ABSTRACT
Brain development involves multiple levels of molecular coordination in forming a functional nervous system. The hippocampus is a brain area that is important for memory formation and spatial reasoning. During early postnatal development of the hippocampal circuit, Fibroblast growth factor 22 (FGF22) and FGF7 act to establish a balance of excitatory and inhibitory tone. Both FGFs are secreted from CA3 dendrites, acting on excitatory or inhibitory axon terminals formed onto CA3 dendrites, respectively. Mechanistically, FGF22 utilizes FGFR2b and FGFR1b to induce synaptic vesicle recruitment within axons of dentate granule cells (DGCs), and FGF7 utilizes FGFR2b to induce synaptic vesicle recruitment within interneuron axons. FGF signaling eventually induces gene expression in the presynaptic neurons; however, the effects of FGF22-induced gene expression within DGCs and FGF7-induced gene expression within interneurons in the context of a developing hippocampal circuit have yet to be explored. Here, we propose one hypothetical mechanism of FGF22-induced gene expression in controlling adult neurogenesis.
KEYWORDS: adult neurogenesis, excitatory/inhibitory, FGF22, FGF7, FGFR1b, FGFR2b, Fibroblast growth factor receptors, hippocampus, presynaptic differentiation, synapse development
Development of the nervous system is a complex process with many intertwined steps, beginning with neurogenesis and neural differentiation and culminating in synaptogenesis and synaptic differentiation. Coordination of molecular signaling throughout development ensures that the brain develops into a well-balanced neural network—i.e. one with balanced excitatory and inhibitory tone. Excitatory/inhibitory balance is determined in part by the number, strength, and distribution of excitatory and inhibitory synapses and is important for brain function. In our recent paper,1 we described fibroblast growth factor receptors (FGFRs) and downstream signaling that guide excitatory and inhibitory presynaptic differentiation. In this brief commentary, we will describe these results and propose potential implications of synaptogenic FGFR signaling for adult neurogenesis.
The hippocampus is part of the limbic system and is important for learning, memory, and spatial reasoning.2 Grossly, the basic hippocampal neural circuit connectivity is akin to a feed-forward loop: inputs from entorhinal cortex synapse onto dentate granule cells (DGCs) in the dentate gyrus, and DGCs project onto CA3 pyramidal neurons which in turn project onto CA1 pyramidal neurons, with CA1 pyramidal neurons projecting out to the entorhinal cortex. Interspersed throughout are local interneurons that provide inhibitory tone and coordinate neural oscillations in the circuit.3 Another important and unique feature of hippocampal circuitry is that the subgranular zone (SGZ) in the dentate gyrus is one of at least 2 areas in the brain where neurogenesis continues into adulthood.4 The rate at which new neurons are born depends on many factors, including exercise, stress, hormones, network activity;5,6 and the DGCs that arise through adult neurogenesis are important in aspects of memory formation.7,8 Disruption of either excitatory/inhibitory balance or rates of adult neurogenesis causes neuropsychiatric disease: abnormal excitatory/inhibitory balance can lead to epilepsy, autism spectrum disorders, schizophrenia, cognitive processing, and anxiety disorders;9,10,11,12 and abnormal neurogenesis is seen in depression, cognitive impairment, and epilepsy.6,13 Together, these unique features of the hippocampal circuit demonstrate the importance of appropriate synapse development and maintenance of appropriate levels of neurogenesis into adulthood.
Fibroblast growth factor (FGF) signaling during early postnatal development provides an important mechanism regulating balanced excitatory and inhibitory synaptic tone in the CA3 of the hippocampus.14 FGF22 and FGF7 are secreted from the dendrites of CA3 pyramidal neurons at the sites of nascent synapses, where FGF22 promotes excitatory presynaptic differentiation and FGF7 promotes inhibitory presynaptic differentiation of incoming axons. Specifically, neurotransmitter-laden synaptic vesicles fail to accumulate at excitatory or inhibitory presynaptic-terminals in FGF22 knockout (KO) and FGF7KO mice, respectively, which in turn leads to decreased excitatory or inhibitory synaptic efficacy. Dysregulation of FGF22 or FGF7 has pathological consequences—FGF22KO mice have decreased excitatory drive in the CA3 and are resistant to developing epileptic seizures using a kindling protocol,14,15 while FGF7KO mice have decreased inhibitory tone and have increased susceptibility to kindled epileptic seizures.14,16 The specificity of FGF22 and FGF7 in directing excitatory versus inhibitory presynaptic differentiation is regulated on numerous levels, including through the postsynaptic localization of FGF22 at nascent excitatory and FGF7 at nascent inhibitory synapses along CA3 dendrites,14,17 and through utilization of distinct sets of FGF receptors (FGFRs) in the presynaptic axon-terminals.1
FGFRs comprise a family of transmembrane receptor tyrosine kinases encoded for by 4 genes—FGFR1 to FGFR4.18 FGFR1 to FGFR3 have 2 splice isoforms, the “b” and “c” splice isoforms, which differ in their ligand-binding specificity, expression profile, and developmental function. In in vitro mitogenic assays, FGF22 was shown to preferentially activate FGFR2b and FGFR1b, while FGF7 was shown to preferentially activate FGFR2b.19 Now we have shown these ligand-receptor pairings occur in vivo during synapse development: FGF22 utilizes both FGFR2b and FGFR1b to induce excitatory presynaptic differentiation, and FGF7 utilizes FGFR2b to induce inhibitory presynaptic differentiation.1 To show FGFR involvement in excitatory/inhibitory synapse formation, we immunostained hippocampal sections from mice constitutively lacking FGFR2b or FGFR1b, or conditionally (during the postnatal synaptogenic period) lacking FGFR2 or FGFR1 (both b and c isoforms). Receptor-KO mice were investigated at postnatal day 8, when early synapse formation peaks and FGF22 and FGF7 are maximally expressed in the CA3.14 To assess presynaptic differentiation, we stained sections for vesicular glutamate transporter 1 (VGLUT1), a marker of excitatory synaptic vesicles, and for vesicular GABA transporter (VGAT), a marker of inhibitory synaptic vesicles, that cluster at nerve terminals as excitatory or inhibitory synapses develop. FGFR2bKO, FGFR2KO, FGFR1bKO, and FGFR1KO mice have decreased excitatory presynaptic differentiation, and FGFR2bKO and FGFR2KO (but not FGFR1bKO or FGFR1KO) have decreased inhibitory presynaptic differentiation. To demonstrate that the receptors are required for neurons to respond to FGF22 or FGF7 with excitatory or inhibitory presynaptic differentiation, hippocampal neurons were cultured from the FGFR-KO mice and FGF22 or FGF7 was applied: FGF22 failed to induce excitatory presynaptic differentiation in neurons lacking FGFR2b, FGFR2, FGFR1b, or FGFR1 as measured by VGLUT1 clustering on CA3 neuron dendrites in culture, and FGF7 failed to induce inhibitory presynaptic differentiation in neurons lacking FGFR2b or FGFR2 as measured by VGAT clustering on CA3 neuron dendrites. Furthermore, by deleting the receptors selectively in presynaptic neurons in culture and simultaneously fluorescently marking the synaptic vesicles in those same neurons, we showed that FGFR2 and FGFR1 are required in DGCs (a main excitatory input to CA3) to respond to FGF22 in terms of excitatory synaptic vesicle accumulation, and FGFR2 (but not FGFR1) is required in inhibitory interneurons to respond to FGF7. Finally, we addressed what downstream signaling is required to induce synaptic vesicle accumulation. Because FGFRs are receptor tyrosine kinases, ligand binding induces dimerization, trans-autophosphorylation and kinase activation, which leads to activation of downstream signaling pathways.18,20 By performing rescue experiments with FGFR2b constructs containing point mutations that affect kinase activity or adaptor protein binding sites, we showed that within DGCs, FGF22 requires FGFR2b kinase activity, FRS2-binding (which is upstream of ERK and AKT signaling), and PI3K binding (which is upstream of AKT signaling), but not PLC-gamma binding (which is upstream of calcium signaling). These findings are summarized in Figure 1.
Figure 1.
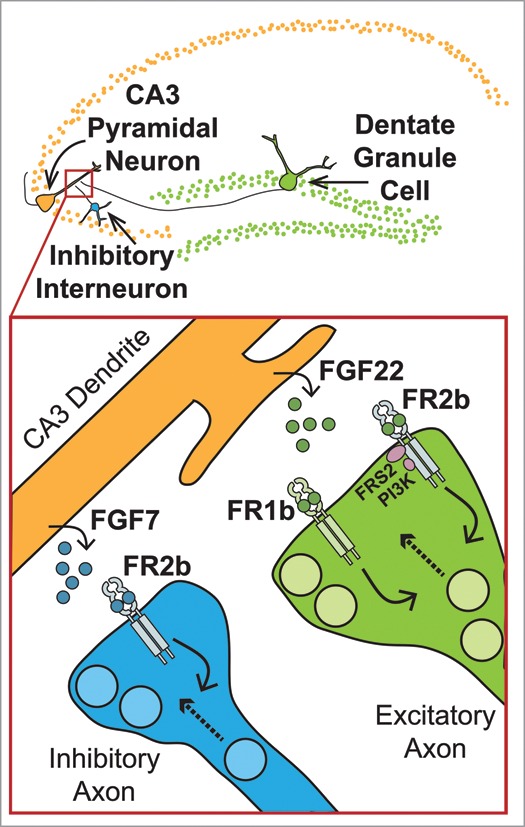
Target-derived FGFs induce excitatory and inhibitory presynaptic differentiation. Top: In the hippocampal circuit, dentate granule cells (DGCs) provide a major excitatory input to the CA3, and interneurons provide the inhibitory input. Bottom: FGF22 and FGF7 are secreted at excitatory and inhibitory nascent synapses on CA3 dendrites in the hippocampus.14,17 FGF22 acts on FGFR2b and FGFR1b within DGCs to induce synaptic vesicle accumulation in excitatory axon terminals, and FGF7 acts on FGFR2b within interneurons to induce synaptic vesicle accumulation within inhibitory axoN-terminals.1,14 FGFR2b activated by FGF22 utilizes kinase activity and signaling downstream of FRS2 and PI3K, but not PLC-gamma, to induce accumulation of excitatory synaptic vesicles.1
FGF signaling ultimately induces gene expression. Indeed, throughout development, FGFs are known to direct important morphological changes through regulation of gene expression.18 FGF-induced gene expression is highly dependent on cellular and developmental context, and no unique target gene to the FGF pathway is known. This is in contrast to signaling pathways such as the Sonic hedgehog pathway, which reproducibly induces Gli expression in any cell type.21 Thus, an interesting line of inquiry stemming from our work is i) whether FGF22 induces gene expression in DGCs and whether FGF7 induces gene expression in interneurons, ii) what the FGF-induced genes are in these 2 types of neurons, and iii) how FGF-induced gene expression affects the developing neural circuit. We speculate that one potential function of FGF22-induced genes in the dentate gyrus is control of adult neurogenesis. Our lab found that FGF22KO animals have lower levels of adult neurogenesis as measured by the number of doublecortin-positive cells in the SGZ (which marks immature granule cells), without a significant decrease in proliferating cells (as measured by Ki67) or in the gross morphology of the dentate gyrus.15 This suggests that FGF22 is required for differentiation or maintenance of newly born neurons in the adult dentate gyrus.
Adult neurogenesis in the SGZ is a highly regulated process in many ways distinct from embryonic neurogenesis.22,23,24 During development, the dentate gyrus arises in a multi-step process, with 3 cycles of proliferation and migration; first at the dentate notch in the cortical plate, then in the hilus, and finally in the SGZ. In the adult, the neural precursor cells reside in the SGZ and can give rise to either neurons or astrocytes. FGF signaling is important for both developmental and adult neurogenesis, from initial specification of neural progenitors to maintenance of adult neurogenesis.25 During development, gradients of FGF8, FGF17, and FGF18 induce neuroepithelial cell fates in the emerging cortex; FGF2, FGF8, FGF10, FGF15, and FGFR3 interplay to control proliferation and differentiation of neural precursors; and FGF2/FGFR1 signaling is necessary for embryonic neurogenesis in the cortex both in vivo and in vitro.25 As for adult neurogenesis, the roles of FGF signaling in the mature brain are also becoming apparent. FGF10 (which is closely related to FGF7 and FGF22) has been hypothesized to maintain adult but not embryonic neurogenesis, although this has yet to be demonstrated directly;26 FGF10 has been implicated in adult neurogenesis in the murine hypothalamus.27 Deletion of FGFR1 in the central nervous system by using Nestin promoter-driven Cre decreased the number of proliferating cells in the SGZ, as measured by BrdU incorporation, and decreased the number of cells co-expressing BrdU and NeuN (a neuronal marker) to a greater degree than those co-expressing BrdU and GFAP (a glial marker), suggesting the importance of FGFR1 in proliferation and neuronal maturation or survival during adult neurogenesis of the SGZ.28 Loss of FGFR2 from radial glial cells (neural precursors) and astrocytes leads to decreased proliferating cells, as measured by BrdU, and decreased doublecortin expression.29 Recent work in which FGFRs were deleted or activated in Nestin+ cells (neural precursors) in adult mice demonstrated that FGF signaling is required for maintaining SGZ stem cells and sufficient to induce neurogenesis.30 Altogether, FGF signaling is important for neurogenesis from the earliest stages of development through control of adult neurogenesis.
How does FGF22 fit into this picture? One possibility is that FGF22 secreted from CA3—the same FGF22 that induces excitatory synapses—induces expression of genes in the presynaptic DGC, which in turn, help promote adult neurogenesis in the dentate gyrus (Fig. 2). Indeed, we compared gene expression in DGCs from 28-day-old mice and found that several genes encoding for secreted proteins that have been implicated in adult neurogenesis are downregulated in FGF22KO mice compared to WT (unpublished data). Thus, FGF22 may indirectly control adult neurogenesis by upregulating genes for secreted proteins in DGCs that once secreted, affect neighboring SGZs to regulate adult neurogenesis as the hippocampal circuit matures. Indeed, the SGZ forms between postnatal days 7 and 14,31 when FGF22 and FGF7 exert their synaptogenic function, and mature DGCs are in close contact with the neurogenic niche. Secreted growth factors and extracellular matrix proteins from mature DGCs are poised to influence the developing neurogenic niche in the SGZ and control the rate or neurogenesis and DGC differentiation. In this way, FGF22 could be acting as a target-derived (CA3-derived) signal to the dentate gyrus to modulate the amount of neurogenesis depending on the needs of the maturating hippocampal circuit. An analogous mechanism occurs in the peripheral nervous system during development, where target organ-derived nerve growth factor modulates cell survival and/or proliferation of the sympathetic nervous system.32 In the hippocampus, target-derived cues such as FGF22 could be anticipating the amount of activity that will occur in the mature circuit, promoting an appropriate amount of neurogenesis in the dentate gyrus to match. Future experiments, including use of a CA3-specific conditional FGF22KO, will prove whether CA3-derived FGF22 is indeed modulating adult neurogenesis.
Figure 2.
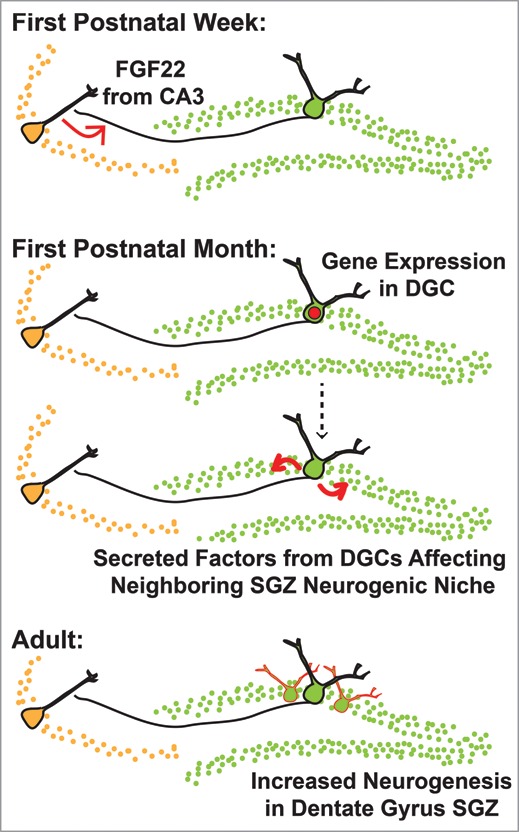
A model for the control of adult neurogenesis in the dentate gyrus by CA3-derived FGF22. One potential effect of CA3-derived FGF22, in addition to excitatory presynaptic differentiation, may be the regulation of adult neurogenesis in the dentate gyrus, thus balancing levels of adult neurogenesis with the potential for excitatory synapse formation in the CA3. Top: During the first postnatal week, FGF22 is highly expressed in the CA3 and induces excitatory presynaptic differentiation.14 FGF22 activates FGFR2b and FGFR1b in DGCs to induce presynaptic differentiation.1 Middle: FGF22 induces gene expression in DGCs, some of which encode for secreted and extracellular factors implicated in control of adult neurogenesis (unpublished data) through sculpting the developing subgranular zone (SGZ) neurogenic niche. Bottom: We hypothesize that gene expression induced by CA3-derived FGF22 regulates adult neurogenesis, through secreted factors influencing the SGZ neurogenic niche. Indeed, FGF22KO mice have decreased adult neurogenesis.15
Many interdependent factors are required to bolster the development of a balanced neural circuit. We have shown the contribution of FGF signaling to excitatory vs. inhibitory synapse formation, and here we proposed a mechanism through which synaptogenesis could control levels of adult neurogenesis. Thus, FGF signaling may not only serve as local synaptogenic signals, but also as global regulators of neural circuit development throughout life.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- [1].Dabrowski A, Terauchi A, Strong C, Umemori H. Distinct sets of FGF receptors sculpt excitatory and inhibitory synaptogenesis. Development 2015; 142:1818-30; PMID:25926357; http://dx.doi.org/ 10.1242/dev.115568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Basu J, Siegelbaum SA. The Corticohippocampal Circuit, Synaptic Plasticity, and Memory. Cold Spring Harb Perspect Biol 2015; 7: pii: a021733; PMID:26525152; http://dx.doi.org/ 10.1101/cshperspect.a021733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci 2007; 8:45-56; PMID:17180162; http://dx.doi.org/ 10.1038/nrn2044 [DOI] [PubMed] [Google Scholar]
- [4].Kempermann G, Song H, Gage FH. Neurogenesis in the Adult Hippocampus. Cold Spring Harb Perspect Biol 2015; 7:a018812; PMID:26330519; http://dx.doi.org/ 10.1101/cshperspect.a018812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].van Praag H. Neurogenesis and exercise: past and future directions. Neuromolecular Med 2008; 10:128-40; PMID:18286389; http://dx.doi.org/ 10.1007/s12017-008-8028-z [DOI] [PubMed] [Google Scholar]
- [6].Braun SM, Jessberger S. Adult neurogenesis and its role in neuropsychiatric disease, brain repair and normal brain function. Neuropathol Appl Neurobiol 2014; 40:3-12; PMID:24308291; http://dx.doi.org/ 10.1111/nan.12107 [DOI] [PubMed] [Google Scholar]
- [7].Aimone JB, Deng W, Gage FH. Resolving new memories: a critical look at the dentate gyrus, adult neurogenesis, and pattern separation. Neuron 2011; 70:589-96; PMID:21609818; http://dx.doi.org/ 10.1016/j.neuron.2011.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Sahay A, Wilson DA, Hen R. Pattern separation: a common function for new neurons in hippocampus and olfactory bulb. Neuron 2011; 70:582-8; PMID:21609817; http://dx.doi.org/ 10.1016/j.neuron.2011.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Cossart R, Dinocourt C, Hirsch JC, Merchan-Perez A, De Felipe J, Ben-Ari Y, Esclapez M, Bernard C. Dendritic but not somatic GABAergic inhibition is decreased in experimental epilepsy. Nat Neurosci 2001; 4:52-62; PMID:11135645; http://dx.doi.org/ 10.1038/82900 [DOI] [PubMed] [Google Scholar]
- [10].Gogolla N, Leblanc JJ, Quast KB, Südhof TC, Fagiolini M, Hensch TK. Common circuit defect of excitatory-inhibitory balance in mouse models of autism. J Neurodev Disord 2009; 1:172-81; PMID:20664807; http://dx.doi.org/ 10.1007/s11689-009-9023-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kehrer C, Maziashvili N, Dugladze T, Gloveli T. Altered Excitatory-Inhibitory Balance in the NMDA-Hypofunction Model of Schizophrenia. Front Mol Neurosci 2008; 1:6; PMID:18946539; http://dx.doi.org/ 10.3389/neuro.02.006.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Yizhar O, Fenno LE, Prigge M, Schneider F, Davidson TJ, O'Shea DJ, Sohal VS, Goshen I, Finkelstein J, Paz JT, Stehfest K, Fudim R, Ramakrishnan C, Huguenard JR, Hegemann P, Deisseroth K. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature 2011; 477:171-8; PMID:21796121; http://dx.doi.org/ 10.1038/nature10360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Jessberger S, Parent JM. Epilepsy and Adult Neurogenesis. Cold Spring Harb Perspect Biol 2015; 7: pii: a020677; PMID:26552418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Terauchi A, Johnson-Venkatesh EM, Toth AB, Javed D, Sutton MA, Umemori H. Distinct FGFs promote differentiation of excitatory and inhibitory synapses. Nature 2010; 465:783-7; PMID:20505669; http://dx.doi.org/ 10.1038/nature09041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lee CH, Umemori H. Suppression of epileptogenesis-associated changes in response to seizures in FGF22-deficient mice. Front Cell Neurosci 2013; 7:43. PMID:23616746. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [16].Lee CH, Javed D, Althaus AL, Parent JM, Umemori H. Neurogenesis is enhanced and mossy fiber sprouting arises in FGF7-deficient mice during development. Mol Cell Neurosci 2012; 51:61-7; PMID:22889808; http://dx.doi.org/ 10.1016/j.mcn.2012.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Terauchi A, Timmons KM, Kikuma K, Pechmann Y, Kneussel M, Umemori H. Selective synaptic targeting of the excitatory and inhibitory presynaptic organizers FGF22 and FGF7. J Cell Sci 2015; 128:281-92; PMID:25431136; http://dx.doi.org/ 10.1242/jcs.158337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ornitz DM, Itoh N. The Fibroblast Growth Factor signaling pathway. Wiley Interdiscip Rev Dev Biol 2015; 4:215-66; PMID:25772309; http://dx.doi.org/ 10.1002/wdev.176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Zhang X, Ibrahimi OA, Olsen SK, Umemori H, Mohammadi M, Ornitz DM. Receptor specificity of the fibroblast growth factor family. The complete mammalian FGF family. J Biol Chem 2006; 281:15694-700; PMID:16597617; http://dx.doi.org/ 10.1074/jbc.M601252200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Dabrowski A, Umemori H. Orchestrating the synaptic network by tyrosine phosphorylation signalling. J Biochem 2011; 149:641-53; PMID:21508038; http://dx.doi.org/ 10.1093/jb/mvr047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Dailey L, Ambrosetti D, Mansukhani A, Basilico C. Mechanisms underlying differential responses to FGF signaling. Cytokine Growth Factor Rev 2005; 16:233-47; PMID:15863038; http://dx.doi.org/ 10.1016/j.cytogfr.2005.01.007 [DOI] [PubMed] [Google Scholar]
- [22].Li Y, Mu Y, Gage FH. Development of neural circuits in the adult hippocampus. Curr Top Dev Biol 2009; 87:149-74; PMID:19427519; http://dx.doi.org/ 10.1016/S0070-2153(09)01205-8 [DOI] [PubMed] [Google Scholar]
- [23].Li G, Pleasure SJ. Morphogenesis of the dentate gyrus: what we are learning from mouse mutants. Dev Neurosci 2005; 27:93-9; PMID:16046842; http://dx.doi.org/ 10.1159/000085980 [DOI] [PubMed] [Google Scholar]
- [24].Li G, Pleasure SJ. Genetic regulation of dentate gyrus morphogenesis. Prog Brain Res 2007; 163:143-52; PMID:17765716; http://dx.doi.org/ 10.1016/S0079-6123(07)63008-8 [DOI] [PubMed] [Google Scholar]
- [25].Guillemot F, Zimmer C. From cradle to grave: the multiple roles of fibroblast growth factors in neural development. Neuron 2011; 71:574-88; PMID:21867876; http://dx.doi.org/ 10.1016/j.neuron.2011.08.002 [DOI] [PubMed] [Google Scholar]
- [26].Hajihosseini MK, De Langhe S, Lana-Elola E, Morrison H, Sparshott N, Kelly R, Sharpe J, Rice D, Bellusci S. Localization and fate of Fgf10-expressing cells in the adult mouse brain implicate Fgf10 in control of neurogenesis. Mol Cell Neurosci 2008; 37:857-68; PMID:18329286; http://dx.doi.org/ 10.1016/j.mcn.2008.01.008 [DOI] [PubMed] [Google Scholar]
- [27].Haan N, Goodman T, Najdi-Samiei A, Stratford CM, Rice R, El Agha E, Bellusci S, Hajihosseini MK. Fgf10-expressing tanycytes add new neurons to the appetite/energy-balance regulating centers of the postnatal and adult hypothalamus. J Neurosci 2013; 33:6170-80; PMID:23554498; http://dx.doi.org/ 10.1523/JNEUROSCI.2437-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Zhao M, Li D, Shimazu K, Zhou YX, Lu B, Deng CX. Fibroblast growth factor receptor-1 is required for long-term potentiation, memory consolidation, and neurogenesis. Biol Psychiatry 2007; 62:381-90; PMID:17239352; http://dx.doi.org/ 10.1016/j.biopsych.2006.10.019 [DOI] [PubMed] [Google Scholar]
- [29].Stevens HE, Jiang GY, Schwartz ML, Vaccarino FM. Learning and memory depend on fibroblast growth factor receptor 2 functioning in hippocampus. Biol Psychiatry 2012; 71:1090-8; PMID:22541947; http://dx.doi.org/ 10.1016/j.biopsych.2012.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kang W, Hébert JM. FGF Signaling Is Necessary for Neurogenesis in Young Mice and Sufficient to Reverse Its Decline in Old Mice. J Neurosci 2015; 35:10217-23; PMID:26180198; http://dx.doi.org/ 10.1523/JNEUROSCI.1469-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Nicola Z, Fabel K, Kempermann G. Development of the adult neurogenic niche in the hippocampus of mice. Front Neuroanat 2015; 9:53; PMID:25999820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Harrington AW, Ginty DD. Long-distance retrograde neurotrophic factor signalling in neurons. Nat Rev Neurosci 2013; 14:177-87; PMID:23422909; http://dx.doi.org/ 10.1038/nrn3253 [DOI] [PubMed] [Google Scholar]


