Abstract
Purpose
Chronic pain is a widespread and debilitating condition, encountered by physicians in a variety of practice settings. Although many pharmacologic and behavioral strategies exist for the management of this condition, treatment is often unsatisfactory. Scrambler Therapy is a novel, non-invasive pain modifying technique that utilizes transcutaneous electrical stimulation of pain fibers with the intent of re-organizing maladaptive signaling pathways. This review was conducted to further evaluate what is known regarding the mechanisms and mechanics of Scrambler Therapy and to investigate the preliminary data pertaining to the efficacy of this treatment modality.
Methods
The PubMed/Medline, SCOPUS, EMBASE, and Google Scholar databases were searched for all articles published on Scrambler Therapy prior to November 2015. All case studies and clinical trials were evaluated and reported in a descriptive manner.
Results
To date, 20 reports, of varying scientific quality, have been published regarding this device; all but one small study, published only as an abstract, provided results that appear positive.
Conclusion
The positive findings from preliminary studies with Scrambler Therapy support that this device provides benefit for patients with refractory pain syndromes. Larger, randomized studies are required to further evaluate the efficacy of this approach.
Keywords: Scrambler Therapy, Pain, Chronic pain, Chemotherapy-induced peripheral neuropathy
Introduction
Chronic pain is estimated to affect 100 million people in the USA alone, resulting in up to $635 billion in medical expenses and lost productivity each year. [1] It predisposes to psychiatric comorbidity, and its massive impact is highlighted by the fact that it is the most common cause of long-term disability in the USA [2].
In simplest terms, pain can be defined as a bodily sensation experienced during genuine, or perceived, tissue injury [3]. In the acute setting, this sensation can serve as a protective role by alerting an individual to avoid potentially harmful stimuli and to protect the body during healing. When pain fails to communicate biologically useful or accurate information, it is maladaptive and thereby becomes a disease state in its own right. It is generally agreed that pain becomes “chronic” when it persists beyond the expected period of tissue injury and healing. The specific duration of symptoms required to qualify for a diagnosis of chronic pain is debatable, but generally is considered to be in the range of 3 to 6 months [4].
The perception of noxious stimuli originates from nociceptors of the peripheral nervous system. Nociceptors recognize stimuli in the form of thermal, mechanical, or chemical inputs. The stimulation leads to activation of primary sensory nerve fibers that transmit this information to the central nervous system, via a complex network of interneurons housed predominantly in the dorsal root ganglia, posterior horn of the spinal cord, brain stem, and thalamus. Ultimately, signals reach the forebrain for interpretation of the sensory experience. There are multiple mechanisms that underlie the dysregulation of this system in chronic pain. In the setting of injury, for example, inflammatory changes in the biochemical milieu surrounding peripheral nerves can result in hypersensitization of nociceptors, such that pain signals are communicated in the absence of appropriate stimuli [5]. Neurons surrounding damaged tissue have even shown the ability to develop spontaneous discharges that communicate pain information in the absence of external input [6]. Similarly, spinal cord neurons in the central nervous system exposed to repetitive pain stimuli may undergo changes that result in transmission of action potentials with a reduced threshold of synaptic input [7].
Currently, several treatment modalities exist for the management of chronic pain, including physical therapy, pharmacologic therapy, behavioral medicine, neuromodulation, minimally-invasive interventions, and surgery. Unfortunately, the heterogeneous nature of chronic pain syndromes and the lack of a functional understanding of chronic pain contribute to the absence of a clearly identifiable, appropriate management strategy for many patients. Nonetheless, pharmacologic measures are commonly prescribed as a component of chronic pain management. With many medications available, such as non-steroidal anti-inflammatory agents, anticonvulsants, antidepressants, and opioids, it is exceedingly common for patients to use multiple agents to try to achieve reasonable pain control. [8].
Recognizing the limitations and hazards of polypharmacy, increasing emphasis has been placed on the non-pharmacologic options for management of persistent pain. A strategy combining psychological and physical medicine approaches can provide significant benefit for many patients [9]. Neuromodulatory techniques, particularly since the commercial availability of wearable transcutaneous electrical nerve stimulation (TENS) units in the mid-1970s, have gained popularity as an adjunct to both pharmacological and non-pharmacologic pain managements [10]. While promising in theory, the scientific data supporting such methods remain limited, without consistently-shown benefit, underscoring the need for novel therapeutic options [11, 12].
The aim of this paper is to review what is known about the mechanism of a relatively new neuromodulatory approach, Scrambler Therapy, and discuss the trials and clinical experience, published to date, regarding its use.
Methods
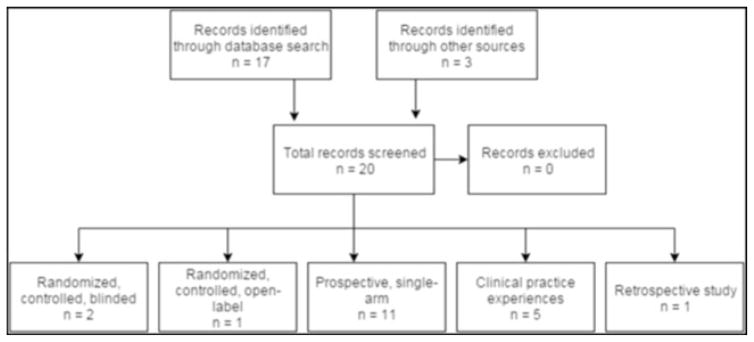
Reports regarding Scrambler Therapy were identified by a combination of database search, communication with investigators, and reviewing bibliographies of previously published manuscripts (Fig. 1). Several databases were utilized in the literature search, including PubMed/Medline, SCOPUS, EMBASE, and Google Scholar. Search terms including “Scrambler Therapy” and “Calmare” were used to identify all articles published prior to November 1, 2015. The search was refined with the use of Boolean terminology, specifically “Scrambler Therapy OR Calmare,” which yielded the largest number of articles. Results of these studies were reviewed and reported with an analytic intent that was primarily descriptive.
Fig 1.
Flowchart depicting search methodology and records included in qualitative analysis
Scrambler Therapy development and mechanisms
Giuseppe Marineo, a biophysicist who developed an interest in treating chronic pain, developed Scrambler Therapy and conducted basic and applied research related to its use. Marineo claims that chronic pain is the consequence of a phenomenon produced by the persistence in time of pain pathway activation, a typical condition of neuropathies. This process results in a loss of the linearity in the cause–effect relation that characterizes the physiological acute pain (which is protective) and creates a new type of nonlinear behavior of the pain system, that tends to self-sustain an anomalous response to painful and non-painful stimuli. Marineo proposes that the entire chronic pain process can be controlled by intervening on the afferent information aspects of pain, the variable that characterizes and mainly regulates every activity of the nervous system and represents its natural cybernetic expression [13]. In short, Scrambler Therapy’s active principle is information control that manipulates the modulation or re-modulation of the pain system, and its physiological or pathological responses, in line with plastic properties of the nervous system. More specifically, a Scrambler Therapy unit is composed of five electrical stimulation channels that, through the surface receptors of C fibers, replace the endogenous pain information with a synthetic one of “non-pain” or “normal-self” that travels through the same pain pathways to the brain. Through plasticity within brain networks mediating the perception of pain, a series of treatments “retrain” the brain so that the area of concern is no longer considered painful. Marineo proposes that his functioning principle, like its neurophysiological target that uses receptors of C fibers, replaces the chronic pain information, rather than attempting to block its ascending path. An in-depth analysis on these differences is described in the International Patent PCT/IT2007/000647 and U.S. Patent No. 8,380,317.
Scrambler Therapy has also drawn comparisons to spinal cord stimulation, which is another interventional technique that has been utilized in the treatment of refractory chronic pain. Spinal cord stimulation has been proven to be efficacious in a diverse array of pain syndromes, including refractory angina, failed back syndrome, and complex regional pain syndrome (CRPS), with the ability to reduce pain intensity in some cases by over 50 % [14]. The drawback of this approach has largely been its invasiveness and cost.
What is the normal course of Scrambler Therapy?
Several authors of the present manuscript utilize Scrambler Therapy in clinical practice. Information in the following section is derived from their experience in treating hundreds of patients for a variety of pain syndromes. A patient treated with Scrambler Therapy has the area of pain identified and then has electrodes placed on normal tissue around the painful site. The electrodes are not placed at the site of actual pain, but, instead, placed at a nearby location of preserved sensation. The dermatomal location is to feed this “non-pain” confusing information into the regular nervous circuit using peripheral nerves, rather than accessing the spinal cord. The intensity of stimulation is adjusted according to patient comfort and, if the placement is correct, pain will usually be replaced by the Scrambler device sensation, which is often described as “pleasant, vibratory, and/or humming”. Up to the full set of five sets of electrodes can be used to treat the area(s) of pain. The device is allowed to run for a total of 30–45 min once the electrodes have been optimally positioned and stimulation intensity correctly regulated. After a session’s completion, patients may report a soothing sensation and note that the pain has been markedly reduced or has disappeared entirely.
The benefit from Scrambler Therapy, after the first treatment, generally lasts for a relatively short period of time. When treatment is reinitiated the next day, the same process happens, but the benefit generally lasts longer, e.g., for a few hours. In most cases, if the treatment has been given properly, with each treatment session, the non-pain (or meaningful relief) timeframe is extended. The duration of posttreatment relief classically lengthens with continued treatments until, ideally, the benefit is maintained throughout the entire day. Usually, Scrambler Therapy is given for a total of ten treatment sessions on consecutive weekdays, if feasible, although some patients need fewer and some patients need more treatments. Pain relief can be expected to persist for weeks to months after treatment is stopped. When patients relapse, booster sessions can be administered. It may only take one or two booster sessions to re-establish the benefit that previously occurred, and this benefit may last for a substantial period of time (oftentimes months or longer).
Scrambler Therapy is an operator-dependent methodology. Treatment success is highly dependent on the ability of the operator to eliminate pain during each single treatment without any significant patient discomfort. Failure to completely resolve pain in a treated area (or have a Visual Analog Score < =1) during each treatment session may lead to less satisfactory results. Experience has confirmed that more expert operators can eliminate pain during Scrambler Therapy when less experienced ones have failed. This may explain, in part, why data coming from different publications are relatively heterogeneous.
Scrambler Therapy clinical trials
To date, 20 trials/reports of Scrambler Therapy are available for review (Table 1) [13, 15–33]. Eighteen have been published as manuscripts [13, 17–33] and two only as abstracts [15, 16]. One is a retrospective study [33], five deal with clinical practice experiences [19, 21, 23, 26, 27], 11 are prospective single-arm clinical trials [13, 15, 17, 18, 22, 24, 25, 28, 30–32], one is a randomized open-label controlled trial [20], and two are randomized, blinded, placebo-controlled trials [16, 29].
Table 1.
Summary of Scrambler Therapy trials
| Reports, by first author | Year | Patients | Condition | Results | Trial type | Comments | |
|---|---|---|---|---|---|---|---|
| 1 | Marineo [13] | 2003 | 11 | Drug-resistant visceral pain | Substantial pain reduction | Prospective trial | |
| 2 | Sabato [24] | 2005 | 226 | Multiple chronic pain syndromes | 80 % of patients with greater than a 50 % pain reduction | Prospective trial | |
| 3 | Smith [30] | 2010 | 18 | Chemotherapy-induced neuropathy | Over 50 % reduction in pain | Prospective trial | 16 evaluable |
| 4 | Abdi [15] | 2011 | 10 | Back pain | 28 % reduction in pain | Prospective trial | Abstract only |
| 5 | Marineo [20] | 2011 | 52 | Post-herpetic neuralgia, spinal canal stenosis, and postsurgical neuropathic pain | Pain reduced more in Scrambler arm, than the control arm at 1 and 3 months (P < 0.0001) | Randomized, controlled trial | Open-label trial |
| 6 | Ricci [28] | 2012 | 82 | Various cancer and non-cancer pains | Mean pain scores dropped from 6.2/10 prior to treatment to 1.6 just after completing 10 treatment days to 2.9, 2 weeks after finishing treatment. | Prospective trial | 73 evaluable patients |
| 7 | Ghatak [18] | 2011 | 8 | Chronic low back pain | Pain score drop from 8.12 to 6.93; Drop in Oswestry Disability Index from 49.88 to 18.44 | Prospective trial | Open label |
| 8 | Sparadeo [27] | 2012 | 173 | Chronic pain >6 months | Marked pain reduction | Clinical practice experience | 91 provided 3–6 months follow-up |
| 9 | Coyne [17] | 2013 | 39 | Cancer pain syndromes, including chemotherapy-induced neuropathy | Significant pain reduction with 10 treatment days that largely lasted for 3 months | Prospective trial | |
| 10 | Smith [25] | 2013 | 10 | Post-herpetic neuralgia | 95 % pain reduction, that largely lasted for 3 months | Prospective trial data | Some patients were the same as in a previous trial [18] |
| 11 | Ko [19] | 2013 | 3 | Post-herpetic neuralgia | Marked pain reduction | Clinical practice experience | |
| 12 | Park [23] | 2013 | 3 | Cancer bone metastases | Marked pain reduction | Clinical practice experience | |
| 13 | Campbell [16] | 2013 | 14 | Chemotherapy-induced neuropathy | No differences between active and placebo arms | Prospective, double- blind, placebo-controlled trial | Abstract only |
| 14 | Pachman [22] | 2014 | 37 | Chemotherapy-induced neuropathy | Average pain decreased by 53 % at end of treatment and benefit largely remained for 10 weeks after completion. | Prospective trial | Decrease in tingling and numbness, too. |
| 15 | Sparadeo [26] | 2014 | 91 | Variety of pain syndromes | Substantial pain reduction | Clinical practice experience | Consecutive patients; Some patients were the same as in a previous trial [30] |
| 16 | Moon [21] | 2014 | 147 | Variety of pain syndromes | Clinical practice experience | ||
| 17 | Starkweather [29] | 2015 | 30 | Low back pain | Significant improvements in active vs control group for: (1) worse pain and pain interference states; (2) pain sensitivity measures, and (3) differential mRNA expression of 17 pain genes | Prospective, double-blind, placebo-controlled trial | |
| 18 | Notaro [31] | 2015 | 25 | Bone and visceral metastases | All patients experienced at least a 50 % drop in pain scores, with average duration of response of 7.7 weeks; improved sleep performance | Prospective trial | |
| 19 | Compagnone [33] | 2015 | 201 | Variety of pain syndromes | Reduction from mean pain score of 7.41 at baseline to 1.6 following treatment | Retrospective cohort | |
| 20 | Lee [32] | 2016 | 20 | Various cancer-related pain syndromes | Mean pain score decreased from 7.4 to 3.7 by visit 3 | Prospective, single-arm |
The first trial was authored by the Scrambler Therapy developer, Marineo, in 2003 and reported the results of the treatment of 11 patients with cancer-associated, drug-resistant, visceral pain [13]. This manuscript noted that pain was quickly and markedly reduced in the studied patients, with 9 of 11 patients stopping the use of pharmacologic pain therapy altogether after the first five sessions, without any associated side effects. Pain scores were reported to have decreased from approximately 8.5 out of 10, at study initiation, to approximately 0.5 out of 10, after 10 treatments. No adverse effects were reported.
A second trial was published in 2005, with Marineo as a co-author [24]. A total of 226 patients with neuropathic pain were treated, including patients with failed back surgery, brachial plexus neuropathy, and other chronic pain conditions. This trial, while also uncontrolled, was impressively large and reported that 80 % of subjects had at least a 50 % pain reduction and 10 % experienced a reduction of 25–49 %. Ten percent (10 %) had no appreciable response. No adverse effects were reported.
Additional groups became involved in the clinical evaluation of this therapy with the publication in 2010 of the first study that did not include Marineo as a co-author [30]. This was a pilot trial in 16 evaluable patients with chronic chemotherapy-induced peripheral neuropathy, conducted at Virginia Commonwealth University. The findings from this study were in line with the success seen with the previously reported trials. After ten treatments, the average reported pain score dropped nearly 60 %, with four patients achieving complete resolution of pain. Patients with recurrent pain were successfully retreated with 1–3 subsequent treatments.
The next trial, currently only available as an abstract, involved ten patients with failed back surgery treated by an anesthesiology-trained pain physician [15]. While it only noted a 28 % mean pain reduction, there were patients on this trial who had substantial relief after multiple other therapies had failed to provide benefit. The author of this abstract, a coauthor on the present manuscript, notes that there are three reasons why his success rate might have been relatively low: (1) he had limited operator experience; (2) he included study subjects with multifactorial intractable pain despite intensive polypharmacy; and (3) treatment while adjuvant anticonvulsants were continued. Empiric observations have suggested less than optimal outcomes if these medications are not discontinued prior to treatment [21].
Marineo and colleagues published the first randomized, controlled trial in 2011, which involved 52 patients with chronic neuropathic pain related to postsurgical causes, post-herpetic neuralgia, or spinal cord stenosis [20]. Scrambler Therapy was compared to a control arm that utilized standard pharmacologic guideline-based recommendations, including frequent phone calls to modify analgesics. The pain reduction, after finishing 10 days of treatment, was 28 % in the control group (pain scores dropped from 8.0 to 5.8 out of 10) compared to a 91 % reduction with the Scrambler group (pain scores dropped from 8.1 to 0.7; p < 0.0001). Pain scores in the control arm were 5.7 and 5.9 at 2 and 3 months, respectively, as opposed to 1.4 and 2.0 in the Scrambler group (p < 0.0001). Analgesic consumption, including opioids, antidepressants, and anticonvulsants, decreased by 72 % in the Scrambler group. Allodynia also was reduced in the Scrambler patients, from 77 % at baseline to 15 % at 3 months. Benefit was obtained relatively equally amongst patients of all of the three diagnostic categories.
The sixth trial involved 82 (73 evaluable) prospectively-treated patients, about half of whom had cancer-related pain [28]. Mean pain scores reduced from 6.2/10 before to 1.6/10 at the end of treatment and were 2.9/10 1 month after treatment was finished. Similar results were seen in patients with and without cancer. When patients were asked whether they would repeat this treatment, 97 % (71/73) responded affirmatively.
The seventh trial involved a cohort of eight patients treated with Scrambler Therapy for chronic low back pain [18]. Patients were treated for six consecutive days; pain scores were recorded prior to initiation of treatment and after each session. The mean pain score was 8.12/10 at baseline, dropping to 6.93/10 after the first treatment. The mean pain score dropped to 3.63/10 in day 6. The group also recorded the Oswestry Disability Index (ODI) and found that mean score dropped from 49.88/100 to 18.44/100 by the end of the study, signifying an average drop from severe to minimal disability.
The eighth investigation was a prospective trial that reported on a series of 39 patients with cancer pain syndromes, including 33 with chemotherapy-induced peripheral neuropathy [17]. Scrambler Therapy was associated with significant positive changes from baseline for a large number of outcomes, including degree of pain, interference with normal activities, and sensory neuropathy symptoms. The benefit persisted up to 3 months.
A small prospective trial published in 2013 involved 10 patients with post-herpetic neuralgia and included some data previously reported in another publication [25]. The work reported a 95 % reduction in pain scores at 1 month, with sustained benefit observed at 2 and 3 month follow-up times.
In 2014, a prospective pilot trial experience was published, involving the treatment of 37 patients with chemotherapy-induced peripheral neuropathy, noting about a 50 % reduction in pain, tingling, and numbness [22]. The increase in Scrambler benefit over the course of the trial suggested that, despite initial operator training in the administration of Scrambler Therapy, a learning curve was evident in this trial. The last 25 % of patients entered on this clinical trial did substantially better than did the first 25 % of patients, likely a reflection of improved technique afforded by greater experience.
The first attempt to compare Scrambler Therapy to a sham control was presented as an abstract at the 2013 Annual Meeting of the American Society of Clinical Oncology, involving 14 patients who were treated in a randomized, controlled, and double-blind manner [16]. Results from this study have not been published as a manuscript. While the authors did note that the sham treatment from this particular trial was believable, in that the patients could not more often detect which of the two procedures was the true one, the authors did not observe any real improvements in neuropathy in the patients treated with the sham procedure versus Scrambler Therapy. This may well have been because this group had little experience with the technique prior to conducting their study. This finding fits with above-noted work that observed that there is a learning curve for the appropriate application of this therapy for treating chemotherapy neuropathy [22], which likely also applies to the treatment of other conditions. Additionally, the results of this trial support that there was not much of a placebo effect in this trial, as no benefit was noted in either trial arm. Paradoxically, this would support the argument that the positive results reported in other chemotherapy neuropathy Scrambler Therapy trials are not just ascribable to a placebo effect.
In 2015, a single-blind, sham-controlled, randomized clinical trial involving 30 patients with low back pain was reported from Virginia Commonwealth University [29]. These authors noted significant decreases in the Brief Pain Inventory (BPI) back pain scores and pain interference scores (P ≤ 0.05). They also noted improvements in pain sensitivity, as measured by participants’ thresholds for pain in the initially painful area. Of note, the group randomizedtoScramblerTherapyhadsubstantialdecreasesin10 serum messenger RNAs (mRNAs) associated with nerve pain such as nerve growth factor (NGF) and glial derived nerve factor (GDNF), compared to no decreases in the sham group, understanding that these mRNAs have not yet been established as correlates for pain.
More recently, two subsequent single-arm prospective trials have been published which support therapeutic benefit. A pilot study was reported from an Italian hospital, evaluating outcomes of Scrambler Therapy in 25 patients with pain related to bony and visceral metastases [31]. Each patient was scheduled for 10 daily sessions of treatment, and pain outcomes were measured by the use of a numeric pain scale. All patients were reported to have experienced at least a 50 % decrease in pain scores, with a mean pain score of 8.4 at baseline dropping to 2.9 after completion of the treatment course. The average duration of “pain control” (defined as >50 % reduction from baseline pain score) was 7.7 +/− 5.3 weeks. Sleep performance was also noted to improve significantly for the cohort. In Korea, Lee et al. performed an open-label, single-arm, exploratory study involving 20 patients with CIPN, metastatic bone pain, and postsurgical neuropathic pain [32]. Pain scores decreased significantly, as did consumption of rescue opioid medication.
Clinical Practice Experiences
Two case series, published in 2013, each included three patients with cancer pain or post-herpetic pain [19, 23]. Both of these reports came from different authors and both reported positive benefits in the patients who were treated.
Sparadeo et al. reported their clinical practice experience regarding 91 of their initial 173 patients, representing all of those for whom they had collected data. These patients had a variety of pain syndromes, including CRPS, spine pain, neuralgias (such as post-herpetic or post-chemotherapy), and multi-focal pain problems [27]. As part of their practice, with these 91 patients, they collected visual pain scores before and after each treatment for all of them and BPI questionnaires, in a subset of them, prior to treatment initiation and at 3- and 6-month follow-up times. The mean pain score prior to the first treatment was 7.2/10; it was 3.0/10 on the 10th day, prior to that day’s treatment. Relatively similar results were seen for the different pain syndromes. BPI scores at 3 to 6 months of follow-up were reported to be improved by more than 50 %.
In a second manuscript, Sparadeo and D’Amato [26] analyzed the pre- and posttreatment data of 95 individuals (some of whom had been reported in the previous publication) entering their Scrambler Therapy program for treatment of chronic neuropathic pain, divided into two groups: CRPS and chronic spine-based pain. All patients were weaned from opioids and anticonvulsants being used for pain control. The data analysis revealed that 70 % of the entire sample was still reporting significant improvement 3 to 6 months following treatment. The two studied groups had similar levels of pain and degrees of lifestyle impact. Additionally, the 3–6-month successes were similar in the two treatment groups.
Another clinical practice experience report involved 147 patients treated at two United States military sites and one South Korean site. They noted that 38 % of patients had at least a 50 % pain reduction that lasted for more than a month [21].
Retrospective Study
Lastly, one retrospective report on Scrambler Therapy, involving 201 patients across multiple centers, was recently reported [33]. Patients were treated for a variety of chronic pain syndromes; the most common indications included post-herpetic neuralgia, chronic low back pain, and polyneuropathy/ peripheral neuropathy. Patients were treated for a mean number of 10 sessions, with 39 patients experiencing complete resolution of pain symptoms sooner than this. The mean pain scores were 7.41 prior to treatment and 1.6 following treatment (P < 0.0001). Achieving a pain score of 0 during treatment was observed to associate with durability of pain control, prompting the authors to advocate for complete response as a target of therapy sessions.
Does Scrambler Therapy actually work?
Arguments against Scrambler Therapy certainly exist, with critics attributing much of the benefit to a placebo effect. Some of the positive endorsements in social media and on the Internet are only anecdotal. Additionally, the developer of Scrambler Therapy participated in the initial clinical trials, and this could be perceived as a potential conflict of interest even though it is scientifically desirable and logical to expect the device inventor to report the first set of results. Additionally, some of the reports claim that there is a phenomenal benefit that lasts for a long time, which sounds too good to be true. Lastly, there are no large, placebo-controlled, double-blinded clinical trials to estimate the effectiveness of Scrambler Therapy.
On the other hand, while some reports [13, 20, 24, 25] involved the inventor of the Scrambler device, these positive findings have been independently replicated by diverse groups [15–19, 21–23, 26–30] in nearly all of the reported studies, involving over 900 patients in total. In some cases, the benefit achieved has been substantial, with some patients achieving complete pain resolution and substantially reduced dependence on pharmacologic therapy. There has been only one report of a negative experience [16]. This was from one small, placebo-controlled trial in patients with chemotherapy-induced peripheral neuropathy. This was published only as an abstract, did not show much of a reduction in either study arm (arguing against a placebo effect), and was produced by a group that did not have much experience using Scrambler Therapy. This raises concerns regarding the validity of this trial, as data have supported that there is an extended learning curve with the provision of Scrambler Therapy, particularly for chemotherapy-induced neuropathy [22]. At the same time, it must be noted that although this is the only negative trial published on Scrambler Therapy, the possibility of publication bias cannot be excluded. Negative experiences may not be put into publication form for various reasons, and so the currently available literature may be overestimating the positive experience with this technology.
The downsides of trying Scrambler Therapy
The downsides of trying Scrambler Therapy for chronic pain primarily relate to the time and expense associated with its administration, in addition to noting that many proposed treatments for chronic pain have not withstood the rigors of time and/or well conducted randomized trials. Additionally, the therapy is not yet widely available and some insurance companies will not pay for it due to lack of evidence or will reimburse it at very low rates. However, some insurance companies are covering this treatment as they have started to note the benefit of this therapy in allowing patients to return to work, with decreased use of medications and procedures. Scrambler Therapy relies on practitioner skill and familiarity with technique, which can influence outcomes, as has been noted in the literature. This might impede rapid integration into practice, especially in the absence of formalized training.
Additional Research
Additional work is needed to better understand the mechanism of Scrambler Therapy and to conduct larger randomized clinical trials investigating the efficacy of Scrambler Therapy in a number of chronic pain states. A large, multi-center, randomized, sham-controlled double-blinded trial, involving patients with a variety of chronic pain syndromes, would strengthen the conclusions from initial studies. The data compiled, to date, support the feasibility and value of such an undertaking. Multiple other research lines of investigation would be helpful for further defining the worth of Scrambler Therapy. Such work could better evaluate the types of patients who benefit, the best means for teaching operators, and the compatibility of this approach with other treatment approaches. For example, as indicated above, there are recommendations to titrate down and discontinue anticonvulsant medications prescribed for pain management prior to initiating Scrambler Therapy, based on the theory and empiric clinical experience that these agents may interfere with the therapeutic mechanisms involved. Whether this is truly necessary could be a focus of future research. To better define the mechanisms of action, studies of brain reactivity (functional MRI) and peripheral nerve function (changes in epidermal nerve fiber density or electrophysiological measures or quantitative sensory nerve testing) would be useful [34].
Acknowledgments
Financial support Research reported in the publication was supported, in part, by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Numbers UL1TR000135 and KL2TR000136-09 (Ruddy). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Compliance with Ethical Standards
Conflict of interest Potential conflict of interest: Competitive Technologies provided Scrambler devices and supplies to Mayo Clinic, Virginia Commonwealth University, and Johns Hopkins for conducting research.
References
- 1.Simon L. Relieving pain in America: A blueprint for transforming prevention, care, education, and research. J Pain Pall Care Phmaracotherapy. 2012;26:197–198. [Google Scholar]
- 2.Stewart WF, Ricci JA, Chee E, Morganstein D, Lipton R. Lost productive time and cost due to common pain conditions in the US workforce. JAMA. 2003;290:2443–2454. doi: 10.1001/jama.290.18.2443. [DOI] [PubMed] [Google Scholar]
- 3.Merskey H, Bogduk N. Classification of chronic pain. 2. IASP Press; Seattle: 1994. [Google Scholar]
- 4.Merskey H, Harold E. Classification of chronic pain: descriptions of chronic pain syndromes and definitions of pain terms. Pain. 1986;3:S1–226. [PubMed] [Google Scholar]
- 5.Woolf CJ, Doubell TP. The pathophysiology of chronic pain—increased sensitivity to low threshold A beta-fibre inputs. Curr Opin Neurobiol. 1994;4:525–534. doi: 10.1016/0959-4388(94)90053-1. [DOI] [PubMed] [Google Scholar]
- 6.Zimmermann M. Pathobiology of neuropathic pain. Eur J Pharmacol. 2001;429:23–37. doi: 10.1016/s0014-2999(01)01303-6. [DOI] [PubMed] [Google Scholar]
- 7.Ji RR, Kohno T, Moore KA, Woolf CJ. Central sensitization and LTP: do pain and memory share similar mechanisms? Trends Neurosci. 2003;26:696–705. doi: 10.1016/j.tins.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 8.Freynhagen R, Bennett MI. Diagnosis and management of neuropathic pain. BMJ. 2009;339:b3002. doi: 10.1136/bmj.b3002. [DOI] [PubMed] [Google Scholar]
- 9.Allegrante JP. The role of adjunctive therapy in the management of chronic nonmalignant pain. Am J Med. 1996;101:33S–39S. doi: 10.1016/s0002-9343(96)00136-2. [DOI] [PubMed] [Google Scholar]
- 10.Maurer D. Transcutaneous stimulator and stimulation method. 1974 Google Patents. [Google Scholar]
- 11.Cruccu G, Aziz TZ, Garcia-Larrea L, Hansson P, Jensen TS, Lefaucheur JP, Simpson BA, Taylor RS. EFNS guidelines on neurostimulation therapy for neuropathic pain. Eur J Neurol. 2007;14:952–970. doi: 10.1111/j.1468-1331.2007.01916.x. [DOI] [PubMed] [Google Scholar]
- 12.Nnoaham KE, Kumbang J. Transcutaneous electrical nerve stimulation (TENS) for chronic pain. Cochrane Database Syst Rev. 2008:CD003222. doi: 10.1002/14651858.CD003222.pub2. [DOI] [PubMed] [Google Scholar]
- 13.Marineo G. Untreatable pain resulting from abdominal cancer: new hope from biophysics? JOP. 2003;4:1–10. [PubMed] [Google Scholar]
- 14.Deer TR, Mekhail N, Provenzano D, Pope J, Krames E, Leong M, Levy RM, Abejon D, Buchser E, Burton A, Buvanendran A, Candido K, Caraway D, Cousins M, DeJongste M, Diwan S, Eldabe S, Gatzinsky K, Foreman RD, Hayek S, Kim P, Kinfe T, Kloth D, Kumar K, Rizvi S, Lad SP, Liem L, Linderoth B, Mackey S, McDowell G, McRoberts P, Poree L, Prager J, Raso L, Rauck R, Russo M, Simpson B, Slavin K, Staats P, Stanton-Hicks M, Verrills P, Wellington J, Williams K, North R. The appropriate use of neurostimulation of the spinal cord and peripheral nervous system for the treatment of chronic pain and ischemic diseases: the Neuromodulation Appropriateness Consensus Committee. Neuromodulation. 2014;17:515–550. doi: 10.1111/ner.12208. [DOI] [PubMed] [Google Scholar]
- 15.Abdi S, Lakkimsetty VM, Barrera J, Candiotti K, Lubarsky D. The Use of “Scrambler Therapy” for Failed Back Surgery Syndrome. Pain Physician. 2011;14:E465–E491. [Google Scholar]
- 16.Campbell T, Nimunkar AJ, Retseck J, Eickhoff JC, Backonja M, Cleary JF, Kwekkeboom KL, Yen TY. A randomized, double-blind study of “Scrambler” therapy versus sham for painful chemotherapy-induced peripheral neuropathy (CIPN) J Clin Oncol. 2013;31(suppl) abstr 9635. [Google Scholar]
- 17.Coyne PJ, Wan W, Dodson P, Swainey C, Smith TJ. A Trial of Scrambler Therapy in the Treatment of Cancer Pain Syndromes and Chronic Chemotherapy-Induced Peripheral Neuropathy. J Pain Palliat Care Pharmacother. 2013;27:359–364. doi: 10.3109/15360288.2013.847519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghatak RK, Nandi SN, Bhakta A, Mandal GC, Bandyopadhyay M, Kumar S. Prospective study of application of biological communication (cybernatics) in management of chronic low back pain—a preliminary report. Nepal Med Coll J. 2011;13:257–260. [PubMed] [Google Scholar]
- 19.Ko YK, Lee HY, Lee WY. Clinical experiences on the effect of scrambler therapy for patients with postherpetic neuralgia. Korean J Pain. 2013;26:98–101. doi: 10.3344/kjp.2013.26.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marineo G, Iorno V, Gandini C, Moschini V, Smith TJ. Scrambler Therapy May Relieve Chronic Neuropathic Pain More Effectively Than Guideline-Based Drug Management: Results of a Pilot, Randomized, Controlled Trial. J Pain Symptom Manage. 2011;43:87–95. doi: 10.1016/j.jpainsymman.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 21.Moon JY, Kurihara C, Beckles JP, Williams KE, Jamison DE, Cohen SP. Predictive factors associated with success and failure for calmare (Scrambler) therapy: a multi-center analysis. Clin J Pain. 2014;31:750–756. doi: 10.1097/AJP.0000000000000155. [DOI] [PubMed] [Google Scholar]
- 22.Pachman DR, Weisbrod BL, Seisler DK, Barton DL, Fee-Schroeder KC, Smith TJ, Lachance DH, Liu H, Shelerud RA, Cheville AL, Loprinzi CL. Pilot evaluation of Scrambler therapy for the treatment of chemotherapy-induced peripheral neuropathy. Support Care Cancer. 2014;23:943–951. doi: 10.1007/s00520-014-2424-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park HS, Sin WK, Kim HY, Moon JY, Park SY, Kim YC, Lee SC. Scrambler therapy for patients with cancer pain - case series. Korean J Pain. 2013;26:65–71. doi: 10.3344/kjp.2013.26.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sabato AF, Marineo G, Gatti A. Scrambler therapy. Minerva Anestesiol. 2005;71:479–482. [PubMed] [Google Scholar]
- 25.Smith TJ, Marineo G. Treatment of postherpetic pain with scrambler therapy, a patient-specific neurocutaneous electrical stimulation device. Am J Hosp Palliat Care. 2013 doi: 10.1177/1049909113494002. [DOI] [PubMed] [Google Scholar]
- 26.Sparadeo F, Kaufman C, D’Amato S. Scrambler Therapy: Effective use of artificial neurons for the treatment of neuropathic pain. J Life Care Plan. 2014;14(4):19–30. [Google Scholar]
- 27.Sparadeo FCK, D’Amato S. Scrambler Therapy: An Innovative and Effective Treatment for Chronic Neuropathic Pain. J Life Care Plan. 2012;11(3):3–15. [Google Scholar]
- 28.Ricci M, Pirotti S, Scarpi E, Burgio M, Maltoni M, Sansoni E, Amadori D. Managing chronic pain: results from an open-label study using MC5-A Calmare(R) device. Support Care Cancer. 2011;20:405–412. doi: 10.1007/s00520-011-1128-6. [DOI] [PubMed] [Google Scholar]
- 29.Starkweather AR, Coyne P, Lyon DE, Elswick RK, Jr, An K, Sturgill J. Decreased low back pain intensity and differential gene expression following calmare(R): results from a double-blinded randomized sham-controlled study. Res Nurs Health. 2015;38:29–38. doi: 10.1002/nur.21632. [DOI] [PubMed] [Google Scholar]
- 30.Smith TJ, Coyne PJ, Parker GL, Dodson P, Ramakrishnan V. Pilot trial of a patient-specific cutaneous electrostimulation device (MC5-A Calmare(R)) for chemotherapy-induced peripheral neuropathy. J Pain Symptom Manage. 2010;40:883–891. doi: 10.1016/j.jpainsymman.2010.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Notaro P, Dell’Agnola CA, Dell’Agnola AJ, Amatu A, Bencardino KB, Siena S. Pilot evaluation of scrambler therapy for pain induced by bone and visceral metastases and refractory to standard therapies. Support Care Cancer. 2015 doi: 10.1007/s00520-015-2952-x. [DOI] [PubMed] [Google Scholar]
- 32.Lee SC, Park KS, Moon JY, Kim EJ, Kim YC, Seo H, Kyung J, Lee DJ. An exploratory study on the effectiveness of “Calmare therapy” in patients with cancer-related neuropathic pain: A pilot study. Eur J Oncol Nurs. 2016;21:1–7. doi: 10.1016/j.ejon.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 33.Compagnone C, Tagliaferri F. Chronic pain treatment and scrambler therapy: a multicenter retrospective analysis. Acta Biomed. 2015;86:149–156. [PubMed] [Google Scholar]
- 34.Gustin SM, Peck CC, Wilcox SL, Nash PG, Murray GM, Henderson LA. Different pain, different brain: thalamic anatomy in neuropathic and non-neuropathic chronic pain syndromes. J Neurosci. 2011;31:5956–5964. doi: 10.1523/JNEUROSCI.5980-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]