Abstract
A large number of susceptibility genes have been implicated in psychiatric disorders with a developmental origin, yet their biological roles and signaling mechanisms in neurodevelopment are largely unknown. Disrupted-In-Schizophrenia 1 (DISC1), a susceptibility gene for several major psychiatric disorders, regulates the development of newborn neurons in the adult hippocampus. Systemic pharmacological inhibition of mTOR signaling with rapamycin has been shown to rescue DISC1 deficiency-induced neurodevelopmental defects, as well as cognitive and affective deficits. Whether mTOR signaling plays a cell-autonomous and/or non-cell-autonomous role in DISC1-dependent regulation of neuronal development is not clear. Here we provide genetic evidence that hyper-activation of mTOR activator Rheb1 (Ras homolog enriched in brain 1) in newborn neurons recapitulates DISC1 deficiency-induced neurodevelopmental defects, including neuronal morphogenesis and migration. We further show that genetic deletion of Rheb1 rescues those defects in a cell-autonomous fashion in developing newborn neurons in the adult hippocampus. Our genetic and functional studies demonstrate that Rheb1 acts as a key mediator of DISC1-dependent regulation of mTOR signaling and neuronal development during adult hippocampal neurogenesis.
Keywords: adult neurogenesis, neurodevelopment, DISC1, Rheb1
Introduction
One of the greatest challenges in treating complex neurodevelopmental disorders, such as schizophrenia (SCZ) and autism spectrum disorders (ASD), is identifying determinative pathophysiology and potential targets for effective intervention. While genome-wide association studies have revealed a vast number of candidate risk genes associated with SCZ and ASD, much less is known about how these genetic risk factors could impact the developing nervous system.1-3 Determining the functional role of risk genes in normal neuronal development and identifying their signaling pathways will not only lead to a better understanding of the pathogenesis of SCZ and ASD, but also the identification of potential targets for therapeutic intervention.
Disrupted-In-Schizophrenia 1 (DISC1) is a rare risk gene originally identified at the breakpoint of a balanced chromosomal translocation t(1;11)(q42;q14) that co-segregates with major mental illness in a unique Scottish pedigree.4 Subsequent genetic linkage and association studies identified additional variants of DISC1 that are associated with SCZ, affective disorders, and ASD.5,6 DISC1 encodes a scaffold protein with multiple binding partners that have a broad impact on intracellular signaling pathways and neurodevelopmental processes,7,8 including neural progenitor proliferation,9 neuronal migration,10 axonal and dendritic growth, and synapse formation.11-16 During adult hippocampal neurogenesis, DISC1 knockdown decreases proliferation in neural progenitors,9 but accelerates dendritic growth and leads to soma hypertrophy, ectopic dendrites and aberrant positioning of newborn neurons.11 Interestingly, DISC1 deficiency in hippocampal adult-born neurons alone is sufficient to induce specific behavioral deficits.17 Converging evidence from biochemical and genetic studies suggest a model in which DISC1 regulates adult-born neuron development through regulation of intracellular Akt activation.18,19 Notably, systemic inhibition of mTOR signaling by rapamycin during early stages of neuronal development rescues specific behavioral impairments as well as cellular deficits induced by DISC1-deficiency, but rescues only behavior deficits, without changing the cellular phenotype, if applied during later stages.17 Thus, mTOR signaling may have both a direct and an indirect impact on neuronal development and circuitry function. It is unknown how mTOR is activated in this model and whether mTOR is required in a cell-autonomous manner to mediate DISC1-dependent regulation of neuronal development.
The small GTPase Rheb1 (Ras homolog enriched in the brain 1) has been shown to be a specific activator of mTOR complex 1 via direct binding to mTOR and it stimulates the kinase activity of mTOR when loaded with GTP in vitro.20-23 The role of Rheb1 in the brain has just begun to be elucidated. One recent study showed that Rheb1 is both necessary and sufficient for mTOR complex 1 activation in vivo in the brain and that Rheb1 is essential for embryonic brain development and myelination24 and for hippocampal dependent plasticity.25 In another study, deletion of Rheb1 in hypothalamic neurons leads to reduced food intake and hypoglycemia, a phenotype also found when hypothalamus mTOR complex 1 activity is dysregulated.26,27
Here, using Rheb1 conditional knockout and conditional overexpression mouse models in combination with retrovirus-mediated expression of Cre to specifically manipulate Rheb1 expression in newborn neurons in the adult hippocampus, we provide evidence that Rheb1 is essential for mTOR signaling and development of newborn neurons. Furthermore, Rheb1 overexpression mimics the cellular phenotypes induced by DISC1 knockdown, while Rheb1 knockout rescues those deficits. Therefore, our studies suggest that Rheb1 is an important regulator of mTOR signaling and a downstream mediator of DISC1 to regulate neuronal development during adult neurogenesis.
Materials and Methods
In vivo birth-dating, genetic manipulation of neural progenitors with retroviruses
Engineered self-inactivating murine onco-retroviruses were used to co-express shRNA under the U6 promoter and a fluorescent marker (GFP or DsRed) under EF1a promoter, or Cre recombinase and GFP under the ubiquitin promoter specifically in proliferating cells and their progeny, as previously described28 (Fig. 1A and 2A). Specific shRNAs against mouse Disc1 (sh-D1) and control shRNA have been previously characterized and validated via rescue experiments with shRNA-resistant wild-type DISC1 expression.11,18,19 High titers of engineered retroviruses were produced by co-transfection of viral vectors and vesicular stomatitis viral envelope (VSVP) into 293gp cells followed by ultra-centrifugation of viral supernatant as previously described.11
Figure 1.
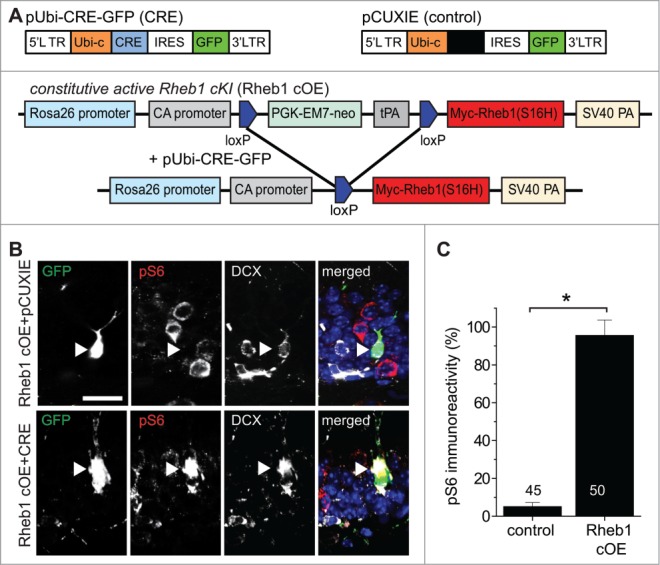
Hyper-activation of Rheb1 is sufficient to activate mTOR signaling in newborn neurons in the adult hippocampus. (A) Schematic diagrams of retroviral vectors used for expressing GFP (pCUXIE), co-expressing GFP and Cre recombinase (pUbi-CRE-GFP) (upper panel). Also shown is a schematic diagram for strategies of expressing Rheb1-S16H specifically in proliferating neural progenitors in the adult dentate gyrus (lower panel). Retroviruses co-expressing GFP and Cre were stereotaxically injected into the dentate gyrus of adult Rheb1 cOE mice. (B and C) Increased mTOR signaling in newborn neurons expressing Rheb1-S16H at 14 dpi. Shown in (B) are sample confocal images of immunostaining of GFP, pS6-Ser235/236 and immature neuron marker DCX. Arrowheads point to GFP+DCX+ newborn neurons. Note the high pS6 levels in a GFP+ neuron expressing Cre (lower panel), and very low levels in GFP+ only neurons (upper panel). Scale bar: 20 μm. Shown in (C) is a summary of quantification of pS6 levels in GFP+ newborn neurons under different conditions. The immunofluorescence intensity of pS6 in GFP+DCX+ cells was normalized to that of GFP−DCX+ neurons in the same brain sample. Numbers associated with the bar graph indicate numbers of GFP+ neurons examined. Values represent mean + SEM (*: p < 0.01; Student's t-test).
The following adult mice were used (6–8 weeks old): WT (C57BL/6) mice, ROSA-Rheb1-S16Hf/f and Rheb1f/f mice.24 Animals housed under standard conditions were anaesthetized and concentrated retroviruses were stereotaxically injected into the dentate gyrus at 4 sites (0.5 μl per site at 0.25 μl/min) with following coordinates (from Bregma in mm): posterior = 2, lateral = + 1.6, ventral = 2.5; posterior = 3, lateral = + 2.6, ventral = 3.2, as previously described.11 All animal procedures used in this study were performed in accordance with the protocol approved by the Institutional Animal Care and Use Committee.
Immunohistology, confocal imaging and analysis
Coronal brain sections (40 μm in thickness) were prepared from injected mice and processed for immunostaining as described.29,30 The sections were incubated for 30 min in 4′,6′-diaminodino-2-phenylindole (DAPI, 1:5000) before washing and mounting. Images were acquired on a confocal system (Zeiss LSM 710) using a multi-track configuration.
For analysis of cell morphology, Z-series stacks of confocal images were taken and a single confocal image slice with the largest soma area for individual GFP+ or DsRed+ or GFP+DsRed+ neurons was used for quantification using NIH ImageJ program. For analysis of neuronal positioning, single section confocal images of GFP+ or DsRed+ or GFP+DsRed+ neurons with DAPI staining were used to determine the soma localization within 4 areas defined in Figure 3A. For analysis of dendritic development, 3D reconstructions of entire dendritic processes of each newborn neuron were made from Z-series stacks of confocal images. The 2D projection images were traced with NIH ImageJ. All newborn dentate granule neurons with largely intact dendritic trees were analyzed for total dendritic length as described.11 The measurements did not include corrections for inclinations of dendritic process and therefore represent projected lengths. Sholl analysis for dendritic complexity was carried out by counting the number of dendrites that cross a series of concentric circles at 10 μm intervals from the soma as previously described.11 For analysis of pS6 levels, primary antibodies against pS6-Ser235/236 (rabbit, 1:1000, Cell Signaling) were used. Effective immunostaining of pS6 required an antigen retrieval protocol as previously described.18 All analyses were carried out by personnel who were blind to experimental conditions. Multiple animals for each condition were analyzed. Statistical significance was determined by ANOVA or Student's t-test as indicated.
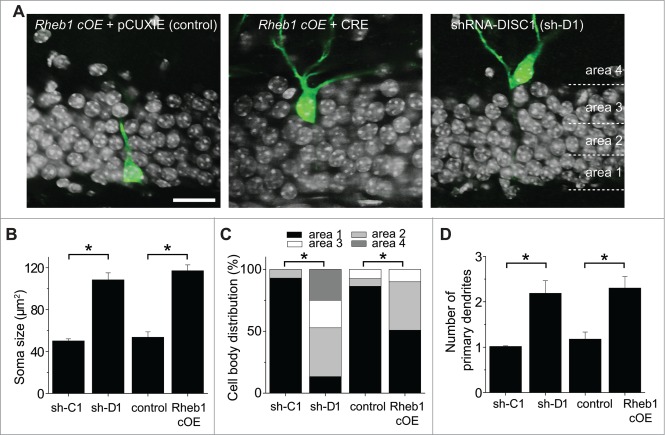
Figure 3.
Rheb1 hyper-activation recapitulates DISC1 knockdown-induced cell positioning and morphological defects of newborn neurons in the adult hippocampus. (A–C) Soma size and cell body position of newborn neurons after different manipulations at 14 dpi. Shown in (A) are sample single-section confocal images to show the soma size and localization within the defined 4 areas in the dentate gyrus. Scale bar: 20 μm. Shown in (B) is a summary of soma size. The same groups of cells as in Figure 2C were analyzed. Values represent mean + SEM. (*: p< 0.01; Student's t-test). Shown in (C) is a summary of distribution of GFP+ cells within each area as defined in (A). (D) A summary of the number of primary dendrites. The same groups of cells as in Figure 1C were analyzed. Values represent mean + SEM. (*: p < 0.01; Student's t-test).
Results
Hyper-activation of Rheb1 is sufficient to activate mTOR signaling in newborn neurons in the adult hippocampus
To examine the function of Rheb1 in newborn granule neurons in the adult hippocampus, we first tested whether Rheb1 hyper-activation specifically in newborn neurons is sufficient to activate the downstream mTOR signaling pathway. In combination with genetically modified mouse conditional lines, we employed a retrovirus-mediated “single-cell” genetic approach for birth-dating and genetic manipulation of proliferating neural progenitors in the adult mouse hippocampus in vivo.28,31 Retroviruses co-expressing Cre recombinase and GFP or those expressing GFP alone were stereotaxically injected into the dentate gyrus of adult conditional Rheb1 overexpression (cOE) mice, in which a constitutively active form of Rheb1 (S16H) is expressed from the ROSA26 locus in a Cre-dependent fashion24,32 (Fig. 1A). Immunostaining of phosphorylated forms of ribosomal S6 (pS6), an indicator of mTOR activation,33 and the immature neuronal marker doublecortin (DCX) showed a dramatic increase of pS6 levels in GFP+CRE+DCX+ neurons, but not in control GFP+CRE−DCX+ neurons at 14 d post viral injection (dpi), compared to unlabeled GFP−DCX+ neurons in the same preparation, in which the pS6 level is very low (Fig. 1B and C). This result confirms that Cre-mediated constitutively active Rheb1-S16H expression is sufficient to activate mTOR signaling in newborn neurons in the adult hippocampus.
Rheb1 hyper-activation recapitulates DISC1 knockdown-induced developmental defects in newborn neurons in the adult hippocampus
Given that DISC1 knockdown (KD) leads to increased mTOR signaling in newborn neurons during adult hippocampal neurogenesis,18 we examined whether Rheb1 hyper-activation in newborn neurons produces similar neurodevelopmental defects as DISC1 KD. Retroviruses co-expressing Cre recombinase and GFP or GFP alone were stereotaxically injected into the dentate gyrus of Rheb1 cOE mice, while retroviruses co-expressing GFP with either shRNA against mouse Disc1 (sh-D1) or scrambled shRNA (sh-C1) were stereotaxically injected into the dentate gyrus of wild type mice (Fig. 1A and 2A). Consistent with previous results,11,18 newborn neurons co-expressing GFP and a previously characterized potent shRNA against mouse Disc1 (sh-D1) exhibited significantly accelerated dendritic growth at 14 dpi (Fig. 2B and C). Interestingly, GFP+ newborn neurons expressing Rheb1-S16H displayed similarly precocious dendritic developmental defects (Fig. 2B and C). Sholl analysis also revealed increased dendritic complexity of newborn neurons with either DISC1 KD or Rheb1-S16H overexpression (Fig. 2D).
Figure 2.
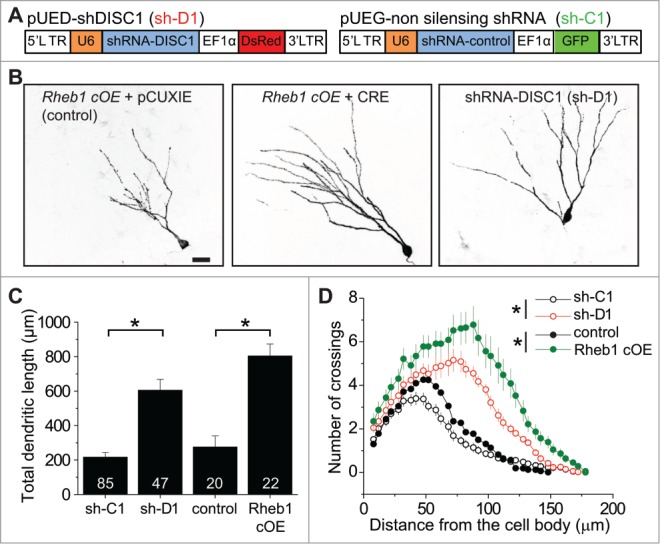
Rheb1 hyper-activation recapitulates DISC1 knockdown-induced dendritic developmental defects of newborn neurons in the adult hippocampus. (A) Schematic diagrams of retroviral vectors used for co-expressing GFP and sh-C1 or DsRed and sh-D1. (B–D) Dendritic length and complexity of newborn neurons at 14 d post retroviral injection (dpi) to co-express GFP and sh-C1 or sh-D1 in proliferating neural progenitors in the dentate gyrus of adult WT mice, or to express GFP alone (control) or co-express GFP and Cre (Rheb1 cOE) in conditional Rheb1-S16H overexpression mice. Shown in (B) are sample projection confocal images under different conditions. Scale bar: 20 μm. Shown in (C) is a summary of total dendritic length. Numbers associated with bar graphs indicate total numbers of GFP+ cells examined from 4 animals for each condition. Values represent mean + SEM. (*: p< 0.01; Student's t-test). Shown in (D) is a summary of Sholl analysis of dendritic complexity of newborn neurons. The same groups of neurons as in Figure 2C were examined. Values represent mean + SEM (*: p < 0.01; 2-way ANOVA).
We next examined cell positioning and morphology. Consistent with previous studies,11,18 newborn neurons with DISC1 deficiency exhibited enlarged soma, ectopic primary dendrites, and aberrant cell positioning into the outer granule cell and molecular layers at 14 dpi (Fig. 3). GFP+Cre+ newborn neurons expressing Rheb1-S16H displayed very similar neuronal developmental defects in soma size and formation of ectopic primary dendrites as DISC1 KD (Fig. 3A, B and D), whereas the neuronal positioning defect appeared to be less severe (Fig. 3C). Together, these data suggest that Rheb1 hyper-activation specifically in newborn neurons is sufficient to induce neuronal developmental defects similar to those observed following DISC1 KD during adult hippocampal neurogenesis.
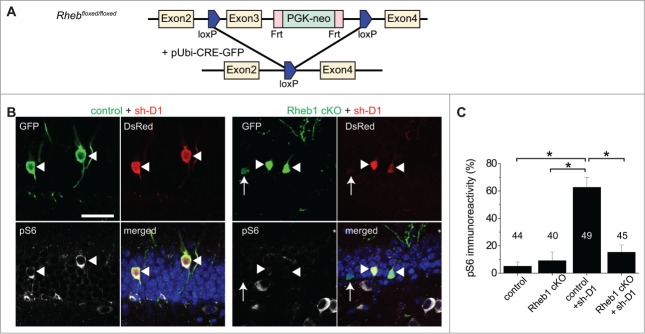
Rheb1 is required for the DISC1 knockdown-induced increase of mTOR signaling in newborn neurons during adult hippocampal neurogenesis
To investigate whether Rheb1 mediates the increase in mTOR signaling elicited by DISC1 KD, we used Rheb1 conditional knockout mice (cKO; Fig. 4A). Retroviruses co-expressing GFP and Cre were stereotaxically injected into the dentate gyrus of adult Rheb1 cKO. No significant change in the immunoreactivity of pS6 was detected for newborn neurons co-expressing GFP and CRE at 14 dpi in the dentate gyrus of adult hippocampus, as the basal level of pS6 was very low (Fig. 4C). We further employed a double retroviral manipulation approach for simultaneous Rheb1 deletion and DISC1 KD using a mixture of retroviruses co-expressing GFP/CRE and those co-expressing DsRed/sh-D1 in adult Rheb1 cKO mice. Rheb1 deletion normalized the DISC1 KD-induced increase of pS6 levels in newborn neurons while control cells with DISC1 KD alone expressed significantly higher levels of pS6 (Fig. 4B and C). This result suggests that endogenous Rheb1 is required for DISC1 KD-induced over-activation of mTOR signaling in newborn neurons in the adult hippocampus in vivo.
Figure 4.
Rheb1 activation is required for DISC1 knockdown-induced increase of mTOR signaling in newborn neurons during adult hippocampal neurogenesis. (A) A schematic diagram showing the strategy of knocking out Rheb1 specifically in proliferating neural progenitors in the adult dentate gyrus. Retroviruses co-expressing GFP and Cre were stereotaxically injected into the dentate gyrus of adult Rheb1 cKO mice. (B and C) Rheb1 knockout abolished DISC1 KD-induced increase of pS6-Ser235/236 levels in newborn neurons in the adult Rheb1 cKO mice. Shown in (B) are sample confocal images of immunostaining of GFP and pS6. Arrowheads point to GFP+DsRed+ newborn neurons; arrows point to GFP+ only newborn neurons. Note high pS6 levels in GFP+ neurons expressing sh-D1 (left panel), but not in those expressing sh-D1 and Cre (right panel). Scale bar: 20 μm. Shown in (C) is a summary of quantification of pS6 levels in GFP+ neurons under different conditions. Numbers associated with the bar graph indicate numbers of GFP+DsRed+ neurons examined. Values represent mean + SEM (*: p < 0.01; Student's t-test).
Rheb1 is required for DISC1 knockdown-induced neurodevelopmental defects
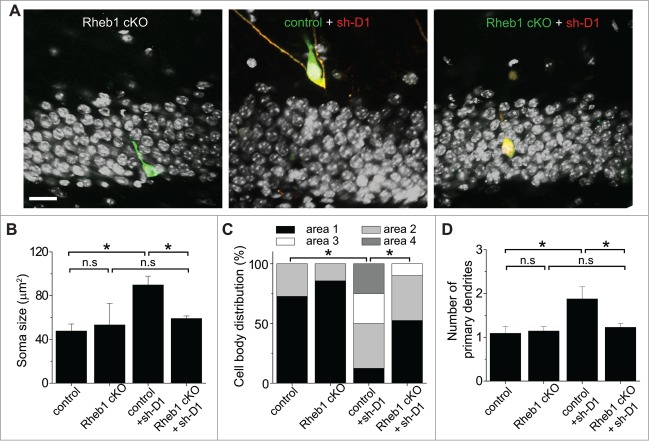
We next examined whether Rheb1 mediates DISC1 signaling during regulation of neuronal development using Rheb1 cKO. Unlike Rheb1 hyper-activation, no significant developmental defects were detected for newborn neurons co-expressing GFP and CRE at 14 dpi in the dentate gyrus of adult Rheb1 cKO mice (Figs. 5 and 6), similar to what we found with DISC1 overexpression.11 For simultaneous Rheb1 deletion and DISC1 KD, a mixture of retroviruses co-expressing GFP/CRE and those co-expressing DsRed/sh-D1 were stereotaxically injected into the dentate gyrus of adult Rheb1 cKO mice. Newborn neurons with only DISC1 KD exhibited enhanced total dendritic growth and increased dendritic complexity and these DISC1 KD-induced aberrant dendritic phenotypes were rescued by loss of function of Rheb1 in the same animal (Fig. 5). We further analyzed the cell positioning and morphology of DISC1-deficient newborn neurons with or without Rheb1 expression. Rheb1 deletion rescued the enlarged soma, abnormal cell positioning and ectopic primary dendrites resulting from DISC1 KD (Fig. 6). Thus, endogenous Rheb1 function is required for DISC1 KD-induced neuronal developmental defects in vivo.
Figure 5.
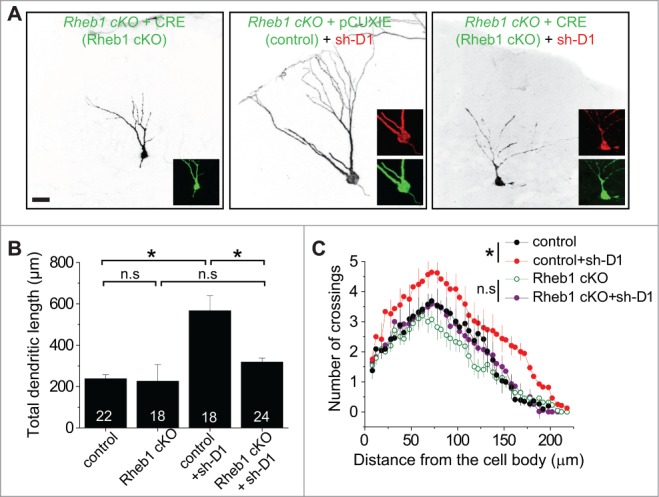
Rheb1 is required for DISC1 knockdown-induced dendritic developmental defects of newborn neurons in the adult hippocampus. (A–C) Dendritic length and complexity of newborn neurons at 14 d post retroviral injection (dpi) to co-express GFP alone (control) or co-express GFP and Cre (Rheb1 cKO) and DsRed/sh-D1 in adult conditional Rheb1 KO mice. Shown in (A) are sample projection confocal images of newborn neurons under different conditions. Scale bar: 20 μm. Shown in (B) is a summary of total dendritic length. Numbers associated with bar graphs indicate total numbers of GFP+ cells examined from 4 animals for each condition. Values represent mean + SEM. (*: p < 0.01; Student's t-test). Shown in (C) is a summary of Sholl analysis of dendritic complexity of newborn neurons. The same groups of neurons as in Figure 5B were examined. Values represent mean + SEM (*: p < 0.01; 2-way ANOVA).
Figure 6.
Rheb1 function is required for DISC1 knockdown-induced cell positioning and morphological defects of newborn neurons in the adult hippocampus. (A–C) Soma size and cell body position of newborn neurons after different manipulations at 14 dpi. Shown in (A) are sample single-section confocal images to show soma size and localization of newborn neurons within the defined 4 areas in the dentate gyrus. Scale bar: 20 μm. Shown in (B) is a summary of soma size. The same groups of cells as in Figure 5B were analyzed. Values represent mean + SEM. (*: p < 0.01; Student's t-test). Shown in (C) is a summary of the distribution of GFP+ cells within each area as defined in Figure 3A. (D) A summary of the number of primary dendrites. The same groups of cells as in Figure 5B were analyzed. Values represent mean + SEM. (*: p < 0.01; Student's t-test).
Taken together, our genetic gain- and loss-of-function experiments established Rheb1 as a key mediator of DISC1-dependent regulation of mTOR signaling and neuronal development during adult hippocampal neurogenesis in a cell-autonomous fashion.
Discussion
The scaffold protein DISC1 has many binding partners and thus plays a broad and complex role in neuronal development.7,34 The mechanism through which DISC1 regulates distinct aspects of neuronal development in vivo is not well understood. The mTOR signaling pathway, as a major pathway controlling cellular metabolic homeostasis, has been proposed to function as a signaling hub to integrate external stimuli for the regulation of many biological processes, including neural development and neuropsychiatric diseases.35 However, due to the complexity of this pathway, with multiple upstream regulators and downstream effectors,33 it has been difficult to dissect the components that regulate neuronal development. Although previous results have demonstrated that systemic administration of the mTOR antagonist rapamycin could ameliorate effects of DISC1 knockdown in neuronal development,17-19 it was not known whether the relevant changes in mTOR signaling were operating in a cell-autonomous manner. Using in vivo “single-cell” genetic analyses in conditional knockout and conditional overexpression mouse models to selectively manipulate Rheb1 in adult-born neurons, we demonstrate here a cell-autonomous role of Rheb1 in mediating DISC1 regulation of neuronal development.
Adult hippocampal neurogenesis is an exceptionally advantageous in vivo cellular model system for investigating functions and signaling pathways of risk genes for mental disorders in neuronal development.36-38 It recapitulates the complete process of embryonic neuronal development, including proliferation, fate determination, migration, axon/dendrite development and synaptic integration, with a prolonged time course, allowing for high resolution analysis of each stage of neuronal development and its associated molecular events. In this study we have identified Rheb1 as a key mediator linking DISC1 to the mTOR pathway in regulating neuronal development. Rheb1 was originally identified by differential cloning as a small GTPase in the Ras family that is induced by synaptic activity in dentate granule cells in the context of seizures and long-term potentiation.21 The physiological role of Rheb1 in vivo has only recently been a target of investigation because of the availability of newly developed conditional genetic mouse models, as a direct knockout is embryonic lethal. For example, specific deletion of Rheb1 in the oligodendrocyte precursor cells using Olig1/2-Cre, or in the brain using nestin-Cre, results in defects in oligodendrocyte precursor proliferation and myelination during brain development, respectively, and both processes are dependent on signaling through mTOR complex 1, but not TORC2.24,32 In the current study, combining in vivo single-cell manipulation using a retrovirus to express Cre and conditional Rheb1 overexpression mouse lines, we provided evidence that phenotypes resulting from over-activation of Rheb1 closely resemble those seen with DISC1 knockdown. More importantly, we found that deletion of Rheb1 normalizes mTOR activity and rescues cellular phenotypes induced by DISC1 knockdown. Thus, Rheb1 is both required and sufficient to mediate DISC1-dependent regulation of neural development in newborn neurons in the adult hippocampus (Fig. 7A).
Figure 7.
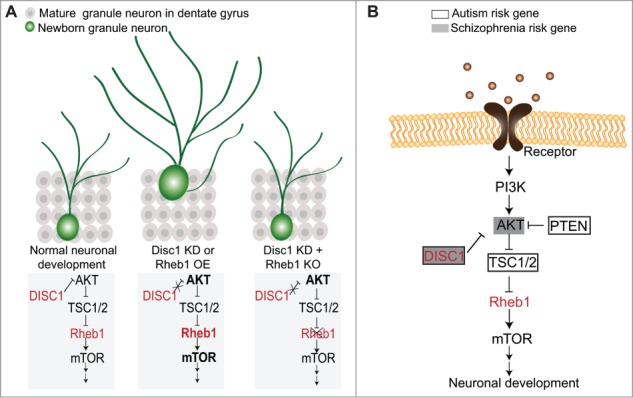
A summary model of DISC1 signaling mediated by Rheb1 in regulating neuronal development during adult hippocampal neurogenesis.
In addition to its putative relevance in neurodevelopmental disorders, such as ASD and SCZ, Rheb1 is a direct target of TSC1/2, the protein complex encoded by the TSC1 and TSC2 genes. Heterozygous mutations in TSC1 or TSC2 are the cause of tuberous sclerosis, a neurocutaneous disorder with a high incidence of brain involvement, intellectual disability and co-morbid diagnoses of ASD.39 Independently, genetic association studies have identified TSC1 and TSC2 as risk genes for ASD.40 Our results implicate TSC1/2 in the Rheb1-mediated effect of DISC1 knockdown in adult neurogenesis, potentially linking another ASD risk gene to the DISC1 signaling cascade (Fig. 7B), which may subsequently reveal common targets for intervention, diagnostic markers and treatment of multiple mental disorders.
Furthermore, the signaling pathway we have delineated in the development of adult newborn neurons may have direct relevance to SCZ, as disruptions in post-natal neurogenesis have been implicated in the pathogenesis of this disorder. Postmortem brains from patients with SCZ show a reduced number of proliferating neural progenitors,41 immaturity of dentate gyrus granule neurons42 and diminished volume of the hippocampus.43 While it remains an interesting idea that disturbances in adult hippocampal neurogenesis may contribute to psychiatric disorders, the signaling mechanism discovered here provides a foundation to understand how multiple risk genes functionally converge into common pathways that may be responsible for the etiology of psychiatric disorders and could be potential targets for therapeutic interventions.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank D. Weinberger, D. St. Clair and D. Valle for discussion, Jaden Shin for gene expression analyses, members of Ming and Song Laboratories for help and critical comments, L. Liu, Y. Cai, Q. Hussaini, and M. Jardine-Alborz for technical support.
Funding
This work was supported by NIH (NS048271, MH105128), NARSAD, and MSCRF to G-l.M., by NIH (NS047344 and NS093772) and MSCRF to H.S., by NARSAD and NIH (NS093772) to K.C., and by NARSAD to E.K.
References
- 1.Lewis DA, Levitt P. Schizophrenia as a disorder of neurodevelopment. Annu Rev Neurosci 2002; 25:409-32; PMID:12052915; http://dx.doi.org/ 10.1146/annurev.neuro.25.112701.142754 [DOI] [PubMed] [Google Scholar]
- 2.Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry 1987; 44:660-9; PMID:3606332; http://dx.doi.org/ 10.1001/archpsyc.1987.01800190080012 [DOI] [PubMed] [Google Scholar]
- 3.Geschwind DH. Advances in autism. Annu Rev Med 2009; 60:367-80; PMID:19630577; http://dx.doi.org/ 10.1146/annurev.med.60.053107.121225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Millar JK, Wilson-Annan JC, Anderson S, Christie S, Taylor MS, Semple CA, Devon RS, Clair DM, Muir WJ, Blackwood DH, et al.. Disruption of two novel genes by a translocation co-segregating with schizophrenia. Hum Mol Genet 2000; 9:1415-23; PMID:10814723; http://dx.doi.org/ 10.1093/hmg/9.9.1415 [DOI] [PubMed] [Google Scholar]
- 5.Sachs NA, Sawa A, Holmes SE, Ross CA, DeLisi LE, Margolis RL. A frameshift mutation in Disrupted in Schizophrenia 1 in an American family with schizophrenia and schizoaffective disorder. Mol Psychiatry 2005; 10:758-64; PMID:15940305; http://dx.doi.org/ 10.1038/sj.mp.4001667 [DOI] [PubMed] [Google Scholar]
- 6.Chubb JE, Bradshaw NJ, Soares DC, Porteous DJ, Millar JK. The DISC locus in psychiatric illness. Mol Psychiatry 2008; 13:36-64; PMID:17912248; http://dx.doi.org/ 10.1038/sj.mp.4002106 [DOI] [PubMed] [Google Scholar]
- 7.Porteous DJ, Millar JK, Brandon NJ, Sawa A. DISC1 at 10: connecting psychiatric genetics and neuroscience. Trends Mol Med 2011; 17:699-706; PMID:22015021; http://dx.doi.org/ 10.1016/j.molmed.2011.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomson PA, Malavasi EL, Grunewald E, Soares DC, Borkowska M, Millar JK. DISC1 genetics, biology and psychiatric illness. Front Biol (Beijing) 2013; 8:1-31; PMID:23550053; http://dx.doi.org/ 10.1007/s11515-012-1254-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mao Y, Ge X, Frank CL, Madison JM, Koehler AN, Doud MK, Tassa C, Berry EM, Soda T, Singh KK, et al.. Disrupted in schizophrenia 1 regulates neuronal progenitor proliferation via modulation of GSK3beta/β-catenin signaling. Cell 2009; 136:1017-31; PMID:19303846; http://dx.doi.org/ 10.1016/j.cell.2008.12.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kamiya A, Kubo K, Tomoda T, Takaki M, Youn R, Ozeki Y, Sawamura N, Park U, Kudo C, Okawa M, et al.. A schizophrenia-associated mutation of DISC1 perturbs cerebral cortex development. Nat Cell Biol 2005; 7:1167-78; PMID:16299498; http://dx.doi.org/ 10.1038/ncb1328 [DOI] [PubMed] [Google Scholar]
- 11.Duan X, Chang JH, Ge S, Faulkner RL, Kim JY, Kitabatake Y, Liu XB, Yang CH, Jordan JD, Ma DK, et al.. Disrupted-In-Schizophrenia 1 regulates integration of newly generated neurons in the adult brain. Cell 2007; 130:1146-58; PMID:17825401; http://dx.doi.org/ 10.1016/j.cell.2007.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Faulkner RL, Jang MH, Liu XB, Duan X, Sailor KA, Kim JY, Ge S, Jones EG, Ming GL, Song H, et al.. Development of hippocampal mossy fiber synaptic outputs by new neurons in the adult brain. Proc Natl Acad Sci U S A 2008; 105:14157-62; PMID:18780780; http://dx.doi.org/ 10.1073/pnas.0806658105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayashi-Takagi A, Takaki M, Graziane N, Seshadri S, Murdoch H, Dunlop AJ, Makino Y, Seshadri AJ, Ishizuka K, Srivastava DP, et al.. Disrupted-in-Schizophrenia 1 (DISC1) regulates spines of the glutamate synapse via Rac1. Nat Neurosci 2010; 13:327-32; PMID:20139976; http://dx.doi.org/ 10.1038/nn.2487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Q, Charych EI, Pulito VL, Lee JB, Graziane NM, Crozier RA, Revilla-Sanchez R, Kelly MP, Dunlop AJ, Murdoch H, et al.. The psychiatric disease risk factors DISC1 and TNIK interact to regulate synapse composition and function. Mol Psychiatry 2011; 16:1006-23; PMID:20838393; http://dx.doi.org/ 10.1038/mp.2010.87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kang E, Burdick KE, Kim JY, Duan X, Guo JU, Sailor KA, Jung DE, Ganesan S, Choi S, Pradhan D, et al.. Interaction between FEZ1 and DISC1 in regulation of neuronal development and risk for schizophrenia. Neuron 2011; 72:559-71; PMID:22099459; http://dx.doi.org/ 10.1016/j.neuron.2011.09.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wen Z, Nguyen HN, Guo Z, Lalli MA, Wang X, Su Y, Kim NS, Yoon KJ, Shin J, Zhang C, et al.. Synaptic dysregulation in a human iPS cell model of mental disorders. Nature 2014; 515(7527):414-8; PMID:25132547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou M, Li W, Huang S, Song J, Kim JY, Tian X, Kang E, Sano Y, Liu C, Balaji J, et al.. mTOR Inhibition ameliorates cognitive and affective deficits caused by Disc1 knockdown in adult-born dentate granule neurons. Neuron 2013; 77:647-54; PMID:23439118; http://dx.doi.org/ 10.1016/j.neuron.2012.12.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim JY, Duan X, Liu CY, Jang MH, Guo JU, Pow-anpongkul N, Kang E, Song H, Ming GL. DISC1 regulates new neuron development in the adult brain via modulation of AKT-mTOR signaling through KIAA1212. Neuron 2009; 63:761-73; PMID:19778506; http://dx.doi.org/ 10.1016/j.neuron.2009.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim JY, Liu CY, Zhang F, Duan X, Wen Z, Song J, Feighery E, Lu B, Rujescu D, St Clair D, et al.. Interplay between DISC1 and GABA signaling regulates neurogenesis in mice and risk for schizophrenia. Cell 2012; 148:1051-64; PMID:22385968; http://dx.doi.org/ 10.1016/j.cell.2011.12.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Long X, Lin Y, Ortiz-Vega S, Yonezawa K, Avruch J. Rheb binds and regulates the mTOR kinase. Curr Biol 2005; 15:702-13; PMID:15854902; http://dx.doi.org/ 10.1016/j.cub.2005.02.053 [DOI] [PubMed] [Google Scholar]
- 21.Yamagata K, Sanders LK, Kaufmann WE, Yee W, Barnes CA, Nathans D, Worley PF. rheb, a growth factor- and synaptic activity-regulated gene, encodes a novel Ras-related protein. J Biol Chem 1994; 269:16333-9; PMID:8206940 [PubMed] [Google Scholar]
- 22.Sato T, Nakashima A, Guo L, Tamanoi F. Specific activation of mTORC1 by Rheb G-protein in vitro involves enhanced recruitment of its substrate protein. J Biol Chem 2009; 284:12783-91; PMID:19299511; http://dx.doi.org/ 10.1074/jbc.M809207200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang Q, Inoki K, Kim E, Guan KL. TSC1/TSC2 and Rheb have different effects on TORC1 and TORC2 activity. Proc Natl Acad Sci U S A 2006; 103:6811-6; PMID:16627617; http://dx.doi.org/ 10.1073/pnas.0602282103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zou J, Zhou L, Du XX, Ji Y, Xu J, Tian J, Jiang W, Zou Y, Yu S, Gan L, et al.. Rheb1 is required for mTORC1 and myelination in postnatal brain development. Dev Cell 2011; 20:97-108; PMID:21238928; http://dx.doi.org/ 10.1016/j.devcel.2010.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goorden SM, Abs E, Bruinsma CF, Riemslagh FW, van Woerden GM, Elgersma Y. Intact neuronal function in Rheb1 mutant mice: implications for TORC1-based treatments. Hum Mol Genet 2015; 24:3390-8; PMID:25759467; http://dx.doi.org/ 10.1093/hmg/ddv087 [DOI] [PubMed] [Google Scholar]
- 26.Cota D, Proulx K, Smith KA, Kozma SC, Thomas G, Woods SC, Seeley RJ. Hypothalamic mTOR signaling regulates food intake. Science 2006; 312:927-30; PMID:16690869; http://dx.doi.org/ 10.1126/science.1124147 [DOI] [PubMed] [Google Scholar]
- 27.Yang W, Jiang W, Luo L, Bu J, Pang D, Wei J, Du C, Xia X, Cui Y, Liu S, et al.. Genetic deletion of Rheb1 in the brain reduces food intake and causes hypoglycemia with altered peripheral metabolism. Int J Mol Sci 2014; 15:1499-510; PMID:24451134; http://dx.doi.org/ 10.3390/ijms15011499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ge S, Goh EL, Sailor KA, Kitabatake Y, Ming GL, Song H. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature 2006; 439:589-93; PMID:16341203; http://dx.doi.org/ 10.1038/nature04404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bonaguidi MA, Wheeler MA, Shapiro JS, Stadel RP, Sun GJ, Ming GL, Song H. In vivo clonal analysis reveals self-renewing and multipotent adult neural stem cell characteristics. Cell 2011; 145:1142-55; PMID:21664664; http://dx.doi.org/ 10.1016/j.cell.2011.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Song J, Zhong C, Bonaguidi MA, Sun GJ, Hsu D, Gu Y, Meletis K, Huang ZJ, Ge S, Enikolopov G, et al.. Neuronal circuitry mechanism regulating adult quiescent neural stem-cell fate decision. Nature 2012; 489:150-4; PMID:22842902; http://dx.doi.org/ 10.1038/nature11306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature 2002; 415:1030-4; PMID:11875571; http://dx.doi.org/ 10.1038/4151030a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zou Y, Jiang W, Wang J, Li Z, Zhang J, Bu J, Zou J, Zhou L, Yu S, Cui Y, et al.. Oligodendrocyte precursor cell-intrinsic effect of Rheb1 controls differentiation and mediates mTORC1-dependent myelination in brain. J Neurosci 2014; 34:15764-78; PMID:25411504; http://dx.doi.org/ 10.1523/JNEUROSCI.2267-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoeffer CA, Klann E. mTOR signaling: at the crossroads of plasticity, memory and disease. Trends Neurosci 2009; 33:67-75; PMID:19963289; http://dx.doi.org/ 10.1016/j.tins.2009.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brandon NJ, Sawa A. Linking neurodevelopmental and synaptic theories of mental illness through DISC1. Nat Rev Neurosci 2011; 12:707-22; PMID:22095064; http://dx.doi.org/ 10.1038/nrn3120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Costa-Mattioli M, Monteggia LM. mTOR complexes in neurodevelopmental and neuropsychiatric disorders. Nat Neurosci 2013; 16:1537-43; PMID:24165680; http://dx.doi.org/ 10.1038/nn.3546 [DOI] [PubMed] [Google Scholar]
- 36.Christian K, Song H, Ming GL. Adult neurogenesis as a cellular model to study schizophrenia. Cell Cycle 2010; 9:636-7; PMID:20107312; http://dx.doi.org/ 10.4161/cc.9.4.10932 [DOI] [PubMed] [Google Scholar]
- 37.Ming GL, Song H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron 2011; 70:687-702; PMID:21609825; http://dx.doi.org/ 10.1016/j.neuron.2011.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Christian KM, Song H, Ming GL. Functions and dysfunctions of adult hippocampal neurogenesis. Annu Rev Neurosci 2014; 37:243-62; PMID:24905596; http://dx.doi.org/ 10.1146/annurev-neuro-071013-014134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ehninger D, Silva AJ. Rapamycin for treating Tuberous sclerosis and Autism spectrum disorders. Trends Mol Med 2011; 17:78-87; PMID:21115397; http://dx.doi.org/ 10.1016/j.molmed.2010.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kelleher Iii RJ, Geigenmuller U, Hovhannisyan H, Trautman E, Pinard R, Rathmell B, Carpenter R, Margulies D. High-Throughput Sequencing of mGluR Signaling Pathway Genes Reveals Enrichment of Rare Variants in Autism. PLoS One 2012; 7:e35003; PMID:22558107; http://dx.doi.org/ 10.1371/journal.pone.0035003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reif A, Fritzen S, Finger M, Strobel A, Lauer M, Schmitt A, Lesch KP. Neural stem cell proliferation is decreased in schizophrenia, but not in depression. Mol Psychiatry 2006; 11:514-22; PMID:16415915; http://dx.doi.org/ 10.1038/sj.mp.4001791 [DOI] [PubMed] [Google Scholar]
- 42.Walton NM, Zhou Y, Kogan JH, Shin R, Webster M, Gross AK, Heusner CL, Chen Q, Miyake S, Tajinda K, et al.. Detection of an immature dentate gyrus feature in human schizophrenia/bipolar patients. Transl Psychiatry 2012; 2:e135; PMID:22781168; http://dx.doi.org/ 10.1038/tp.2012.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Steen RG, Mull C, McClure R, Hamer RM, Lieberman JA. Brain volume in first-episode schizophrenia: systematic review and meta-analysis of magnetic resonance imaging studies. Br J Psychiatry 2006; 188:510-8; PMID:16738340; http://dx.doi.org/ 10.1192/bjp.188.6.510 [DOI] [PubMed] [Google Scholar]