Abstract
The hippocampus has long been known as a brain structure fundamental for memory formation and retrieval. Recent technological advances of cellular tracing techniques and optogenetic manipulation strategies have allowed to unravel important aspects of the cellular origin of memory, and have started to shed new light on the neuronal networks involved in encoding, consolidation and retrieval of memory in the hippocampus. In particular, memory traces, or engrams, that are formed during encoding in the dentate gyrus and CA3 region are crucial for memory retrieval and amenable to modulation by neuroplastic mechanisms, including adult hippocampal neurogenesis. Here, we will discuss how memory traces are being encoded at the cellular level, how they may contribute to pattern separation and pattern completion in the hippocampus, and how they can be associated with different experiences to express memories of opposite valence. We propose a mechanism by which adult hippocampal neurogenesis may contribute to the formation of engrams, which may be relevant not only for the encoding of contextual information, but also for mood abnormalities, such as anxiety and depression.
Keywords: amygdala, BDNF, dentate gyrus, entorhinal cortex, fear conditioning, immediate early genes, optogenetics, stress, stem cells
The major task of a functional memory system is to encode discrete information without interference and to be able to correctly recall memories when being presented with only partial cues of that memory. Recent work has demonstrated that the hippocampus is able to carry out this task by assigning different computational steps to its individual subregions. Using single unit activity recordings from the dentate gyrus and CA3 region of the rat, Neunuebel and Knierim (2014) demonstrated that subtle changes in local and global cue positions on a circular track disrupt the coherence in the firing rate of dentate granule cells with the spatial position of the rat, suggesting that small differences in the environment can change the place fields of neurons in the dentate gyrus. These changes in dentate gyrus activity in response to subtle variations in the environment occur despite the fact that firing fields in the entorhinal cortex remain stable, supporting the notion that the dentate gyrus performs a pattern separation computation on its input signals from the entorhinal cortex (Leutgeb et al., 2007). In contrast to this ability of the dentate gyrus to disambiguate small differences in mnemonic input, firing rates of downstream CA3 pyramidal cells remain highly correlated with spatial position, and this correlation only degrades when large changes in cue positions are introduced (Neunuebel and Knierim, 2014). Moreover, downstream neuronal representations in CA3 resemble more closely the original entorhinal cortex input, suggesting that CA3 is able to perform a pattern completion computation on the degraded input that it receives from the dentate gyrus (Neunuebel et al., 2013). Therefore, during encoding of (spatial) memory, similar contextual inputs from the entorhinal cortex are first disambiguated by the dentate gyrus as a gateway into the memory storage of the hippocampus. This information is subsequently relayed onto CA3 neurons, which store these different representations and are able to retrieve the memory when neural inputs of incomplete aspects of that memory are being detected (Kheirbek et al., 2012).
Anatomically, the dentate gyrus is ideally evolved to perform pattern separation: The number of granule cells in the dentate gyrus is around 5 times larger than the number of entorhinal cortex cells that project onto them (∼200,000 entorhinal cortex cells vs. ∼1,000,000 dentate granule cells), and the dense network of granule neurons is only sparsely activated by its entorhinal cortex inputs (Amaral et al., 1990; Stone et al., 2011a). This organization of the dentate gyrus facilitates the activation of discrete neuronal populations during experiences of different, but similar spatial contexts (Kheirbek et al., 2012; Liu et al., 2012). In order to understand how a memory trace is formed within the hippocampus in vivo, recent studies have employed novel transgenic techniques, in which promoters of immediate early genes, such as Arc or c-fos (Link et al., 1995; Lyford et al., 1995), control reporter genes during an experimentally defined time window. This technique has allowed indelible labeling of the individual neurons that are recruited during memory encoding of a specific experience, such as during contextual fear conditioning. In line with the aforementioned sparse activation of the dentate gyrus, approximately only 1–5% of granule cells are labeled when mice receive a footshock in a specific context, while levels of activated neurons in CA3 and CA1 are substantially higher (∼10% and ∼15%, respectively) (Liu et al., 2012; Tayler et al., 2013). Interestingly, when mice are fear conditioned by receiving footshocks in a specific context A, and are subsequently re-exposed to that same context 2–5 d later, a small proportion of previously recruited cells in the dentate gyrus and CA3 are reactivated, even though no footshocks are being delivered during the second exposure. In contrast, when mice are exposed to a distinct context B, which they had never experienced before, a different population of neurons in the dentate gyrus and CA3 are activated, suggesting that a fraction of the cells that are active during memory encoding are also recruited during retrieval of that memory (Deng et al., 2013; Denny et al., 2014; Tayler et al., 2013). When mice are exposed to context A 2 weeks after contextual fear conditioning, the number of neurons that are reactivated in the dentate gyrus is reduced compared to the number of neurons that are reactivated 2 days after fear conditioning, suggesting that 2 weeks after encoding, the memory has either started to move out of the dentate gyrus or that memory expression is less reliant on the dentate gyrus (Tayler et al., 2013). Moreover, 30 d after memory encoding in context A, reactivation in the dentate gyrus and CA3 are further decreased, while the amount of freezing, which is measured as an index of successful retrieval of the conditioned fear, remains high and is similar in context A and in context B (Denny et al., 2014) (Fig. 1). The similar behavioral response likely reflects behavioral generalization at this remote time point. However, it is important to note that the hippocampus is not the only region where an entire engram is stored. Indeed, during encoding, neurons are also recruited in cortical areas, such as the retrosplenial cortex, the lateral entorhinal cortex and the posterior parietal cortex, and reactivation of these cortical cells of the memory trace remains stable over time (Wang et al., 2006; Tayler et al., 2013). Moreover, one month after memory acquisition, memory retrieval is not as dependent on the hippocampus, and predominantly reliant on cortical areas (Tayler et al., 2013).
The functional implications of the hippocampal engram for the expression of a memory have been demonstrated mainly by optogenetic studies, which have stimulated or inhibited the population of neurons that were recruited during memory encoding. Specifically, granule cells in the dentate gyrus that were recruited during contextual fear conditioning in context A were labeled with the light-sensitive ion channel, channelrhodopsin-2 (ChR2), and mice were subsequently exposed to a novel context B. Light-induced activation of the same cells that were labeled during encoding of context A, increased freezing levels in the new context B, in which a footshock was never delivered, demonstrating that activation of the same set of granule neurons that are recruited during the formation of a fear memory is sufficient for the expression of that memory (Liu et al., 2012). Accordingly, optogenetic inhibition of these engram-bearing cells in the dentate gyrus impairs context-elicited freezing after fear conditioning in context A, demonstrating that reactivation of the memory trace is indeed necessary for memory expression (Denny et al., 2014). Interestingly, when dentate granule neurons are labeled with ChR2 during exposure to a neutral context, and are then activated when mice receive footshocks in a distinct context, the memory of the fear for the footshock can be stored in these artificially activated cells and later on retrieved when mice are re-exposed to the neutral context in which they never received a foot shock (Garner et al., 2012; Liu et al., 2012; Ramirez et al., 2013). These data demonstrate that the memory engram of a neutral experience can be experimentally associated with an event of high valence, such as fear. This ‘false’ memory is stable, recruits downstream fear-processing structures such as the basolateral amygdala (BLA), and can be re-activated in a completely novel context to express the false fear memory (Ramirez et al., 2013). Moreover, a memory of positive or negative valence can be manipulated to become a memory of the opposite valence. Redondo and colleagues (2014) demonstrated that the valence of a fearful memory (i.e., foot shocks in context A) can be transformed into a rewarding memory when the engram-bearing cells that encode the fearful memory are activated during the experience of a rewarding stimulus, for example, during exposure to a female conspecific. As a result, mice whose fearful memory engram has been associated with reward now show less freezing in the aversive context. Similarly, the memory of a rewarding situation can be replaced by an aversive memory, when the engram of a reward is activated during exposure to a fearful context (Redondo et al., 2014). Together, these findings demonstrate that the memory trace is plastic and can be transformed when neurons of the dentate gyrus that encode this memory are active during encoding of a different context. On a circuit level, this transformation in memory valence is mediated by changes in connectivity of the hippocampus with different memory engrams in the BLA. However, while the dentate gyrus memory engram is malleable and can be associated with different valences, the engram in the BLA is stable. Therefore, re-association of one and the same dentate gyrus engram with memories of different valences will subsequently recruit different neuronal populations in the BLA, suggesting that the BLA retains the fear-related information belonging to that engram, but this information is no longer being accessed due to plastic changes that have occurred at the level of the dentate gyrus (Redondo et al., 2014).
How the dentate gyrus performs these critical steps in memory encoding, consolidation, and retrieval at the cellular and molecular level is still largely unknown. The unique ability of the dentate gyrus to continuously generate new neurons throughout adulthood has repeatedly been shown to be involved in hippocampus-dependent learning and memory (reviewed in Koehl and Abrous, 2011, Kheirbek et al., 2012; Drew et al., 2013) as well as in forgetting of previously formed memories (Akers et al., 2014). Adult hippocampal neurogenesis is specifically important for encoding weak memories, but not for encoding strong memories, as mice without neurogenesis are impaired in contextual fear conditioning when only brief training (one shock) is provided, while mice without neurogenesis are not impaired in contextual fear conditioning when strong training (multiple shocks) is provided (Drew et al., 2010). Moreover, adult neurogenesis is necessary for the dentate gyrus to perform behavioral pattern separation, as neurogenesis ablation by x-irradiation impairs spatial discrimination when cues are presented with only little spatial separation (Clelland et al., 2009; Swan et al., 2014). Accordingly, increasing neurogenesis improves behavioral pattern separation in a contextual discrimination task (Sahay et al., 2011), demonstrating the involvement of neurogenesis in maintaining this crucial function of the dentate gyrus during memory formation.
The question remains how adult hippocampal neurogenesis contributes to the formation of the memory trace and to the reactivation of neurons that are recruited during memory encoding. Initial studies suggested that adult-born neurons might be preferentially recruited to the memory trace during encoding because they exhibit lower excitation thresholds than their mature counterparts (Kee et al., 2007). However, this model is controversial, as recent studies have demonstrated that adult-born granule cells are integrated into spatial memory networks only after 5 weeks of age, and that these neurons are not more or less likely to be activated during encoding, than granule cells that were generated during embryonic or early postnatal development (Stone et al., 2011b). Surprisingly, although neurogenesis ablation impairs context-elicited freezing after one shock contextual fear conditioning, it does not affect reactivation of engram-bearing cells in the dentate gyrus. Instead, although adult hippocampal neurogenesis is restricted to the dentate gyrus, ablation of adult-born neurons decreases reactivation only downstream in CA3 (Drew et al., 2010; Denny et al., 2012; Denny et al., 2014), suggesting a modulatory role of adult-born neurons for overall hippocampal function and engram formation. Indeed, adult hippocampal neurogenesis has been shown to regulate overall neuronal activity in the dentate gyrus (Burghardt et al., 2012; Ikrar et al., 2013, Lacefield et al., 2012) and ablation of neurogenesis prolongs the maintenance of LTP in the dentate gyrus and thereby retains hippocampus-dependency of memory retrieval. These data suggest that adult-born neurons are necessary to promote a gradual decay of dentate gyrus activity and determine how long a memory remains dependent on the hippocampus before it is relayed to extra-hippocampal regions, including cortical structures (Kitamura et al., 2009). Disturbed connectivity within the dentate gyrus network without neurogenesis may thus ultimately result in inaccurate activation of downstream neurons in CA3, and consequently cause the recruitment of a less defined population of neurons to the memory engram. Such disturbances may reduce the ability of the dentate gyrus to disambiguate similar contextual representations and could be independent of changes in the engram of the dentate gyrus. Instead, adult-born neurons may compete with established synaptic connections to reconfigure existing neuronal circuits and projections to CA3. The role of adult-born hippocampal neurons in memory processing may thus not predominantly lie in their action as independent cellular encoding units, but rather in regulating neural activity of dentate granule cells, which causes remodeling of downstream hippocampal neural networks (Sahay and Hen, 2007; Piatti et al., 2013).
To unambiguously address the question if and how adult-born neurons may regulate overall dentate gyrus activity, it will be necessary for future studies to visualize and functionally manipulate adult-born neurons and mature granule cells separately while they are fully connected within the neurogenic niche. Modern in vivo microscopy techniques (Barretto et al., 2011; Ziv et al., 2013) and electrophysiological approaches, (Pernía-Andrade et al., 2014) paired with transgenic techniques to specifically label either adult-born cells or mature neurons, will be crucial to determine how these individual cell populations interact physiologically to contribute to memory formation in awake behaving animals. Such in vivo approaches will allow addressing crucial questions of connectivity as they relate to neural network function and behavior in a more natural way than electrophysiological measurements of acute slices. In addition, novel cellular tracing techniques, such as monosynaptic rabies virus-mediated retrograde tracers to label synaptic connections of adult-born neurons, will be invaluable tools to map the in vivo connectivity of adult-born granule cells. (Vivar et al., 2012) Together, these techniques may ultimately be able provide unprecedented insight into the functional role of adult-born neurons in regulating the neural circuitry of the dentate gyrus, and in hippocampal memory processing.
How these complex cellular computations are regulated at the molecular level has not yet been widely explored. Only two recent studies have examined the role of brain-derived neurotrophic factor (BDNF) as a molecular mediator for pattern separation in the dentate gyrus. Infusions of a BDNF blocking antibody into the dentate gyrus, either 15 min before or 5 min after training in a spatial location discrimination task, impaired discrimination of 2 nearby positions 24 hours later, suggesting that BDNF may be necessary for memory encoding and early consolidation. Importantly, blocking BDNF only impaired discrimination of locations that were very close in space, but not when the distance between the same locations was larger, suggesting a role for BDNF specifically in behavioral pattern separation. Importantly, and in line with the above-described role of the dentate gyrus engram in retrieval of that same memory, blocking BDNF 15 min before the retrieval test did not impair the ability to distinguish 2 nearby locations, confirming that BDNF-dependent pattern separation takes place during encoding, and not during retrieval. Moreover, blocking BDNF 6 hrs after encoding does also not impair pattern separation, indicating that the effect of BDNF is either acute, or limited to only the first phase of the consolidation phase (Bekinschtein et al., 2013). Importantly, local infusion of BDNF into the dentate gyrus during encoding also improves pattern separation, and this effect is absent in animals in which neurogenesis is ablated (Bekinschtein et al., 2014). While these findings suggest that BDNF may act specifically on adult-born neurons to enable pattern separation in the dentate gyrus, it remains to be elucidated how this acute action of BDNF may regulate the activity of adult-born neurons. The tropomyosin-related kinase B (TrkB) receptor, which is expressed in neural progenitors, is a likely target and has previously been shown to be necessary for proliferation and differentiation of adult hippocampal progentior cells (Li et al., 2008), as well as for neurogenesis-dependent LTP (Bergami et al., 2008), which, as described above, may be of particular relevance for the formation of engrams not only in the dentate gyrus, but also downstream in CA3.
In summary, while labeling and functionally manipulating engrams has greatly improved our understanding of the cellular basis of memory, many questions remain to be answered. Multiple lines of evidence point toward a crucial role for adult hippocampal neurogenesis in memory formation, but how the population of adult-born neurons contributes to reactivation of the memory engram in CA3, but not in the dentate gyrus, is still a matter of debate. Moreover, all studies discussed in this review have exclusively investigated engrams in the dorsal dentate gyrus. While the encoding of contextual fear memories is specifically dependent on the dorsal, but not the ventral portion of the dentate gyrus (Kheirbek et al., 2013), it remains to be elucidated whether different types of memories form also in the ventral dentate gyrus, where they may be particularly relevant for emotional behavior, anxiety, and regulation of the neuroendocrine system. Indeed, chronic stress predominantly decreases neurogenesis in the ventral dentate gyrus and also impairs memory retrieval and neuronal reactivation in CA3 (Denny et al., 2014), pointing toward stress-induced disturbances in engram formation. It remains to be determined whether the same cellular and molecular mechanisms that control memory formation and pattern separation may also operate to encode stress-related information that ultimately precipitate depression and anxiety. If this was the case, it may provide important new insight into the cellular basis of mood disorders and their treatment. Indeed, a previous report has shown that conditioning to a safety cue can inhibit conditioned fear memory and elicit an antidepressant-like response in behavioral tasks of depression and anxiety. Moreover, the antidepressant-like effects of learned safety are dependent on neurogenesis and associated with BDNF release in the dentate gyrus (Pollak et al., 2008), further supporting a role for BDNF-dependent effects on adult-born hippocampal neurons in regulating the plasticity of the memory trace in the hippocampus. Considering the aforementioned role of neurogenesis specifically in encoding weak, but not strong memories, a reduction in neurogenesis as a result of chronic stress may impair encoding of weak stimuli of positive valence, thereby biasing the perception of the environment toward events of potentially more negative valence, ultimately facilitating the development of depressive mood. Therefore, it may be conceivable to enhance neurogenesis and activate engrams of safety or positive valence in order to inhibit learned fear or cellular ensembles that encode aversive, possibly depressogenic information.
The same innovative technology that has recently enabled the groundbreaking discovery of hippocampal memory traces, and the possibility of their modulation, will likely continue to answer many of the remaining questions about the functional role of neurogenesis in memory formation. Addressing these important questions may ultimately allow us to harness the potential of adult-born neurons as crucial regulators of hippocampal function, memory, and mood.
Figure 1.
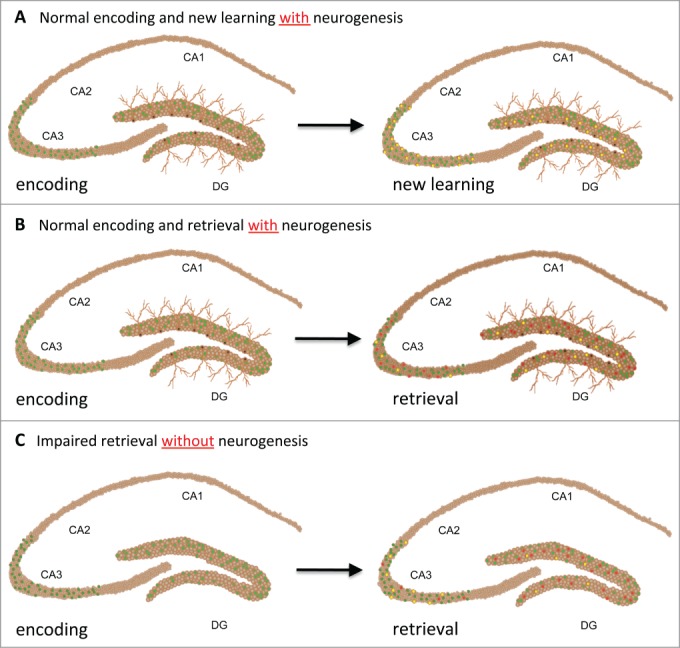
Figure showing encoding, retrieval and new learning with and without neurogenesis. (A) left: immediate early gene activation (Arc, c-fos) in the dentate gyrus and CA3 region of the hippocampus during memory encoding (green cells). Right: During new learning, predominantly new cells are being activated in the dentate gyrus and CA3 (yellow cells). (B) During memory retrieval, a subset of cells that were activated during encoding are recruited (red cells), and new cells are being activated (yellow cells). (C) Without neurogenesis, memory retrieval is impaired and reactivation is decreased in CA3 but not in the dentate gyrus, as indicated by less co-labeling (red cells) than in (B).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Akers KG, Martinez-Canabal A, Restivo L, Yiu AP, De Cristofaro A, Hsiang HL, Wheeler AL, Guskjolen A, Niibori Y, Shoji H, et al.. Hippocampal neurogenesis regulates forgetting during adulthood and infancy. Science 2014: 344:598-602; PMID:24812394; http://dx.doi.org/ 10.1126/science.1248903 [DOI] [PubMed] [Google Scholar]
- Amaral DG, Ishizuka N, Claiborne B. Neurons, numbers and the hippocampal network. Prog Brain Res 1990; 83:1-11; PMID:2203093; http://dx.doi.org/ 10.1016/S0079-6123(08)61237-6 [DOI] [PubMed] [Google Scholar]
- Barretto RP, Ko TH, Jung JC, Wang TJ, Capps G, Waters AC, Ziv Y, Attardo A, Recht L, Schnitzer MJ. Time-lapse imaging of disease progression in deep brain areas using fluorescence microendoscopy. Nat Med 2011; 17(2):223-8; PMID:21240263; http://dx.doi.org/ 10.1038/nm.2292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekinschtein P, Kent BA, Oomen CA, Clemenson GD, Gage FH, Saksida LM, Bussey TJ. BDNF in the dentate gyrus is required for consolidation of “pattern-separated” memories. Cell Rep 2013; 5:759-68; PMID:24209752; http://dx.doi.org/ 10.1016/j.celrep.2013.09.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekinschtein P, Kent BA, Oomen CA, Clemenson GD, Gage FH, Saksida LM, Bussey TJ. Brain-derived neurotrophic factor interacts with adult-born immature cells in the dentate gyrus during consolidation of overlapping memories. Hippocampus 2014; 24:905-11; PMID:24825389; http://dx.doi.org/; http://dx.doi.org/ 10.1002/hipo.22304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergami M, Rimondini R, Santi S, Blum R, Götz M, Canossa M. Deletion of TrkB in adult progenitors alters newborn neuron integration into hippocampal circuits and increases anxiety-like behavior. Proc Natl Acad Sci U S A 2008; 105:15570-5; PMID:18832146; http://dx.doi.org/ 10.1073/pnas.0803702105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burghardt NS, Park EH, Hen R, Fenton AA. Adult-born hippocampal neurons promote cognitive flexibility in mice. Hippocampus 2012; 22:1795-808; PMID:22431384; http://dx.doi.org/ 10.1002/hipo.22013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clelland CD, Choi M, Romberg C, Clemenson GD, Fragniere A, Tyers P, Jessberger S, Saksida LM, Barker RA, Gage FH, Bussey TJ. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science 2009; 325:210-3; PMID:19590004; http://dx.doi.org/ 10.1126/science.1173215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Mayford M, Gage FH. Selection of distinct populations of dentate granule cells in response to inputs as a mechanism for pattern separation in mice. Elife 2013; 2:e00312; PMID:23538967; http://dx.doi.org/ 10.7554/eLife.00312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny CA, Burghardt NS, Schachter DM, Hen R, Drew MR. 4- to 6-week-old adult-born hippocampal neurons influence novelty-evoked exploration and contextual fear conditioning. Hippocampus 2012; 22:1188-201; PMID:21739523; http://dx.doi.org/ 10.1002/hipo.20964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny CA, Kheirbek MA, Alba EL, Tanaka KF, Brachman RA, Laughman KB, Tomm NK, Turi GF, Losonczy A, Hen R. Hippocampal memory traces are differentially modulated by experience, time, and adult neurogenesis. Neuron 2014; 83:189-201; PMID:24991962; http://dx.doi.org/ 10.1016/j.neuron.2014.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew MR, Denny CA, Hen R. Arrest of adult hippocampal neurogenesis in mice impairs single- but not multiple-trial contextual fear conditioning. Behav Neurosci 2010; 124:446-54; PMID:20695644; http://dx.doi.org/ 10.1037/a0020081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew LJ, Fusi S, Hen R. Adult neurogenesis in the mammalian hippocampus: why the dentate gyrus? Learn Mem 2013; 20:710-29; PMID:24255101; http://dx.doi.org/ 10.1101/lm.026542.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner AR, Rowland DC, Hwang SY, Baumgaertel K, Roth BL, Kentros C, Mayford M. Generation of a synthetic memory trace. Science 2012; 335:1513-16; PMID:22442487; http://dx.doi.org/ 10.1126/science.1214985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikrar T, Guo N, He K, Besnard A, Levinson S, Hill A, Lee HK, Hen R, Xu X, Sahay A. Adult neurogenesis modifies excitability of the dentate gyrus. Front Neural Circuits 2013; 7:204; PMID:24421758; http://dx.doi.org/ 10.3389/fncir.2013.00204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kee N, Teixeira CM, Wang AH, Frankland PW. Preferential incorporation of adult-generated granule cells into spatial memory networks in the dentate gyrus. Nat Neurosci 2007; 10:355-362; PMID:17277773; http://dx.doi.org/ 10.1038/nn1847 [DOI] [PubMed] [Google Scholar]
- Kheirbek MA, Drew LJ, Burghardt NS, Costantini DO, Tannenholz L, Ahmari SE, Zeng H, Fenton AA, Hen R. Differential control of learning and anxiety along the dorsoventral axis of the dentate gyrus. Neuron 2013; 77:955-68; PMID:23473324; http://dx.doi.org/ 10.1016/j.neuron.2012.12.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kheirbek MA, Klemenhagen KC, Sahay A, Hen R. Neurogenesis and generalization: a new approach to stratify and treat anxiety disorders. Nat Neurosci 2012; 15:1613-20; PMID:23187693; http://dx.doi.org/ 10.1038/nn.3262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura T, Saitoh Y, Takashima N, Murayama A, Niibori Y, Ageta H, Sekiguchi M, Sugiyama H, Inokuchi K. Adult neurogenesis modulates the hippocampus-dependent period of associative fear memory. Cell 2009; 139:814-27; PMID:19914173; http://dx.doi.org/ 10.1016/j.cell.2009.10.020 [DOI] [PubMed] [Google Scholar]
- Koehl M, Abrous DN. A new chapter in the field of memory: adult hippocampal neurogenesis. Eur J Neurosci 2011; 33:1101-14; PMID:21395854; http://dx.doi.org/ 10.1111/j.1460-9568.2011.07609.x [DOI] [PubMed] [Google Scholar]
- Lacefield CO, Itskov V, Reardon T, Hen R, Gordon JA. Effects of adult-generated granule cells on coordinated network activity in the dentate gyrus. Hippocampus 2012; 22:106-16; PMID:20882540; http://dx.doi.org/ 10.1002/hipo.20860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leutgeb JK, Leutgeb S, Moser MB, Moser EI. Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science 2007; 315:961-6; PMID:17303747; http://dx.doi.org/ 10.1126/science.1135801 [DOI] [PubMed] [Google Scholar]
- Li Y, Luikart BW, Birnbaum S, Chen J, Kwon CH, Kernie SG, Bassel-Duby R, Parada LF. TrkB regulates hippocampal neurogenesis and governs sensitivity to antidepressive treatment. Neuron 2008; 59:399-412; PMID:18701066; http://dx.doi.org/ 10.1016/j.neuron.2008.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link W, Konietzko U, Kauselmann G, Krug M, Schwanke B, Frey U, Kuhl D. Somatodendritic expression of an immediate early gene is regulated by synaptic activity. Proc Natl Acad Sci U S A 1995; 92:5734-8; PMID:7777577; http://dx.doi.org/ 10.1073/pnas.92.12.5734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Ramirez S, Pang PT, Puryear CB, Govindarajan A, Deisseroth K, Tonegawa S. Optogenetic stimulation of a hippocampal engram activates fear memory recall. Nature 2012; 484:381-5; PMID:22441246; http://dx.doi.org/ 10.1038/484410a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyford GL, Yamagata K, Kaufmann WE, Barnes CA, Sanders LK, Copeland NG, Gilbert DJ, Jenkins NA, Lanahan AA, Worley PF. Arc, a growth factor and activity-regulated gene, encodes a novel cytoskeleton-associated protein that is enriched in neuronal dendrites. Neuron 1995; 14:433-45; PMID:7857651; http://dx.doi.org/ 10.1016/0896-6273(95)90299-6 [DOI] [PubMed] [Google Scholar]
- Neunuebel JP, Knierim JJ. CA3 retrieves coherent representations from degraded input: direct evidence for CA3 pattern completion and dentate gyrus pattern separation. Neuron 2014; 81:416-27; PMID:24462102; http://dx.doi.org/ 10.1016/j.neuron.2013.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neunuebel JP, Yoganarasimha D, Rao G, Knierim JJ. Conflicts between local and global spatial frameworks dissociate neural representations of the lateral and medial entorhinal cortex. J Neurosci 2013; 33:9246-58; PMID:23719794; http://dx.doi.org/ 10.1523/JNEUROSCI.0946-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernía-Andrade AJ, Jonas P. Theta-gamma-modulated synaptic currents in hippocampal granule cells in vivo define a mechanism for network oscillations. Neuron 2014; 81(1):140-52; PMID:24333053; http://dx.doi.org/ 10.1016/j.neuron.2013.09.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piatti VC, Ewell LA, Leutgeb JK. Neurogenesis in the dentate gyrus: carrying the message or dictating the tone. Front Neurosci 2013; 7:50; PMID:23576950; http://dx.doi.org/ 10.3389/fnins.2013.00050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak DD, Monje FJ, Zuckerman L, Denny CA, Drew MR, Kandel ER. An animal model of a behavioral intervention for depression. Neuron 2008; 60:149-61; PMID:18940595; http://dx.doi.org/ 10.1016/j.neuron.2008.07.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez S, Liu X, Lin PA, Suh J, Pignatelli M, Redondo RL, Ryan TJ, Tonegawa S. Creating a false memory in the hippocampus. Science 2013; 341:387-91; PMID:23888038; http://dx.doi.org/ 10.1126/science.1239073 [DOI] [PubMed] [Google Scholar]
- Redondo RL, Kim J, Arons AL, Ramirez S, Liu X, Tonegawa S. Bidirectional switch of the valence associated with a hippocampal contextual memory engram. Nature 2014; 513:426-30; PMID:25162525; http://dx.doi.org/ 10.1038/nature13725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahay A, Hen R. Adult hippocampal neurogenesis in depression. Nat Neurosci 2007; 10:1110-5; PMID:17726477; http://dx.doi.org/ 10.1038/nn1969 [DOI] [PubMed] [Google Scholar]
- Sahay A, Scobie KN, Hill AS, O'Carroll CM, Kheirbek MA, Burghardt NS, Fenton AA, Dranovsky A, Hen R. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature 2011; 472:466-70; PMID:21460835; http://dx.doi.org/ 10.1038/nature09817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone SS, Teixeira CM, Devito LM, Zaslavsky K, Josselyn SA, Lozano AM, Frankland PW. Stimulation of entorhinal cortex promotes adult neurogenesis and facilitates spatial memory. J Neurosci 2011a; 31:13469-84; PMID:21940440; http://dx.doi.org/ 10.1523/JNEUROSCI.3100-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone SS, Teixeira CM, Zaslavsky K, Wheeler AL, Martinez-Canabal A, Wang AH, Sakaguchi M, Lozano AM, Frankland PW. Functional convergence of developmentally and adult-generated granule cells in dentate gyrus circuits supporting hippocampus-dependent memory. Hippocampus 2011b; 21:1348-62; PMID:20824726; http://dx.doi.org/ 10.1002/hipo.20845 [DOI] [PubMed] [Google Scholar]
- Swan AA, Clutton JE, Chary PK, Cook SG, Liu GG, Drew MR. Characterization of the role of adult neurogenesis in touch-screen discrimination learning. Hippocampus 2014; 24:1581-91; PMID:25074617; http://dx.doi.org/ 10.1002/hipo.22337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tayler KK, Tanaka KZ, Reijmers LG, Wiltgen BJ. Reactivation of neural ensembles during the retrieval of recent and remote memory. Curr Biol 2013; 23:99-106; PMID:23246402; http://dx.doi.org/ 10.1016/j.cub.2012.11.019 [DOI] [PubMed] [Google Scholar]
- Vivar C, Potter MC, Choi J, Lee JY, Stringer TP, Callaway EM, Gage FH, Suh H, van Praag H. Monosynaptic inputs to new neurons in the dentate gyrus. Nat Commun 2012; 3:1107; PMID:23033083; http://dx.doi.org/ 10.1038/ncomms2101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang KH, Majewska A, Schummers J, Farley B, Hu C, Sur M, Tonegawa S. In vivo two-photon imaging reveals a role of arc in enhancing orientation specificity in visual cortex. Cell 2006; 126:389-402; PMID:16873068; http://dx.doi.org/ 10.1016/j.cell.2006.06.038 [DOI] [PubMed] [Google Scholar]
- Ziv Y, Burns LD, Cocker ED, Hamel EO, Ghosh KK, Kitch LJ, El Gamal A, Schnitzer MJ. Long-term dynamics of CA1 hippocampal place codes. Nat Neurosci 2013; 16(3):264-6; PMID:23396101; http://dx.doi.org/ 10.1038/nn.3329 [DOI] [PMC free article] [PubMed] [Google Scholar]


