Abstract
Purpose
Stereotactic body radiation therapy (SBRT) is associated with excess toxicity following treatment of central lung tumors. Risk-adapted fractionation appears to have mitigated this risk, but it remains unclear whether SBRT is safe for all tumors within the central lung zone, especially those abutting the proximal bronchial tree (PBT). We investigated the dependence of toxicity on tumor proximity to PBT and whether tumors abutting the PBT had greater toxicity than other central lung tumors after SBRT.
Materials and methods
A total of 108 patients receiving SBRT for central lung tumors were reviewed. Patients were classified based on closest distance from tumor to PBT. Primary endpoint was SBRT-related death. Secondary endpoints were overall survival, local control, and grade 3+ pulmonary adverse events. We compared tumors abutting the PBT to nonabutting and those ≤1 cm and >1 cm from PBT.
Results
Median follow-up was 22.7 months. Median distance from tumor to PBT was 1.78 cm. Eighty-eight tumors were primary lung and 20 were recurrent or metastatic; 23% of tumors were adenocarcinoma and 71% squamous cell. Median age was 77.5 years. Median dose was 4500 cGy in 5 fractions prescribed to the 100% isodose line. Eighteen patients had tumors abutting the PBT, 4 of whom experienced SBRT-related death. No other patients experienced death attributed to SBRT. Risk of SBRT-related death was significantly higher for tumors abutting the PBT compared with nonabutting tumors (P < .001). Two patients with SBRT-related death received anti-vascular endothelial growth factor therapy and experienced pulmonary hemorrhage. Patients with tumors ≤1 cm from PBT had significantly more grade 3+ events than those with tumors >1cm from PBT (P = .014).
Conclusions
Even with risk-adapted fractionation, tumors abutting PBT are associated with a significant and differential risk of SBRT-related toxicity and death. SBRT should be used with particular caution in central-abutting tumors, especially in the context of anti-vascular endothelial growth factor therapy.
Introduction
Surgical excision is the gold standard therapy for early-stage non-small cell lung cancer (NSCLC), but stereotactic body radiation therapy (SBRT) has been shown to provide high-quality outcomes among inoperable patients1,2 and is under investigation as an acceptable alternative to surgery among operable patients.3,4 SBRT is also effective in the treatment of lung metastases.5
However, excessive toxicity was reported among patients treated with SBRT for tumors in the central lung zone when using doses on the order of 20 Gy per fraction.6 This led to description of a 2-cm perimeter around the proximal bronchial tree (PBT) called the “no-fly zone” in which high-dose fractions are not recommended. Investigation of a reduced dose-per-fraction SBRT schedule for central NSCLC is under way in Radiation Therapy Oncology Group (RTOG) study 0813. Because the results of RTOG 0813 are pending, many centers have adopted a less aggressive fractionation schedule in treating central NSCLC with SBRT, which is believed to be safer while maintaining adequate efficacy.7–9
Despite encouraging results about the safety of reduced dose-per-fraction SBRT in central lung tumors, several publications have still reported instances of striking toxicity such as bronchial stricture, bronchial necrosis, and fatal hemoptysis.10–13 Moreover, the definition of “central” is somewhat arbitrary and not consistent between studies, with RTOG 0813 adopting a broader definition than RTOG 0236. We therefore sought to investigate whether the risk of toxicity within the central lung zone is uniform. In particular, we hypothesized that tumors close to or abutting the PBT may represent a special subset of central tumors that are at particularly high risk of severe toxicity.
In addition, SBRT can be delivered to central lung tumors in the context of metastatic disease; whether SBRT toxicity is affected by exposure to chemotherapeutic or biologically targeted agents is largely unknown. Treatment with bevacizumab, a targeted vascular endothelial growth factor (VEGF) inhibitor, in the absence of radiation is associated with major life-threatening bleed in central lung tumors.14 Most lung SBRT studies focus on early-stage NSCLC, which is not typically treated with systemic therapy. The safety profile of lung SBRT for metastatic patients exposed to biological therapy such as bevacizumab is largely unknown. We therefore updated our SBRT experience for central lung tumors7 and analyzed anatomical variables to investigate the dependence of toxicity on distance between the tumor and PBT and on anti-VEGF exposure.
Methods and materials
A retrospective, institutional review board-approved evaluation was undertaken of 108 patients who received SBRT for centrally located tumors with biologically effective dose (BED)10 > 85 Gy from 2007 to 2013. The most common fractionation was 9 Gy × 5 (n = 60) followed by 10 Gy × 5 (n = 19) and 12 Gy × 4 (n = 18); treatment was delivered every other day. Patients underwent simulation with a 4-dimensional computed tomography scan and immobilization with an alpha cradle or other customized immobilization device. The gross tumor volume (GTV) was contoured and expanded to generate an internal target volume based on respiratory excursion. A clinical target volume was generated with a 2–3 mm expansion of the internal target volume, and the clinical target volume was expanded 5 mm in all directions to generate the PTV. All patients were treated with intensity modulated radiation therapy, and treatment plans were generated using our in-house treatment planning system. Dose was prescribed to the 100% isodose line with the following dose constraints: total lung percentage of normal tissue receiving 20 Gy or greater (V20) < 12%, ipsilateral lung V20 < 25%, and maximum point dose to the PBT was kept <110% of prescription dose. Patients were treated with 4–7 coplanar, intensity modulated 6-MV beams.
“Central” was defined as GTV <2 cm from the PBT or intersection between PTV and the mediastinal envelope, as per the RTOG 0813 trial. We define “abutting” tumors as those that touch or invade the trachea, a main stem bronchus, or a lobar bronchus. Each patient was classified according to the closest distance from GTV to PBT. The primary endpoint was SBRT-related death, which was defined as a respiratory death potentially attributable to SBRT. Secondary endpoints were overall survival (OS), local control (LC), and grade 3+ pulmonary adverse events potentially attributable to SBRT, including cough, dyspnea, pneumonitis, and hemoptysis. We compared patients with abutting tumors to those with nonabutting tumors, and also compared patients with GTV ≤1 cm from PBT to those >1 cm from PBT. Mortality in abutting versus nonabutting tumors was compared with Fisher exact test; incidence of adverse events was compared with log-rank analysis. Kaplan-Meier analysis was used to estimate grade 3+ toxicity-free survival, OS, and LC. Patient and SBRT characteristics were compared using Mann-Whitney U test for continuous variable and χ2 test for categorical variables.
Results
Median follow-up was 22.7 months (range, 1.5–71.5). Median distance from GTV to PBT was 1.78 cm (range, 0–7.58 cm). Patient characteristics are listed in Table 1. Eighteen patients had tumors abutting the PBT. Overall incidence of SBRT-related death was 3.7% (n = 4). All 4 patients had tumors abutting the PBT and experienced toxicity within 1 year of SBRT (Table 2, Fig 1). Dosimetric parameters for patients with toxic death are also provided in Table 2. Incidence of SBRT-related death was significantly higher for tumors abutting the PBT compared to nonabutting tumors (22.2% vs 0%, P < .001). Grade 3+ toxicity-free survival at 1 year for patients with and without tumors abutting the PBT was 75.2% and 93.0%, respectively (not statistically significant, P = .11). Patients with abutting tumors were significantly younger (P = .013) and received lower BED10 (P = .012) than patients with nonabutting tumors. There was also a significant difference in lung lesion site (P = .043) and tumor histology (P = .022) between the clinical groupings. No significant difference between abutting and nonabutting tumors was found for gender distribution, Karnofsky Performance Status, primary site, TNM status, or tumor size.
Table 1.
Patient and SBRT characteristics
| All patients | Central-abutting tumors |
Nonabutting central tumors |
GTV ≤1 cm from PBT |
GTV >1 cm from PBT |
|
|---|---|---|---|---|---|
| Value or number (%) | |||||
| Number of patients | 108 | 18 | 90 | 26 | 82 |
| Age, median, y (range) | 77.5 (49–95) | 69 (49–84) | 79 (52–95) | 69 (49–87) | 79 (52–95) |
| Sex | |||||
| Male | 53 (49) | 7 (39) | 46 (51) | 9 (35) | 44 (54) |
| Female | 55 (51) | 11 (61) | 44 (49) | 17 (65) | 38 (46) |
| Karnofsky Performance Status | |||||
| ≥80% | 78 (72) | 15 (83) | 63 (70) | 21 (81) | 57 (70) |
| <80% | 30 (28) | 3 (17) | 27 (30) | 5 (19) | 25 (30) |
| Primary site | |||||
| Lung | 101 (93) | 17 (94) | 84 (93) | 22 (85) | 80 (98) |
| Colorectal | 2 (2) | 1 (6) | 1 (1) | 1 (4) | 0 (0) |
| Uterine | 2 (2) | 0 (0) | 2 (2) | 2 (8) | 0 (0) |
| Head and neck | 2 (2) | 0 (0) | 2 (2) | 0 (0) | 2 (2) |
| Hepatobiliary | 1 (1) | 0 (0) | 1 (1) | 1 (4) | 0 (0) |
| Lung lesion site | |||||
| RUL | 29 (27) | 4 (22) | 25 (28) | 7 (27) | 22 (27) |
| RML | 8 (7) | 2 (11) | 6 (7) | 3 (12) | 5 (6) |
| RLL | 17 (16) | 4 (22) | 13 (14) | 5 (19) | 12 (15) |
| R hilar | 2 (2) | 2 (11) | 0 (0) | 2 (8) | 0 (0) |
| LUL | 27 (25) | 2 (11) | 25 (28) | 3 (12) | 24 (29) |
| LLL | 24 (22) | 4 (22) | 20 (22) | 5 (19) | 19 (23) |
| L Hilar | 1 (1) | 0 (0) | 1 (1) | 1 (4) | 0 (0) |
| Tumor histology | |||||
| Adenocarcinoma | 77 (71) | 8 (44) | 69 (77) | 14 (54) | 63 (77) |
| Squamous cell | 25 (23) | 8 (44) | 17 (19) | 9 (35) | 16 (20) |
| Other | 6 (6) | 2 (11) | 4 (4) | 3 (12) | 3 (4) |
| TNM status (n = 88) a | |||||
| T1N0M0 | 66 (75) | 8 (67) | 58 (76) | 11 (65) | 55 (77) |
| T2N0M0 | 22 (25) | 4 (33) | 18 (24) | 6 (35) | 16 (23) |
| Lung lesion greatest dimension | |||||
| ≤ 2 cm | 43 (40) | 5 (28) | 38 (42) | 8 (31) | 35 (43) |
| >2 to ≤ 3 cm | 36 (33) | 7 (39) | 29 (32) | 9 (35) | 27 (33) |
| >3 to ≤ 5 cm | 27 (25) | 6 (33) | 21 (23) | 9 (35) | 18 (22) |
| >5 cm | 2 (2) | 0 (0) | 2 (2) | 0 (0) | 2 (2) |
| Prescription dose | |||||
| 4500 cGy in 5 fx | 60 (56) | 14 (78) | 46 (51) | 21 (81) | 39 (48) |
| 5000 cGy in 5 fx | 19 (18) | 4 (22) | 15 (17) | 4 (15) | 15 (18) |
| 4800 cGy in 4 fx | 18 (17) | 0 (0) | 18 (20) | 1 (4) | 17 (21) |
| 5400 cGy in 3 fx | 7 (7) | 0 (0) | 7 (8) | 0 (0) | 7 (9) |
| 6000 cGy in 3 fx | 2 (2) | 0 (0) | 2 (2) | 0 (0) | 2 (2) |
| 4400 cGy in 4 fx | 1 (1) | 0 (0) | 1 (1) | 0 (0) | 1 (1) |
| 3600 cGy in 2 fx | 1 (1) | 0 (0) | 1 (1) | 0 (0) | 1 (1) |
| BED10, median (range) | 85.5 (85.5–180) | 85.5 (85.5–100) | 85.5 (85.5–180) | 85.5 (85.5–105.6) | 100 (85.5–180) |
| VEGF inhibitor therapy | 10 (9) | 4 (22) | 6 (7) | 6 (23) | 4 (5) |
BED = biologically effective dose; cGy = centigray; fx = fractions; L, left; LUL, left upper lobe; R, right; RLL, right lower lobe; RML, right middle lobe; RUL, right upper lobe; VEGF = vascular endothelial growth factor.
For 88 patients with primary lesions, classified according to the seventh edition (2010) of the American Joint Committee on Cancer staging manual.
Table 2.
Characteristics of patients experiencing SBRT-related death
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | |
|---|---|---|---|---|
| Clinical scenario | 75-year-old woman T1bN0 NSCLC |
67-year-old man T2aN0 NSCLC |
54-year-old man Oligometastatic NSCLC |
49 year-old man Oligometastatic colorectal cancer |
| Tumor location | LLL | LLL | LLL | LLL |
| Tumor size | 2.4 × 2.1 cm | 4.1 × 3.6 cm | 2.9 × 1.7 cm | 2.4 × 2.3 cm |
| History of anti-VEGF therapy | No | No | Yes | Yes |
| Prescription dose | 4500 cGy (9 Gy × 5) |
4500 cGy (9 Gy × 5) |
4500 cGy (9 Gy × 5) |
5000 cGy (10 Gy × 5) |
| Cause of death | Sepsis from pneumonia, 8 months after SBRT |
Acute respiratory failure and pneumonia, 7 months after SBRT |
Acute pulmonary hemorrhage, 10 months after SBRT |
Acute pulmonary hemorrhage, 9 months after SBRT |
| GTV/PTV size (mL) | 24.2/93.3 | 74.4/181 | 16.0/99.6 | 20.2/94.7 |
| Max point dose to PBT/NFZ (cGy) | 4488/4776 | 4501/4526 | 4720/4940 | 5144/5406 |
| Ipsilateral/bilateral lung V20 (%) | 19.4/8.8 | 22.0/8.2 | 18.4/7.4 | 24.5/11.0 |
GTV, gross tumor volume; L, left; LLL, left lower lobe; NFZ, no-fly zone; NSCLC, non-small cell lung cancer; PBT, proximal bronchial tree; PTV, planning target volume; SBRT, stereotactic body radiation therapy; V20, percentage of normal tissue receiving 20 Gy or greater; VEGF, vascular endothelial growth factor.
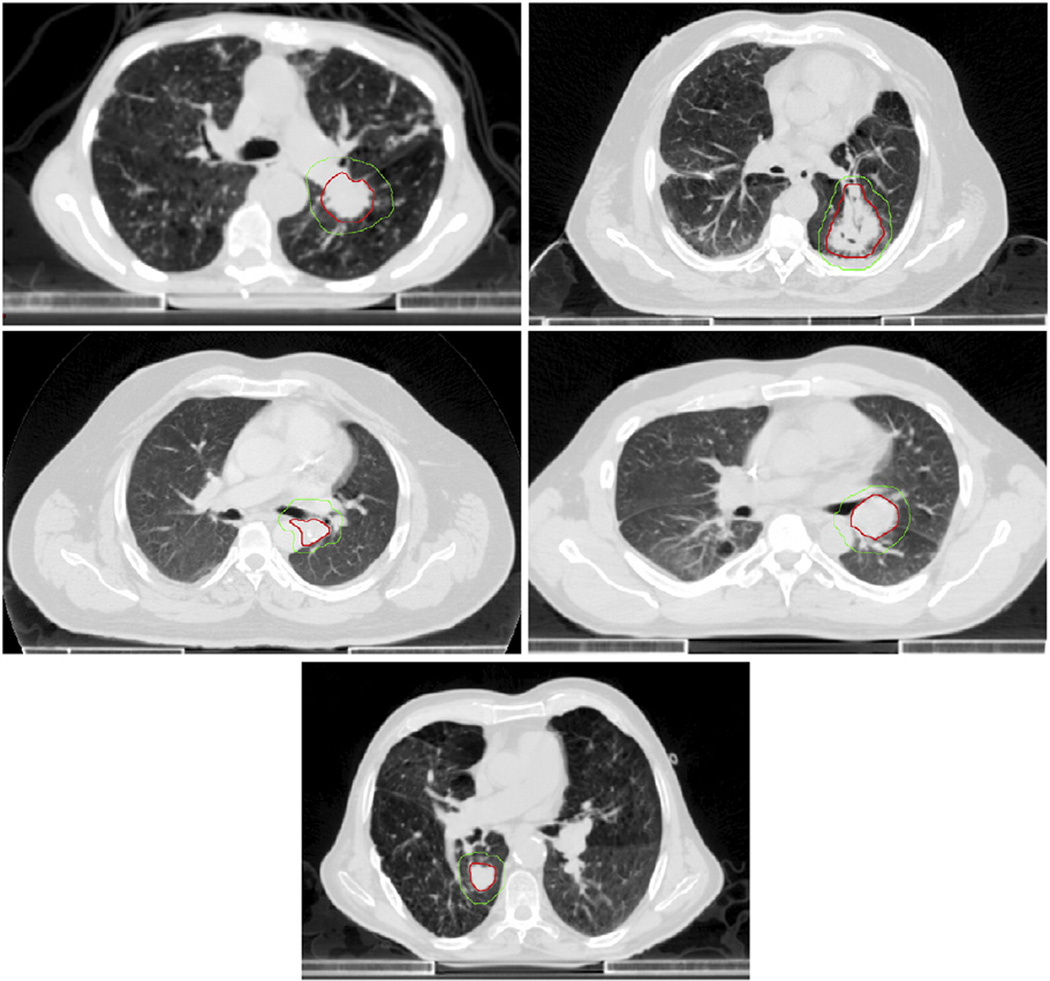
Figure 1.
Representative cross-sectional images of 4 tumors in patients with stereotactic body radiation therapy–related death and a patient with nonabutting tumor. Gross and planning target volume contours overlaid in red and green, respectively. Shown left to right, top to bottom: patient 1, patient 2, patient 3, patient 4, and nonabutting tumor patient.
The 4 cases of potentially SBRT-related death occurred between 7 and 10months after SBRT. Three patients received 45 Gy in 5 fractions and a fourth received 50 Gy in 5 fractions. One patient developed gram-negative rod pneumonia and died of sepsis. Another also developed an apparent pneumonia and died of acute respiratory failure. In both cases, a definitive etiology for their respiratory complication was not reached, but there was a significant clinical suspicion that history of radiation therapy was a contributing factor. The 2 other patients died of acute pulmonary hemorrhage. Both had a history of bevacizumab exposure. One was in the context of metastatic NSCLC; bevacizumab and pemetrexed were given in the 3 years leading up to SBRT, held during SBRT, and continued afterwards. The other was in the context of metastatic colon cancer; bevacizumab, 5-fluorouracil, and irinotecan were given beginning 20 months before SBRT, held during SBRT, and continued afterwards. Bronchoscopy was not performed on either patient with pulmonary hemorrhage. There was no evidence of disease progression at the time of death for any of the 4 patients with toxic death; no autopsies were performed. Of note, there were no respiratory deaths of any kind (whether attributable to SBRT or not) in the patients with nonabutting tumors.
Two other patients with abutting tumors and history of bevacizumab treatment experienced no grade 3+ toxicity: 1 started bevacizumab (in combination with carboplatin and pemetrexed) 10 months after SBRT and the other had received bevacizumab (in combination with pemetrexed) until 5 months before SBRT. Six patients with nonabutting tumors received treatment with a VEGF inhibitor (bevacizumab, sorafenib, or pazopanib). All 6 patients were treated with anti-VEGF therapy over a year after SBRT, and none of these patients experienced grade 3+ toxicity. Overall, 12 patients received cytotoxic chemotherapy, 8 with anti-VEGF therapy and 4 without. Eight of the cytotoxic regimens were for stage IV lung cancer and included carboplatin, pemetrexed, gemcitabine, erlotinib, paclitaxel, and docetaxel. Other regimens included 5-fluorouracil and irinotecan for metastatic colorectal cancer and temozolomide, carboplatin, and paclitaxel for endometrial cancer.
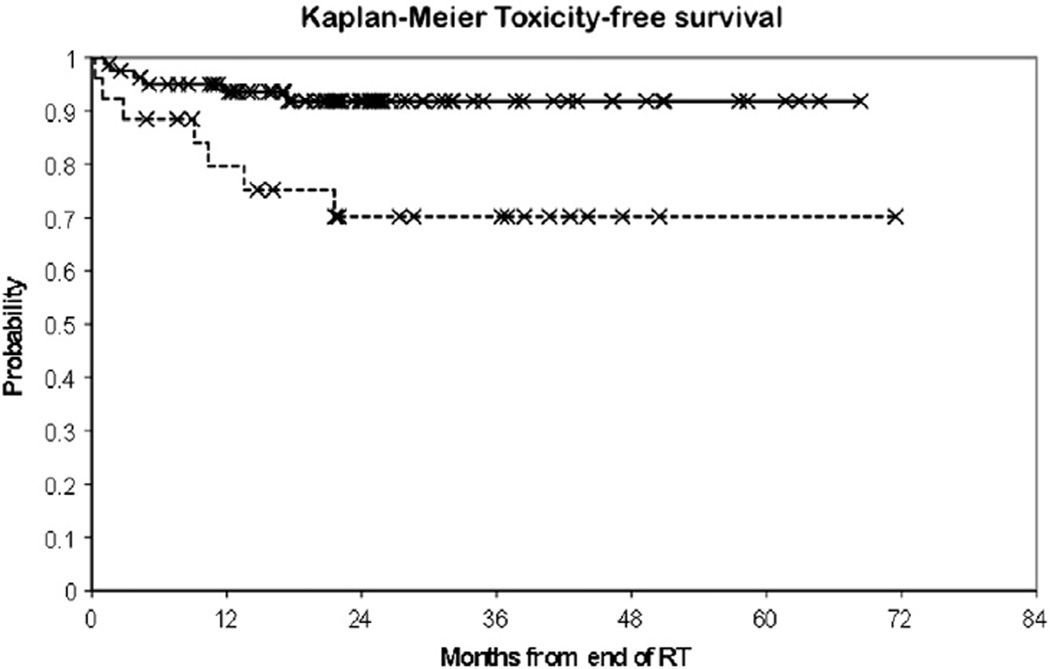
Overall incidence of grade 3+ adverse events was 12.0% (n = 13). Twenty-six patients (24.0%) had GTV ≤1 cm from PBT. There were significantly more grade 3+ events among these patients (30.7% [n = 7] vs 7.3% [n = 6], P = .014) and their 1-year grade 3+ toxicity-free survival was significantly lower (79.6% vs 93.6%,P = .008, Fig 2). Patients with tumors ≤1 cm from PBT were significantly younger (P < .001) and received lower BED10 (P < .001) than patients with tumors >1 cm from PBT. There was also a significant difference in primary site (P = .009) and lung lesion site (P = .041) between the clinical groupings. No significant difference between abutting and nonabutting tumors was found for gender distribution, Karnofsky Performance Status, tumor histology, TNM status, or tumor size. Overall 2-year LC was 77.4%and 2-year OS was 63.9%; neither OS nor LC was significantly different for patients ≤1 cm versus >1 cm from PBT.
Figure 2.
Grade 3+ toxicity-free survival for tumors ≤1 cm from the proximal bronchial tree (dashed line) versus ≥1 cm from the proximal bronchial tree (solid line). RT, radiation therapy.
Discussion
Even in a cohort using risk-adapted fractionation of 9–10 Gy per fraction, we observed a 3.7% fatal toxicity rate with SBRT for central lung tumors. Notably, 4 of 18 patients (22.2%) with tumors abutting the PBT experienced fatal complications attributed to SBRT, whereas none of the other 90 patients did. Maximum point dose to the PBT in these 4 patients did not exceed 105% of prescription, and the bilateral lung V20 did not exceed 11%; these metrics would have met the constraints specified in RTOG 0813. We also observed that the risk of severe (grade 3+) toxicity was higher in patients with tumors closer to the PBT.
Retrospective attribution of pulmonary toxicity has limitations, as previously described for radiation pneumonitis.15,16 Bronchoscopy and autopsy were not performed in the 4 patients with respiratory death; therefore, attribution had to be inferred from clinical history. There was significant clinical suspicion that SBRT contributed to the toxicity in each of the 4 patients with respiratory death.
LC in this study is lower than that reported in other studies of SBRT for lung tumors.1,2 This is likely because the median BED10 of the cohort was lower than 100 Gy because a prescription dose of 45 Gy in 5 fractions was standard practice for central tumors at our institution until recently. No difference in LC was observed between patients with tumors ≤1 cm versus >1 cm from PBT. Prospective dose-escalation studies such as RTOG 0813 are examining higher BED regimens for central lung tumors and should better define the LC that can be achieved with SBRT in these patients.
The present study builds upon our previous analysis,7 which found that pulmonary toxicity was not significantly associated with any studied dose-volume metrics including the lung, PBT, or no-fly zone. Our observations suggest that toxicity risk in the central lung zone may be better predicted by the anatomic relationship of tumor to the PBT, rather than dosimetric values. In particular, it may be possible that irrespective of dosimetric constraints, central-abutting tumors have a unique predilection for toxicity, which may be related to the physical effect of the tumor abutment on the adjacent structures.
Chaudhuri et al17 recently published an analysis comparing SBRT outcomes for 34 central and 34 peripheral lung tumors. Among the central tumors, they defined an “ultra-central” subset of tumors directly abutting the PBT. Seven tumors in their analysis were categorized as “ultra-central”; none of these patients experienced grade 2 toxicity or greater, and no SBRT-related deaths were noted in any of the 68 patients in the study. The authors noted that the small sample size limited their power to observe differences among treatment groups. Their findings stand in stark contrast to the severe and differential toxicity observed in the present analysis. The reason for this difference is unclear, but because they only included 7 patients with central-abutting tumors, it is possible that their sample size was too small to identify an underlying susceptibility for severe toxicity.
In the present analysis, 2 of the 4 patients with SBRT-related death also had a history of treatment with bevacizumab, a VEGF inhibitor, preceding SBRT and within weeks of it. Death in these 2 patients was related to pulmonary hemorrhage. Treatment with VEGF inhibitor appears to be a risk factor for SBRT-related toxicity in the gastrointestinal tract.18 We therefore also suggest that the combination of anti-VEGF therapy and central lung lesions may represent an especially high-risk scenario for SBRT. Furthermore, it is noteworthy that the other patients with history of anti-VEGF exposure (2 abutting and 6 nonabutting) had more significant temporal separation between SBRT and anti-VEGF exposure, and in some cases did not receive anti-VEGF therapy until after SBRT. This suggests that anti-VEGF therapy may be particularly risky when used immediately before or after SBRT.
Compared with patients with nonabutting tumors, central-abutting patients were younger and received no more than 10 Gy per fraction and 50 Gy total, whereas some nonabutting patients received more aggressive fractionation schemes (Table 1). Therefore, the difference in toxicity is not likely explained by baseline differences in patient characteristics or radiation dose.
Overall, our analysis suggests that the risk of toxicity among central lung tumors is not uniform: tumors closer to the PBT—particularly those abutting the PBT—may be at especially high risk for SBRT-related toxicity. The reason for this susceptibility is unclear, but we speculate that tumor abutment or invasion may alter the integrity and radiation response of large airways and mediastinal structures. Our results additionally suggest that anti-VEGF therapy may amplify toxicity risk in lung SBRT, especially when the 2 treatments are given in close temporal proximity. Though increasing experience indicates that moderate-dose SBRT is generally safe for central lung tumors, this may not apply to the subset of “central-abutting” tumors, particularly in patients with history of anti-VEGF therapy. Until more is known, great caution should be employed when considering SBRT in these situations.
Footnotes
Conflicts: None.
References
- 1.Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA. 2010;303:1070–1076. doi: 10.1001/jama.2010.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baumann P, Nyman J, Hoyer M, et al. Outcome in a prospective phase II trial of medically inoperable stage I non-small-cell lung cancer patients treated with stereotactic body radiotherapy. J Clin Oncol. 2009;27:3290–3296. doi: 10.1200/JCO.2008.21.5681. [DOI] [PubMed] [Google Scholar]
- 3.Onishi H, Shirato H, Nagata Y, et al. Stereotactic body radiotherapy (SBRT) for operable stage I non-small-cell lung cancer: Can SBRT be comparable to surgeryz. Int J Radiat Oncol Biol Phys. 2011;81:1352–1358. doi: 10.1016/j.ijrobp.2009.07.1751. [DOI] [PubMed] [Google Scholar]
- 4.Timmerman RD, Paulus R, Pass HI, et al. RTOG 0618: Stereotactic body radiation therapy (SBRT) to treat operable early-stage lung cancer patients. ASCO Meet Abstr. 2013;31(Suppl 15):7523. [Google Scholar]
- 5.Rusthoven KE, Kavanagh BD, Burri SH, et al. Multi-institutional phase I/II trial of stereotactic body radiation therapy for lung metastases. J Clin Oncol. 2009;27:1579–1584. doi: 10.1200/JCO.2008.19.6386. [DOI] [PubMed] [Google Scholar]
- 6.Timmerman R, McGarry R, Yiannoutsos C, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol. 2006;24:4833–4839. doi: 10.1200/JCO.2006.07.5937. [DOI] [PubMed] [Google Scholar]
- 7.Modh A, Rimner A, Williams E, Foster A, Shah M, Shi W, et al. Local control and toxicity in a large cohort of central lung tumors treated with stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys. 2014;90(5):1168–1176. doi: 10.1016/j.ijrobp.2014.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang JY, Li Q-Q, Xu Q-Y, et al. Stereotactic ablative radiation therapy for centrally located early stage or isolated parenchymal recurrences of non-small cell lung cancer: How to fly in a “no fly zone.”. Int J Radiat Oncol Biol Phys. 2014;88:1120–1128. doi: 10.1016/j.ijrobp.2014.01.022. [DOI] [PubMed] [Google Scholar]
- 9.Senthi S, Haasbeek CJA, Slotman BJ, Senan S. Outcomes of stereotactic ablative radiotherapy for central lung tumours: A systematic review. Radiother Oncol. 2013;106:276–282. doi: 10.1016/j.radonc.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 10.Milano MT, Chen Y, Katz AW, Philip A, Schell MC, Okunieff P. Central thoracic lesions treated with hypofractionated stereotactic body radiotherapy. Radiother Oncol. 2009;91:301–306. doi: 10.1016/j.radonc.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 11.Song SY, Choi W, Shin SS, et al. Fractionated stereotactic body radiation therapy for medically inoperable stage I lung cancer adjacent to central large bronchus. Lung Cancer. 2009;66:89–93. doi: 10.1016/j.lungcan.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 12.Rowe BP, Boffa DJ, Wilson LD, Kim AW, Detterbeck FC, Decker RH. Stereotactic body radiotherapy for central lung tumors. J Thorac Oncol. 2012;7:1394–1399. doi: 10.1097/JTO.0b013e3182614bf3. [DOI] [PubMed] [Google Scholar]
- 13.Corradetti MN, Haas AR, Rengan R. Central-airway necrosis after stereotactic body-radiation therapy. N Engl J Med. 2012;366:2327–2329. doi: 10.1056/NEJMc1203770. [DOI] [PubMed] [Google Scholar]
- 14.Johnson DH, Fehrenbacher L, Novotny WF, et al. Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol. 2004;22:2184–2191. doi: 10.1200/JCO.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 15.Kocak Z, Evans ES, Zhou S-M, et al. Challenges in defining radiation pneumonitis in patients with lung cancer. Int J Radiat Oncol Biol Phys. 2005;62:635–638. doi: 10.1016/j.ijrobp.2004.12.023. [DOI] [PubMed] [Google Scholar]
- 16.Yirmibesoglu E, Higginson DS, Fayda M, et al. Challenges scoring radiation pneumonitis in patients irradiated for lung cancer. Lung Cancer. 2012;76:350–353. doi: 10.1016/j.lungcan.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chaudhuri AA, Tang C, Binkley MS, et al. Stereotactic ablative radiotherapy (SABR) for treatment of central and ultra-central lung tumors. Lung Cancer. 2015;89:50–56. doi: 10.1016/j.lungcan.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 18.Pollom EL, Deng L, Pai RK, et al. Gastrointestinal toxicities with combined antiangiogenic and stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys. 2015;92:568–576. doi: 10.1016/j.ijrobp.2015.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]