Abstract
It is widely known that neurogenesis, brain function and cognition decline with aging. Increasing evidence suggests that cerebrovascular dysfunction is a major cause of cognitive impairment in the elderly but is also involved in age-related neurodegenerative diseases. Finding ways and molecules that reverse this process and ameliorate age- and disease-related cognitive impairment by targeting vascular and neurogenic deterioration would be of great therapeutic value. In Katsimpardi et al. we reported that young blood has a dual beneficial effect in the aged brain by restoring age-related decline in neurogenesis as well as inducing a striking remodeling of the aged vasculature and restoring blood flow to youthful levels. Additionally, we identified a youthful systemic factor, GDF11 that recapitulates these beneficial effects of young blood. We believe that the identification of young systemic factors that can rejuvenate the aged brain opens new roads to therapeutic intervention for neurodegenerative diseases by targeting both neural stem cells and neurogenesis as well as at the vasculature.
Keywords: Alzheimer's disease, blood vessels, neural stem cells, neurogenesis, neurodegenerative diseases, rejuvenation
Neurogenesis in the adult brain occurs primarily in 2 areas: the dentate gyrus of the hippocampus and the subventricular zone (SVZ) of the lateral ventricles.1,2 The capacity of these areas to give rise to new neurons throughout life depends on the existence of neural stem cells (NSCs) and their progenitors. In the SVZ, NSCs are part of a specialized niche that creates a special microenvironment, providing the necessary regulatory cues for proliferation and differentiation.3-6 NSCs are in immediate contact with the cerebrospinal fluid and blood vessels, which are responsible for delivering external regulatory signals.7,8 Because of the intimate link between NSCs and blood vessels the niche is also called the neurovascular niche. The prevalence of NSCs and their capacity to produce new neurons greatly declines with aging, as NSCs become more quiescent,9 possibly because the volume of the vascular niche is decreased.10 This change in NSC behavior is associated with reduced neuroplasticity and cognitive functioning.11-13 At the same time, aging results in rarefaction of the microvasculature in some regions of the brain as well as morphological and physiological changes leading to decreased cerebral blood flow (CBF), which consequently leads to seizures, stroke, decreased neurogenesis and cognitive decline.14,15 This shows that the role of blood vessels is crucial at multiple levels and in may regions of the brain, on one hand by providing support and regulatory cues to stem cells and on the other hand because it is involved in several pathologies related to aging. Reversing the age-related alterations in the morphology and function of blood vessels could then be a way to ameliorate both neurogenesis defects as well as pathological conditions related to deteriorated vasculature and blood flow.
Restoration of neurogenesis and neurodegenerative diseases
Young blood contains factors that are able to rejuvenate aged tissues, including muscle, liver, heart and the nervous system,13,16-18 albeit via different mechanisms of action in each tissue. In Katsimpardi et al. we hypothesized that the age-related decline in neurogenesis and vascular functioning can also be reversed by young systemic factors.19 To test this hypothesis we used the model of heterochronic parabiosis where 2 animals of different age are surgically attached to achieve common blood circulation (Fig. 1). This system is very useful when examining the effects of systemic factors from one animal to another because it does not require continuous transfusions of blood. More importantly, parabiosis is a powerful tool in understanding the mechanisms of aging and rejuvenation since it provides a biological model where the effects of aging and rejuvenation can be studied in real time, as the heterochronic “rejuvenated” mouse is genetically identical to the old mouse, but the signaling pathways are altered to permit rejuvenation. This suggests that triggering pathways that are active in the young, but not in the old mouse, can lead to restoration of age-related phenotypes.
Figure 1.
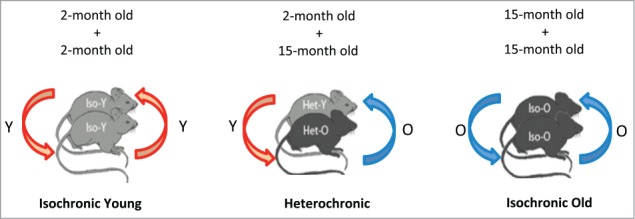
Layout of the parabiosis experiment. Mice of same (isochronic) or different (heterochronic) age are surgically joined to share a common blood circulation.
Initially we observed that exposure of the aged SVZ neurovascular niche to young blood resulted in an increase of neural stem cell and progenitor cells, marked by Sox2 and Olig2 markers, as well as an expansion of the SVZ itself, seen as an increase in Ki67+ proliferating cells. This observation was further demonstrated in in vitro cultures of neurospheres, derived from the rejuvenated old mouse, which were also more proliferative. This suggests that NSCs, which are heterogeneous in their differentiation potential 20–22 and are subject to environmental cues in order to acquire their fate 23,24 can be manipulated by systemic factors in order to be activated. This is critical in the context of aging where NSCs have reduced self-renewal and differentiation potential, but also in the case of injury where activation of NSCs can be key for repair. Furthermore, we observed that the increase in neural stem/progenitor cell population in the aged niche after heterochronic parabiosis was accompanied by an increase in new neurons populating the olfactory bulb in the aged brain. This increase in new olfactory bulb neurons also resulted in a functional improvement since heterochronic old mice exhibited amelioration in olfactory discrimination. These results are in accordance with relevant studies showing that young blood has the capacity to improve synaptic plasticity in the hippocampus of the aged brain and to reverse the age-related impairment in cognitive functions.25
The possibility of regulating the proliferation and increasing neurogenesis could be very beneficial in the context of age-related neurodegenerative diseases. Neurogenesis is impaired in diseases like human immunodeficiency virus (HIV)-associated cognitive disorders,26 Parkinson27 and Huntington's28 disease as well as epilepsy.29 In the case of Alzheimer's disease (AD), where the plaques and neurofibrillary tangles exist in both SVZ and hippocampus neurogenic areas, compromised neurogenesis is associated with impairments in learning and memory.30 However, an enriched environment and exercise can positively regulate neurogenesis in some AD models31,32 showing the importance of systemic influence. Moreover, the restoration of neurogenesis in AD mice by exposure to a neurogenic factor results in a restoration of cognitive functions in AD mice,33 suggesting that it is possible to treat the symptoms, if not the pathology, of the disease by restoring the age- or disease- related decline in neurogenesis. This suggests that restoring neurogenesis with young systemic factors could be used as a therapeutic tool in AD or other models of neurodegenerative diseases.
Vascular remodeling as a therapeutic tool for neurodegeneration
In the normal brain, neural stem cells and blood vessels are closely associated in the SVZ4-6 and in the hippocampus.34 In the SVZ, NSCs interact with endothelial cells by extending end-feet to the blood vessels, which regulate NSC self-renewal, proliferation and differentiation by secreting soluble factors.35-38 However, aging does not only affect NSCs but also greatly impacts blood vessels and CBF. A substantial rarefaction of the arterioles,15 with a decrease in smooth muscle and elastin and an increase in collagen and basement membrane39 has been described. In addition to that, there are changes in capillary endothelial cells, pericytes as well as the astrocytic endfeet.14 These age-related changes in blood vessels result in decreased CBF and reduced delivery of oxygen and nutrients in the brain parenchyma, which affects not only NSC behavior and neurogenesis as explained above, but also the blood-brain barrier thus impairing proper neuronal function and activity in the whole brain.
Because of the close association between NSCs, neurons and blood vessels, we hypothesized that the aged vasculature might also be affected by young factors. In vitro exposure of endothelial cells to young serum showed an increase in proliferation, whereas old serum induced a decrease. This shows that factors in the young blood act on endothelial cells to promote proliferation and expansion of the blood vessels. However, this does not exclude the possibility that these or other factors may also act on other types of cells. When we analyzed the blood vessels in brains of heterochronic old mice, we found that blood vessel volume in the aged SVZ was increased after exposure of the area to young blood. After testing other areas of the aged brain we found that vascular remodeling also occurred in the hippocampus and cortex. This prompted us to test if not only the morphology but also the functionality of the vasculature was restored after parabiosis. Using magnetic resonance imaging (MRI) in both the whole brain and in the SVZ we found that CBF was increased reaching young levels after exposure of an old brain to young blood. These findings showed for the first time that exposure of the aged brain to young blood results in a brain-wide vascular remodeling followed by restoration of the blood flow to the youthful levels. We believe that this finding can have an important impact in the case of neurodegenerative diseases since increasing evidence from experimental studies suggests that cerebrovascular dysfunction is a major cause of cognitive impairment in age-related and neurodegenerative diseases such as AD.40-42 Although AD is usually linked with neuronal degeneration, the role of the vascular pathology is also very important, as the pathological phenotype of amyloid plaques and neurofibrillary tangles correlates with vascular deterioration.42-44 It is interesting to note that the vascular phenotype of AD includes reduction of CBF,45-47 together with a breakdown of the blood brain barrier,48-50 loss of tight and adherens junctions51 and alterations in cerebral capillaries.14 Since young blood can restore the age-related deterioration of the vasculature as well as the CBF back to young levels, we believe that this could potentially have a beneficial effect in the context of AD or other neurodegenerative diseases.
GDF11 as a possible therapeutic agent
GDF11, a member of the transforming growth factor-β (TGF-β) superfamily of secreted signaling molecules, is a youthful factor that is highly expressed in the young blood but not in the old.18 GDF11 was shown to be able to reverse age-related cardiac hypertrophy18 restore genomic integrity in aged muscle satellite cells and improve physical fitness in aged mice.52 In our studies, GDF11 was also systemically injected in aged mice and the brains were analyzed for its possible effect on NSCs and blood vessels. To our surprise we found that GDF11 was able to increase the numbers of NSCs in the aged SVZ compared to untreated aged SVZ thereby recapitulating the effect of young blood, although to a lesser extent since the increase was half of that was observed with parabiosis. When we looked at the effect on blood vessels we also found an induction of vascular remodeling in the treated aged mice. We also found that in vitro GDF11 treatment of endothelial cells induced an increase in proliferation via the SMAD2/3 pathway suggesting that it acts directly on endothelial cells.
The fact that a single factor can restore age-relate defects in both neurogenesis and vasculature is very encouraging. Moreover, it was recently found that ALK5-dependent TGF-β signaling via Smad2/3 is involved in hippocampal neurogenesis,53 which correlates with our observation that GDF11 acts through the same pathway in the SVZ, suggesting an important role for the TGF-β singaling pathways in both neurogenesis and vascular remodeling in the adult and aged brain.
Additional experiments are needed to understand the mechanism of action of GDF11, but finding a molecule that can target vascular deterioration and restore age-related decline in neurogenesis and potentially ameliorate cognitive impairment can be of great therapeutic value.
Conclusion
In this work we provide evidence of a dual effect of young blood and GDF11 on neurogenesis and vascular remodeling in the aged brain. However, it still remains unknown if the 2 effects are related. We could imagine both scenarios to be true. On one hand, we know that the blood-brain barrier of the SVZ is slightly looser than other areas6,54 so we could imagine youthful systemic factors, like GDF11, affecting NSC directly. On the other hand, it is known that neurogenesis is affected by changes in the levels of nitric oxide and oxygen following changes in local blood flow55,56 and that the vasculature regulates NSC behavior by locally secreting factors4,37 so there is a possibility that the effect of young blood and GDF11 is primarily on the vasculature.
Although both cases could be made, we believe that young blood and GDF11 act primarily on the vasculature and that the positive regulation of NSC and neurogenesis is an indirect effect (Fig. 2). The restoration of the aged vasculature could activate a youthful cellular mechanism, like the secretion of local factors that are usually secreted in the young brain to regulate neurogenesis. The restoration of CBF, which brings more oxygen and nutrients, could lead to a restored microenvironment, which is crucial for proper NSC proliferation and differentiation cues, and a more youthful neurogenic niche. However additional experiments need to be performed to prove this hypothesis.
Figure 2.
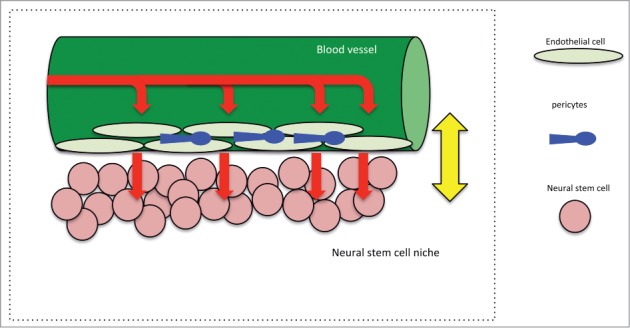
Schematic depicting the multi-level regulation of the neurovascular niche in the adult brain. Blood vessels receive systemic signals and secrete regulatory factors to modulate neural stem cell behavior.
In any case, the fact that young systemic factors can restore neurogenesis, cerebrovascular dysfunction and cerebral blood flow in a brain-wide manner is a novel finding that stresses the important role of the vasculature in the brain. Since the etiology of neurodegenerative diseases like AD shows an increasingly prominent role for cerebral blood flow and vascular abnormalities as major disease factors, we believe that our findings could open new possibilities for treatment, especially by using a single youthful factor like GDF11.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1. Kempermann G, Kuhn HG, Gage FH. Genetic influence on neurogenesis in the dentate gyrus of adult mice. Proc Natl Acad Sci U S A. 1997;94:10409-14; PMID:9294224; http://dx.doi.org/ 10.1073/pnas.94.19.10409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gritti A, Bonfanti L, Doetsch F, Caille I, Alvarez-Buylla A, Lim DA, Galli R, Verdugo JM, Herrera DG, Vescovi AL. Multipotent neural stem cells reside into the rostral extension and olfactory bulb of adult rodents. J Neurosci. 2002;22:437-45; PMID:11784788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sawamoto K, Wichterle H, Gonzalez-Perez O, Cholfin JA, Yamada M, Spassky N, Murcia NS, Garcia-Verdugo JM, Marin O, Rubenstein JL, et al. . New neurons follow the flow of cerebrospinal fluid in the adult brain. Science. 2006;311:629-32; PMID:16410488; http://dx.doi.org/ 10.1126/science.1119133 [DOI] [PubMed] [Google Scholar]
- 4. Kokovay E, Goderie S, Wang Y, Lotz S, Lin G, Sun Y, Roysam B, Shen Q, Temple S. Adult svz lineage cells home to and leave the vascular niche via differential responses to sdf1/cxcr4 signaling. Cell Stem Cell. 2010;7:163-73; PMID:20682445; http://dx.doi.org/ 10.1016/j.stem.2010.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shen Q, Wang Y, Kokovay E, Lin G, Chuang SM, Goderie SK, Roysam B, Temple S. Adult svz stem cells lie in a vascular niche: A quantitative analysis of niche cell-cell interactions. Cell Stem Cell. 2008;3:289-300; PMID:18786416; http://dx.doi.org/ 10.1016/j.stem.2008.07.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tavazoie M, Van der Veken L, Silva-Vargas V, Louissaint M, Colonna L, Zaidi B, Garcia-Verdugo JM, Doetsch F. A specialized vascular niche for adult neural stem cells. Cell Stem Cell. 2008;3:279-88; PMID:18786415; http://dx.doi.org/ 10.1016/j.stem.2008.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sawada M, Matsumoto M, Sawamoto K. Vascular regulation of adult neurogenesis under physiological and pathological conditions. Front Neurosci. 2014;8:53; PMID:24672424; http://dx.doi.org/ 10.3389/fnins.2014.00053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ihrie RA, Alvarez-Buylla A. Lake-front property: A unique germinal niche by the lateral ventricles of the adult brain. Neuron. 2011;70:674-86; PMID:21609824; http://dx.doi.org/ 10.1016/j.neuron.2011.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lugert S, Basak O, Knuckles P, Haussler U, Fabel K, Gotz M, Haas CA, Kempermann G, Taylor V, Giachino C. Quiescent and active hippocampal neural stem cells with distinct morphologies respond selectively to physiological and pathological stimuli and aging. Cell Stem Cell.6:445-56; PMID:20452319; http://dx.doi.org/ 10.1016/j.stem.2010.03.017 [DOI] [PubMed] [Google Scholar]
- 10. Hattiangady B, Shetty AK. Aging does not alter the number or phenotype of putative stem/progenitor cells in the neurogenic region of the hippocampus. Neurobiol Aging. 2008;29:129-47; PMID:17092610; http://dx.doi.org/ 10.1016/j.neurobiolaging.2006.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: Age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16:2027-33; PMID:8604047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Seki T, Arai Y. Age-related production of new granule cells in the adult dentate gyrus. Neuroreport. 1995;6:2479-82; PMID:8741746; http://dx.doi.org/ 10.1097/00001756-199512150-00010 [DOI] [PubMed] [Google Scholar]
- 13. Villeda SA, Luo J, Mosher KI, Zou B, Britschgi M, Bieri G, Stan TM, Fainberg N, Ding Z, Eggel A, et al. . The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature. 2011;477:90-4; PMID:21886162; http://dx.doi.org/ 10.1038/nature10357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Farkas E, Luiten PG. Cerebral microvascular pathology in aging and alzheimer's disease. Prog Neurobiol. 2001;64:575-611; PMID:11311463; http://dx.doi.org/ 10.1016/S0301-0082(00)00068-X [DOI] [PubMed] [Google Scholar]
- 15. Sonntag WE, Lynch CD, Cooney PT, Hutchins PM. Decreases in cerebral microvasculature with age are associated with the decline in growth hormone and insulin-like growth factor 1. Endocrinology. 1997;138:3515-20; PMID:9231806 [DOI] [PubMed] [Google Scholar]
- 16. Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433:760-4; PMID:15716955; http://dx.doi.org/ 10.1038/nature03260 [DOI] [PubMed] [Google Scholar]
- 17. Ruckh JM, Zhao JW, Shadrach JL, van Wijngaarden P, Rao TN, Wagers AJ, Franklin RJ. Rejuvenation of regeneration in the aging central nervous system. Cell Stem Cell. 2012;10:96-103; PMID:22226359; http://dx.doi.org/ 10.1016/j.stem.2011.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Loffredo FS, Steinhauser ML, Jay SM, Gannon J, Pancoast JR, Yalamanchi P, Sinha M, Dall'Osso C, Khong D, Shadrach JL, et al. . Growth differentiation factor 11 is a circulating factor that reverses age-related cardiac hypertrophy. Cell. 2013;153:828-39; PMID:23663781; http://dx.doi.org/ 10.1016/j.cell.2013.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Katsimpardi L, Litterman NK, Schein PA, Miller CM, Loffredo FS, Wojtkiewicz GR, Chen JW, Lee RT, Wagers AJ, Rubin LL. Vascular and neurogenic rejuvenation of the aging mouse brain by young systemic factors. Science.344:630-4; PMID:24797482; http://dx.doi.org/ 10.1126/science.1251141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Doetsch F, Petreanu L, Caille I, Garcia-Verdugo JM, Alvarez-Buylla A. Egf converts transit-amplifying neurogenic precursors in the adult brain into multipotent stem cells. Neuron. 2002;36:1021-34; PMID:12495619; http://dx.doi.org/ 10.1016/S0896-6273(02)01133-9 [DOI] [PubMed] [Google Scholar]
- 21. Merkle FT, Fuentealba LC, Sanders TA, Magno L, Kessaris N, Alvarez-Buylla A. Adult neural stem cells in distinct microdomains generate previously unknown interneuron types. Nat Neurosci.17:207-14; PMID:24362763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lledo PM, Merkle FT, Alvarez-Buylla A. Origin and function of olfactory bulb interneuron diversity. Trends Neurosci. 2008;31:392-400; PMID:18603310; http://dx.doi.org/ 10.1016/j.tins.2008.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fuchs E, Tumbar T, Guasch G. Socializing with the neighbors: stem cells and their niche. Cell. 2004;116:769-78; PMID:15035980; http://dx.doi.org/ 10.1016/S0092-8674(04)00255-7 [DOI] [PubMed] [Google Scholar]
- 24. Alvarez-Buylla A, Lim DA. For the long run: Maintaining germinal niches in the adult brain. Neuron. 2004;41:683-6; PMID:15003168; http://dx.doi.org/ 10.1016/S0896-6273(04)00111-4 [DOI] [PubMed] [Google Scholar]
- 25. Villeda SA, Plambeck KE, Middeldorp J, Castellano JM, Mosher KI, Luo J, Smith LK, Bieri G, Lin K, Berdnik D, et al. . Young blood reverses age-related impairments in cognitive function and synaptic plasticity in mice. Nat Med. 2014;20:659-63; PMID:24793238; http://dx.doi.org/ 10.1038/nm.3569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Krathwohl MD, Kaiser JL. Hiv-1 promotes quiescence in human neural progenitor cells. J Infect Dis. 2004;190:216-26; PMID:15216454; http://dx.doi.org/ 10.1086/422008 [DOI] [PubMed] [Google Scholar]
- 27. Winner B, Rockenstein E, Lie DC, Aigner R, Mante M, Bogdahn U, Couillard-Despres S, Masliah E, Winkler J. Mutant alpha-synuclein exacerbates age-related decrease of neurogenesis. Neurobiol Aging. 2008;29:913-25; PMID:17275140; http://dx.doi.org/ 10.1016/j.neurobiolaging.2006.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mangiarini L, Sathasivam K, Seller M, Cozens B, Harper A, Hetherington C, Lawton M, Trottier Y, Lehrach H, Davies SW, et al. . Exon 1 of the hd gene with an expanded cag repeat is sufficient to cause a progressive neurological phenotype in transgenic mice. Cell. 1996;87:493-506; PMID:8898202; http://dx.doi.org/ 10.1016/S0092-8674(00)81369-0 [DOI] [PubMed] [Google Scholar]
- 29. Hattiangady B, Rao MS, Shetty AK. Chronic temporal lobe epilepsy is associated with severely declined dentate neurogenesis in the adult hippocampus. Neurobiol Dis. 2004;17:473-90; PMID:15571983; http://dx.doi.org/ 10.1016/j.nbd.2004.08.008 [DOI] [PubMed] [Google Scholar]
- 30. Ashe KH. Learning and memory in transgenic mice modeling alzheimer's disease. Learn Mem. 2001;8:301-8; PMID:11773429; http://dx.doi.org/ 10.1101/lm.43701 [DOI] [PubMed] [Google Scholar]
- 31. Lazarov O, Marr RA. Neurogenesis and alzheimer's disease: at the crossroads. Exp Neurol 2010; 223:267-81; PMID:19699201; http://dx.doi.org/ 10.1016/j.expneurol.2009.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mirochnic S, Wolf S, Staufenbiel M, Kempermann G. Age effects on the regulation of adult hippocampal neurogenesis by physical activity and environmental enrichment in the app23 mouse model of alzheimer disease. Hippocampus. 2009;19:1008-18; PMID:19219917; http://dx.doi.org/ 10.1002/hipo.20560 [DOI] [PubMed] [Google Scholar]
- 33. Wang JM, Singh C, Liu L, Irwin RW, Chen S, Chung EJ, Thompson RF, Brinton RD. Allopregnanolone reverses neurogenic and cognitive deficits in mouse model of alzheimer's disease. Proc Natl Acad Sci U S A. 2010;107:6498-6503; PMID:20231471; http://dx.doi.org/ 10.1073/pnas.1001422107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425:479-94; PMID:10975875; http://dx.doi.org/ 10.1002/1096-9861(20001002)425:4%3c479::AID-CNE2%3e3.0.CO;2-3 [DOI] [PubMed] [Google Scholar]
- 35. Ramirez-Castillejo C, Sanchez-Sanchez F, Andreu-Agullo C, Ferron SR, Aroca-Aguilar JD, Sanchez P, Mira H, Escribano J, Farinas I. Pigment epithelium-derived factor is a niche signal for neural stem cell renewal. Nat Neurosci. 2006;9:331-9; PMID:16491078; http://dx.doi.org/ 10.1038/nn1657 [DOI] [PubMed] [Google Scholar]
- 36. Jin K, Zhu Y, Sun Y, Mao XO, Xie L, Greenberg DA. Vascular endothelial growth factor (vegf) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci U S A. 2002;99:11946-50; PMID:12181492; http://dx.doi.org/ 10.1073/pnas.182296499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shen Q, Goderie SK, Jin L, Karanth N, Sun Y, Abramova N, Vincent P, Pumiglia K, Temple S. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304:1338-40; PMID:15060285; http://dx.doi.org/ 10.1126/science.1095505 [DOI] [PubMed] [Google Scholar]
- 38. Ottone C, Krusche B, Whitby A, Clements M, Quadrato G, Pitulescu ME, Adams RH, Parrinello S. Direct cell-cell contact with the vascular niche maintains quiescent neural stem cells. Nat Cell Biol.16:1045-56; PMID: 25283993; http://dx.doi.org/ 10.1038/ncb3045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hajdu MA, McElmurry RT, Heistad DD, Baumbach GL. Effects of aging on cerebral vascular responses to serotonin in rats. Am J Physiol. 1993;264:H2136-40; PMID:8322944 [DOI] [PubMed] [Google Scholar]
- 40. de la Torre JC, Mussivand T. Can disturbed brain microcirculation cause alzheimer's disease? Neurol Res. 1993;15:146-53; PMID:8103579 [DOI] [PubMed] [Google Scholar]
- 41. Iadecola C, Gorelick PB. Converging pathogenic mechanisms in vascular and neurodegenerative dementia. Stroke. 2003;34:335-7; PMID:12574528; http://dx.doi.org/ 10.1161/01.STR.0000054050.51530.76 [DOI] [PubMed] [Google Scholar]
- 42. Iadecola C. The overlap between neurodegenerative and vascular factors in the pathogenesis of dementia. Acta Neuropathol. 2010;120:287-96; PMID:20623294; http://dx.doi.org/ 10.1007/s00401-010-0718-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fotuhi M, Hachinski V, Whitehouse PJ. Changing perspectives regarding late-life dementia. Nat Rev Neurol. 2009;5:649-58; PMID:19918254; http://dx.doi.org/ 10.1038/nrneurol.2009.175 [DOI] [PubMed] [Google Scholar]
- 44. Jellinger KA, Attems J. Prevalence and pathology of vascular dementia in the oldest-old. J Alzheimers Dis. 2010;21:1283-93; PMID:21504129 [DOI] [PubMed] [Google Scholar]
- 45. Bateman GA, Levi CR, Schofield P, Wang Y, Lovett EC. Quantitative measurement of cerebral haemodynamics in early vascular dementia and alzheimer's disease. J Clin Neurosci. 2006;13:563-8; PMID:16540327; http://dx.doi.org/ 10.1016/j.jocn.2005.04.017 [DOI] [PubMed] [Google Scholar]
- 46. Tanaka M, Fukuyama H, Yamauchi H, Narita M, Nabatame H, Yokode M, Fujimoto N, Kita T, Murakami M. Regional cerebral blood flow abnormalities in nondemented patients with memory impairment. J Neuroimaging. 2002;12:112-8; PMID:11977904; http://dx.doi.org/ 10.1111/j.1552-6569.2002.tb00106.x [DOI] [PubMed] [Google Scholar]
- 47. Johnson KA, Albert MS. Perfusion abnormalities in prodromal ad. Neurobiol Aging. 2000;21:289-92; PMID:10867213; http://dx.doi.org/ 10.1016/S0197-4580(00)00137-8 [DOI] [PubMed] [Google Scholar]
- 48. Farrall AJ, Wardlaw JM. Blood-brain barrier: Ageing and microvascular disease–systematic review and meta-analysis. Neurobiol Aging. 2009;30:337-52; PMID:17869382; http://dx.doi.org/ 10.1016/j.neurobiolaging.2007.07.015 [DOI] [PubMed] [Google Scholar]
- 49. Zipser BD, Johanson CE, Gonzalez L, Berzin TM, Tavares R, Hulette CM, Vitek MP, Hovanesian V, Stopa EG. Microvascular injury and blood-brain barrier leakage in alzheimer's disease. Neurobiol Aging. 2007;28:977-86; PMID:16782234; http://dx.doi.org/ 10.1016/j.neurobiolaging.2006.05.016 [DOI] [PubMed] [Google Scholar]
- 50. Paul J, Strickland S, Melchor JP. Fibrin deposition accelerates neurovascular damage and neuroinflammation in mouse models of alzheimer's disease. J Exp Med. 2007;204:1999-2008; PMID:17664291; http://dx.doi.org/ 10.1084/jem.20070304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Iadecola C. Neurovascular regulation in the normal brain and in alzheimer's disease. Nat Rev Neurosci. 2004;5:347-60; PMID:15100718; http://dx.doi.org/ 10.1038/nrn1387 [DOI] [PubMed] [Google Scholar]
- 52. Sinha M, Jang YC, Oh J, Khong D, Wu EY, Manohar R, Miller C, Regalado SG, Loffredo FS, Pancoast JR, et al. . Restoring systemic gdf11 levels reverses age-related dysfunction in mouse skeletal muscle. Science. 2014;344:649-52; PMID:24797481; http://dx.doi.org/ 10.1126/science.1251152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. He Y, Zhang H, Yung A, Villeda SA, Jaeger PA, Olayiwola O, Fainberg N, Wyss-Coray T. Alk5-dependent tgf-beta signaling is a major determinant of late-stage adult neurogenesis. Nat Neurosci. 2014;17:943-52; PMID:24859199; http://dx.doi.org/ 10.1038/nn.3732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Katsimpardi L, Litterman NK, Schein PA, Miller CM, Loffredo FS, Wojtkiewicz GR, Chen JW, Lee RT, Wagers AJ, Rubin LL. Vascular and neurogenic rejuvenation of the aging mouse brain by young systemic factors. Science. 2014;344:630-4; PMID:24797482; http://dx.doi.org/ 10.1126/science.1251141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Moreno-Lopez B, Romero-Grimaldi C, Noval JA, Murillo-Carretero M, Matarredona ER, Estrada C. Nitric oxide is a physiological inhibitor of neurogenesis in the adult mouse subventricular zone and olfactory bulb. J Neurosci. 2004;24:85-95; PMID:14715941; http://dx.doi.org/ 10.1523/JNEUROSCI.1574-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Panchision DM. The role of oxygen in regulating neural stem cells in development and disease. J Cell Physiol. 2009;220:562-8; PMID:19441077; http://dx.doi.org/ 10.1002/jcp.21812 [DOI] [PubMed] [Google Scholar]


