Abstract
Captive rearing of insect pests is necessary to understand their biology and to develop control methods. The avian nest fly, Philornis downsi Dodge and Aitken, is a blood-sucking parasite during its larval stage and a serious threat to endemic birds in the Galapagos Islands where it is considered invasive. In order to procure large numbers of flies for biological studies, rearing media and diets were trialed for rearing the larval stage of P. downsi under controlled conditions in the absence of its avian host. P. downsi eggs were obtained from field-caught female flies, and once eggs hatched they were reared on chicken blood for the first 3 d. Following this, three diets were tested on second- and third-instar larvae: 1) chicken blood only; 2) chicken blood, hydrolyzed protein and dried milk powder; and 3) chicken blood, hydrolyzed protein and brewer’s yeast. Out of 385 P. downsi larvae tested, we were able to rear 50 larvae to the adult stage. The highest level of mortality was found in the first-instar larvae. Survivorship of second- and third-instar larvae was similar irrespective of diet and diet did not significantly influence larval or pupal development times; though larvae fed the diet with brewer’s yeast developed marginally faster. Pupal weights were similar to those of larvae that had developed on bird hosts in the field. To our knowledge, this is the first effective protocol for rearing a hematophagous parasitic avian fly from egg to adult in the absence of a living host.
Keywords: Muscidae, Diptera, invasive species, ectoparasites, insect rearing
The development of methods for rearing insects in the laboratory is fundamental to understanding the biology and ecology of a species, as well as for developing control techniques in the event that the species in question affects the well-being of humans or biodiversity (Cohen 2001, Parra 2012). To successfully rear a species under artificial conditions, requirements for temperature, humidity, space, photoperiod, food, and other physiological and environmental conditions must be understood (Huho et al. 2007, Sorensen et al. 2012). The replication of relationships of obligate parasites that feed on living, vertebrate hosts in the laboratory is particularly challenging and it often takes many years of research to find the correct laboratory conditions and optimal diet (Hunt and Schmidtmann 2005, Chen et al. 2014, Medone et al. 2015). To our knowledge, few attempts have been made to rear flies belonging to the genera Protocalliphora (Calliphoridae), Passeromyia, and Philornis (Muscidae) whose larvae feed on the blood of young birds and who have a synchronized relationship with their hosts (Gold and Dalhsten 1983, Bennett and Whitworth 1991). Several species from these groups affect the health or survivorship of threatened bird species (Little 2008, Wunderle and Arendt 2011, Jais 2014, Domínguez et al. 2015, Woolaver et al. 2015) and captive rearing in the laboratory would facilitate studies to understand the biology of these species in order to develop methods for reducing parasite numbers in bird nests.
The development of a rearing method for Philornis downsi Dodge and Aitken (Diptera: Muscidae) is a high priority for conservation in the Galapagos Islands where this introduced parasitic fly is seriously affecting populations of at least 16 endemic bird species or subspecies (Causton et al. 2013, Kleindorfer et al. 2014). Some of these species have highly restricted distributions and are on the brink of extinction; such as the Mangrove Finch, Camarhynchus heliobates (Snodgrass and Heller) (∼80 individuals remaining) (Cunninghame et al. 2015). The parasite P. downsi, reported in Brazil (Mendonça and Couri 1999), Trinidad (Dodge and Aitken 1968), Argentina (Silvestri et al. 2011), and recently mainland Ecuador (Bulgarella et al. 2015), was first observed in the nests of birds in the Galapagos Islands in 1997 (Fessl et al. 2001). Specimens from Galapagos dating back to 1964 were discovered later (Causton et al. 2006), but the exact date of introduction of the fly is unknown.
Adult P. downsi are free-living, feeding on decaying organic matter, fruit, and nectar (Couri 1999). However, in its larval stage P. downsi is an obligate parasite feeding on the blood of hatchlings of landbirds, principally passerines (Couri 1985, 1999, Fessl and Tebbich 2002, Bulgarella and Heimpel 2015). Upon emerging from eggs laid at the base of the nest, the first-instar larvae typically move into the nares of the hatchlings where they feed on blood and tissues (Fessl et al. 2006a). Up to 200 eggs have been reported in a single nest in Galapagos (Lincango et al. 2015). After 3–4 d, the second-instar larvae move to the base of the nest and feed externally on hatchlings at night (Dudaniec and Kleindorfer 2006, Fessl et al. 2006a). Up to 100% of hatchlings in a nest can die from larval feeding and in some cases before the Philornis larvae have completed development (O’Connor et al. 2010a, Kleindorfer et al. 2014, Cunninghame et al. 2015). Larvae have been observed feeding on the internal and external tissues of dead chicks on these occasions (saprophagy) (Huber 2008, O’Connor et al. 2010b). The engorged larvae pupate at the base of the nest where they remain until adults emerge (Fessl et al. 2006a). Little is known about the development time of P. downsi in the field. Larval development has been estimated at 4–7 d and the pupal stage lasts ∼10–14 d (Fessl et al. 2006b, Causton et al. 2013, Kleindorfer et al. 2014). These estimates are within the range of the larval (5–8 d) and pupal (5–20 d) development times reported for other Philornis species that are blood feeders (Oniki 1983, Arendt 1985, Delannoy and Cruz 1991, Young 1993, Teixeira 1999, Rabuffetti and Reboreda 2007, Quiroga and Reboreda 2013).
P. downsi has been found in 13 of the 15 larger islands in the archipelago (Causton et al. 2013). The success of mitigating the impacts of this fly depends in part on being able to rear P. downsi under laboratory conditions, which will provide sufficient specimens to study the biology and ecology of this species and test potential control options, such as classical biological control and the Sterile Insect Technique (SIT) release methods which necessitate mass-rearing on a non-host diet (Sorensen et al. 2012).
In 2008–2009, a first attempt was made to rear P. downsi under laboratory conditions in the Galapagos Islands (Lincango and Causton 2008, Hellman and Fierke 2009). Researchers experimented with a wide range of environmental conditions and artificial diets. Ingredients of these diets included chicken blood, plasma, serum, or throat mucus, powdered egg white, powdered milk, lamb blood, ground beef, beef liver; blood agar, and psyllium. Over a period of one year and many trials to find a suitable diet and rearing medium, three adults were reared from 477 larvae (Lincango and Causton 2008). These three larvae were reared on a chicken blood diet. Adult flies completed their life cycle in an average of 36 d (∼4 d egg, ∼18 d larva, and ∼12 d pupa) (Lincango and Causton 2008).
In 2013–2014, environmental conditions and artificial diets were further developed and tested formally. Due to these efforts, we report here the development of a novel rearing method that enabled the successful production of P. downsi from egg to adult in the absence of a living host.
Materials and Methods
Experimental Conditions
Studies were conducted between May 2013 and July 2014 at the Insect Containment Facility of the Charles Darwin Research Station (CDRS) on Santa Cruz Island, in the Galapagos Islands, Ecuador. P. downsi eggs, pupae, and adults were reared in the laboratory at an average temperature of 26.5 ± 5°C with an average relative humidity of 66 ± 15% and a photoperiod of 12:12(L:D) h (light source was Philips: TL80 F32T8/TL841, USA 8H, 11W). Larvae were kept in mini-incubators (Brinsea Mini-Eco incubator 115 V, 18 W max, Titusville, FL USA) and set at 36°C with a relative humidity between 80 and 95%. The incubator was permanently covered with 2 mm thick black craft foam to produce total darkness because it had been observed that larvae show signs of negative phototaxis.
Collection of Adults
P. downsi females were collected on Santa Cruz Island, Ecuador. Flies were collected from an arid, lowland site at El Barranco (0° 44′ 34.1″S, 90° 18′10.4″W, elevation 15–41 m) and from the humid highlands at Los Gemelos (0° 37′82.0″S, 90° 23′44.4″W, elevation 589–616 m). At each location, McPhail traps (Naturquim, Guayaquil, Ecuador) were hung in trees ∼3 m above the ground. These traps have a clear plastic top and a yellow plastic bottom with a 9 cm entrance in the middle through which flies enter. The bottom of the trap was filled with a blended papaya-based baiting mixture that was composed of 78 g ripe papaya fruit, 18 g sugar, and 120 ml water, which is known to be attractive to P. downsi (Lincango and Causton 2009). Live adult female flies were collected from the McPhail traps and brought to the laboratory.
Maintenance of Adult Flies
In the laboratory, flies were placed in clear plastic containers of 10 cm in diameter and 6 cm in height with up to 15 females in each container. Each container had three openings covered with a fine mesh (two on the sides and one on the lid). Three inverted caps from 1.5 ml microcentrifuge tubes were glued to the base of the container and filled with food through a 0.5 cm hole cut in the mesh from the lid of the container. A cotton plug was used to cover the hole when it was not in use. All flies were fed once a day at 0800 h. Adult flies were fed a diet consisting of: water (120 ml), ripe papaya (78 g), white sugar (18 g), hydrolyzed protein (Only Natural-Collagen Hydrolyzed, Natural Protein, Guayaquil, Ecuador) (6 g), and milk powder fortified with iron, calcium, zinc, and vitamin C and D (Nestlé, Quito, Ecuador) (6 g). All containers with adults were sprayed with 1.5 ml water five times a day (0800, 1000, 1200, 1500, and 1700 h).
Egg Collection and Larval Eclosion
Field-caught P. downsi females readily laid eggs on the inside surfaces of the containers. Containers were checked daily for eggs, which were removed with a moistened sterile, fine paintbrush. The eggs were placed on 7 cm2 pieces of 2 mm thick black craft foam at a maximum density of 60 eggs per square of foam. The eggs were covered with an inverted transparent plastic petri dish measuring 5 cm in diameter × 1.7 cm in height. Since the larvae are very mobile, a weight was placed on top of each petri dish to prevent larvae from escaping. To ensure that food and water were readily available to newly hatched larvae, holes were made in the inverted petri dish and two glass tubes measuring 2.5 cm × 0.5 cm fitted into these holes. On the day eggs were collected, cotton moistened with pre-boiled water was placed into each glass tube. From the day after egg collection until egg hatch, a cotton plug with 0.5 ml chicken blood was added to the inner end of one of the tubes, while at the same time, avoiding direct contact with the cotton moistened with water in the same tube (Fig. 1A). The plugs with blood were replaced twice a day and the cotton moistened with water twice daily until all larvae had hatched. The eggs were checked twice daily at 0800 and 1600 h until all eggs had hatched or for up to 6 d, after which time they were considered infertile. The time to egg hatch was estimated for 132 eggs.
Fig. 1.
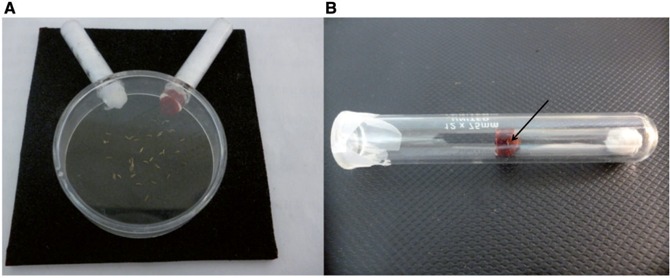
Rearing media for eggs and first-instar larvae of P. downsi. (A) Eggs were placed on squares of black craft foam covered with an inverted plastic petri dish with two glass tubes inserted into the petri dish, one with a cotton plug with chicken blood and the other with cotton moistened with pre-boiled water. (B) First-instar larvae (see arrow) were maintained in small, open-ended glass tubes; one end with a cotton plug moistened with 1 drop of pre-boiled water, the other end with chicken blood. To prevent escape, each tube was placed inside a sealed glass test tube. Photo credit: Paola Lahuatte.
Larval Rearing
Larvae were fed chicken blood that was obtained from a poultry farm located on Santa Cruz Island. Blood was collected in 1.5 ml microcentrifuge tubes that were placed directly below the necks of 3-month-old chickens as they were killed for commercial purposes. The tubes were shaken, and then placed immediately in a cooler with ice, and transported to the insect containment facility at CDRS where the tubes were stored at 1°C for up to 15 d. In preparation for feeding, the blood was heated to 50°C in a heating block (Benchmark myblock Mini Dry Bath, Benchmark Scientific, Edison, NJ) for 10 min to eliminate contaminants. The blood was then cooled to 36°C before being presented to P. downsi larvae. Preliminary studies had shown that first- and early second-instar larvae are able to digest only chicken blood while the later instars are more versatile in their dietary requirements. Because of this, for the first 3 d larvae were offered chicken blood only. Following this, larvae were given either a chicken–blood-only diet or one of two enriched chicken blood diets (see Second- and Third-Instar Larval Stages).
First-Instar Larvae
Newly hatched larvae were placed in 3 × 0.5 cm glass tubes with open ends using a sterile, fine paint brush. One of the ends received a cotton plug moistened with one drop of pre-boiled water, the other end was plugged with cotton moistened with 0.3 ml of chicken blood; these plugs were replaced twice daily (0800 and 1600 h). Due to the high mobility of the larvae and to prevent their escape, each tube was placed inside a larger glass test tube 7 cm × 1.2 cm and the tube opening sealed with Parafilm (Bemis Co., inc., Oshkosh, WI) (Fig. 1B). The Parafilm was perforated with 0.3 mm holes to facilitate airflow. The glass tubes and cotton were replaced daily. For these trials three larvae were placed in each tube, but for rearing purposes it is possible to rear up to 10 larvae in each tube.
Second- and Third-Instar Larval Stages
On day 4 the larvae were placed individually in 7 cm × 1.2 cm glass test tubes half-filled with cotton moistened with 1.5 ml purified water using sterile, fine forceps. At 1600 h each day, a 1 cm piece of 0.5 diameter plastic drinking straw, containing cotton with 0.5 ml diet, was placed into each tube. Larvae were randomly assigned to one of the following diets:
Diet 1: 0.3 ml chicken blood only;
Diet 2: 0.3 ml chicken blood + 4 mg hydrolyzed protein + 6 mg of milk powder fortified with iron, calcium, zinc and vitamin C and D;
Diet 3: 0.3 ml of chicken blood + 4 mg hydrolyzed protein + 6 mg of brewer's yeast powder (Ecuanatu, Quito - Ecuador).
The diets were mixed using a vortex mixer (Model VX-200, Labnet International, Edison, NJ, USA) for one minute after which the food was heated to 36°C for 1 minute. Between 29 and 31 larvae were offered each diet upon reaching day 4. The test tubes were sealed with Parafilm and perforated with 0.3 mm holes. Larvae were checked daily and all food and containers were changed daily.
Weighing and Maintenance of Pupae
Approximately 12 h after larvae pupated, the puparia were weighed using an analytical balance (Ohaus Adventurer digital laboratory scale model AP110S, resolution 0.0001–110 g, OHAUS, Parsippany, NJ) to the nearest 0.1 mg. In order to compare the weight of laboratory-reared pupae with pupae that developed under natural conditions, 43 pupae were randomly selected from a Warbler Finch (Certhidea olivacea Gould) and Small Tree finch (Camarhynchus parvulus [Gould]) nest that had been collected from Los Gemelos (see Cimadom et al. 2014 for methodology) and weighed.
After measurements were taken, the pupae were placed individually in clear plastic cups (4 × 3.5 cm), which were covered with muslin held in place by rubber bands. Pupae were maintained at an average temperature of 26.5 ± 5°C and 66 ± 15% average humidity and checked daily until all adults had emerged or a minimum of three months had passed without emergence.
Data Analysis
The effect of larval diet on development times and pupal weight for the laboratory treatments was estimated using an analysis of variance (ANOVA) after ensuring that assumptions of ANOVA were met, followed by Tukey HSD contrasts in the event that significant differences were found. For the analysis comparing the weight of pupae collected in the field with those reared on the various diets in the laboratory, assumptions of ANOVA were not met since the variance in weight of the field-collected pupae greatly exceeded that of the laboratory-reared pupae. We therefore used a Generalized Linear Model with Normal variance distribution and an Identity link function. Effects of larval diet on larval mortality and pupal eclosion rates were analyzed using contingency table analyses. All analyses were performed using JMP v.11 (SAS Institute Inc., Cary, NC, USA, 2012) software.
Results
Following the procedures described earlier, 41 fully formed adults were obtained from 400 eggs collected from field-caught females. Of those 400 eggs, 385 hatched (96% viability), and larval survival to the second instar was 23.6% leaving 91 larvae that molted to second instar and that were assigned to one of three diet options. Mortality rates for the second- and third-instar larvae together was 19.3% in diet 1 (n = 31), 29.0% in larvae fed diet 2 (n = 31), and 27.6% in diet 3 (n = 29) and; these differences were not significantly different from each other (Likelihood Ratio χ2 = 0.903; P = 0.631). Differences in adult eclosion rates were not statistically significant either (Table 1; Likelihood Ratio χ2 = 0.520; P = 0.773). A total of 50 adult individuals emerged from pupae (44% males and 56% females); nine of these adults were unable to inflate their wings fully and had a short life span for unknown reasons. Five of the adults with vestigial wings had been fed on the control diet.
Table 1.
Mean duration of developmental phases of P. downsi under laboratory conditions
| Larva died/escaped | No pupae | Mean days larva–pupa (±SEM) | Mean days pupa–adult (±SEM) | Adult eclosion (% success) | Mean days larva–adult (±SEM) | |
|---|---|---|---|---|---|---|
| Diet 1 | 6/2 | 23 | 10 (0.3) | 10.1 (0.3) | 17 (74%) | 19.6 (0.3) |
| Diet 2 | 9/1 | 21 | 9.9 (0.4) | 10.1 (0.2) | 17 (81%) | 19.6 (0.4) |
| Diet 3 | 8/1 | 20 | 9.0 (0.3) | 9.8 (0.1) | 16 (80%) | 18.6 (0.2) |
Larvae were fed chicken blood for days 1–3 and from day 4 onwards received either chicken blood only (diet 1), chicken blood + hydrolyzed protein + milk powder (diet 2), or chicken blood + hydrolyzed protein + brewers yeast (diet 3).
The mean time to egg hatch was 3.5 d (SEM ± 0.1, n = 132) with a range of <24 h to 12 d and a mode of 3 d. Mean development time for larvae that reached the pupal phase was fastest on diet 3 (Table 1), but differences between diets were only marginally significant (F = 25.077; df = 2; P = 0.089). The pupal development times were not significantly different between treatments in which larvae were fed enriched or chicken–blood-only diets (F = 1.001; df = 2; P = 0.375) (Table 1).
Pupal Weights
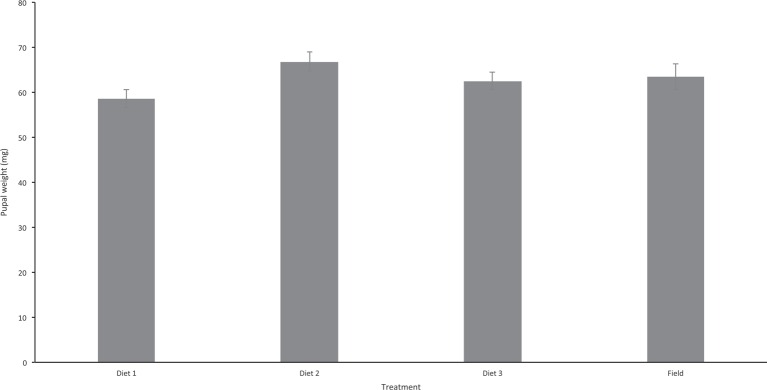
Pupal weight differed significantly among treatments depending on the diet fed to second-instar larvae (Fig. 2; F = 4.237; df = 2; P = 0.019). The highest average pupal weight was obtained with diet 2 (chicken blood + hydrolyzed protein + milk powder), followed by diet 3 (chicken blood + hydrolyzed protein + brewer’s yeast); while diet 1 (chicken-blood-only) resulted in the lowest pupal weight. Diet 2 differed significantly from diet 1, but diet 3 did not differ significantly from diets 1 or 2 (Tukey-Kramer HSD). The average weight of pupae reared in the laboratory was within the range of the weight of pupae collected in the field (range of 21 – 112 mg, mean 63.4 mg ± SEM 2.8, n = 43; Fig. 2), and there was no significant difference between the weight of field-collected and any of the groups of laboratory-reared pupae (χ2 = 4.03, d.f. = 3; P = 0.258).
Fig. 2.
Comparison of Philornis pupal weights among artificial diet treatments (chicken blood only [diet 1], chicken blood + hydrolyzed protein + milk powder [diet 2] or chicken blood + hydrolyzed protein + brewers yeast [diet 3]).
Discussion
In this study, a novel method was developed for rearing P. downsi from egg to adult in the absence of its bird host. To our knowledge, this is the first effective protocol for rearing larvae of P. downsi or any other species of free-living dipteran ectoparasite of birds on an artificial diet. The success rate for rearing adults from eggs was ∼10%, considerably higher than the first attempts (0.6% success rate rearing first-instar larvae through to adult) (Lincango and Causton 2008). Furthermore, egg-to-adult development time of laboratory-reared P. downsi averaged between 22 and 24 d, within the timeframe estimated for P. downsi in nests from the field; 15–24 d (Fessl et al. 2006b, Causton et al. 2013, Kleindorfer et al. 2014). Laboratory rearing on a domestic passerine bird, the society finch, Lonchura striata (L.) also produced development times within this range (M. Bulgarella and G.E.H., unpublished data).
Egg hatch rates were high (96%) showing that laboratory conditions were suitable for egg development. In the wild, P. downsi infests a variety of nest types in a wide range of habitats (from dense, moss-filled nests in cool, wet forests to nests built of twigs in arid habitats) (Lack 1947, Grant 1986). On the other hand, the high mortality (77%) of first-instar larvae in this study was a major impediment to rearing larvae in the laboratory. Recently eclosed larvae typically spend the first few days of development feeding and living inside the nares of young chicks (Fessl et al. 2006a), and may require a more specialized environment. Mortality may also be due to contamination of the chicken blood or diet ingredients by bacteria. Serratia, Pseudomonas, and Enterobacter were found in some P. downsi larvae in the laboratory (T. J. Kurtti, personal communication). Furthermore, too much handling may be an issue. It is possible that by using sterile blood or blood containing anti-microbial agents, that a longer-lasting diet (such as the diets used to rear screwworm larvae [Chaudury and Skoda 2013]) could be developed. This might increase survivorship of early instar larvae.
Survival rates of later instars fed on enriched diets or a diet of chicken blood only were higher with more than two-thirds of the larvae forming pupae, suggesting that environmental conditions were adequate for rearing mature larvae. Late-instar larvae migrate from the inner lining of the nest to feed on chicks (Fessl et al. 2006a) and are probably able to withstand greater environmental variation than are early instar larvae.
Diet did not significantly influence larval or pupal development times, although there was a marginally significant trend for larvae offered the diet incorporating brewer’s yeast (diet 3) to develop 1 d faster than larvae fed pure chicken blood. However, larvae that were given a diet of chicken blood, hydrolyzed protein and enriched milk achieved a significantly greater pupal weight than larvae in the chicken-blood-only diet suggesting that it is advantageous to provide larvae with enriched artificial diets. And while pupae were significantly heavier when larvae were fed a richer diet, the range of pupal weights obtained by using all of our three diets was within the range of what is seen in the field.
The health of insects is influenced by the availability of food resources during the feeding stages with the quantity and quality of food ingested by larvae during development determining when pupation takes place, the consequent size of the adults, and also the health of reproductive organs (Scriber and Slansky 1981, Simpson et al. 2015). Dipteran larvae with nutritionally poor diets or with limited access to food, e.g., produce smaller pupae (Ullyett 1950, Gold and Dahlsten 1989, Amano 1998, Nash and Chapman 2014). This effect has been observed in the Galapagos Islands with P. downsi when chick mortality occurs early (Kleindorfer et al. 2014). For insects, including Philornis species, the size of the larvae and pupae is directly correlated with the size of adults (Davidowitz et al. 2003, Quiroga and Reboreda 2013). A larger body size in adults may provide a reproductive advantage and has also been linked with greater fecundity (Honêk 1993, Taylor and Yuval 1999, Kaspi et al. 2000, Byrne and Rice 2006). Insect size is especially important when being reared for SIT control programs (Sorensen et al. 2012, Pérez-Staples et al. 2013).
Our study demonstrates that it is possible to rear the larvae of an obligate nest parasitic fly on an artificial diet in the absence of its living host and that pupae have equivalent weights to those found in nests in natural conditions. Additional studies are required to demonstrate whether fly health extends to reproductive health and fecundity. These results indicate that the techniques developed for laboratory rearing the larvae of P. downsi have potential for rearing larger numbers of flies. By maintaining colonies of P. downsi outside the breeding season of birds, studies on the biology and control of this invasive pest will be made significantly easier. Rearing the larvae of an obligate nest parasite in the laboratory successfully may also facilitate studies on other species of nest flies that are affecting endangered endemic birds elsewhere in the world such as the critically endangered Yellow cardinal (Gubernatrix cristata [Vieillot]) in Argentina (Domínguez et al. 2015), Ridgway’s hawk (Buteo ridgwayi [Cory]) in the Dominican Republic (Jais 2014, Woolaver et al. 2015) and Puerto Rican Sharp-shinned hawk (Accipiter striatus venator Wetmore) (Wunderle and Arendt 2010).
Acknowledgments
Thanks to D. Cedeño and X. Pilataxi for their collaboration in the field collection of live flies and to H. Boris, M. Bulgarella, J. Castañeda, A. Cimadom, J. Delgado, N. Filek, S. Garcia, J. Halatas, D. Mosquera, A. Sagubay, B. Tutiven, Y. Tutiven for input into the development of rearing technologies and help in the laboratory. We also thank T.J. Kurtti for identifying bacteria within dead larvae and for giving advice on sterile technique, Arno Cimadom for supplying the field-collected pupae and G. Keller and M. Bulgarella for providing valuable input on the manuscript. We thank the Central University of Ecuador, the Charles Darwin Foundation and the Galapagos National Park Directorate for support. This work was supported by funding from the Galapagos Conservancy and the International Community Foundation (with a grant awarded by The Leona M. and Harry B. Helmsley Charitable Trust.) Permission to conduct this study in protected areas of Galapagos National Park was granted by the Galapagos National Park Directorate (Project: PC-02-14: Control of the Invasive Parasite, P. downsi and its impact on biodiversity). This is contribution number 2136 of the Charles Darwin Foundation for the Galapagos Islands.
References Cited
- Amano K. 1998. Studies on the intraspecific competition in dung-breeding flies: IV. effect of larval density on the development of Orthellia pacifica Zimin (Diptera: Muscidae). Ann. Entomol. Soc. Am. 23: 367–372. [Google Scholar]
- Arendt W. J. 1985. Philornis ectoparasitism of pearly-eyed thrashers. II. Effects on adults and reproduction. Auk. 102: 281–292. [Google Scholar]
- Bennett G. F., Whitworth T. L. 1991. Studies on the life history of some species of Protocalliphora (Diptera: Calliphoridae). Can. J. Zool. 69: 2048–2058. [Google Scholar]
- Bulgarella M., Quiroga M. A., Brito-Vera G. A., Dregni J. S., Cunninghame F., Mosquera-Muñoz D. A., Monje L. D., Causton C. E., Heimpel G. E. 2015. Philornis downsi (Diptera: Muscidae), an avian nest parasite invasive to the Galápagos Islands, in Mainland Ecuador. Ann. Entomol. Soc. Am. 108: 242–250. [Google Scholar]
- Bulgarella M., Heimpel G. E. 2015. Host range and community structure of avian nest parasites in the genus Philornis (Diptera: Muscidae) on the island of Trinidad. Ecol. Evol. 5: 3695–3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne P. G., Rice W. R. 2006. Evidence for adaptive male mate choice in the fruit fly Drosophila melanogaster. Proc. R. Soc. B. 273: 917–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Causton C. E., Peck S. B., Sinclair B. J., Roque-Albelo L., Hodgson C. J., Landry B. 2006. Alien insects: threats and implications for conservation of Galapagos islands. Ann. Entomol. Soc. Am. 99: 121–143. [Google Scholar]
- Causton C. E., Cunninghame F., Tapia W. 2013. Management of the avian parasite Philornis downsi in the Galapagos Islands: a collaborative and strategic action plan, pp. 167–173. In Galapagos Report 2011–2012. GNPD, CGREG, CDF and GC; Puerto Ayora, Galapagos, Ecuador. [Google Scholar]
- Chaudhury M. F., Skoda S. R. 2013. An artificial diet for rearing Cochliomyia macellaria (Diptera: Calliphoridae). J. Econ. Entomol. 106: 1927–1931. [DOI] [PubMed] [Google Scholar]
- Chen H., Chaudhury M. F., Sagel A., Phillips P. L., Skoda S. R. 2014. Artificial diets used in mass production of the New World Screwworm, Cochliomyia hominivorax. J. Appl. Entomol. 138: 708–714. [Google Scholar]
- Cimadom A., Ulloa A., Meidl P., Zöttl M., Zöttl E., Fessl B., Nemeth E., Dvorak M., Cunninghame F., Tebbich S. 2014. Invasive parasites, habitat change and heavy rainfall reduce breeding success in Darwin’s finches. PloS One. 9: e107518.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen A. C. 2001. Formalizing insect rearing and artificial diet technology. Am. Entomol. 47: 198–206. [Google Scholar]
- Couri M. S. 1985. Considerações sobre as relações ecológicas das larvas de Philornis Meinert, 1890 (Diptera, Muscidae) com aves. Rev. Brasil. Entomol. 29: 17–20. [Google Scholar]
- Couri M. S. 1999. Myiasis caused by obligatory parasites Ia. Philornis Meinert (Muscidae), pp. 44–70. In Guimarães J. H., Papavero N. (eds.), Myiasis in man and animals in the Neotropical region; Editora Pleiade, São Paulo, Brazil. [Google Scholar]
- Cunninghame F., Switzer R., Parks B., Young G., Carrión A., Medranda P., Sevilla C. 2015. Conserving the critically endangered mangrove finch: head-starting to increase population size, pp. 151–157. In Galapagos Report 2013-2014. GNPD, CGREG, CDF and GC; Puerto Ayora, Galapagos, Ecuador. [Google Scholar]
- Davidowitz G., D’Amico L. J., Nijhout H. F. 2003. Critical weight in the development of insect body size. Evol. Develop. 5: 188–197. [DOI] [PubMed] [Google Scholar]
- Delannoy C. A., Cruz A. 1991. Philornis parasitism and nestling survival of the Puerto Rican Sharp-shinned Hawk, pp. 93–103. In Loye J. E., Zuk M. (eds.), Bird–parasite interactions: ecology, evolution, and behavior. Oxford University Press, Oxford, United Kingdom. [Google Scholar]
- Dodge H. R., Aitken T. H. G. 1968. Philornis flies from Trinidad (Diptera: Muscidae). J. Kans. Entomol. Soc. 41: 134–154. [Google Scholar]
- Domínguez M., Reboreda J. C., Mahler B. 2015. Impact of Shiny Cowbird and botfly parasitism on the reproductive success of the globally endangered Yellow Cardinal Gubernatrix cristata. Bird Conserv. Int. 25: 294–305. [Google Scholar]
- Dudaniec R. Y., Kleindorfer S. 2006. Effects of the parasitic flies of the genus Philornis (Diptera: Muscidae) on birds. Emu. 106: 13–20. [Google Scholar]
- Fessl B., Couri M. S., Tebbich S. 2001. Philornis downsi Dodge & Aitken, new to the Galapagos Islands (Diptera, Muscidae). Stud. Dipterol. 8: 317–322. [Google Scholar]
- Fessl B., Tebbich S. 2002. Philornis downsi – a recently discovered parasite on the Galapagos archipelago a threat for Darwin’s finches? Ibis. 144: 445–451. [Google Scholar]
- Fessl B., Sinclair B. J., Kleindorfer S. 2006a. The life cycle of Philornis downsi (Diptera: Muscidae) parasitizing Darwin’s finches and its impacts on nestling survival. Parasitology. 133: 739–747. [DOI] [PubMed] [Google Scholar]
- Fessl B., Kleindorfer S., Tebbich S. 2006b. An experimental study on the effects of an introduced parasite in Darwin’s finches. Biol. Conserv. 127: 55–61. [Google Scholar]
- Gold C. S., Dahlsten D. L. 1983. Effects of parasitic flies (Protocalliphora spp.) on nestlings of mountain and chestnut-backed chickadees. Wilson Bull. 95: 560–572. [Google Scholar]
- Gold C. S., Dahlsten D. L. 1989. Prevalence, habitat selection, and biology of Protocalliphora (Diptera: Calliphoridae) found in nests of mountain and chestnut-backed chickadees in California. Hilgardia. 57: 1–19. [Google Scholar]
- Grant P. R. 1986. Ecology and Evolution of Darwin’s Finches, 2nd edn Princeton Univerrsity Press, Princeton, USA. [Google Scholar]
- Hellman W., Fierke M. 2009. Philornis downsi Trapping and Rearing. Technical report Charles Darwin Foundation, Puerto Ayora, Galapagos, Ecuador. [Google Scholar]
- Honěk A. 1993. Intraspecific variation in body size in fecundity in insects: a general relationship. Oikos. 66: 483–492. [Google Scholar]
- Huber S. K. 2008. Effects of the introduced parasite Philornis downsi on nestling growth and mortality in the medium ground finch (Geospiza fortis). Biol. Conserv. 141: 601–609. [Google Scholar]
- Huho B. J., Ng’habi K. R., Killeen G. F., Nkwengulila G., Knols B. G. J., Ferguson H. M. 2007. Nature beats nurture: a case study of the physiological fitness of free-living and laboratory-reared male Anopheles gambiae s.l. J. Exp. Biol. 210: 2939–2947. [DOI] [PubMed] [Google Scholar]
- Hunt G. J., Schmidtmann E. T. 2005. Care, maintenance and experimental infection of biting midges, pp. 741–745. In Marquardt W.C. (ed.). Biology of disease vectors, 2nd edn Elsevier Academic Press, Burlington, MA. [Google Scholar]
- Jais T. 2014. Conversaciones de campo. Spizaetus. 17: 35–39. [Google Scholar]
- Kaspi R., Taylor P. W., Yuval B. 2000. Diet and size influence sexual advertisement and copulatory success of males in Mediterranean fruit fly leks. Ecol. Entomol. 25: 279–284. [Google Scholar]
- Kleindorfer S., Peters K. J., Custance G., Dudaniec R. Y., O’Connor J. A. 2014. Changes in Philornis infestation behavior threaten Darwin’s finch survival. Curr. Zool. 60: 542–550. [Google Scholar]
- Lack D. 1947. Darwin’s Finches. Cambridge University Press; Cambridge, UK. [Google Scholar]
- Lincango P., Causton C. 2008. Crianza en cautiverio de Philornis downsi, en las Islas Galápagos. Technical report Charles Darwin Foundation, Puerto Ayora, Galapagos, Ecuador. [Google Scholar]
- Lincango P., Causton C. 2009. Ensayos de atrayentes para la captura de la mosca parásito, Philornis downsi (Diptera: Muscidae) en las Islas Galápagos. Technical Report Charles Darwin Foundation, Puerto Ayora, Galapagos, Ecuador. [Google Scholar]
- Lincango P., Causton C., Cedeño D., Castañeda J., Hillstrom A., Freund D. 2015. Interactions between the avian parasite, Philornis downsi (Diptera: Muscidae) and the Galapagos Flycatcher, Myiarchus magnirostris Gould (Passeriformes: Tyrannidae). J. Wild. Dis. 51: 907–910. [DOI] [PubMed] [Google Scholar]
- Little S. E. 2008. Myiasis in wild birds, pp. 546–556 In Atkinson C. T., Thomas N. J., Hunter D. B. (eds), Parasitic diseases of wild birds. Wiley-Blackwell, Ames, Iowa. [Google Scholar]
- Medone P., Balsalobre A., Rabinovich J. E., Marti G. A., Menu F. 2015. Life history traits and demographic parameters of Triatoma infestans (Hemiptera: Reduviidae) fed on human blood. J. Med. Ent. 52: 1282–1290. [DOI] [PubMed] [Google Scholar]
- Mendonça E., de C., Couri M. S. 1999. New associations between Philornis Meinert (Diptera, Muscidae) and Thamnophilidae (Aves, Passeriformes). Rev. Bras. Zool. 16: 1223–1225. [Google Scholar]
- Nash W. J., Chapman T. 2014. Effect of dietary components on larval life history characteristics in the Medfly (Ceratitis capitata: Diptera, Tephritidae). PLoS One. 9: e86029. doi:10.1371/journal.pone.0086029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor J. A., Sulloway F. J., Robertson J., Kleindorfer S. 2010a. Philornis downsi parasitism is the primary cause of nestling mortality in the critically endangered Darwin's medium tree finch Camarhynchus pauper. Biodivers. Conserv. 9: 853–866. [Google Scholar]
- O’Connor J. A., Robertson J., Kleindorfer S. 2010b. Video analysis of host–parasite interactions in nests of Darwin’s finches. Oryx. 44: 588–94. [Google Scholar]
- Oniki Y. 1983. Notes on fly (Muscidae) parasitism of nestlings of South American birds. Gerfaut. 73: 281–286. [Google Scholar]
- Parra J. R. 2012. The evolution of artificial diets and their interactions in science and technology, pp. 51–92. In Panizzi A. R., Parra J. R. (eds.), Insect bioecology and nutrition for integrated pest management. CRC Press London, New York, USA. [Google Scholar]
- Pérez-Staples D., Shelly T. E., Yuval B. 2013. Female mating failure and the failure of ‘mating’ in sterile insect programs. Entomol. Exp. Appl. 146: 66–78. [Google Scholar]
- Quiroga M. A., Reboreda J. C. 2013. Sexual differences in life history traits of Philornis seguyi (Diptera: Muscidae) parasitizing house wrens Troglodytes aedon. Ann. Entomol. Soc. Am. 106: 222–227. [Google Scholar]
- Rabuffetti F. L., Reboreda J. C. 2007. Early infestation by bot flies (Philornis seguyi) decreases chick survival and nesting success in chalk-browed mockingbirds (Mimus saturninus). Auk. 124: 898–906. [Google Scholar]
- Scriber J. M., Slansky F. 1981. The nutritional ecology of immature arthropods. Ann. Rev. Entomol. 26: 183–211. [Google Scholar]
- Silvestri L., Antoniazzi L. R., Couri M. S., Monje L. D., Beldomenico P. M. 2011. First record of the avian ectoparasite Philornis downsi Dodge & Aitken, 1968 (Diptera: Muscidae) in Argentina. Syst. Parasitol. 80: 137–140. [DOI] [PubMed] [Google Scholar]
- Simpson S. J., Clissold F. J., Lihoreau M., Ponton F., Wilder S. M., Raubenheimer D. 2015. Recent advances in the integrative nutrition of arthropods. Ann. Rev. Entomol. 60: 293–311. [DOI] [PubMed] [Google Scholar]
- Sorensen J. G., Addison M. F., Terblanche J. S. 2012. Mass-rearing of insects for pest management: challenges, synergies and advances from evolutionary physiology. Crop Prot. 38: 87–94. [Google Scholar]
- Taylor P. W., Yuval B. 1999. Postcopulatory sexual selection in Mediterranean fruit flies: advantages for large and protein-fed males. Anim. Behav. 58: 247–254. [DOI] [PubMed] [Google Scholar]
- Teixeira D. M. 1999. Myiasis caused by obligatory parasites. Ib. General observations on the biology of species of the genus Philornis Meinert, 1890 (Diptera: Muscidae), pp. 71–96. In Guimarães J. H., Papavero N. (eds.), Myiasis in man and animals in the Neotropical Region. Editora Pleiade, São Paulo, Brazil. [Google Scholar]
- Ullyett G. C. 1950. Competition for food and allied phenomena in sheep blowfly populations. Phil. Trans. Roy. Soc. London B. 234: 77–174. [Google Scholar]
- Woolaver L. G., Nichols R. K., Morton E. S., Stutchbury B. J. 2015. Breeding ecology and predictors of nest success in the Critically Endangered Ridgway’s Hawk Buteo ridgwayi. Bird Conserv. Int. 25: 385–398. [Google Scholar]
- Wunderle J. M., Arendt W. J. 2011. Avian studies and research opportunities in the Luquillo Experimental Forest: A tropical rain forest in Puerto Rico. Forest. Ecol. Manag. 262: 33–48. [Google Scholar]
- Young B. E. 1993. Effects of the parasitic botfly Philornis carinatus on nestling house wrens, Troglodytes aedon, in Costa Rica. Oecologia. 93: 256–262. [DOI] [PubMed] [Google Scholar]