-Win. An improved method for the coupling of aryl and vinyl bromides with alkyl halides is presented that achieves high yields for a variety of substrates while lowering the catalyst loading and temperature of the reactions. Alkenyl bromides furnish the desired olefins with near complete stereoretention. Reactions can be run with as low as 0.5 mol% catalyst loading or reactions with a higher loading finish in as little as 20 min.
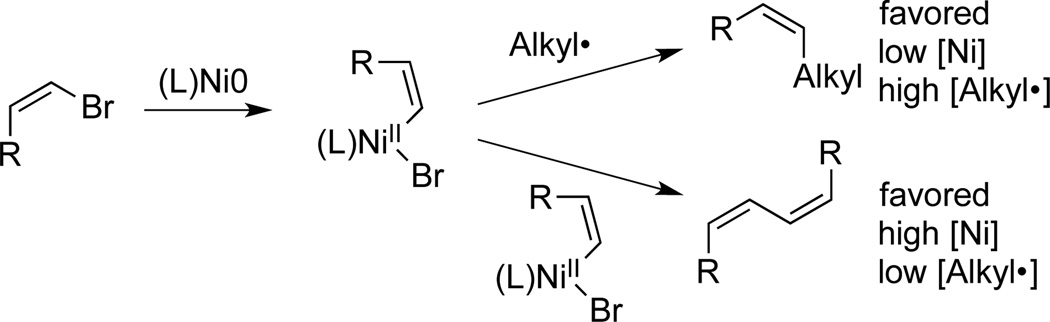
The cross-electrophile coupling of aryl halides with alkyl halides has seen rapid development in the previous six years.[1] This work has been motivated both by the large number of available organic halides and the realization that, in some cases, cross-electrophile coupling can succeed where cross-coupling with organometallic reagents had failed.[2] While a wide variety of functionalized aryl bromides have been coupled in high yield,[3] fewer examples of vinyl bromides have appeared.[4] We reported reasonable yields with 1,2-disubstituted vinyl bromides,[4b] but less hindered vinyl bromides coupled in lower yield because of competing diene formation (Scheme 1). Indeed, formation of dimers is the major challenge in cross-electrophile coupling.[1b, 1c] To date, this challenge had been addressed through ligand design.
Scheme 1.
Challenges in the cross-electrophile coupling of vinyl bromides with alkyl bromides.
In contrast to these previous efforts, our recent mechanistic studies demonstrated that less vinyl dimer would be formed at lower catalyst concentration.[5] However, there were no reports of these couplings below 12.5 mM (5 mol%) nickel concentration because at lower concentrations the reactions became slow and did not reach completion. Previously reported conditions have required stoichiometric additives (MgCl2, pyridine), long reaction times (24 h), or higher temperatures (40–80 °C), even with relatively high catalyst loadings (7–10 mol% nickel).[4b, 6] During our mechanistic studies,[5] we had found that the addition of TMS-Cl to the metallic reductant (Mn powder) accelerated the rate of cross-electrophile coupling reactions while maintaining selectivity at a given catalyst concentration.[7] We report here modified conditions that allow the selective coupling of a variety of vinyl bromides with primary and secondary alkyl halides.
Starting from our reported conditions in DMF,[4b, 5] we found that trace benzonitrile[8] in DMA solvent provided more consistent results. While initial reactions provided primarily the dimeric diene product 4a (entries 1–3), additional sodium iodide improved the yield (entry 4), presumably by forming a more reactive alkyl iodide in situ.[4b] Lowering the catalyst loading to 1 mol% (entry 5) and changing the solvent from DMA to DMPU further improved the yield (entries 5–8).
While we had previously found that reactions performed with 4,4’-di-tert-butyl-2,2’-bipyridine (dtbbpy) and 4,4’-dimethoxy-2,2’-bipyridine (dmbpy) formed less dimeric product than reactions performed with simple 2,2’-bipyridine (bpy),[4b, 5, 9] this difference was not apparent under these conditions (entries 5 and 6), perhaps because of the low catalyst loading. This is convenient because bpy is readily available at low cost.[10] We also found that reactions performed without ligand present were low-yielding and formed unidentified side products (see Supporting Information, Table S1), but reactions with a small excess of ligand worked as well as those with a 1:1 nickel/ligand ratio. We found that pre-formed (bpy)NiI2 (containing a small excess of ligand) was a useful pre-catalyst because it is not appreciably hygroscopic, while NiI2 is deliquescent. This proved crucial since these reactions could be set up on the bench using standard equipment (25 mL flask with septum under argon).
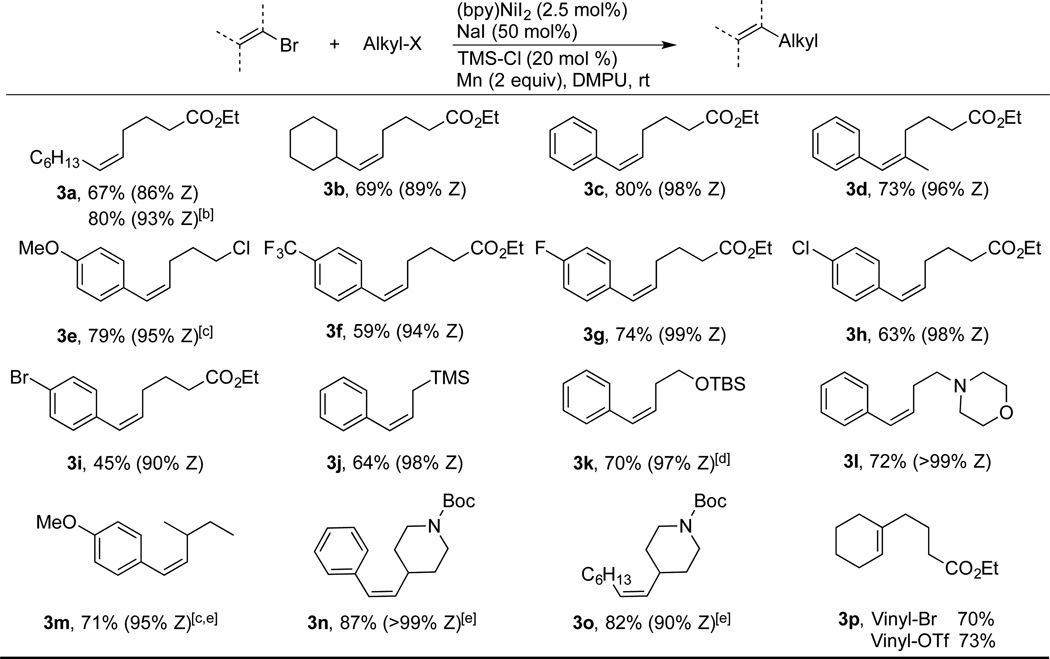
These conditions were then applied to a variety of (Z)-vinyl bromides with a catalyst loading of 1–2.5 mol%. Primary and secondary alkyl substituted vinyl bromides coupled without event (3a–b, Table 2). While benzonitrile improved selectivity for substrate 3a (Table 2), there was no improvement with styrenyl bromides 3c and 3f. Because benzonitrile slowed down the rate of reactions with 3c and 3f, it was omitted for the rest of the substrates. Additionally, we found that reactions of most vinyl bromides provided similar yields at 1 mol% and 2.5 mol% catalyst loading, but reactions at 2.5 mol% were more robust. Substitution at the 1-position is well tolerated (3d), as well as electron donating and withdrawing substituents on the aromatic ring (3e–f)[11]. Styrenyl bromides bearing halogens on the aryl ring also coupled efficiently and selectively (3g–i), although at prolonged reaction times dimerization of the brominated product 3i was observed to give the symmetrical biaryl. This is not surprising as these conditions can effectively couple aryl bromides (vida infra), but it demonstrates that vinyl bromides are more reactive than aryl bromides. Finally, the coupling of vinyl triflates with alkyl halides is presented for the first time. The triflate provided results similar to those obtained with the corresponding vinyl bromide (3p).[4c, 4d]
Table 2.
Cross-electrophile Coupling of Vinyl and Alkyl Halides[a].
Reactions performed on 2.0 mmol scale (0.25 m) using a 1:1 ratio of starting materials. Yields are of isolated product mixtures.
0.5 mmol scale (0.25 m), 1 mol% (bpy)NiI2, 5 mol% PhCN.
Starting material was 91% (Z).
1.5 equivalents of alkyl bromide used.
Alkyl iodide used. NaI omitted.
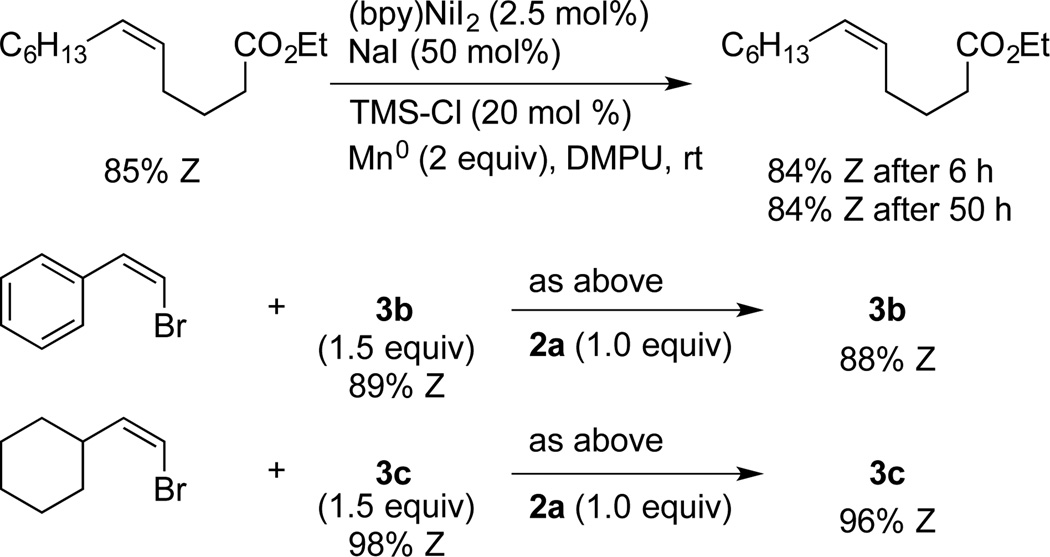
These modified conditions remain compatible with a range of functional groups, including some new examples (Table 2). Coupling with (bromomethyl)trimethylsilane furnishes allylsilane 3j with good stereocontrol.[4f] In addition to the utility of the resulting allylsilicon reagent,[12] the TMS-CH2 group can serve as a net methyl electrophile after protiodesilylation.[13] While methylation of acyl and alkyl electrophiles has been demonstrated,[14] vinyl and aryl electrophiles have not been reported. The protiodesilylation of the allyl TMS group would provide allylbenzene,[15] a synthetically useful transposition. An alkyl bromide containing a β-silyloxy group provided a reasonable yield of 3k (70%). Couplings of this type can be challenging by other methods due to competing β-silyloxy elimination, resulting in tedious workarounds.[16] In addition, a tertiary amine was well tolerated (3l). Secondary alkyl bromides reacted slowly under these conditions, resulting mostly in dimerization of the vinyl bromide. We suspected this was due to slow conversion of the secondary alkyl bromide to the more reactive alkyl iodide. Indeed, starting with the alkyl iodide rather than forming it in-situ resulted in high yields (3m–3o).
When the conditions that were optimized for the coupling of (Z)-1-bromoalkenes were applied to an (E)-1-bromoalkene, a low yield of cross-coupled product was obtained due to competing dimerization of the less-hindered bromoalkene. Here again, a lower loading (0.5 mol% rather than 2.5 mol%) was beneficial and the use of a slightly different ligand (dmbpy) provided the highest yield (Scheme 2).
Scheme 2.
Coupling of (E)-vinyl Halides
In addition to vinyl bromides, these new conditions were also effective for aryl bromides. We found that reactions with 1–2.5 mol% of catalyst at room temperature afforded yields and rates comparable to reactions previously reported with 5–10 mol% catalyst at 60 °C (entries 1 and 2).[4b] In cases where catalyst loading is not important, but short reaction times are critical, the use of higher loadings and elevated temperature allowed reactions to complete in 20 min (entry 5).
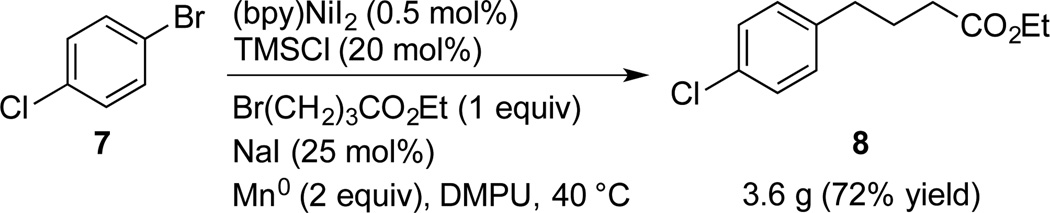
For large-scale reactions,[17] loadings down to 0.5 mol% were effective and 3.6 g of product 8 was produced with 51 mg of the lower-cost (bpy)NiI2 (Scheme 3). This represents a 10-fold decrease in catalyst loading over the state of the art. If the cost of DMPU is a concern, reactions run in DMA provided comparable yields (Table 1).
Scheme 3.
Large Scale Coupling of Aryl and Alkyl Halides
Table 1.
Reaction Optimization[a].
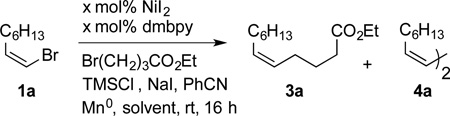 | |||||
|---|---|---|---|---|---|
| Entry | x | NaI [mol %] |
Solvent | Yield 3a [%][b] |
Yield 4a [%][b] |
| 1[c] | 5.0 | - | DMA | 12 | 27 |
| 2 | 5.0 | - | DMA | 15 | 19 |
| 3 | 5.0 | 25 | DMA | 36 | 14 |
| 4 | 5.0 | 50 | DMA | 57 | 15 |
| 5 | 1.0 | 50 | DMA | 62 | 10 |
| 6[d] | 1.0 | 50 | DMA | 71 | 7 |
| 7[d] | 1.0 | 50 | DMF | 61 | 11 |
| 8[d] | 1.0 | 50 | DMPU | 88 (80)[e] | 4 |
| 9[f] | 5.0 | 25 | DMPU | 44 | n.d. |
Reactions performed on 0.5 mmol scale (0.25 m) using a 1:1 ratio of starting materials, benzonitrile (5 mol%), chlorotrimethylsilane (20 mol%), manganese metal (2 equiv).
Determined by GC using dodecane as an internal standard. 4a yields are uncorrected.
60 °C, 2h.
(bpy)NiI2 used as a precatalyst.
Isolated yield.
As in reference [4b].
n.d. = not determined, dmbpy = 4,4’-dimethoxy-2,2’-bipyridine, DMA = N,N-dimethylacetamide, DMF = N,N-dimethylformamide, DMPU = N,N'-dimethylpropyleneurea.
In the couplings of vinyl bromides (Table 2), we observed a small amount of E-product when starting with pure Z-vinyl bromides. Three possibilities for this loss of stereochemistry were considered: isomerization of vinyl bromide, isomerization of product, and isomerization of a vinylnickel intermediate on the way to forming product. We were able to rule out isomerization of the product by resubjection of isolated products 3a, 3b, and 3c to the reaction conditions (Scheme 4). In all three cases, only a small amount of isomerization was observed, suggesting that loss of stereochemistry occurs before C-C bond formation. Isomerization of the vinyl bromide, if it happens, was not observed when analyzing reaction mixtures by GC analysis. However, this does not rule out isomerization of the vinyl bromide because E-vinyl bromides react with nickel faster than Z-vinyl bromides (vide supra). In this scenario, no accumulation of the E vinyl bromide would be expected. For this reason, we cannot yet be certain of the mechanism of isomerization.
Scheme 4.
Isomerization Studies.
In conclusion, an improved method for the reductive cross-electrophile coupling of vinyl and aryl halides with alkyl halides has been developed. The identification of conditions with faster turnover allowed for the use of lower catalyst concentrations, minimizing dimerization of unhindered vinyl bromides. Lower catalyst concentration also allowed the use of an inexpensive pre-catalyst, (bpy)NiI2. The coupling of primary (Z)- and (E)-vinyl bromides was demonstrated as well as extension to a vinyl triflate and other vinyl bromides. These refined conditions can also be applied to the coupling of aryl bromides with alkyl bromides, furnishing the alkylated arene in yields comparable to the best available conditions. We also demonstrated how higher catalyst loading can enable fast reactions (20 min) and how a very low loading can be used for economical gram-scale reactions. We anticipate that these conditions, that require no specialized equipment or rigorous exclusion of air and moisture, will constitute a general starting point for researchers interested in applying cross-electrophile coupling in synthesis.
Experimental Section
To a 25 mL round-bottom flask containing a teflon-coated stir-bar was sequentially added: nickel pre-catalyst (23.4 mg of (bpy)NiI2, 0.050 mmol), Mn0 dust (220 mg, 4.00 mmol), and sodium iodide (148 mg, 1.00 mmol). The reaction flask was sealed with a rubber septum, and the septum was fitted with a needle connected to an oil bubbler and a needle connected to an argon manifold. The headspace of the flask was purged with argon gas for two minutes and then the needle to the bubbler was removed. DMPU (8.0 mL), ethyl-4-bromobutyrate (390 mg, 2.00 mmol), (Z)-(2-bromovinyl)benzene (366 mg, 2.00 mmol), and trimethylsilyl chloride (51 µL, 0.40 mmol) were sequentially added via syringe and the resulting mixture was stirred on the benchtop (~1200 rpm) at ambient temperature under Ar. After the reaction was judged complete (~16 h), the reaction mixture was poured into water (30 mL). This aqueous mixture was then extracted with diethyl ether (3 × 30 mL). The organic layers were combined, washed with 25 mL of brine, and dried over anhydrous MgSO4. After filtration of the organic layer, the volatile materials were removed on a rotary evaporator under vacuum (10 mmHg/20 °C). The residue was purified by flash chromatography on SiO2 (10 % Et2O/hexanes) to afford 351 mg of 3c (80% yield).
Supplementary Material
Table 3.
Improved Coupling of Aryl and Alkyl Halides[a].
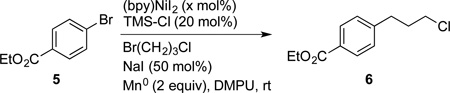 | |||
|---|---|---|---|
| Entry | bpyNiI2 [mol %] | Time | Yield 6 [%][b] |
| 1 | 1.0 | 18 h | 85 |
| 2 | 2.5 | 14 h | 78 |
| 3 | 5.0 | 3 h | 78 |
| 4 | 10.0 | 60 min | 84 |
| 5[c] | 10.0 | 20 min | 79 |
Reactions performed on 0.5 mmol scale using a 1:1 ratio of starting materials.
Determined by GC using dodecane as an internal standard.
60 °C.
Acknowledgments
Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under award number GM097243. KAJ is supported by an NSF Graduate Fellowship (NSF DGE-1419118). DJW is a Camille Dreyfus Teacher-Scholar and a Novartis Early Career Awardee. We thank Alex Callahan (Univ. of Rochester) for experimental assistance, Dr. Nicholas Gower (Univ. of Rochester) for preliminary studies on the effect of benzonitrile and TMS-Cl on reactions, and Yang Zhao (Univ. of Rochester) for helpful discussions.
Footnotes
Supporting information for this article is given via a link at the end of the document.
References
- 1.a.) Weix DJ. Acc. Chem. Res. 2015;48:1767–1775. doi: 10.1021/acs.accounts.5b00057. [DOI] [PMC free article] [PubMed] [Google Scholar]; b.) Gu J, Wang X, Xue W, Gong H. Org. Chem. Front. 2015;2:1411–1421. [Google Scholar]; c.) Everson DA, Weix DJ. J. Org. Chem. 2014;79:4793–4798. doi: 10.1021/jo500507s. [DOI] [PMC free article] [PubMed] [Google Scholar]; d.) Knappke CEI, Grupe S, Gärtner D, Corpet M, Gosmini C, Jacobi von Wangelin A. Chem. Eur. J. 2014;20:6828–6842. doi: 10.1002/chem.201402302. [DOI] [PubMed] [Google Scholar]
- 2.Molander GA, Traister KM, O’Neill BT. J. Org. Chem. 2014;79:5771–5780. doi: 10.1021/jo500905m. [DOI] [PubMed] [Google Scholar]
- 3.a.) Ackerman LKG, Anka-Lufford LL, Naodovic M, Weix DJ. Chem. Sci. 2015;6:1115–1119. doi: 10.1039/c4sc03106g. [DOI] [PMC free article] [PubMed] [Google Scholar]; b.) Wang S, Qian Q, Gong H. Org. Lett. 2012;14:3352–3355. doi: 10.1021/ol3013342. [DOI] [PubMed] [Google Scholar]; c.) Amatore M, Gosmini C. Chem. Eur. J. 2010;16:5848–5852. doi: 10.1002/chem.201000178. [DOI] [PubMed] [Google Scholar]; d.) Gomes P, Gosmini C, Périchon J. Org. Lett. 2003;5:1043–1045. doi: 10.1021/ol0340641. [DOI] [PubMed] [Google Scholar]
- 4.a.) Cherney AH, Reisman SE. J. Am. Chem. Soc. 2014;136:14365–14368. doi: 10.1021/ja508067c. [DOI] [PMC free article] [PubMed] [Google Scholar]; b.) Everson DA, Jones BA, Weix DJ. J. Am. Chem. Soc. 2012;134:6146–6159. doi: 10.1021/ja301769r. [DOI] [PMC free article] [PubMed] [Google Scholar]; c.) Zhao Y, Weix DJ. J. Am. Chem. Soc. 2014;136:48–51. doi: 10.1021/ja410704d. [DOI] [PMC free article] [PubMed] [Google Scholar]; d.) Zhao Y, Weix DJ. J. Am. Chem. Soc. 2015;137:3237–3240. doi: 10.1021/jacs.5b01909. [DOI] [PMC free article] [PubMed] [Google Scholar]; e.) Krasovskaya V, Krasovskiy A, Bhattacharjya A, Lipshutz BH. Chem. Commun. 2011;47:5717–5719. doi: 10.1039/c1cc11087j. [DOI] [PMC free article] [PubMed] [Google Scholar]; f.) Krasovskiy A, Duplais C, Lipshutz BH. Org. Lett. 2010;12:4742–4744. doi: 10.1021/ol101885t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biswas S, Weix DJ. J. Am. Chem. Soc. 2013;135:16192–16197. doi: 10.1021/ja407589e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.a.) Anka-Lufford LL, Prinsell MR, Weix DJ. J. Org. Chem. 2012;77:9989–10000. doi: 10.1021/jo302086g. [DOI] [PMC free article] [PubMed] [Google Scholar]; b.) Czaplik WM, Mayer M, Jacobi von Wangelin A. Synlett. 2009;2009:2931–2934. [Google Scholar]
- 7.Takai K, Ueda T, Hayashi T, Moriwake T. Tetrahedron Lett. 1996;37:7049–7052. [Google Scholar]
- 8.The benzonitrile might be acting to stabilize a low-valent nickel intermediate, see: Ge S, Green RA, Hartwig JF. J. Am. Chem. Soc. 2014;136:1617–1627. doi: 10.1021/ja411911s. Yin G, Kalvet I, Englert U, Schoenebeck F. J. Am. Chem. Soc. 2015;137:4164–4172. doi: 10.1021/jacs.5b00538.
- 9.Everson DA, Shrestha R, Weix DJ. J. Am. Chem. Soc. 2010;132:920–921. doi: 10.1021/ja9093956. [DOI] [PubMed] [Google Scholar]
- 10.2,2’-Bipyridine is about $0.15/mmol and NiI2 is $0.40 to $1.39/mmol depending upon the source and state.
- 11.We believe that the improvement in selectivity observed for 3e is due to rapid dimerization of the E-vinyl bromide under these conditions.
- 12.Chabaud L, James P, Landais Y. Eur. J. Org. Chem. 2004;2004:3173–3199. [Google Scholar]
- 13.a.) Eaborn C, Mahmoud FMS. J. Organomet. Chem. 1981;206:49–58. [Google Scholar]; b.) Mills RJ, Taylor NJ, Snieckus V. J. Org. Chem. 1989;54:4372–4385. [Google Scholar]
- 14.Liang Z, Xue W, Lin K, Gong H. Org. Lett. 2014;16:5620–5623. doi: 10.1021/ol502682q. [DOI] [PubMed] [Google Scholar]
- 15.Fleming I, Langley JA. J. Chem. Soc., Perkin Trans. 1981;1:1421–1423. [Google Scholar]
- 16.Busacca CA, Senanayake CH. In: Transition Metal-Catalyzed Couplings in Process Chemistry. Magano J, Dunetz JR, editors. Wiley-VCH: Weinheim; 2013. pp. 25–38. [Google Scholar]
- 17.Everson DA, George DT, Weix DJ, Buergler JF, Wood JL. Org. Synth. 2013;90:200–214. doi: 10.15227/orgsyn.090.0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.