Abstract
The receptor for advanced glycation end products (RAGE) is a multiligand receptor involved in inflammatory disorders, tumor outgrowth, diabetic complications and Alzheimer's disease (AD). RAGE transports circulating amyloid-β toxins across the blood–brain barrier (BBB) into the brain. RAGE–amyloid-β toxin interaction at the BBB leads to oxidative stress, inflammatory responses and reduced cerebral blood flow. Thus, regulating RAGE activity at the BBB and/or within brain could be beneficial to AD patients. Herein, the structure–function relation for RAGE–ligand interaction and the role of RAGE as a potential target in the development of treatments for AD and other RAGE-associated disorders are discussed. Despite recent setbacks in the development of RAGE-based therapies for AD, a new generation of compounds that regulate RAGE activity could be efficacious. Careful studies are needed in rodent and nonrodent animal models of AD with new the generation of RAGE antagonists to ensure safety and efficacy in chronic treatment before clinical trials.
Background
The receptor for advanced glycation end products (RAGE) is a 35–45 kD pattern recognition multiligand receptor and member of the immunoglobulin superfamily of receptor [1–3]. Since its discovery as a cell surface receptor in bovine lungs [4], it was shown to be ubiquitously expressed in many cell types, including various brain cell types, such as endothelial cells, pericytes, astrocytes and microglia [5–7]. While RAGE is a receptor for advanced glycation end products (which is the product of non-enzymatic glycation and oxidation of proteins and lipids [8]), it also interacts with several other ligands that are structurally unrelated, including HMGB1 (also called amphoterin), S100/calgranulins family of polypeptides (e.g., S100B, S100P, S100A1, S100A2, S100A4, S100A5, S100A6, S100A7, S100A8/A9, S100A12 and S100A13) [2,9,10], macrophage-1 antigen (Mac-1) [11], phosphatidylserine [12], lipopolysaccarides [13], transthyretin [14,15] and amyloid-β toxins (Aβ) (Table 1) [1,5,6,16]. In addition to cell surface RAGE, soluble RAGE (sRAGE) and endogenous secretory RAGE (esRAGE) are present in human plasma but may [17] or may not be present in mouse plasma [18], depending on the methods used to detect RAGE. With the exception of lungs, the basal expression of RAGE is low but increases with the levels of its ligands and at sites of stress and injury [1,4–8].
Table 1.
Receptor for advanced glycation end products main ligands and their functions.
| Ligand | Ligand family | Ligand-binding region | Function | Related disease |
|---|---|---|---|---|
| Advanced glycation end products | AGEs | V-type | Advanced glycation endproducts are present in high levels in diabetes | Diabetes |
| HMGB1 | HMGB1 | V-type | Involved in cell stress mechanisms. It also induces cellular migration | Tumor outgrowth |
| S100b | S100 calgranulins | V-type | Calcium-binding protein that is related to Alzheimer's disease | Alzheimer's disease |
| S100A6 | S100 calgranulins | C2-type | Calcium-binding protein that binds to a different domain | Alzheimer's disease |
| Aβ | Aβ | Aβ/oligomer – V-type Aggregate – C1 type | Oligomer species appears more pathogenic. Aβ transport at the blood–brain barrier, cerebral blood flow reduction and neuroinflammation | Alzheimer's disease |
| Mac-1 | β2-integrin | VC1 subunit | Involved with leukocyte recruitment relevant in inflammatory disorders | Diabetes |
| Transthyretin | Transthyretin | VC1 subunit | Associated with extracellular amyloid deposits that lead to degeneration of neurons in the peripheral nerve | Alzheimer's disease |
| LPS | LPS | VC1 subunit | A bioactive component of bacterial cell walls, it can bind with RAGE to regulate inflammatory responses | LPS-induced septic shock |
| Phosphatidylserine | Phospholipid | Bind on soluble RAGE | Assists in the clearance of apoptotic cells | Diabetes and atherosclerosis |
Aβ: Amyloid-β; LPS: Lipopolysaccaride; RAGE: Receptor for advanced glycation end products.
RAGE is a cargo transporter [5,19] and a transmembrane cell-signaling receptor [1,20–23]. RAGE binds free Aβ and mediates pathophysiological cellular responses [1,5,21,22,24], including transport of circulating plasma Aβ across the blood–brain barrier (BBB) into the brain, oxidative stress and reduction in the cerebral blood flow (CBF) by increasing cerebral ET-1 levels and increasing levels of proinflammatory cytokines, such as TNF-α and IL-6 [5]. Thus, compounds that block or regulate Aβ/RAGE interaction at the BBB and/or cells within the brain may have multiple effects, such as reducing levels of oxidative stress, neuroinflammation and brain Aβ levels; this may improve CBF and learning and memory, all of which should have benefcial therapeutic effects in Alzheimer's disease (AD).
RAGE is also associated with other chronic diseases, including diabetes [24,25], cancer growth and metastasis [26], cardiovascular disease [27,28] and inflammation [29,30]. Pharmaceutical agents that antagonize RAGE or RAGE-knockout mice have suggested that blocking RAGE-mediated effects can slow the progression of neurodegeneration, chronic inflammation and oxidative stress [5,6]. Therefore, RAGE is a major therapeutic target for inhibiting pathophysiological consequences of ligand/RAGE interactions. Indeed, to antagonize ligand binding to RAGE, small compounds (FP-04494700; mono- and bi-cyclicazole derivatives) have been used in Phase II clinical trials for mild to moderate AD (ClinicalTrials. gov identifer: NCT00566397 [101]) and diabetes nephropathy (Clinicaltrials.gov identifier: NCT00287183 [102]). However, recently it was suggested that these studies in AD were ineffective but the reasons are unclear, at present. The results are not posted on ClinicalTrials.gov nor published in peer-reviewed journals. Despite this set-back, other RAGE blockers with a possible different site of interaction with RAGE, and therefore different mode of action, may be effective in regulating RAGE activity in a safe and efficacious manner.
Structure–function relationship of RAGE
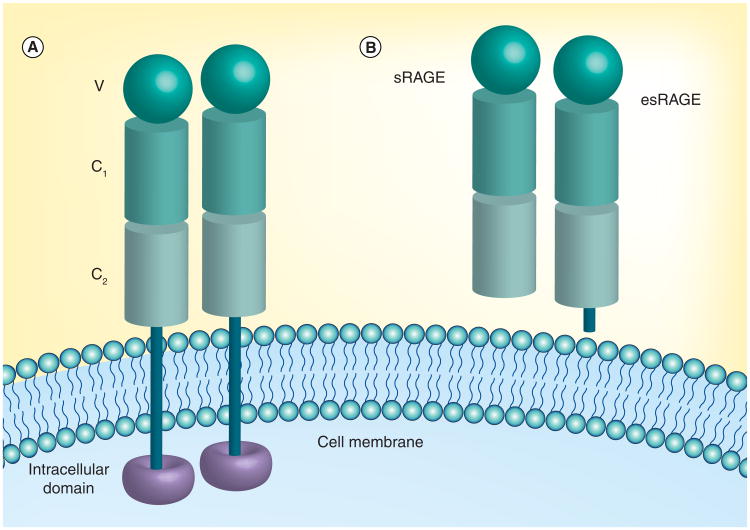
RAGE consists of three main regions: the extracellular domain (approximately amino acid residues 23–342) that interacts with the ligands, a transmembrane domain (residues 343–363) and a short intracellular domain (residues 364–404) involved in transmembrane signaling (Figure 1A). The extracellular region is composed of three immunoglobulin-like domains, a V-type domain and two C-type domains (C1 and C2) [31,32]. The amino acid sequences of the V-domain are residues 23–119, the C1-domain residues 120–233 and the C2-domain residues 234–325. High-resolution x-ray crystallography has shown that the V-C1 domains exist as an elongated structure at an angle (a bend) of 144/145° [2,33]. The V-C1 tandem domain is connected to the C2 domain by a flexible linker with a minimum of 7 amino acids [2,33]. The C2 region is structurally independent of the V-C1 domains. As a result, V-C1 forms a function unit with unique binding sites for most of RAGE ligands [2,3,33].
Figure 1. Structure and location of receptor for advanced glycation end products isoforms.
(A) Structure of RAGE. RAGE consists of three main regions: the extracellular domain that interacts with the ligands, a transmembrane domain and a short intracellular domain involved in transmembrane signaling. The extracellular region consists of three immunoglobulin-like domains, a V-type domain and two C-type domains (C1 and C2). RAGE may be oligomerized via the V-domain. (B) sRAGE. There are splice variants of the gene that encode for several forms of mutant RAGE, such as esRAGE or RAGE_V1, which contains a unique peptide at the C2-immunoglobulin domain. There is an sRAGE formed by ectodomain shedding due to the proteolytic action of matrix metallopeptidases and/or ADAM 10, presumably on the cell surface of RAGE.
esRAGE: Endogenous secretory receptor for advanced glycation end products; sRAGE: Soluble receptor for advanced glycation end products.
The forces involved in protein–protein interaction include hydrophobic, electrostatic, H-bond, disulfide bridge and van der Waals. The structural configuration of the V-C1 domains is held together by several interdomain hydrogen bonds and hydrophobic interactions [2,26]. For example, the amino acid Gln-119 in the V-type domain bind via a pseudo hydrogen bond with Tyr-150 in the C1-type domain. In addition, the carboxylate oxygen of Glu-94 in the V-type domain binds with the hydroxyl hydrogen of Tyr-150 in the C1-type domain via hydrogen bonds [2]. However, hydrogen bonds are not the only force keeping the V-type domain bonded to the C1-type domain; hydrophobic interactions exist as well. There are hydrophobic interactions between the side chain of Pro-215 from the C1-type domain and the side chain of Tyr-118, and between the side chain of Tyr-150 and Ile-91 from another area of the C1-type domain [2]. Thus, the V-C1 complex exists as an integrated rigid structural unit for the interaction with ligands [2,33,34].
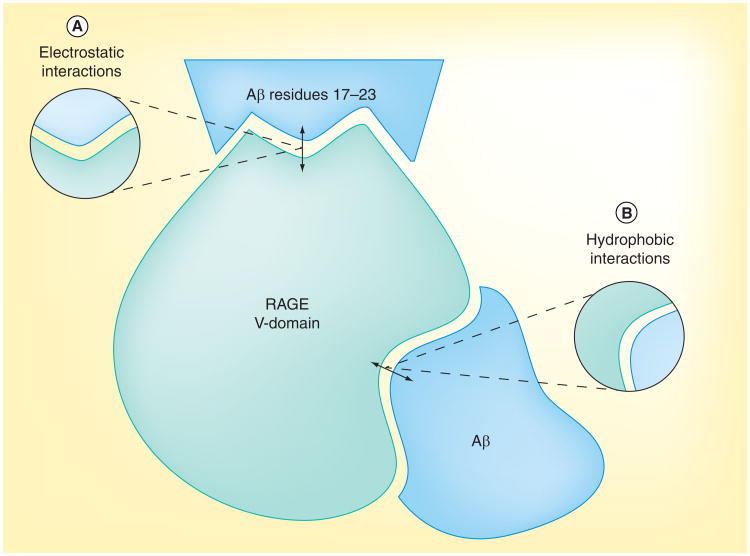
The V-C1 domains exist with some Gly and Pro residues in sequence that results in a loop structure with the occurrence of many Arg and Lys residues in the V-domain [2,3,33]. The large number of Arg and Lys residues means the V-domain is highly positively charged in these regions. Thus, there are elevated levels of ligand interaction in this binding domain that led to the theory that RAGE bind to ligands by electrostatic interaction, recognizing regions that are highly negative charged on the ligands. Thus, RAGE, a pattern recognition receptor [35–37], may recognize negative charge and/or hydrophobicity on its ligands. There are several positively charged patches on the V-domain of RAGE, including Arg-29, Lys-37, Lys-39, Lys-43, Lys-44, Arg-48, Lys-52, Arg-98, Arg-104, Lys-107, Lys-110, Arg-114 and Arg-116 [33,38,39], which may form two main regions [39] that interact with negatively charged regions of RAGE ligands (e.g., Aβ, S100 proteins, age-modified compounds and HMGB1) (Figure 2B). RAGE also has a number of hydrophobic pockets, which include residues Ile-26, Ala-28, Ile-30, Pro-33, Leu-34, Val-35, Leu-36, Trp-61, Val-63, leu-64, Trp-72, Val-75, Val-78, Leu-79, Pro-80, Phe-85, Leu-86, Pro-87, Ala-88, val-89, Ile 91 and Tyr-118 [33,39]. There is one hydrophobic cavity close to the C1 terminal formed by Ile-30, Pro-87, Ala-88, Ile-91 and Tyr-118 (Figure 2) [39].
Figure 2. Interaction sites between receptor for advanced glycation end products and its ligands.
Residues 17–23 (LVFFAED) of Aβ interact with the V-domain of RAGE. This is a highly hydrophobic string of residues flanked by two negatively charged residues (ED) in Aβ. (A) RAGE, a pattern recognition receptor, may recognize negatively charged regions on its ligands. There are several positively charged patches on the V-domain of RAGE, including Arg-29, Lys-37, Lys-39, Lys-43, Lys-44, Arg-48, Lys-52, Arg-98, Arg-104, Lys-107, Lys-110, Arg-114 and Arg-116, which may interact with negatively charged regions on Aβ. (B) RAGE also has a number of hydrophobic pockets, which includes residues Ile-26, Ala-28, Ile-30, Pro-33, Leu-34, Val-35, Leu-36, Trp-61, Val-63, leu-64, Trp-72, Val-75, Val-78, Leu-79, Pro-80, Phe-85, Leu-86, Pro-87, Ala-88, Val-89, Ile-91 and Tyr-118. There is one hydrophobic cavity close to the C1 terminal formed by Ile-30, Pro-87, Ala-88, Ile-91 and Tyr-118.
Aβ: Amyloid-β; RAGE: Receptor for advanced glycation end products.
The main ligands that bind to RAGE in the V-C1 ligand-binding pockets are AGEs, Aβ and S100 proteins [2]. While the molecular basis for the interaction of each ligand to RAGE is unclear, all RAGE ligands have a net negative charge at neutral pH and most tend to oligomerize [2,3]. At physiological pH, Aβ has a net negative charge. The surface of amyloid fibrils has patches of negative charges [2,3,16,35]. Residues 17–23 (LVFFAED) of Aβ interact with the V-domain of RAGE, a 30 amino acid residue N-terminal sequence (Figure 2A). This is a highly hydrophobic string of residues flanked by two negatively charged residues (ED) in Aβ [40]. Thus, compounds that interact with amino acid residues of the RAGE ligand-binding pockets, positively charged area or hydrophobic regions, may prevent Aβ interaction.
In the case of S100B, it is attracted to the receptor entropically by hydrophobic interactions dependent on calcium ions bound to S100B and on residues 54–67 of the V-domain [33,41]. To study the mechanism of RAGE–S100B interaction, isothermal titration calorimetry was used to better understand the thermodynamics of the interaction [33]. The isothermal titration calorimetry showed that the RAGE and S100B interaction features unfavorable positive enthalpy but favorable entropy [33]. Thus, the interaction is entropy driven. There are three factors that contribute to entropy-driven binding reactions: hydrophobic salvation effects, conformational changes in protein structure and changes in rotation and translation. Two of these factors are unfavorable; conformation changes within the protein and changes in rotation and translation. The only favorable factor is binding in the hydrophobic ligand-binding clefts in RAGE that interact with S100B dimer, tetrameric and octameric protein [33]. Thus, RAGE antagonists that penetrate the BBB and achieve effective therapeutic levels in the brain could regulate interaction of all RAGE ligands binding to RAGE, including Aβ and S100B.
In the case of AGEs, RAGE does not recognize the specific amino acids that are modified, but instead it may recognize the negatively charged patches of the protein surface of AGE. In the formation of AGEs, the positive charge located on the protein surface may be removed or the negatively charged area could be introduced [3,33,42]. RAGE can also bind directly to DNA and RNA via the sulfate region within RAGE, which allows the phosphate group on nucleic acids to bind to RAGE [33]. Thus, electrostatic and hydrophobic interactions appear to be the main forces involved in RAGE–ligand binding. Compounds that blocks ligand binding to RAGE may interact with the positive charged regions or the hydrophobic areas.
RAGE isoforms
While RAGE is encoded by one gene, AGER [4], there are splice variants of this gene that encode for several forms of mutant RAGE [32], such as endogenous secretory RAGE (esRAGE or RAGE_V1), which contains a unique peptide at the C2-immunoglobulin domain [6,8,29], and dominant negative RAGE, which is cell surface bound but lacks the cytoplasmic domain [3]. In addition, there is an sRAGE formed by ectodomain shedding due to the proteolytic action of matrix metallopeptidase and/or ADAM 10 presumably on the cell surface of RAGE (Figure 1B) [6,32]. In human plasma/serum, levels of sRAGE and esRAGE may be associated with inflammatory disorders, diabetes and vascular disorders [6,10,17]. Using in vivo models, it was shown that sRAGE can block the effects of RAGE ligands on levels of inflammatory markers and can protect against complications in diabetes and other chronic illnesses [5,6]. In a mouse model of AD, PD-hAPP, sRAGE increased CBF and reduced neuroinflammation and brain Aβ levels by acting as a decoy receptor that sequesters the Aβ and preventing it from interacting with membrane bound RAGE [5]. However, RAGE is known to self-associate and forms oligomers via the C1 domain [2,38]. Ligand binding may stabilize the RAGE oligomers that lead to transmembrane cell signaling. It is also possible that binding of sRAGE to cell membrane bound RAGE, due to RAGE hetero-oligomerization, prevents Aβ binding to the V-C1 domain [2,38]. Levels of sRAGE may play a role in chronic disorders, such as cardiovascular, inflammatory, diabetes and AD. As a result, RAGE blockers and sRAGE may be used to regulate the effect of RAGE activation, and hopefully prevent or delay the onset and progression of these chronic disorders. In addition to a potential therapeutic target, these soluble RAGE molecules could be potential biomarkers, but further work is needed to elucidate their specificity and sensitivity as biomarkers of AD.
RAGE & AD
Accumulation of Aβ in the brain is associated with AD but its role in the etiology of the disease is unclear [43,44]. In the aging brain, faulty Aβ clearance via low-density lipoprotein related-receptor 1 (LRP1) and/or increased Aβ influx into brain from the circulation, via RAGE-mediated transport across the BBB, may be responsible for its progressive accumulation in sporadic AD [45–51]. Thus, there is bidirectional transport of Aβ across the BBB. Transport from blood to brain is mediated by RAGE expressed on the luminal surface of the BBB [5,52,53]. In contrast, transport from brain to blood is mediated by LRP1 expressed mainly on the abluminal surface of the BBB [45,47,50]. The transport of Aβ from blood to brain by LRP2 is mediated by apoJ but this is saturated at blood levels of apoJ, and therefore, plays a negligible role in the control of brain Aβ levels [45,49].
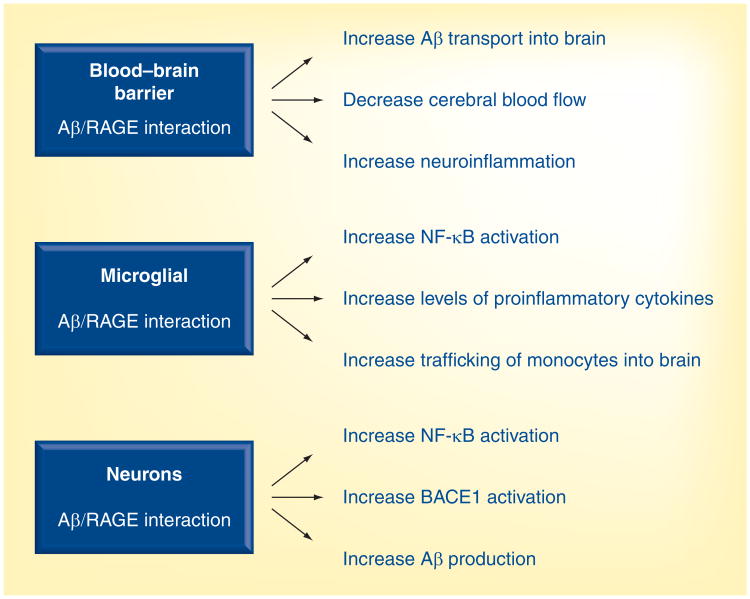
RAGE binds free Aβ in the low nanomolar range and mediates pathophysiological cellular responses [1,5,20–23]. These responses include transport of circulating plasma Aβ into the brain, oxidative stress, reduction in the CBF by increasing cerebral ET-1 levels and increasing levels of proinflammatory cytokines, such as TNF-α and IL-6 (Figure 3) [5]. In the brains of AD patients and mouse models of AD, RAGE expression is increased on cells of the neurovascular unit, particularly in an Aβ-rich environment [5,51–53]. Thus, compounds that block Aβ–RAGE interaction at the BBB and/or cells within the brain may have multiple actions, such as reducing neuroinflammation, improving CBF and cognitive decline, and reducing brain Aβ levels, which should have beneficial therapeutic effects in AD (Figure 3). In addition, RAGE antagonists that cross the BBB may reduce RAGE-mediated neuroinflammation, mainly via microglia, and Aβ generation, mainly by neurons, via inactivation of NF-κB [54] and BACE1 expression (Figure 3) [55].
Figure 3. Amyloid β/receptor for advanced glycation end products-mediated effects at the blood–brain barrier, microglia and neuron.
These effects will be regulated with new second-generation RAGE antagonists.
Aβ: Amyloid β RAGE: Receptor for advanced glycation end products.
In APPsw+/− mice and in AD patients, the increased levels of proinflammatory mediators in the brain are associated with the degree of Aβ deposits [56–59]. Microglia activity and their respond to Aβ deposits may be regulated by inflammatory cytokines [59,60–63]. While the role of microglia in the clearance of Aβ deposits is unclear, they are a major source of these cytokines [61,64,65]. Reducing RAGE-mediated activity in microglia may reduce the secretion of proinflammatory cytokines, via inhibition of NF-κB activation [1,54]. Consequently, microglia activity is reduced in the vicinity of Aβ deposits [64,65]. In addition to residential microglia, trafficking of blood-derived bone marrow stem cells or monocytes, which differentiate into microglia, would be affected by reducing the activity of RAGE at the BBB [66] or decreasing the secretion of chemotactic-attractant cytokines by microglia. In addition, reducing the levels of cytokines, such as TNF-α, a major proinflammatory cytokine secreted by microglia in AD and APPsw+/− mice, may reduce neuronal cell death [67,68]. Taken together, reducing neuroinflammation may improve neuronal function, which in turn, improves learning and memory [69]. Thus RAGE antagonists that enter the brain may be efficacious in regulating microglia activity. Studies are needed to establish the level of microglia activity that is needed to control the accumulation of Aβ. However, RAGE antagonists that regulate both the production of Aβ and microglia activity might be effective in controlling levels of brain Aβ (Figure 3).
RAGE may also induce AD-like effects through astrocytes and another RAGE ligand, S100B. Activated astrocytes produce acute-phase reactants and proinflammatory molecules (such as S100B), which decreases neuronal survival. The overexpression of S100B acts to accelerate AD [70,71]. S100B can cause neuronal damage through overexpression of NO as seen with elevated levels in AD models. S100B also influence neurite extension [71]. Thus, RAGE blockers may act on several cell types within the brain to reduce AD pathology and improve learning and memory.
RAGE as a therapeutic target
While RAGE is associated with many vascular and inflammatory disorders as well as AD, its normal function is unclear. Thus, regulating RAGE activity could be more efficacious than blocking its action. This can be achieved by using antagonists that interact with cell surface RAGE or the use of sRAGE to modulate RAGE activity. sRAGE may act as a decoy receptor, which reduces ligand binding to cell surface RAGE, and thereby regulating the activity of RAGE. It may also form hetro-oligomers by interacting with cell surface bound RAGE [2,38]. While the use of sRAGE as a potential therapeutic approach has been tested in rodent modes of AD and diabetes, its efficacy and safety in larger animal models is yet to be determined. The effects of oligmerization between membrane bound RAGE and sRAGE is unclear [2,38].
Recently, a small compound (FP-04494700) has been used to antagonize ligand binding to RAGE in Phase II clinical trials for mild to moderate AD (ClinicalTrials.gov identifier: NCT00566397 [101]), but this clinical trial was terminated. It was suggested that this RAGE blocker was ineffective but the reasons are unclear. It is important that the data are published so that second generation compounds or other antagonist can be developed that could regulate RAGE activity without toxic effects.
There are two groups of small molecules that have been used as blockers of RAGE–Aβ interaction. One compound is an azole derivative (FP-04494700) that was used in the Phase II clinical. The molecule weight for one of these compounds is approximately 532 Da, and it has one H-bond donor (assuming the tertiary amine is protonated at the physiological pH), three H-bond acceptors, a logP of 7.46 and a 2D polar surface area of 40.72 at physiological pH. These properties were estimated since details of the structure of this compound is not published. The other compound is a tertiary amide that is still in preclinical development. The molecule weight is 327 Da, and it has one H-bond acceptor, a logP of 4.309, 2D polar surface area of 20.31 Å2 at pH 7.4 and is uncharged at physiological pH. It appears as if the tertiary amide contains an electron-rich aromatic group, an electron-poor aromatic group and a hydrophobic group. It is possible that the hydrophobic group and/or the electron-rich aromatic group interact with RAGE. The IC50 for inhibiting Aβ–RAGE interaction for the azole compound is in the high nanomolar range, while it is in the low nanomolar range for the tertiary amide. Thus, the properties of these compounds are different, which may indicate that their mode of action is different. Details on their molecular basis for inhibition of ligand binding to RAGE are needed.
Recently, it was shown that a type of tertiary amide, identified by library screening, competitively antagonizes Aβ–RAGE interaction at the BBB, it also improves functional CBF responses to brain stimulation and cognition, and reduces brain Aβ levels in a mouse model of AD, APPsw−/+ mice. A smaller second generation analog, with greater BBB permeability and potency than the parent compound, was more effective in reducing the levels of proinflammatory cytokines and Aβ production by inhibiting NF-κB nuclear translocation and BACE1 activity, respectively. In turn, this improved CBF responses to brain stimulation and cognitive deficits, especially in APPsw+/− mice with prominent Aβ deposits. These multiple beneficial effects of this class of compound may have therapeutic applications, especially in AD [72-76]. During production of this review on RAGE an article was published on the preclinical studies using the tertiary amide in a mouse model of AD [77].
Small organic chemical molecules may be more feasible given the cost associated with GMP production. Oral administration is convenient and compliance is good. The major unmet need in the AD therapeutic market is for disease-modification and -prevention drugs. RAGE is still a potential target in the development of therapies to regulate levels of brain Aβ, and in AD.
Future perspective
The following approaches should be applied in order to further develop RAGE as a therapeutic target:
While the clinical trials (Phase II) with an azole derivative were terminated, it does not mean that RAGE blockers, in general, would not work. The reasons for the publicized failure are unknown, since the data were not published. Nevertheless, given the complexities of the RAGE structure and its ability to bind ligands that are structurally unrelated, it is possible that other RAGE blockers or second-generation compounds with a different mode of action may work. Further work is needed to explore the use of RAGE blockers. It is possible that a second-generation derivative of the azole compound or new generation of chemical compounds may be effective. A type of tertiary amide, which has different chemical properties to the azole, blocks Aβ–RAGE interaction with higher affinity and therefore needs further investigation. The search for a treatment of AD must continue, although the risk of failure in clinical trials seems high;
The recent studies using x-ray crystallography to identify the molecular basis of ligand/RAGE interaction should lead to a better understanding on the mechanism for binding of multiple ligands to RAGE. This technique could be used to identify the site or sites of interaction of RAGE blockers to RAGE, and therefore the molecular basis for controlling RAGE activity. This should lead to the development of more specific and effective RAGE antagonists of Aβ;
Detailed pharmacokinetics are needed to establish bioavailability, metabolism and half-life (t½) in plasma and tissues, such as brain;
Further studies are needed in larger animal models of AD to demonstrate safety and efficacy before progression to clinical trials;
In addition, the normal role of RAGE needs to be addressed further. RAGE knockout mice appear to have increased home cage activity and enhanced sensitivity to auditory stimulation [78]. Rather than blocking Aβ–RAGE interaction, it might be possible to modulate its interaction;
Role of small organic molecules on oligomerization of RAGE and its effect on transmembrane cell signaling may help in determining the effectiveness of this therapy;
Since activation of microglia may be a good and bad thing, its levels may need to be regulated but not blocked. The levels of RAGE blockers that enter the brain may need to be controlled to ensure that microglia activity is not completely blocked. This may also be the case for Aβ production by neurons, since the normal function of brain Aβ remains unclear.
Executive summary.
Background
Receptor for advanced glycation end products (RAGE) is a multiligand receptor that binds structurally unrelated ligands. It is present as a cell surface bound receptor and as soluble RAGE (sRAGE) in plasma.
The extracellular region is made up of three domains; V, C1 and C2. Raised levels of RAGE are associated with Alzheimer's disease (AD), inflammatory disorders and diabetes.
At the blood-brain barrier, amyloid-β (Aβ)-RAGE interaction causes a reduction in cerebral blood flow,neuroinflammation and increased levels of brain Aβ.
Thus, compounds that regulate Aβ–RAGE interaction could be beneficial in AD patients. However, a recent Phase II clinical trial was terminated, but the reason is unclear since the data are not published.
Structure–function relationship of RAGE
Recently, x-ray crystallography studies have revealed details of RAGE structure and possible sites for ligand interaction. These studies have shown that the V- and C1-domains function as a single fixed unit (V-C1 domain) while the C2-domain functions independently.
These studies have also shown that RAGE has patches of positive charge and regions of hydrophobicity that may interact with ligands. Thus, ligands with patches of negative charge or containing regions of hydrophobicity may interact with RAGE by pattern recognition.
Aβ has regions of negative charge and areas of hydrophobicity that may interact with RAGE. Thus, compounds that block the positive-charge patches on RAGE that interact with Aβ may prevent Aβ–RAGE-mediated effects. Also, compounds that block the hydrophobic regions on RAGE that interacts with Aβ may prevent Aβ–RAGE-mediated effects.
These are at least two ways by which RAGE blockers may interfere with the pattern recognition mechanism of Aβ/RAGE interaction.
RAGE isoform
There are many isoforms of RAGE. Cell membrane bound RAGE may interact, via the C1 domain, with itself forming self oligomerization, or with sRAGE forming hetero-oligomers. These oligomerizations may be enhanced with ligand-RAGE interactions that could stabilize the complex and mediate transmembrane cell signaling.
sRAGE maybe a possible biomarker.
The role of RAGE blockers on RAGE oligomerization is unclear.
RAGE & AD
Levels of RAGE are increased with aging and in AD brains. This would increase the transport of Aβ across the blood-brain barrier into brain, reduce cerebral blood flow and increase neuroinflammation. In the brain, RAGE may increase Aβ production by neuron, and neuroinflammation mainly by microglia.
Thus regulating Aβ/RAGE interaction with RAGE blockers may be beneficial in AD.
RAGE as a therapeutic target
To date there are two classes of small organic compound that have been shown to block Aβ–RAGE interaction: azole-derivatives and a type of tertiary amide.
Clinical trials (Phase II) with the azole compound were terminated, but the reasons for this are not published.
Preclinical studies with the tertiary amide in a mouse model of AD have shown that this compound was effective, but further work is needed before it should be used in clinical trials.
These two compounds are chemically different, and therefore there mechanism of action may also be different.
sRAGE may act as a decoy receptor that prevents ligands binding to the cell surface RAGE. It may do so by forming hetero-oligomers with cell surface bound RAGE, which could prevent ligand binding to cell surface RAGE.
Acknowledgments
The author wishes to thank BL Miller (University of Rochester, Departments of Biochemistry and Biophysics, and Department of Dermatology, USA) for information on the chemical properties of the azole and tertiary amide compounds. The author also wishes to thank R Deane (University of Rochester, Department of Neurosurgery, USA) for evaluation and suggestions on the manuscript.
Key Terms
- Blood–brain barrier
A cellular barrier that separates blood and brain by restricting the transport of polar molecules
- Neuroinflammation
An inflammatory response in brain that mobilizes the body immune mechanisms
- Alzheimer's disease
An irreversible, progressive brain disease that slowly destroys nerve cells, which leads to a loss in memory, thinking and behavior
- Neurodegeneration
Structural and functional loss of nerve cells
- RAGE blockers
Compounds that block the cell surface receptor called RAGE
Footnotes
For reprint orders, please contact reprints@future-science.com
Financial & competing interests disclosure: The author received funding from the NIH (grant number AG029481). The author has no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
■ of interest
- 1■.Yan SD, Chen X, Fu J, et al. RAGE and amyloid-beta peptide neurotoxicity in Alzheimer's disease. Nature. 1996;382(6593):685–691. doi: 10.1038/382685a0. Key paper on receptor for advanced glycation end products (RAGE)–amyloid-β (Aβ) in Alzheimer's disease. [DOI] [PubMed] [Google Scholar]
- 2■.Koch M, Chitayat S, Dattilo BM, et al. Structural basis for ligand recognition and activation of RAGE. Structure. 2010;18(10):1342–1352. doi: 10.1016/j.str.2010.05.017. Key paper on the molecular structure of RAGE and its interaction with ligands. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3■.Fritz G. RAGE: a single receptor fits multiple ligands. Trends Biochem Sci. 2011;36(12):625–632. doi: 10.1016/j.tibs.2011.08.008. Good review on RAGE–ligand interaction. [DOI] [PubMed] [Google Scholar]
- 4.Neeper M, Schmidt AM, Brett J, et al. Cloning and expression of a cell surface receptor for advanced glycosylation end products of proteins. J Biol Chem. 1992;267(21):14998–15004. [PubMed] [Google Scholar]
- 5■.Deane R, Du Yan S, Submamaryan RK, et al. RAGE mediates amyloid-beta peptide transport across the blood–brain barrier and accumulation in brain. Nat Med. 2003;9(7):907–913. doi: 10.1038/nm890. Key paper on RAGE/blood–brain barrier interaction. [DOI] [PubMed] [Google Scholar]
- 6■.Yan SF, Ramasamy R, Schmidt AM. Soluble RAGE: therapy and biomarker in unraveling the RAGE axis in chronic disease and aging. Biochem Pharmacol. 2010;79(10):1379–1386. doi: 10.1016/j.bcp.2010.01.013. Reviews RAGE as a therapeutic target. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmidt AM, Vianna M, Gerlach M, et al. Isolation and characterization of two binding proteins for advanced glycosylation end products from bovine lung which are present on the endothelial cell surface. J Biol Chem. 1992;267(21):14987–14997. [PubMed] [Google Scholar]
- 8.Ramasamy R, Yan SF, Schmidt AM. Advanced glycation endproducts: from precursors to RAGE: round and round we go. Amino Acids. 2010;42(4):1151–1161. doi: 10.1007/s00726-010-0773-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leclerc E, Fritz G, Vetter SW, Heizmann CW. Binding of S100 proteins to RAGE: an update. Biochim Biophys Acta. 2009;1793(6):993–1007. doi: 10.1016/j.bbamcr.2008.11.016. [DOI] [PubMed] [Google Scholar]
- 10■.Ramasamy R, Yan SF, Schmidt AM. RAGE: therapeutic target and biomarker of the inflammatory response – the evidence mounts. J Leukoc Biol. 2009;86(3):505–512. doi: 10.1189/jlb.0409230. Develops the possibility of RAGE as a therapeutic target. [DOI] [PubMed] [Google Scholar]
- 11.Pullerits R, Brisslert M, Jonsson IM, Tarkowski A. Soluble receptor for advanced glycation end products triggers a proinflammatory cytokine cascade via beta2 integrin Mac-1. Arthritis Rheum. 2006;54(12):3898–3907. doi: 10.1002/art.22217. [DOI] [PubMed] [Google Scholar]
- 12.He M, Kubo H, Morimoto K, et al. Receptor for advanced glycation end products binds to phosphatidylserine and assists in the clearance of apoptotic cells. EMBO J. 2011;12(4):358–364. doi: 10.1038/embor.2011.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamamoto Y, Harashima A, Saito H, et al. Septic shock is associated with receptor for advanced glycation end products ligation of LPS. J Immunol. 2011;186(5):3248–3257. doi: 10.4049/jimmunol.1002253. [DOI] [PubMed] [Google Scholar]
- 14.Sousa MM, Yan SD, Stern D, Saraiva MJ. Interaction of the receptor for advanced glycation end products (RAGE) with transthyretin triggers nuclear transcription factor κB (NF-κB) activation. Lab Invest J Tech Methods Pathol. 2000;80(7):1101–1110. doi: 10.1038/labinvest.3780116. [DOI] [PubMed] [Google Scholar]
- 15.Monteiro FA, Cardoso I, Sousa MM, Saraiva MJ. In vitro inhibition of transthyretin aggregate-induced cytotoxicity by full and peptide derived forms of the soluble receptor for advanced glycation end products (RAGE) FEBS Lett. 2006;580(14):3451–3456. doi: 10.1016/j.febslet.2006.05.020. [DOI] [PubMed] [Google Scholar]
- 16■.Sturchler E, Galichet A, Weibel M, Leclerc E, Heizmann CW. Site-specific blockade of RAGE-Vd prevents amyloid-beta oligomer neurotoxicity. J Neurosci. 2008;28(20):5149–5158. doi: 10.1523/JNEUROSCI.4878-07.2008. Reports that V domain interacts with Aβ. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17■.Raucci A, Cugusi S, Antonelli A, et al. A soluble form of the receptor for advanced glycation endproducts (RAGE) is produced by proteolytic cleavage of the membrane-bound form by the sheddase a disintegrin and metalloprotease 10 (ADAM10) FASEB J. 2008;22(10):3716–3727. doi: 10.1096/fj.08-109033. Discusses soluble RAGE. [DOI] [PubMed] [Google Scholar]
- 18.Kalea AZ, Reiniger N, Yang H, Arriero M, Schmidt AM, Hudson BI. Alternative splicing of the murine receptor for advanced glycation end-products (RAGE) gene. FASEB J. 2009;23(6):1766–1774. doi: 10.1096/fj.08-117739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mackic JB, Stins M, Mccomb JG, et al. Human blood–brain barrier receptors for Alzheimer's amyloid-beta 1–40 Asymmetrical binding, endocytosis, and transcytosis at the apical side of brain microvascular endothelial cell monolayer. J Clin Invest. 1998;102(4):734–743. doi: 10.1172/JCI2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arancio O, Zhang HP, Chen X, et al. RAGE potentiates Abeta-induced perturbation of neuronal function in transgenic mice. EMBO J. 2004;23(20):4096–4105. doi: 10.1038/sj.emboj.7600415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yan SD, Zhu H, Zhu A, et al. Receptor-dependent cell stress and amyloid accumulation in systemic amyloidosis. Nat Med. 2000;6(6):643–651. doi: 10.1038/76216. [DOI] [PubMed] [Google Scholar]
- 22.Yan SD, Bierhaus A, Nawroth PP, Stern DM. RAGE and Alzheimer's disease: a progression factor for amyloid-beta-induced cellular perturbation? J Alzheimers Dis. 2009;16(4):833–843. doi: 10.3233/JAD-2009-1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baiguera S, Fioravanzo L, Grandi C, Di Liddo R, Parnigotto PP, Folin M. Involvement of the receptor for advanced glycation-end products (RAGE) in beta-amyloid-induced toxic effects in rat cerebromicrovascular endothelial cells cultured in vitro. Int J Mol Med. 2009;24(1):9–15. doi: 10.3892/ijmm_00000199. [DOI] [PubMed] [Google Scholar]
- 24.Ramasamy R, Yan SF, Schmidt AM. The RAGE axis and endothelial dysfunction: maladaptive roles in the diabetic vasculature and beyond. Trends Cardiovasc Med. 2005;15(7):237–243. doi: 10.1016/j.tcm.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 25.Liu LP, Hong H, Liao JM, et al. Upregulation of RAGE at the blood–brain barrier in streptozotocin-induced diabetic mice. Synapse. 2009;63(8):636–642. doi: 10.1002/syn.20644. [DOI] [PubMed] [Google Scholar]
- 26.Allmen EU, Koch M, Fritz G, Legler DF. V domain of RAGE interacts with AGEs on prostate carcinoma cells. Prostate. 2008;68(7):748–758. doi: 10.1002/pros.20736. [DOI] [PubMed] [Google Scholar]
- 27.Yamagishi S, Nakamura K, Matsui T. Regulation of advanced glycation end product (AGE)-receptor (RAGE) system by PPAR-gamma agonists and its implication in cardiovascular disease. Pharm Res. 2009;60(3):174–178. doi: 10.1016/j.phrs.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 28■.Yan SF, Ramasamy R, Schmidt AM. The RAGE axis: a fundamental mechanism signaling danger to the vulnerable vasculature. Circ Res. 2010;106(5):842–853. doi: 10.1161/CIRCRESAHA.109.212217. Reviews the RAGE–ligand axis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29■.Yan SF, Naka Y, Hudson BI, et al. The ligand/RAGE axis: lighting the fuse and igniting vascular stress. Curr Atheroscler Rep. 2006;8(3):232–239. doi: 10.1007/s11883-006-0078-9. Discusses RAGE and vascular stress. [DOI] [PubMed] [Google Scholar]
- 30.Schmidt AM, Sahagan B, Nelson RB, Selmer J, Rothlein R, Bell JM. The role of RAGE in amyloid-beta peptide-mediated pathology in Alzheimer's disease. Curr Opin Investig Drugs. 2009;10(7):672–680. [PubMed] [Google Scholar]
- 31.Hudson BI, Hofmann MA, Bucciarelli L, et al. Glycation and diabetes: the RAGE connection. Curr Sci. 2002;83(12):1515–1521. [Google Scholar]
- 32.Hudson BI, Carter AM, Harja E, et al. Identification, classification, and expression of RAGE gene splice variants. FASEB J. 2008;22(5):1572–1580. doi: 10.1096/fj.07-9909com. [DOI] [PubMed] [Google Scholar]
- 33■.Park H, Adsit FG, Boyington JC. The 1.5 Å crystal structure of human receptor for advanced glycation endproducts (RAGE) ectodomains reveals unique features determining ligand binding. J Biol Chem. 2010;285(52):40762–40770. doi: 10.1074/jbc.M110.169276. Describes RAGE structure and its ligand binding. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dattilo BM, Fritz G, Leclerc E, Kooi CW, Heizmann CW, Chazin WJ. The extracellular region of the receptor for advanced glycation end products is composed of two independent structural units. Biochemistry. 2007;46(23):6957–6970. doi: 10.1021/bi7003735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haupt C, Bereza M, Kumar ST, et al. Pattern recognition with a fibril-specific antibody fragment reveals the surface variability of natural amyloid fibrils. J Mol Biol. 2011;408(3):529–540. doi: 10.1016/j.jmb.2011.02.032. [DOI] [PubMed] [Google Scholar]
- 36.Chavakis T, Bierhaus A, Al-Fakhri N, et al. The pattern recognition receptor (RAGE) is a counterreceptor for leukocyte integrins: a novel pathway for inflammatory cell recruitment. J Exp Med. 2003;198(10):1507–1515. doi: 10.1084/jem.20030800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37■.Xie J, Reverdatto S, Frolov A, Hoffmann R, Burz DS, Shekhtman A. Structural basis for pattern recognition by the receptor for advanced glycation end products (RAGE) J Biol Chem. 2008;283(40):27255–27269. doi: 10.1074/jbc.M801622200. Paper on RAGE as a pattern receptor. [DOI] [PubMed] [Google Scholar]
- 38■.Sarkany Z, Ikonen TP, Ferreira-Da-Silva F, Saraiva MJ, Svergun D, Damas AM. Solution structure of the soluble receptor for advanced glycation end products (sRAGE) J Biol Chem. 2011;286(43):37525–37534. doi: 10.1074/jbc.M111.223438. Describes RAGE–ligand interactions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xue J, Rai V, Singer D, et al. Advanced glycation end product recognition by the receptor for AGEs. Structure. 2011;19(5):722–732. doi: 10.1016/j.str.2011.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40■.Gospodarska E, Kupniewska-Kozak A, Goch G, Dadlez M. Binding studies of truncated variants of the Aβ peptide to the V-domain of the RAGE receptor reveal Aβ residues responsible for binding. Biochim Biophys Acta. 2011;1814(5):592–609. doi: 10.1016/j.bbapap.2011.02.011. Discusses RAGE–Aβ interaction. [DOI] [PubMed] [Google Scholar]
- 41.Leclerc E, Sturchler E, Vetter SW. The S100B/RAGE axis in Alzheimer's disease. Cardiovasc Psychiatry Neurol. 2010;2010:539581. doi: 10.1155/2010/539581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yonekura H, Yamamoto Y, Sakurai S, Watanabe T, Yamamoto H. Roles of the receptor for advanced glycation endproducts in diabetes-induced vascular injury. J Pharmacol Sci. 2005;97(3):305–311. doi: 10.1254/jphs.cpj04005x. [DOI] [PubMed] [Google Scholar]
- 43.Querfurth HW, Laferla FM. Alzheimer's disease. N Engl J Med. 2010;362(4):329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- 44.Hardy J. The amyloid hypothesis for Alzheimer's disease: a critical reappraisal. J Neurochem. 2009;110(4):1129–1134. doi: 10.1111/j.1471-4159.2009.06181.x. [DOI] [PubMed] [Google Scholar]
- 45.Deane R, Wu Z, Zlokovic BV. RAGE (yin) versus LRP (yang) balance regulates alzheimer amyloid beta-peptide clearance through transport across the blood–brain barrier. Stroke. 2004;35(11 Suppl. 1):2628–2631. doi: 10.1161/01.STR.0000143452.85382.d1. [DOI] [PubMed] [Google Scholar]
- 46.Zlokovic BV, Yamada S, Holtzman D, Ghiso J, Frangione B. Clearance of amyloid beta-peptide from brain: transport or metabolism? Nat Med. 2000;6(7):718–719. doi: 10.1038/77397. [DOI] [PubMed] [Google Scholar]
- 47.Shibata M, Yamada S, Kumar SR, et al. Clearance of Alzheimer's amyloid-ss(1–40) peptide from brain by LDL receptor-related protein-1 at the blood–brain barrier. J Clin Invest. 2000;106(12):1489–1499. doi: 10.1172/JCI10498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tanzi RE, Moir RD, Wagner SL. Clearance of Alzheimer's Abeta peptide: the many roads to perdition. Neuron. 2004;43(5):605–608. doi: 10.1016/j.neuron.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 49.Zlokovic BV. The blood–brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57(2):178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 50.Deane R, Wu Z, Sagare A, et al. LRP/amyloid beta-peptide interaction mediates differential brain efflux of Abeta isoforms. Neuron. 2004;43(3):333–344. doi: 10.1016/j.neuron.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 51.Donahue JE, Flaherty SL, Johanson CE, et al. RAGE, LRP-1, and amyloid-beta protein in Alzheimer's disease. Acta Neuropathol. 2006;112(4):405–415. doi: 10.1007/s00401-006-0115-3. [DOI] [PubMed] [Google Scholar]
- 52.Miller MC, Tavares R, Johanson CE, et al. Hippocampal RAGE immunoreactivity in early and advanced Alzheimer's disease. Brai Res. 2008;1230:273–280. doi: 10.1016/j.brainres.2008.06.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Silverberg GD, Miller MC, Messier AA, et al. Amyloid deposition and influx transporter expression at the blood–brain barrier increase in normal aging. J Neuropathol Exp Neurol. 2010;69(1):98–108. doi: 10.1097/NEN.0b013e3181c8ad2f. [DOI] [PubMed] [Google Scholar]
- 54.Paris D, Patel N, Quadros A, et al. Inhibition of Abeta production by NF-kappaB inhibitors. Neurosci Lett. 2007;415(1):11–16. doi: 10.1016/j.neulet.2006.12.029. [DOI] [PubMed] [Google Scholar]
- 55.Bourne KZ, Ferrari DC, Lange-Dohna C, Rossner S, Wood TG, Perez-Polo JR. Differential regulation of BACE1 promoter activity by nuclear factor-kappaB in neurons and glia upon exposure to beta-amyloid peptides. J Neurosci Res. 2007;85(6):1194–1204. doi: 10.1002/jnr.21252. [DOI] [PubMed] [Google Scholar]
- 56.Benzing WC, Wujek JR, Ward EK, et al. Evidence for glial-mediated inflammation in aged APP(SW) transgenic mice. Neurobiol Aging. 1999;20(6):581–589. doi: 10.1016/s0197-4580(99)00065-2. [DOI] [PubMed] [Google Scholar]
- 57.Sly LM, Krzesicki RF, Brashler JR, et al. Endogenous brain cytokine mRNA and inflammatory responses to lipopolysaccharide are elevated in the Tg2576 transgenic mouse model of Alzheimer's disease. Brain Res Bull. 2001;56(6):581–588. doi: 10.1016/s0361-9230(01)00730-4. [DOI] [PubMed] [Google Scholar]
- 58.Akiyama H, Arai T, Kondo H, Tanno E, Haga C, Ikeda K. Cell mediators of inflammation in the Alzheimer disease brain. Alzheimer Dis Assoc Disord. 2000;14(Suppl. 1):S47–S53. doi: 10.1097/00002093-200000001-00008. [DOI] [PubMed] [Google Scholar]
- 59.Wyss-Coray T. Inflammation in Alzheimer disease: driving force, bystander or beneficial response? Nat Med. 2006;12(9):1005–1015. doi: 10.1038/nm1484. [DOI] [PubMed] [Google Scholar]
- 60.Frautschy SA, Yang F, Irrizarry M, et al. Microglial response to amyloid plaques in APPsw transgenic mice. Am J Pahol. 1998;152(1):307–317. [PMC free article] [PubMed] [Google Scholar]
- 61.Hickman SE, Allison EK, El Khoury J. Microglial dysfunction and defective beta-amyloid clearance pathways in aging Alzheimer's disease mice. J Neurosci. 2008;28(33):8354–8360. doi: 10.1523/JNEUROSCI.0616-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Grathwohl SA, Kalin RE, Bolmont T, et al. Formation and maintenance of Alzheimer's disease beta-amyloid plaques in the absence of microglia. Nat Neurosci. 2009;12(11):1361–1363. doi: 10.1038/nn.2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Reed-Geaghan EG, Savage JC, Hise AG, Landreth GE. CD14 and toll-like receptors 2 and 4 are required for fibrillar A{beta}-stimulated microglial activation. J Neurosci. 2009;29(38):11982–11992. doi: 10.1523/JNEUROSCI.3158-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Koenigsknecht-Talboo J, Landreth GE. Microglial phagocytosis induced by fibrillar beta-amyloid and IgGs are differentially regulated by proinflammatory cytokines. J Neurosci. 2005;25(36):8240–8249. doi: 10.1523/JNEUROSCI.1808-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.El Khoury J, Toft M, Hickman SE, et al. Ccr2 deficiency impairs microglial accumulation and accelerates progression of Alzheimer-like disease. Nat Med. 2007;13(4):432–438. doi: 10.1038/nm1555. [DOI] [PubMed] [Google Scholar]
- 66.Giri R, Shen Y, Stins M, et al. Beta-amyloid-induced migration of monocytes across human brain endothelial cells involves RAGE and PECAM-1. Am J Physiol Cell Physiol. 2000;279(6):C1772–C1781. doi: 10.1152/ajpcell.2000.279.6.C1772. [DOI] [PubMed] [Google Scholar]
- 67.Janelsins MC, Mastrangelo MA, Park KM, et al. Chronic neuron-specific tumor necrosis factor-alpha expression enhances the local inflammatory environment ultimately leading to neuronal death in 3×Tg-AD mice. Am J Pathol. 2008;173(6):1768–1782. doi: 10.2353/ajpath.2008.080528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Park KM, Yule DI, Bowers WJ. Tumor necrosis factor-alpha-mediated regulation of the inositol 1,4,5-trisphosphate receptor promoter. J Biol Chem. 2009;284(40):27557–27566. doi: 10.1074/jbc.M109.034504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fan R, Xu F, Previti ML, et al. Minocycline reduces microglial activation and improves behavioral deficits in a transgenic model of cerebral microvascular amyloid. J Neurosci. 2007;27(12):3057–3063. doi: 10.1523/JNEUROSCI.4371-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mori T, Koyama N, Arendash GW, Horikoshi-Sakuraba Y, Tan J, Town T. Overexpression of human S100B exacerbates cerebral amyloidosis and gliosis in the Tg2576 mouse model of Alzheimer's disease. Glia. 2010;58(3):300–314. doi: 10.1002/glia.20924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Villarreal A, Aviles Reyes RX, Angelo MF, Reines AG, Ramos AJ. S100B alters neuronal survival and dendrite extension via RAGE-mediated NF-kappaB signaling. J Neurochem. 2011;117(2):321–332. doi: 10.1111/j.1471-4159.2011.07207.x. [DOI] [PubMed] [Google Scholar]
- 72.Deane R, Singh I, Sagare AP, et al. Tertiary amide preferentially blocks Aβ/RAGE interaction without causing toxicity. Presented at: Society for Neuroscience; Washington, DC, USA: Nov, 2011. pp. 12–16. [Google Scholar]
- 73.Deane R, Singh I, Sagare A, et al. Tertiary amide reduced BACE1 activity and Aβ production in a mouse model of AD. Presented at: Society for Neuroscience; San Diego, CA, USA: Nov, 2010. pp. 13–17. [Google Scholar]
- 74.Deane R, Singh I, Love R, et al. Differential action of lipophilic and hydrophilic tertiary amide-based RAGE/Aβ blockers in a mouse model of AD. Presented at: Society for Neuroscience; Chicago, IL, USA: Nov, 2009. pp. 17–21. [Google Scholar]
- 75.Deane R, Sagare A, Paquette N, et al. Tertiary amide improves cerebral blood flow and behavioral performance in a mouse model of AD by blocking RAGE/Aβ interaction. Presented at: Society for Neuroscience; Washington, DC, USA: Nov, 2008. pp. 15–19. [Google Scholar]
- 76.Deane R, Perry S, Sagare A, et al. Tertiary amides block RAGE-mediated Aβ transport into brain in a mouse model of AD. Presented at: Society for Neuroscience; Atlanta, GA, USA: Oct, 2006. pp. 14–18. [Google Scholar]
- 77■.Deane R, Singh I, Sagare AP, et al. A multimodal RAGE-specific inhibitor reduces amyloid β-mediated brain disorder in a mouse model of Alzheimer disease. J Clin Invest. 2012;122(4):1377–1392. doi: 10.1172/JCI58642. Key paper on a type of tertiary amide as a RAGE blocker. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sakatani S, Yamada K, Homma C, et al. Deletion of RAGE causes hyperactivity and increased sensitivity to auditory stimuli in mice. PLoS ONE. 2009;4(12):e8309. doi: 10.1371/journal.pone.0008309. [DOI] [PMC free article] [PubMed] [Google Scholar]
Websites
- 101.A Phase II Study Evaluating the Efficacy and Safety of PF 04494700 in Mild to Moderate Alzheimer's Disease. http://clinicaltrials.gov/ct2/show/NCT00566397.
- 102.6-Month Safety and Efficacy Study of TTP488 in Patients With Type 2 Diabetes and Persistent Albuminuria. http://clinicaltrials.gov/ct2/show/NCT00287183.