Abstract
BACKGROUND
Omega-3 fatty acids from fish oil have been associated with beneficial cardiovascular effects but their role in modifying cardiac structures and tissue characteristics in patients who have suffered an acute myocardial infarction (MI) while receiving current guideline-based therapy remains unknown.
METHODS
In a multicenter, double-blind, placebo-controlled trial, participants presenting with an acute MI were randomized 1:1 to 6-months of high-dose omega-3 fatty acids (n=178) or placebo (n=180). Cardiac magnetic resonance imaging was used to assess cardiac structure and tissue characteristics at baseline and following study therapy. The primary study endpoint was change in left ventricular systolic volume index (LVESVI). Secondary endpoints included change in non-infarct myocardial fibrosis, LVEF, and infarct size.
RESULTS
By intention-to-treat analysis, patients randomized to omega-3 fatty acids experienced a significant reduction of LVESVI (−5.8%, P=0.017), and non-infarct myocardial fibrosis (−5.6%, P=0.026) compared with placebo. Per-protocol analysis revealed that those subjects who achieved the highest quartile increase in RBC omega-3 index experienced a 13% reduction in LVESVI as compared with the lowest quartile. In addition, patients in the omega-3 fatty acid arm underwent significant reductions in serum biomarkers of systemic and vascular inflammation and myocardial fibrosis. There were no adverse events associated with high-dose omega-3 fatty acid therapy.
CONCLUSIONS
Treatment of acute MI patients with high-dose omega-3 fatty acids was associated with reduction of adverse LV remodeling, non-infarct myocardial fibrosis, and serum biomarkers of systemic inflammation beyond current guideline-based standard of care.
CLINICAL TRIAL REGISTRATION
URL: http://www.clinicaltrials.gov. Unique identifier: NCT00729430.
Keywords: Omega-3 fatty acids, left ventricular remodeling, myocardial fibrosis, cardiac magnetic resonance imaging, infarct size
INTRODUCTION
Pre-clinical cardiovascular benefits of omega-3 fatty acids from fish oil1, 2 (O-3FA) have been evaluated in large-scale clinical trials in patients suffering an acute myocardial infarction (MI).3, 4 The Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico (GISSI)-Prevenzione open-label trial randomized 11,324 patients to 1 gram/day of O-3FA versus placebo and observed a 20% mortality reduction for O-3FA therapy.3 However, with advances in acute infarct care, the reported incremental benefits of O-3FA therapy have been inconsistent.4 Cardiac magnetic resonance imaging (CMR) offers accurate serial quantification of left ventricular (LV) structure and function, infarct size, and extracellular matrix expansion within non-infarcted myocardium.5 The Omega-3 Acid Ethyl Esters on Left Ventricular Remodeling After Acute Myocardial Infarction (OMEGA-REMODEL) trial is a prospective, multicenter, double-blind, placebo-controlled trial designed to evaluate the hypothesis that 4 grams/day of O-3FA for 6 months after acute MI attenuates adverse LV remodeling beyond optimal standard of care.
METHODS
Patients
Patients were enrolled across 3 tertiary-care centers in Boston, Massachusetts (Brigham and Women’s, Massachusetts General, and Beth Israel Deaconess Medical Center hospitals) who were > 21 years of age and presented with an acute MI based on a) symptoms consistent with an acute coronary syndrome, b) serial Troponin I (or T) profile consistent with acute injury and peak level > 0.5 ng/ml, and c) significant angiographic coronary stenosis. Patient recruitment occurred between June 2008 to August 2012. Exclusion criteria included MI secondary to cardiac procedure, life expectancy < 1-year, clinical indication for O-3FA treatment, active pregnancy, and absolute contraindications to CMR. All patients received standard medical therapy per discretion of the attending cardiologists. The institutional review board at each enrolling site approved the study and all patients provided informed consent.
Study Design and Randomization
The National Institutes of Health provided sole funding for this study, while GlaxoSmithKline (Research Triangle Park, NC) provided study medication (O-3FA and placebo). The investigational pharmacies of the enrolling centers randomized patients 1:1 to either O-3FA or placebo using a 2×2 blocked randomization scheme for age (> 70 years age) and anterior MI location in double-blinded fashion. Computer generated randomization codes were used by the investigational pharmacies for blocked randomization. Pre-treatment and post-treatment visits occurred at 14–28 days and 6-months after index acute MI, respectively. Study visits included collection of coronary risk profile, detailed events of index infarction, adverse events, standardized lifestyle and dietary questionnaires, contrast-enhanced CMR, and blood samples. All procedures during study visits were conducted or overseen in person by a physician investigator.
Study Intervention and Monitoring
During the pre-treatment visit, enrolled patients received 6-month supplies of study drug and were instructed to take 4 one-gram capsules per day with meals. Study drug was either Lovaza®, containing ethyl esters of eicosapentaenoic acid (EPA, ~465 mg) and docosahexaenoic acid (DHA, ~375 mg) (GlaxoSmithKline, Research Triangle Park, NC) or placebo, containing corn oil (600 mg linoleic acid, no O-3FA, and <0.05% of trans-fatty acids). All patients received lifestyle counseling, including dietary recommendations for standard post-MI care6 but no specific recommendations were given with regards to dietary O-3FA intake. All patients were instructed to refrain from consuming over-the-counter fish oil products. Every 2 months during the 6-month study drug period, an investigator conducted scripted telephone interviews with each subject and assessed for tolerance to study drug, adverse events, and pill counts.
Study Endpoints
Primary study endpoint was adverse LV remodeling measured as change in left ventricular end-systolic volume indexed to body surface area (LVESVI, ml/m2) by CMR after 6 months of study therapy. Secondary endpoints included changes in a) non-infarct myocardial fibrosis measured as the myocardial extracellular volume fraction remote from the acute infarction (ECVRemote), b) total infarct size, and c) left ventricular ejection fraction (LVEF). Sudden cardiac death during follow-up was an additional secondary endpoint at trial commencement, but removal was recommended by the Data Safety and Monitoring Board due to anticipated low number of events.
CMR
CMR studies were performed using 3.0 Tesla scanners (Trio or Verio, Siemens, Erlangen, Germany). The CMR protocol consisted of cine function, native and post-contrast myocardial T1 mapping, and late gadolinium enhancement (LGE) imaging. Myocardial T1 was measured using a look-locker gradient-echo sequence (3 short-axis locations centered mid-ventricle) acquired prior to and 5, 15, and 25 minutes after administration of 0.1 mmol/kg of intravenous gadolinium (Magnevist, Bracco). Image analyses using a commercial software (QMass®, Medis Inc., Raleigh, North Carolina) was performed blinded to clinical data, time order of CMR studies, and treatment assignment. Total infarct size was measured as infarct mass (in grams) and as percentage of total LV mass (from LGE images). Infarct mass was similar between 2 criterion (≥2 standard deviations beyond mean remote myocardial signal intensity and full-width half maximum criteria)7 and infarct mass values using ≥2 standard deviation criteria were then used in all analyses. Short-axis LGE and myocardial T1 images were segmented as per the American Heart Association 16-segment model.8 For each T1 Look-Locker acquisition, T1 was determined by non-linear least squares fitting of a parameterized representation of an inversion recovery (signal intensity = A – B·exp(-TI/T1*)) to the measured average signal intensity values in myocardial segments. T1 was then calculated from the best-fit parameters with the correction formula T1 = T1*·(B/A - 1).9 We derived segmental extracellular volume fraction (ECV) by plotting the reciprocal of T1 (R1=1/T1) for myocardial segments against the simultaneously measured R1 in the blood pool, using both pre- and post-contrast measurements where R1 in the blood pool was below 3.5 s−1. R1 data pairs with higher values of R1 in the blood pool were excluded from a linear regression line fit to the R1 data to avoid an underestimation of ECV as conditions of fast water-exchange may not be met.10 ECV was calculated from the slope of the linear regression line, i.e. the partition coefficient (λ), using the blood hematocrit (HCT): ECV= λ·(1-HCT).11 ECV segments without matching late enhancement were averaged to yield the global ECV of the remote, non-infarcted myocardium (ECVRemote).
Biomarkers and Omega-3 Fatty Acids
Blood samples were assayed for red blood cell (RBC) fatty acid levels (OmegaQuant Analytics, LLC, Sioux Falls, SD) and serum biomarkers (Health Diagnostic Laboratory, Inc., Richmond, Virginia) as follows: inflammation [C-reactive protein (CRP), myeloperoxidase (MPO), Lp-PLA2, Fibrinogen], neurohormonal activation [N-terminal prohormone brain natriuretic peptide (NT-proBNP), cystatin C], and cardiac fibrosis [ST-2, galectin-3]. RBC fatty acid composition, which has been shown to correlate with myocardial O-3FA levels and unbiased by recent dietary intake,12, 13 was evaluated using gas chromatography by flame ionization detection. The omega-3 index was calculated from the sum of DHA and EPA and expressed as a percentage of total RBC fatty acids.
Statistical Analysis
Descriptive statistics were calculated by treatment arm using mean +/− SD and median (1st quartile, 3rd quartile), for normal and skewed continuous variables, respectively. Categorical variables were presented as count (%) for each level. General linear mixed models (GLMM) were used to perform an intention-to-treat analysis that included patients missing follow-up visits for the primary and secondary endpoints.14 Restricted Maximum Likelihood (REML) estimation produces unbiased estimates under the assumption the missing responses are Missing At Random (MAR); i.e. the missing responses may be related to the observed responses, but are independent of the unobserved responses. This method alleviates the need for imputation. A compound symmetry correlation structure was used for the repeated measurements. As a sensitivity analysis, the mixed models included increasing levels of covariate adjustment; the initial model only included the randomization group assignment, an indicator variable for pre- or post-treatment visit, and their interaction. Age, gender, race, and clinical site were added to the model as fixed covariates, with CMR infarct size used to adjust for MI severity; RBC omega-3 index was included for pre-treatment exposure to fish oil. Lastly, medication status, coronary risk factors, and heart rate were added to the model. Residual diagnostics were performed to verify model assumptions. A per-protocol analysis was also conducted for all patients that completed both study visits, and the changes in RBC levels of EPA and DHA (summed and individually) were used as a biomarker-based measures of exposure to treatment. The changes in primary and secondary endpoints were regressed against the changes in RBC O-3FA levels (modeled separately as a continuous factor per 1 standard deviation increase and by quartiles using the first quartile as reference). In an exploratory analysis, fish oil randomization group assignment was used to predict changes in biomarkers of inflammation, neurohormonal activation, and cardiac fibrosis. All statistical analyses were performed using SAS (SAS Institutes, version 9.4, Cary, NC), and a p-value < 0.05 was used to ascribe statistical significance.
Power/Sample Size
The primary endpoint, change in LVESVI, was modeled as a log-normal distribution due to expected positive skewness. The coefficient of variation for LVESV in studies of LV dysfunction was reported as 26%.15 The correlation between measurements 6 months apart was assumed to be 0.7.16, 17 In order to have over 80% power and detect a 5% mean within-subject change in LVESV using a two-sided critical level of 0.05, a minimum of 129 patients were required in each arm. Estimating a 30% loss to follow-up and a 25% non-compliance rate, the recruitment goal was 202 patients per arm (N = 404).
RESULTS
Patients and Baseline Clinical Characteristics
Figure 1 illustrates study enrollment and randomization. Due to logistical issues, 3 patients deviated in study scheduling: 2 had pre-treatment visit at 5 days after index MI and 1 had post-treatment visit at 9 months. Baseline demographics stratified by treatment arm are shown in Table 1. Overall, 91% of patients achieved TIMI 3 flow within the infarct related artery and there was high adherence to all post-MI guideline-recommended18 therapies. In the overall cohort 73% of patients were treated with an angiotensin-converting-enzyme inhibitor or angiotensin II receptor blockers, as compared to 89% of those who had suffered an anterior STEMI (83% in placebo group and 94% in omega-3 group, P=0.20). Baseline CMR characteristics stratified by treatment arm are shown in Table 2, while both fatty acids and biomarker levels are shown in Table 3. Median infarct size (13 grams and 11% total LV mass) were similar in both treatment arms. Compared to published values from healthy controls,19, 20 pre-treatment non-infarct myocardial fibrosis of the total cohort was significantly higher (33.8±5.3, n=358 versus 24.8±2.0, n=14, P<0.0001),20 while pre-treatment mean O-3FA values were similar to those in the Framingham Offspring cohort.21 We examined test-retest reproducibility for measuring infarct size by LGE in 38 randomly selected patients and found a high intra-class correlation of 0.94 (95% CI 0.88–0.97). We have also shown high intra-class correlation for intraobserver, interobserver, and test-retest variability for ECV measurments.22
Figure 1. Enrollment and Randomization.
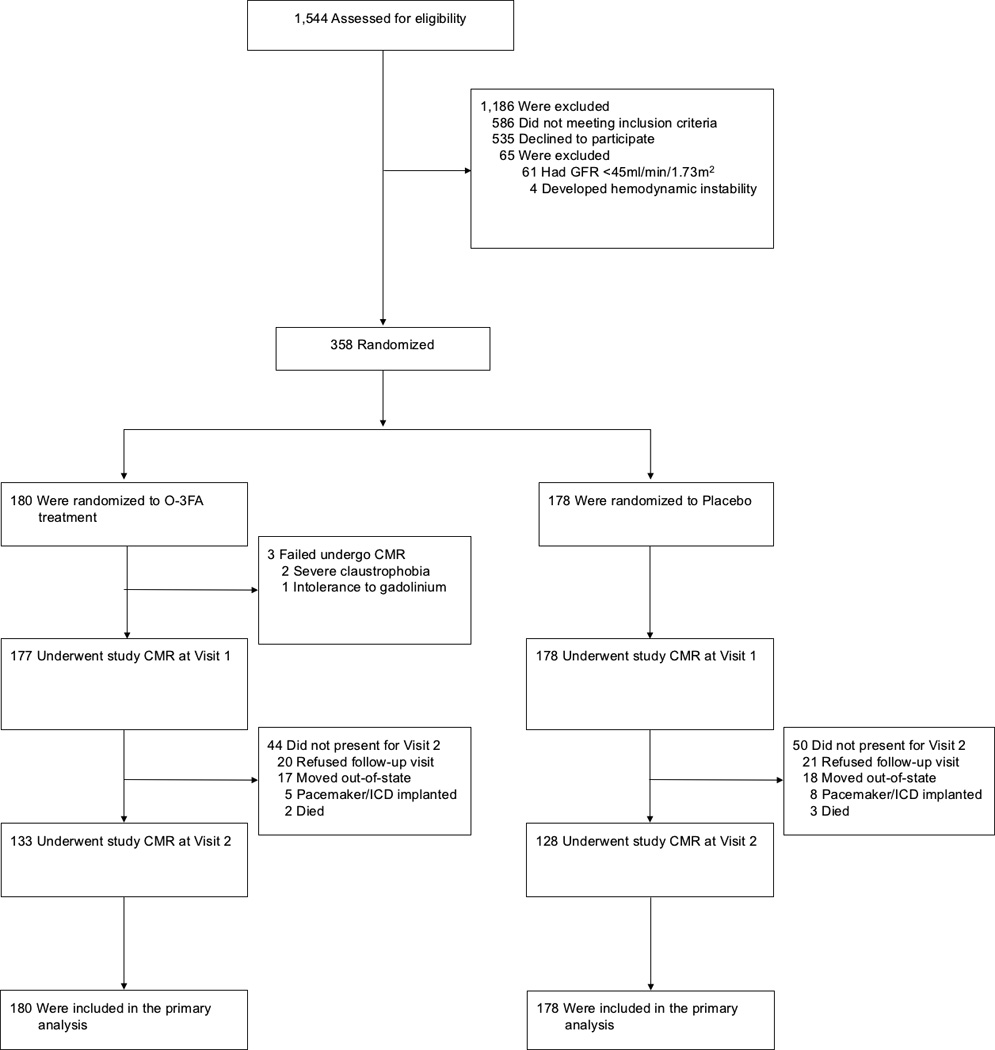
The treatment duration was 6 months for both randomized arms (between study visit 1 and 2). CMR denotes cardiac magnetic resonance imaging, ICD implantable cardioverter-defibrillator, O-3FA omega-3 fatty acids from fish oil.
Table 1.
Baseline Characteristics of the Intention-to-Treat Population*
| Characteristic | Omega-3 Fatty Acids (n = 180) |
Placebo (n = 178) |
P-Value |
|---|---|---|---|
| Demographics | |||
| Age - yr | 60 ± 10 | 58 ± 10 | 0.22 |
| Female sex – no. (%) | 32 (18) | 38 (21) | 0.39 |
| White race – no. (%) | 143 (81) | 146 (82) | 0.68 |
| Body mass index [kg/m2] | 29 ± 5.4 | 29 ± 5.6 | 0.92 |
| Body surface area [m2] | 2.0 ± 0.23 | 2.0 ± 0.22 | 0.82 |
| Heart rate [bpm]† | 64 (60, 71) | 66 (60, 71) | 0.26 |
| Systolic BP [mm Hg] | 121 ± 15 | 120 ± 16 | 0.73 |
| Diastolic BP [mm Hg] | 70 ± 10 | 70 ± 11 | 0.62 |
| Enrolling sites – no. (%) | 0.57 | ||
| Brigham and Women’s | 115 (64) | 109 (61) | |
| Massachusetts General | 38 (21) | 33 (19) | |
| Beth Israel Deaconess | 27 (15) | 36 (20) | |
| Index Event | |||
| STEMI – no. (%) | 102 (57) | 105 (59) | 0.66 |
| Anterior – no. (%) | 48 (27) | 48 (27) | 1.00 |
| TIMI 3 flow achieved – no. (%) | 145 (91) | 156 (91) | 0.99 |
| Troponin-T (peak) [µmol/L]† | 2.8 (0.9, 9.1) | 3.4 (0.8, 10.4) | 0.72 |
| Creatine kinase (peak) [U/L]† | 786 (330, 1608) | 693 (296, 1621) | 0.74 |
| Creatine kinase MB (peak) [U/L] | 61 (26, 152) | 61 (21, 148) | 0.97 |
| Hematocrit (%)† | 39 (36, 42) | 40 (36, 43) | 0.10 |
| Cardiovascular Disease History | |||
| Angina – no. (%) | 44 (25) | 36 (20) | 0.30 |
| Peripheral vascular disease – no. (%) | 7 (4) | 13 (7) | 0.17 |
| Myocardial infarction – no. (%) | 22 (12) | 14 (8) | 0.16 |
| CABG – no. (%) | 24 (13) | 11 (6) | 0.02 |
| PCI – no. (%) | 24 (13) | 23 (13) | 0.91 |
| Congestive heart failure – no. (%) | 4 (2) | 6 (3) | 0.52 |
| NYHA class – no. (%) | 0.37 | ||
| 1 | 167 (94) | 160 (90) | |
| 2 | 10 (5.5) | 17 (9.5) | |
| 3 | 1 (0.5) | 1 (0.5) | |
| Hypercholesterolemia – no. (%) | 134 (75) | 120 (67) | 0.10 |
| Diabetes mellitus – no. (%) | 46 (26) | 45 (25) | 0.90 |
| Hypertension – no. (%) | 118 (66) | 112 (63) | 0.51 |
| Smoker (current) – no. (%) | 23 (13) | 36 (20) | 0.06 |
| Medications | |||
| Dual antiplatelet – no. (%)γ | 174 (98) | 174 (98) | 1.00 |
| Beta-blocker – no. (%) | 163 (92) | 164 (92) | 0.85 |
| Statin – no. (%) | 172 (97) | 171 (96) | 0.78 |
| Calcium channel blocker – no. (%) | 16 (9) | 10 (6) | 0.22 |
| ACE inhibitor or ARB – no. (%) | 134 (75) | 127 (71) | 0.40 |
| Aldosterone antagonists – no. (%) | 0 (0) | 1 (1) | 0.91 |
| Insulin – no. (%) | 18 (10) | 15 (8) | 0.57 |
| Nitroglycerin – no. (%) | 25 (14) | 19 (11) | 0.33 |
| Diuretics – no. (%) | 25 (14) | 18 (10) | 0.33 |
ACE denotes angiotensin converting enzyme, ARB angiotensin receptor blocker, BP blood pressure, NYHA New York Heart Association, PCI percutaneous coronary intervention, and STEMI ST-elevation myocardial infarction. Beta-blocker is defined as β-adrenergic–receptor antagonist, and statin is defined as hydroxymethylglutaryl-coenzyme A reductase inhibitor.
Continuous data are means ± SD if normally distributed, otherwise median (25th, 75th percentile).
Dual antiplatelet therapy included aspirin plus either clopidogrel or prasugrel.
Natural logarithm transformation was used to improve normality and/or homoscedasticity of residuals, prior to performing Student’s t-tests.
Table 2.
Baseline CMR Characteristics of the Intention-to-Treat Population
| Characteristic | Omega-3 Fatty Acids (n = 180) |
Placebo (n = 178) |
P-Value |
|---|---|---|---|
| LVESVI [mL/m2]† | 37 (30, 45) | 35 (27, 82) | 0.29 |
| ECVRemote [%]¶ | 34.3 ± 5.6 | 33.3 ± 4.9 | 0.11 |
| Infarct size [grams] using 2SD† | 13 (6, 23) | 13 (6, 24) | 0.43 |
| Infarct size [grams] using FWHM† | 13 (6, 22) | 12 (5, 23) | 0.45 |
| LVEF [%] | 54 ± 9 | 54 ± 10 | 0.48 |
| LVEDVI [mL/m2] | 84 ± 20 | 82 ± 21 | 0.45 |
| RVEF [%] | 53 ± 6 | 53 ± 8 | 0.85 |
| RVESVI [mL/m2] | 33 ± 9 | 34 ± 11 | 0.48 |
| RVEDVI [mL/m2] | 71 ± 17 | 72 ± 19 | 0.41 |
| Infarct percent (% LV mass) using 2SD† | 11 (6, 21) | 12 (5, 21) | 0.62 |
| Infarct percent (%LV mass) using FWHM† | 12 (6, 19) | 11 (5, 20) | 0.65 |
| LV mass index [g/m2] | 60 ± 14 | 59 ± 15 | 0.34 |
| LV mass/volume [g/mL] | 0.74 ± 0.18 | 0.74 ± 0.20 | 0.95 |
LV was defined as left ventricular, LVEDVI left ventricular end-diastolic volume index, LVEF left ventricular ejection fraction, LVESVI left ventricular end-systolic volume index, RVEDVI right ventricular end-diastolic volume index, RVEF right ventricular ejection fraction, and RVESVI right ventricular end-systolic volume index.
Continuous variables are expressed as means ± SD if normally distributed, otherwise median (25th, 75th percentile).
ECVRemote was the extracellular volume fraction of myocardium remote from the infarction, an estimate of non-infarct fibrosis.
Natural logarithm transformation was used to improve normality and/or homoscedasticity of residuals, prior to performing Student’s t-tests.
Table 3.
Baseline Omega-3 Fatty Acid and Biomarker Levels of Intention-to-Treat Population
| Characteristic | Omega-3 Fatty Acids (n = 180) |
Placebo (n = 178) |
P-Value |
|---|---|---|---|
| Fatty Acids (RBC % of total) | |||
| Omega-3 index | 5.5 ± 1.8 | 5.7 ± 1.7 | 0.45 |
| DHA (C22:6n3) | 4.7 ± 1.3 | 4.9 ± 1.4 | 0.29 |
| EPA (C20:5n3)† | 0.63 (0.47. 0.90) | 0.64 (0.51, 0.89) | 0.67 |
| DPA (C22:5n3) | 2.94 ± 0.48 | 2.94 ± 0.42 | 0.49 |
| α-Linolenic (C18:3n3) | 0.12 ± 0.04 | 0.12 ± 0.04 | 0.71 |
| Arachidonic (C20:4n6) | 17.1 ± 1.7 | 17.2 ± 1.5 | 0.69 |
| Linoleic (C18:2n6) | 9.5 ± 1.5 | 9.4 ± 1.5 | 0.30 |
| Oleic (C18:1) | 13.9 ± 1.1 | 13.9 ± 1.1 | 0.90 |
| Inflammatory Biomarkers | |||
| Fibrinogen [g/L] | 405 (341, 522) | 407 (340, 499) | 0.83 |
| hsCRP [mg/L]† | 2.6 (1.3, 8.5) | 2.4 (1.0, 6.9) | 0.22 |
| Myeloperoxidase [ng/mL]† | 341 (265, 404) | 324 (264, 386) | 0.39 |
| Lp-PLA2 | 171 (140, 200) | 164 (135, 194) | 0.25 |
| Neurohormonal Activation Biomarkers | |||
| NT-proBNP [ng/L]† | 526 (244, 1086) | 460 (224, 881) | 0.27 |
| Cystatin C [mg/dL]† | 1.0 (0.9, 1.2) | 1.0 (0.9, 1.2) | 0.52 |
| GFR [mL/min per 1.73 m2]† | 82 (61, 101) | 84 (66, 102) | 0.54 |
| Cardiac Strain Biomarkers | |||
| ST2 [ng/mL]† | 35 (27, 43) | 36 (29, 43) | 0.23 |
| Galectin-3 [ng/mL]† | 16 (12, 19) | 15 (13, 18) | 0.78 |
| Lipid Levels | |||
| Total Cholesterol [mg/dL] | 129 (107, 148) | 127 (109, 151) | 0.94 |
| LDL-C [mg/dL] | 69 (54, 86) | 66 (54, 84) | 0.46 |
| HDL-C [mg/dL] | 42 (36, 49) | 42 (36, 50) | 0.94 |
| Triglycerides [mg/dL]† | 120 (84, 161) | 121 (92, 183) | 0.26 |
DHA docosahexanoic acid, DPA docosapentaenoic acid, EPA eicosapentanoic acid, GFR glomerular filtration rate, HDL-C high-density lipoprotein cholesterol, hsCRP high sensitivity C-reactive protein, LDL-C low-density lipoprotein cholesterol, Lp-PLA2 lipoprotein-associated phospholipase A2, NT-proBNP N-terminal prohormone brain natriuretic peptide, and RBC red blood cell.
Continuous data are expressed as means ± SD if normally distributed, otherwise median (25th, 75th percentile).
Natural logarithm transformation was used to improve normality and/or homoscedasticity of residuals, prior to performing Student’s t-tests.
Treatment Effects
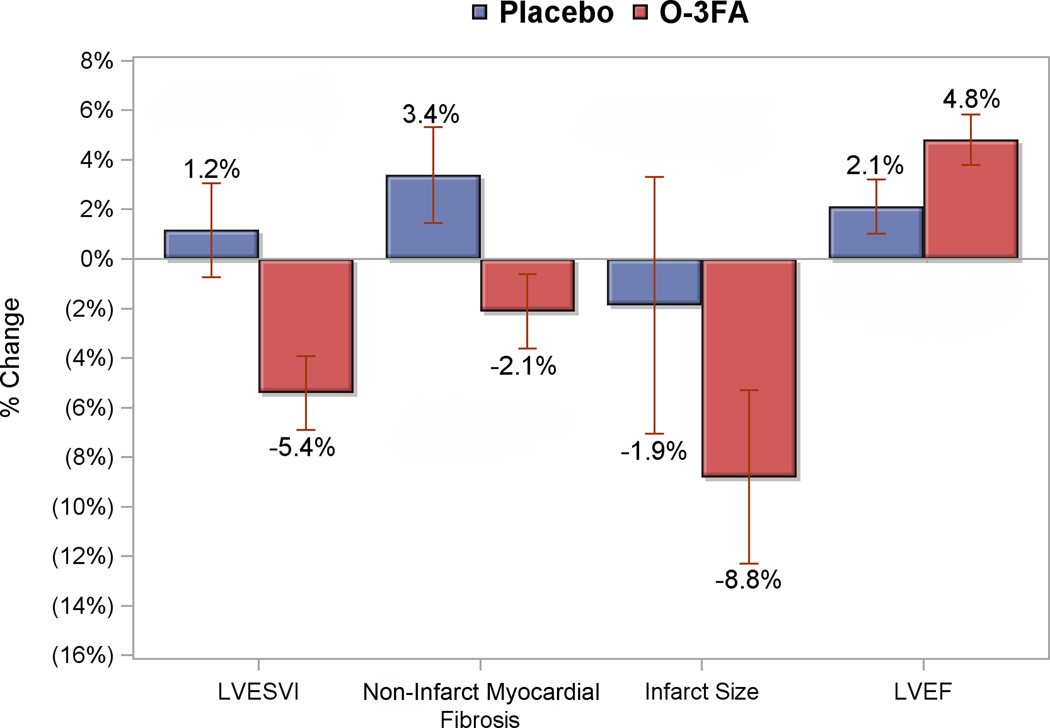
Based on pill counts, compliance to study drug was 96% in both O-3FA and placebo groups (P=0.86). Changes in RBC fatty acid levels are shown in Figure 2. Patients who received O-3FA treatment experienced marked increases in RBC levels of EPA, DHA, and omega-3 index in addition to a decrease in arachidonic acid compared with placebo (all P<0.0001). The greatest impact of O-3FA treatment was on RBC EPA and omega-3 index, which were increased by 256% and 81%, respectively. Figure 3 illustrates the primary and secondary endpoints stratified by treatment assignment. Patients who received O-3FA experienced a mean reduction of LVESVI by 5.4%, compared to a mean 1.2% expansion in the placebo group (P=0.0068). O-3FA patients experienced a mean regression of non-infarct myocardial fibrosis by 2.1%, compared to a mean 3.4% progression in the placebo group (P=0.026). There was a marginally significant difference towards improved LVEF in the O-3FA treated group (4.8 ± 11.3% versus 2.1 ± 12.2%, P=0.073). Although both groups experienced a reduction of infarct size, these reductions were not statistically different between the groups (-8.8 ± 39.9% versus −1.9 ± 57.7%, P=0.27). Intention-to-treat and per-protocol analyses for the mean effects of O-3FA on the primary and secondary endpoints are shown in Table 4. Compared to placebo, O-3FA treatment was associated with a mean −5.8% (95% CI −10.3% to −1.1%, P=0.017) and −6.6% (95% CI −11.3% to −1.8%, P=0.007) reduction in LVESVI by intention-to-treat and per-protocol analyses, respectively. In addition, O-3FA treatment was associated with a significant reduction of non-infarct myocardial fibrosis. Compared to placebo, O-3FA treatment was associated with a mean −5.6% (95% CI −10.4% to −0.9%, P=0.022) and −5.5% (95% CI −10.4% to −0.6%, P=0.026) reduction in non-infarct myocardial fibrosis by intention-to-treat and per-protocol analyses, respectively. There was no significant effect of O-3FA treatment on change in infarct size or LVEF in the intention-to-treat or per-protocol analyses. To remove the potential confounding effect of prior MI, we performed similar intention-to-treat and per-protocol analyses after excluding 36 patients with a history of prior MI. As shown in Supplemental Table 1, O-3FA treatment was associated with a strong and significant reduction of LVESVI and non-infarct myocardial fibrosis in both intention-to-treat and per-protocol analyses in the 322 patients without a history of prior MI.
Figure 2. Percent Change of Fatty Acid Levels from Baseline to Post Treatment.
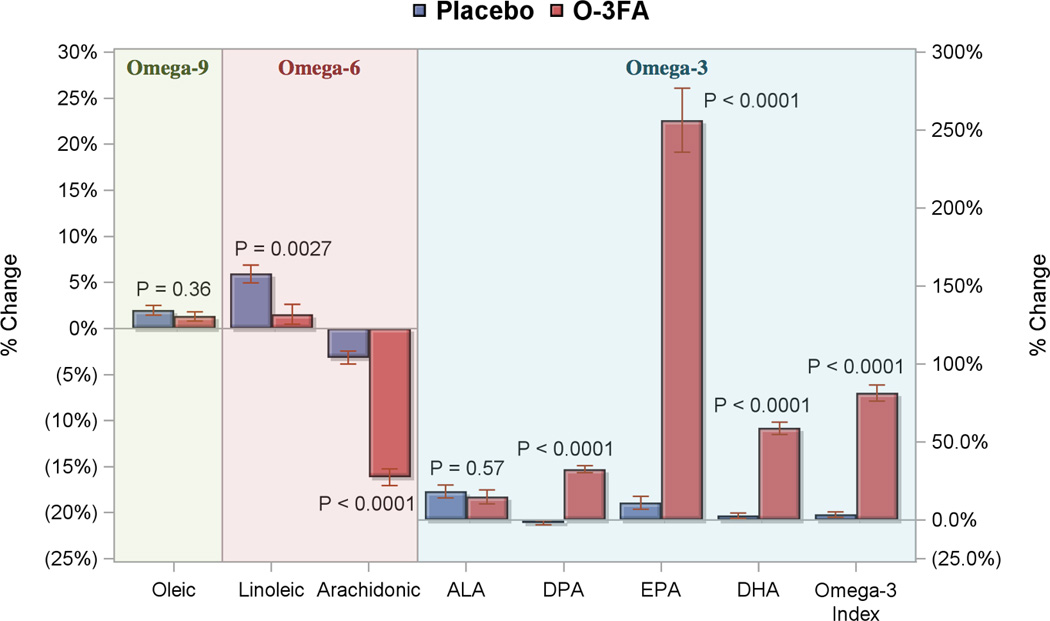
Percent changes from baseline to post treatment levels of red blood cell omega-3 fatty acid are shown for the omega-3 fatty acid treated group (red bars) and placebo arm (blue bars). P values are for comparisons of percent change in red blood cell fatty acid levels between the randomized treatment arms. ALA denoted α-Linolenic acid, DHA docosahexanoic acid, DPA docosapentaenoic acid, EPA eicosapentanoic acid, and O-3FA omega-3 fatty acids from fish oil.
Figure 3. Percent Change of Primary and Secondary Endpoints Post Treatment.
Percent changes from baseline to post treatment of the primary and secondary endpoints are shown for the omega-3 fatty acid treated group (red bars) and placebo arm (blue bars). LVESVI denotes left ventricular end-systolic volume index, and LVEF left ventricular ejection fraction.
Table 4.
Six-month Effect (95% CI) of 4 g/d Lovaza Treatment versus Placebo in Post MI Patients by Intention-to-treat Analysis
| LVESVI | Non-Infarct Myocardial Fibrosis |
Infarct Size | LVEF | |
|---|---|---|---|---|
|
ITT Analysis (GLMM†) |
−5.8% (−10.3%, −1.1%) P = 0.017, N = 358 |
−5.6% (−10.4%, −0.9%) P = 0.022, N = 358 |
−3.4% (−17.8%, 13.6%) P = 0.68, N = 358 |
2.4% (−0.4%, 5.2%) P = 0.094, N = 358 |
|
Per Protocol Analysis (t-test‡) |
−6.6% (−11.3%, −1.8%) P = 0.0068, N = 247 |
−5.5% (−10.4%, −0.6%) P = 0.026, N = 171 |
−6.9% (−19.2%, 5.3%) P = 0.27, N = 254 |
2.7% (−0.3%, 5.6%) P = 0.073, N = 247 |
|
Fish Oil Absolute Change (95% CI) |
−2.6 (−3.8, −1.4) [mL/m2], N = 124 |
−1.3 (−2.5, −0.2) [%], N=84 |
−1.3 (−2.6, 0.0) [%], N=130 |
2.2 (1.3, 3.2) [%], N=124 |
|
Placebo Absolute Change (95% CI) |
−0.5, (−1.8, 0.9) [mL/m2], N=123 |
0.8 (−0.4, 2.1) [%], N=87 |
−1.6 (−2.9, −0.4) [%], N=124 |
0.7 (−0.5, 1.9) [%], N=123 |
CI was defined as confidence interval, ITT intention-to-treat, LVEF left ventricular ejection fraction, and LVESVI left ventricular end-systolic volume index.
The general linear mixed model (GLMM) produces unbiased estimates for responses with missing data (see statistical analysis). LVESVI and Infarct Size were natural logarithm transformed to reduce skewness and/or heteroscedasticity of residuals. Estimates are relative changes.
The per protocol analysis only included patients that attended both visits. No transformations were required, instead Satterthwaite approximation was used for heteroscedasticity. Estimates are relative changes.
The paired absolute changes are calculated on raw data without any transformations.
Additional covariate adjustment of O-3FA effects on primary and secondary outcome measures are shown in Supplemental Table 2. LVESVI reduction by O-3FA therapy remained significant when adjusted for fixed covariates, including age, gender, race, enrolling site, pre-treatment omega-3 index, and pre-treatment log transformed infarct mass by CMR (Model 1, −5.4% relative change from pre-treatment, P=0.03). The effect of O-3FA on change in LVESVI remained significant when guideline-based standard post-MI medical therapies, coronary risk factors, body mass index, and baseline heart rate were added to Model 1 (Model 2, −5.7% relative change from pre-treatment, P=0.021). Non-infarct myocardial fibrosis was also significantly reduced by O-3FA treatment after covariate adjustment for baseline characteristics, O-3FA levels, and infarct size (Model 1, −5.0% relative change from baseline, P=0.046). However, after adjusting for standard post-MI medical therapies, there was only a non-statistically significant trend for the treatment effect of O-3FA on non-infarct myocardial fibrosis (Model 2, −4.7% relative change from baseline, P=0.067).
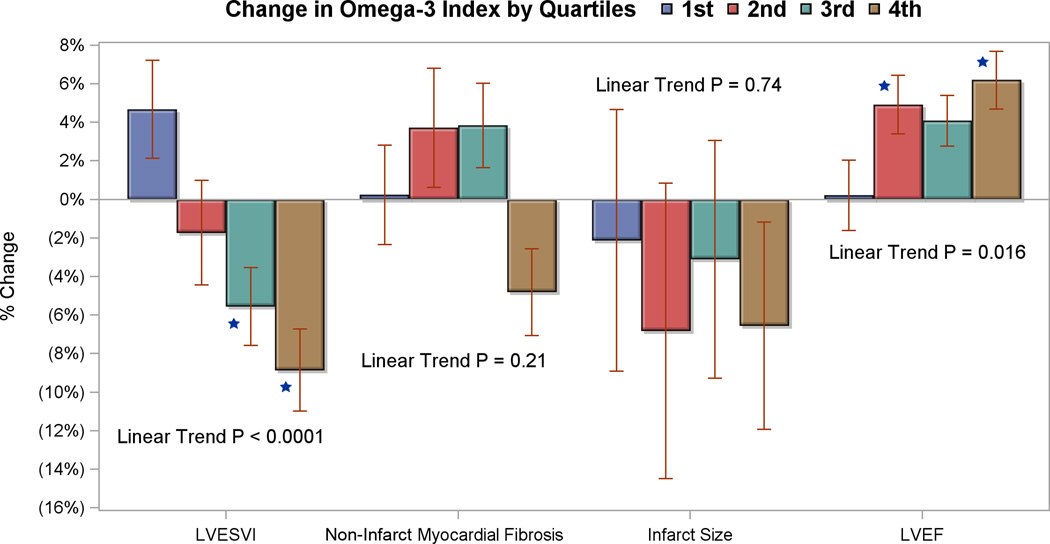
A dose-response relationship for O-3FA treatment was further evaluated in the subgroup of patients who completed both study visits per protocol (Table 5). Change in mean RBC levels of omega-3 index, DHA, and EPA were used as individual biomarkers of exposure to the intervention. For every 1 standard deviation increase in the mean RBC levels of omega-3 index and DHA there were significant reductions in LVESVI and non-infarct myocardial fibrosis, as well as an increase in LVEF. There were no significant associations between change in O-3FA levels and reduction of infarct size. Increases of mean RBC levels of EPA were only associated with a decrease in LVESVI. The strengths of the association between mean RBC levels of omega-3 index and DHA on the primary and secondary endpoints were evaluated using quartile analysis for the % change in RBC omega-3 index levels (Figure 4). Compared to the first quartile as reference, there was a graded significant change in LVESVI (linear trend P<0.0001) and LVEF (linear trend P=0.016), but not for either non-infarct myocardial fibrosis or infarct size.
Table 5.
Mean Percent Change in Primary and Secondary Endpoints per 1 SD Change in RBC Omega-3 Fatty Acids After 6 Months of Treatment
| LVESVI† N = 227 |
Non-Infarct Myocardial Fibrosis N = 157 |
Infarct Size† N = 232 |
LVEF N = 227 |
|
|---|---|---|---|---|
|
Δ Omega-3 Index (% RBC FA) (per 1 SD = 2.6%) |
−4.6% (−6.9%, −2.2%) p = 0.0002 |
−1.0% (−1.9%, −0.1%) p = 0.039 |
2.5% (−5.7%, 11.3%) p = 0.56 |
1.1% (0.3%, 1.9%) p = 0.0087 |
|
Δ DHA (% RBC FA) (per 1 SD = 1.6%) |
−5.2% (−7.5%, −2.8%) p < 0.0001 |
−1.1% (−2.1%, −0.2%) p = 0.013 |
1.0% (−7.0%, 9.7%) p = 0.81 |
1.2% (0.4%, 2.0%) p = 0.0031 |
|
Δ EPA (% RBC FA) (per 1 SD = 1.1%) |
−3.1% (−5.5%, −0.6%) p = 0.015 |
−0.5% (−1.5%, 0.4%) p = 0.25 |
4.4% (−3.9%, 13.4%) p = 0.31 |
0.7% (−0.1%, 1.5%) p = 0.078 |
DHA was defined as docosahexaenoic acid, EPA eicosapentaenoic acid, FA fatty acid, LVEF left ventricular ejection fraction, LVESVI left ventricular end-systolic volume index, RBC red blood cell, and SD standard deviation.
Natural logarithm transformation was used to improve normality and/or homoscedasticity of residuals.
Figure 4. Comparison of Percent Change in Study Endpoints with Quartiles of Change in Omega-3-Index Post Treatment for Patients that Completed Both Study Visits.
Percent changes from pre-treatment to post-treatment of LVESVI, non-infarct myocardial fibrosis, and LVEF versus quartile changes of the red blood cell omega-3 index levels for all patients who completed both study visits (n=227). The quartiles for change in the red blood cell omega-3 index were −0.6% to 0.5%, 0.5 to 2.6%, 2.6 to 5.8%, and > 5.8%. The 5th and 95th percentiles for change in the omega-3 index were −1.0% and 6.8%, respectively. * indicate p-value < 0.05 compared to first quartile (reference), linear trend p-values are also reported.
Effects of O-3FA Treatment on Biomarkers
By intention-to-treat analysis (Table 6), O-3FA treatment was associated with an 8.1% and 7.9% reduction in MPO and ST2, respectively. By per-protocol analysis (Table 6), O-3FA treatment was still associated with similar reductions of MPO and ST2 (9.3% and 8.3%, respectively). Figure 5 displays significant dose-response relationships between quartile increase of omega-3 index and progressive reductions of ST2, Lp-PLA2 and serum triglycerides. In O-3FA patients, reduction of ST2 demonstrated a strong correlation with reduction of non-infarct myocardial fibrosis (Figure 6, r=0.65, P<0.0001).
Table 6.
Six-month Effect of 4 g/d Lovaza Treatment versus Placebo on Blood Biomarkers in Post MI Patients
| Log Response | ITT Analysis N=358 |
P-value | Per Protocol Analysis N=216 |
P-value |
|---|---|---|---|---|
| hsCRP | −25% | 0.089 | −24% | 0.095 |
| Myeloperoxidase | −8.1% | 0.058 | −9.3% | 0.034 |
| LpPLA2 | −3.2% | 0.25 | −4.1% | 0.14 |
| Fibrinogen | −3.6% | 0.29 | −9.7% | 0.27 |
| NT-proBNP | −6.6% | 0.50 | −6.2% | 0.54 |
| CystatinC | 2.3% | 0.24 | 2.1% | 0.29 |
| ST2 | −7.9% | 0.030 | −8.3% | 0.026 |
| Galectin-3 | −4.6% | 0.10 | −4.5% | 0.12 |
| Triglycerides | −4.4% | 0.40 | −3.9% | 0.48 |
hsCRP was defined as high sensitivity C-reactive protein, ITT intention-to-treat, Lp-PLA2 lipoprotein-associated phospholipase A2, and NT-proBNP N-terminal prohormone brain natriuretic peptide.
Figure 5. Comparison of Percent Change in Systemic Biomarkers with Quartiles of Change in Omega-3-Index Post Treatment for Patients that Completed Both Study Visits.
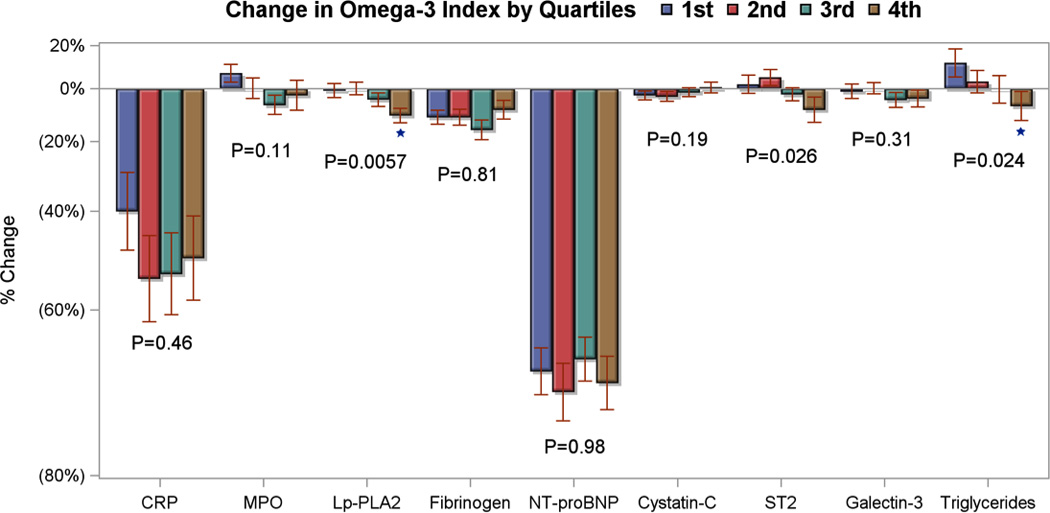
Percent changes from pre-treatment to post-treatment of systemic biomarkers versus quartile changes of the red blood cell omega-3 index levels for all patients who completed both study visits (n=227). The quartiles for change in the red blood cell omega-3 index were −0.6% to 0.5%, 0.5 to 2.6%, 2.6 to 5.8%, and > 5.8%. The 5th and 95th percentiles for change in the omega-3 index were −1.0% and 6.8%, respectively. * indicate p-value < 0.05 compared to first quartile (reference), linear trend p-values are also reported. CRP denotes high sensitivity C-reactive protein, Lp-PLA2 lipoprotein-associated phospholipase A2, and NT-proBNP N-terminal of the prohormone brain natriuretic peptide.
Figure 6. Scatter Plot of Percent Change in Serum Biomarker ST2 versus Percent Change of Non-Infarct Myocardial Fibrosis Post Treatment.
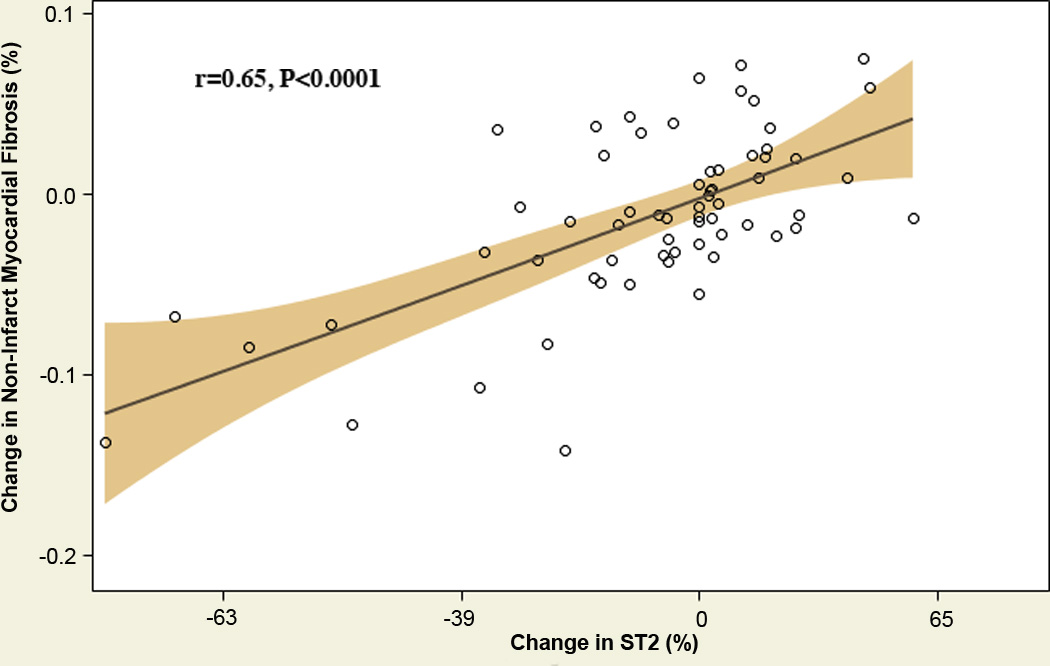
Percent change from baseline to post treatment of the serum biomarker ST2 correlated against percent change in non-infarct myocardial fibrosis following 6 months of treatment with high dose omega-3 fatty acids from fish oil. P-value is for Pearson correlation coefficient.
Patient Outcomes and Study Safety
The most common side effect in this study was nausea, which was reported in 5.9% of the O-3FA treated arm and 5.4% of the placebo arm (P=0.11). Only 4.8% of O-3FA treated patients reported fishy taste, which compared to 1.1% in placebo patients (P=0.04). No subject experienced significant bleeding related to study drug. Amongst the 11 patient who died, 8 who received fish oil treatment died at a median time of 24 months (range 12–37 months) after study enrollment. None of these 8 patients experienced any bleeding during the 6-months of fish oil treatment or experienced any drop in hematocrit during subsequent clinical visits. One O-3FA treated patient experienced tongue swelling one month after enrollment that necessitated study drug termination, which resulted in resolution of the subject’s symptoms. There were 3(2%) and 8(4%) deaths in placebo and O-3FA patients, respectively (P=0.22).
DISCUSSION
Compared to placebo, high-dose O-3FA treatment during the first 6 months following acute MI demonstrated significant reduction of LVESVI and non-infarct myocardial fibrosis in revascularized acute MI patients who are receiving standard guideline-based medical care. We observed that the degree of LVESVI reduction correlated with the degree of O-3FA incorporation into the RBC membrane suggesting RBC omega-3 index may serve as a useful marker of treatment efficacy. The results were highly suggestive of a dose-response relationship with patients in the highest omega-3 index quartile demonstrating the greatest reduction in adverse remodeling (13% reduction of LVESVI). O-3FA treatment was also associated with a significant reduction of both biomarkers of inflammation (MPO, Lp-PLA2) and myocardial fibrosis (ST2). We therefore speculate that O-3FA treatment provides the aforementioned improvement in LV remodeling and non-infarct myocardial fibrosis through suppression of inflammation at both systemic and myocardial levels during the convalescent healing phase following acute MI.
Similar to the OMEGA trial,4 patients in the current study had high adherence to current guideline-based post-MI treatments, including emergent percutaneous coronary revascularization. Contrary to the OMEGA and other O-3FA post-MI trials, the current study utilized a 4-fold higher dose of O-3FA that more closely resembles the doses administered in translational animal studies reporting beneficial cardiovascular effects.23 Numerous studies have reported that improvement of LVESVI during infarct convalescence remains the strongest favorable risk predictor, parallels reduction of post-MI mortality rates, and serves as a common mechanistic pathway for different classes of therapies that reduce mortality, sudden cardiac death, and heart failure incidence.24,25, 26 In the echocardiographic sub-study of the Survival and Ventricular Enlargement (SAVE) Trial, although captopril only reduced post-MI left ventricular end-systolic expansion by 4%, it was associated with a 45% reduction of patient mortality.27 The multicenter CAPRICORN trial reported that carvedilol reduced all-cause post-MI mortality by 20%,28 while the echocardiographic sub-study found only a 5.9% reduction of LVESV at 6-months.29 We hypothesize that the observed improvement in adverse LV remodeling by 5.7% beyond the current guideline-based post-MI therapies may be clinically relevant and requires prospective evaluation in trials adequately powered to assess the therapeutic effects of high dose O-3FA on patient outcomes.
The acute loss of myocardium post MI leads to a complex set of neurohormonal, genetic, and mechanical factors that can trigger adverse left ventricular remodeling within remote non-infarcted myocardium.30 In the early period following MI, inflammatory changes within the non-infarcted myocardium contribute to fibrotic changes, whereas increased wall stress and biomechanical strain in later phases contribute to further myocyte hypertrophy and extracellular matrix expansion. The results of this trial demonstrate potential mechanisms by which O-3FA may attenuate these adverse processes. Our observations that O-3FA treatment was associated with reduction of inflammation are consistent with translational studies that have shown a reduction of inflammatory cytokines by O-3FA exposure in animal and human myocardium post infarction.31–33 Furthermore, O-3FA treatment in this study reduced levels of serum ST, a biomarker that is upregulated in conditions of myocardial necrosis and dysfunction.34 ST2 antagonizes upregulation of interleukin-33, which has antihypertrophic and antifibrotic effects.35, 36 O-3FA treatment has also been shown to directly block cardiac fibroblast transformation, proliferation, and collagen synthesis through activation of the cyclic GMP/protein kinase G pathway.37 These mechanisms may collectively explain the attenuation of post-MI non-infarct myocardial fibrosis and adverse LV remodeling by high-dose O-3FA treatment found in this trial.
This study has several limitations. First, despite efforts of the investigators, a substantial proportion of patients could not return for the post-treatment follow-up visit. While this was distributed relatively evenly in both treatment arms, it remains uncertain where this caused any bias to the main study findings. Second, commercial forms of fish oils are widely available and, therefore, over-the-counter fish oil supplementation by patients could not be reliably eliminated and may have biased our results. However, the dose response relationship between O-3FA therapy and our main study endpoints strongly supported our intention-to-treat analysis. Finally, the absolute percent changes of LVESVI and extracellular volume fraction (a surrogate of non-infarct fibrosis) from O-3FA treatment, started at 2–4 weeks post-MI, were only modest compared to guideline clinical care. Earlier initiation of O-3FA during the first days post-MI may have resulted in a more significant treatment benefit. Prospective trial would be necessary to determine the effect of earlier O-3FA therapy on improving cardiac remodeling, myocardial tissue characteristics, and clinical outcomes.
In conclusion, our study demonstrated a beneficial effect for high dose O-3FA treatment on adverse left ventricular remodeling following acute MI in patients receiving modern, guidelines-based therapies. This finding was supported by the attenuation of concurrent fibrosis within non-infarcted myocardium and lower levels of systemic biomarkers of myocardial inflammation and cardiac fibrosis.
Supplementary Material
Clinical Perspective.
What is new?
Large-scale randomized trials of acute MI patients had reported inconsistent mortality benefits from omega-3 fatty acids (1-g daily). Using cardiac magnetic resonance imaging, the randomized placebo-controlled OMEGA-REMODEL study investigated for cardiac remodeling benefits from O-3FA in acute MI patients who were receiving therapies per current treatment guidelines.
Compared to placebo, patients who received 4-g O-3FA daily experienced significant improvement in both LV end-systolic volume and a surrogate measure of non-infarct myocardial fibrosis during the first 6 months of infarct healing.
These remodeling benefits followed a dose-response relationship with the rise of the in-vivo O-3FA levels quantified by red blood cell index.
What are the clinical implications?
The OMEGA-REMODEL study provides randomized trial evidence that 4-grams daily dose of O-3FA is a safe and effective treatment in improving cardiac remodeling in patients receiving current guideline-based post-MI therapies.
Given that the incidence of heart failure after acute MI remains high despite current therapies, the cardiac remodeling benefits from O-3FA may translate to significant clinical impact and warrants prospective clinical studies.
Acknowledgments
FUNDING SOURCES
The National Heart, Lung, and Blood Institute of the National Institutes of Health funded this study.
Footnotes
DISCLOSURES
No authors have any financial disclosures relevant to the content of this manuscript.
REFERENCES
- 1.Mozaffarian D, Rimm EB. Fish intake, contaminants, and human health: evaluating the risks and the benefits. JAMA. 2006;296:1885–1899. doi: 10.1001/jama.296.15.1885. [DOI] [PubMed] [Google Scholar]
- 2.De Caterina R. n-3 fatty acids in cardiovascular disease. N Engl J Med. 2011;364:2439–2450. doi: 10.1056/NEJMra1008153. [DOI] [PubMed] [Google Scholar]
- 3.Marchioli R, Barzi F, Bomba E, Chieffo C, Di Gregorio D, Di Mascio R, Franzosi MG, Geraci E, Levantesi G, Maggioni AP, Mantini L, Marfisi RM, Mastrogiuseppe G, Mininni N, Nicolosi GL, Santini M, Schweiger C, Tavazzi L, Tognoni G, Tucci C, Valagussa F, Investigators GI-P. Early protection against sudden death by n-3 polyunsaturated fatty acids after myocardial infarction: time-course analysis of the results of the Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico (GISSI)-Prevenzione. Circulation. 2002;105:1897–1903. doi: 10.1161/01.cir.0000014682.14181.f2. [DOI] [PubMed] [Google Scholar]
- 4.Rauch B, Schiele R, Schneider S, Diller F, Victor N, Gohlke H, Gottwik M, Steinbeck G, Del Castillo U, Sack R, Worth H, Katus H, Spitzer W, Sabin G, Senges J, Group OS. OMEGA, a randomized, placebo-controlled trial to test the effect of highly purified omega-3 fatty acids on top of modern guideline-adjusted therapy after myocardial infarction. Circulation. 2010;122:2152–2159. doi: 10.1161/CIRCULATIONAHA.110.948562. [DOI] [PubMed] [Google Scholar]
- 5.Chan W, Duffy SJ, White DA, Gao XM, Du XJ, Ellims AH, Dart AM, Taylor AJ. Acute left ventricular remodeling following myocardial infarction: coupling of regional healing with remote extracellular matrix expansion. JACC Cardiovasc Imaging. 2012;5:884–893. doi: 10.1016/j.jcmg.2012.03.015. [DOI] [PubMed] [Google Scholar]
- 6.Krauss RM, Eckel RH, Howard B, Appel LJ, Daniels SR, Deckelbaum RJ, Erdman JW, Jr, Kris-Etherton P, Goldberg IJ, Kotchen TA, Lichtenstein AH, Mitch WE, Mullis R, Robinson K, Wylie-Rosett J, St Jeor S, Suttie J, Tribble DL, Bazzarre TL. Revision 2000: a statement for healthcare professionals from the Nutrition Committee of the American Heart Association. J Nutr. 2001;131:132–146. doi: 10.1093/jn/131.1.132. [DOI] [PubMed] [Google Scholar]
- 7.Hsu LY, Natanzon A, Kellman P, Hirsch GA, Aletras AH, Arai AE. Quantitative myocardial infarction on delayed enhancement MRI. Part I: Animal validation of an automated feature analysis and combined thresholding infarct sizing algorithm. J Magn Reson Imaging. 2006;23:298–308. doi: 10.1002/jmri.20496. [DOI] [PubMed] [Google Scholar]
- 8.Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, Pennell DJ, Rumberger JA, Ryan T, Verani MS. American Heart Association Writing Group on Myocardial S and Registration for Cardiac I. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105:539–542. doi: 10.1161/hc0402.102975. [DOI] [PubMed] [Google Scholar]
- 9.Deichmann RHA. Quantification of T1 Values by SNAPSHOT-FLASH NMR imaging. Journal of Magnetic Resonance. 1992;96:608–612. [Google Scholar]
- 10.Coelho-Filho OR, Mongeon FP, Mitchell R, Moreno H, Jr, Nadruz W, Jr, Kwong R, Jerosch-Herold M. Role of transcytolemmal water-exchange in magnetic resonance measurements of diffuse myocardial fibrosis in hypertensive heart disease. Circ Cardiovasc Imaging. 2013;6:134–141. doi: 10.1161/CIRCIMAGING.112.979815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jerosch-Herold M, Sheridan DC, Kushner JD, Nauman D, Burgess D, Dutton D, Alharethi R, Li D, Hershberger RE. Cardiac magnetic resonance imaging of myocardial contrast uptake and blood flow in patients affected with idiopathic or familial dilated cardiomyopathy. Am J Physiol Heart Circ Physiol. 2008;295:H1234–H1242. doi: 10.1152/ajpheart.00429.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harris WS, Sands SA, Windsor SL, Ali HA, Stevens TL, Magalski A, Porter CB, Borkon AM. Omega-3 fatty acids in cardiac biopsies from heart transplantation patients: correlation with erythrocytes and response to supplementation. Circulation. 2004;110:1645–1649. doi: 10.1161/01.CIR.0000142292.10048.B2. [DOI] [PubMed] [Google Scholar]
- 13.Harris WS, Von Schacky C. The Omega-3 Index: a new risk factor for death from coronary heart disease? Prev Med. 2004;39:212–220. doi: 10.1016/j.ypmed.2004.02.030. [DOI] [PubMed] [Google Scholar]
- 14.Fitzmaurice GM, Laird NM, Ware JH. Applied longitudinal analysis. 2nd. Hoboken, N.J: Wiley; 2011. [Google Scholar]
- 15.Greenberg B, Quinones MA, Koilpillai C, Limacher M, Shindler D, Benedict C, Shelton B. Effects of long-term enalapril therapy on cardiac structure and function in patients with left ventricular dysfunction. Results of the SOLVD echocardiography substudy. Circulation. 1995;91:2573–2581. doi: 10.1161/01.cir.91.10.2573. [DOI] [PubMed] [Google Scholar]
- 16.Wong M, Staszewsky L, Latini R, Barlera S, Volpi A, Chiang YT, Benza RL, Gottlieb SO, Kleemann TD, Rosconi F, Vandervoort PM, Cohn JN, Val-He FTHFTI. Valsartan benefits left ventricular structure and function in heart failure: Val-HeFT echocardiographic study. J Am Coll Cardiol. 2002;40:970–975. doi: 10.1016/s0735-1097(02)02063-6. [DOI] [PubMed] [Google Scholar]
- 17.Pfeffer MA, Lamas GA, Vaughan DE, Parisi AF, Braunwald E. Effect of captopril on progressive ventricular dilatation after anterior myocardial infarction. N Engl J Med. 1988;319:80–86. doi: 10.1056/NEJM198807143190204. [DOI] [PubMed] [Google Scholar]
- 18.American College of Emergency P, Society for Cardiovascular A, Interventions. O’Gara PT, Kushner FG, Ascheim DD, Casey DE, Jr, Chung MK, de Lemos JA, Ettinger SM, Fang JC, Fesmire FM, Franklin BA, Granger CB, Krumholz HM, Linderbaum JA, Morrow DA, Newby LK, Ornato JP, Ou N, Radford MJ, Tamis-Holland JE, Tommaso CL, Tracy CM, Woo YJ, Zhao DX, Anderson JL, Jacobs AK, Halperin JL, Albert NM, Brindis RG, Creager MA, DeMets D, Guyton RA, Hochman JS, Kovacs RJ, Kushner FG, Ohman EM, Stevenson WG, Yancy CW. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;61:e78–e140. doi: 10.1016/j.jacc.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 19.Mongeon FP, Jerosch-Herold M, Coelho-Filho OR, Blankstein R, Falk RH, Kwong RY. Quantification of extracellular matrix expansion by CMR in infiltrative heart disease. JACC Cardiovasc Imaging. 2012;5:897–907. doi: 10.1016/j.jcmg.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Broberg CS, Chugh SS, Conklin C, Sahn DJ, Jerosch-Herold M. Quantification of diffuse myocardial fibrosis and its association with myocardial dysfunction in congenital heart disease. Circ Cardiovasc Imaging. 2010;3:727–734. doi: 10.1161/CIRCIMAGING.108.842096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harris WS, Pottala JV, Lacey SM, Vasan RS, Larson MG, Robins SJ. Clinical correlates and heritability of erythrocyte eicosapentaenoic and docosahexaenoic acid content in the Framingham Heart Study. Atherosclerosis. 2012;225:425–431. doi: 10.1016/j.atherosclerosis.2012.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neilan TG, Coelho-Filho OR, Shah RV, Abbasi SA, Heydari B, Watanabe E, Chen Y, Mandry D, Pierre-Mongeon F, Blankstein R, Kwong RY, Jerosch-Herold M. Myocardial extracellular volume fraction from T1 measurements in healthy volunteers and mice: relationship to aging and cardiac dimensions. JACC Cardiovasc Imaging. 2013;6:672–683. doi: 10.1016/j.jcmg.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Billman GE, Hallaq H, Leaf A. Prevention of ischemia-induced ventricular fibrillation by omega 3 fatty acids. Proc Natl Acad Sci U S A. 1994;91:4427–4430. doi: 10.1073/pnas.91.10.4427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pfeffer MA, Braunwald E, Moye LA, Basta L, Brown EJ, Jr, Cuddy TE, Davis BR, Geltman EM, Goldman S, Flaker GC, Klein M, Lamas GA, Packer M, Rouleau J, Rouleau JL, Rutherford J, Wertheimer JH, Hawkins CM. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival and ventricular enlargement trial. The SAVE Investigators. N Engl J Med. 1992;327:669–677. doi: 10.1056/NEJM199209033271001. [DOI] [PubMed] [Google Scholar]
- 25.Doughty RN, Whalley GA, Gamble G, MacMahon S, Sharpe N. Left ventricular remodeling with carvedilol in patients with congestive heart failure due to ischemic heart disease. Australia-New Zealand Heart Failure Research Collaborative Group. J Am Coll Cardiol. 1997;29:1060–1066. doi: 10.1016/s0735-1097(97)00012-0. [DOI] [PubMed] [Google Scholar]
- 26.White HD, Norris RM, Brown MA, Brandt PW, Whitlock RM, Wild CJ. Left ventricular end-systolic volume as the major determinant of survival after recovery from myocardial infarction. Circulation. 1987;76:44–51. doi: 10.1161/01.cir.76.1.44. [DOI] [PubMed] [Google Scholar]
- 27.St John Sutton M, Pfeffer MA, Plappert T, Rouleau JL, Moye LA, Dagenais GR, Lamas GA, Klein M, Sussex B, Goldman S, Menapace FJ, Jr, Parker JO, Lewis S, Sestier F, Gordon DF, McEwan P, Bernstein V, Braunwald E, for the SAVE Investigators Quantitative two-dimensional echocardiographic measurements are major predictors of adverse cardiovascular events after acute myocardial infarction. The protective effects of captopril. Circulation. 1994;89:68–75. doi: 10.1161/01.cir.89.1.68. [DOI] [PubMed] [Google Scholar]
- 28.Dargie HJ. Effect of carvedilol on outcome after myocardial infarction in patients with left-ventricular dysfunction: the CAPRICORN randomised trial. Lancet. 2001;357:1385–1390. doi: 10.1016/s0140-6736(00)04560-8. [DOI] [PubMed] [Google Scholar]
- 29.Doughty RN, Whalley GA, Walsh HA, Gamble GD, Lopez-Sendon J, Sharpe N, Investigators CES. Effects of carvedilol on left ventricular remodeling after acute myocardial infarction: the CAPRICORN Echo Substudy. Circulation. 2004;109:201–206. doi: 10.1161/01.CIR.0000108928.25690.94. [DOI] [PubMed] [Google Scholar]
- 30.Pfeffer MA, Braunwald E. Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation. 1990;81:1161–1172. doi: 10.1161/01.cir.81.4.1161. [DOI] [PubMed] [Google Scholar]
- 31.Neschen S, Morino K, Rossbacher JC, Pongratz RL, Cline GW, Sono S, Gillum M, Shulman GI. Fish oil regulates adiponectin secretion by a peroxisome proliferator-activated receptor-gamma-dependent mechanism in mice. Diabetes. 2006;55:924–928. doi: 10.2337/diabetes.55.04.06.db05-0985. [DOI] [PubMed] [Google Scholar]
- 32.Itoh M, Suganami T, Satoh N, Tanimoto-Koyama K, Yuan X, Tanaka M, Kawano H, Yano T, Aoe S, Takeya M, Shimatsu A, Kuzuya H, Kamei Y, Ogawa Y. Increased adiponectin secretion by highly purified eicosapentaenoic acid in rodent models of obesity and human obese subjects. Arterioscler Thromb Vasc Biol. 2007;27:1918–1925. doi: 10.1161/ATVBAHA.106.136853. [DOI] [PubMed] [Google Scholar]
- 33.Oh DY, Talukdar S, Bae EJ, Imamura T, Morinaga H, Fan W, Li P, Lu WJ, Watkins SM, Olefsky JM. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 2010;142:687–698. doi: 10.1016/j.cell.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weinberg EO, Shimpo M, De Keulenaer GW, MacGillivray C, Tominaga S, Solomon SD, Rouleau JL, Lee RT. Expression and regulation of ST2, an interleukin-1 receptor family member, in cardiomyocytes and myocardial infarction. Circulation. 2002;106:2961–2966. doi: 10.1161/01.CIR.0000038705.69871.D9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sabatine MS, Morrow DA, Higgins LJ, MacGillivray C, Guo W, Bode C, Rifai N, Cannon CP, Gerszten RE, Lee RT. Complementary roles for biomarkers of biomechanical strain ST2 and N-terminal prohormone B-type natriuretic peptide in patients with ST-elevation myocardial infarction. Circulation. 2008;117:1936–1944. doi: 10.1161/CIRCULATIONAHA.107.728022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eggers KM, Armstrong PW, Califf RM, Simoons ML, Venge P, Wallentin L, James SK. ST2 and mortality in non-ST-segment elevation acute coronary syndrome. Am Heart J. 2010;159:788–794. doi: 10.1016/j.ahj.2010.02.022. [DOI] [PubMed] [Google Scholar]
- 37.Chen J, Shearer GC, Chen Q, Healy CL, Beyer AJ, Nareddy VB, Gerdes AM, Harris WS, O’Connell TD, Wang D. Omega-3 fatty acids prevent pressure overload-induced cardiac fibrosis through activation of cyclic GMP/protein kinase G signaling in cardiac fibroblasts. Circulation. 2011;123:584–593. doi: 10.1161/CIRCULATIONAHA.110.971853. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.