Abstract
Medium spiny neurons (MSNs) are the main projection neurons of the striatum and are preferentially lost in Huntington's disease (HD). With no current cure for this neurodegenerative disorder, the specificity of neuronal loss in the striatum makes cell transplantation therapy an attractive avenue for its treatment. Also, given that MSNs are particularly vulnerable in HD, it is necessary to understand why these neurons degenerate in order to develop new therapeutic options. Both approaches require access to human MSN progenitors and their mature neuronal derivatives. Human embryonic stem cells and HD patient induced pluripotent stem cells (together referred to as hPSCs) may serve as an unlimited source of such tissue if they can be directed toward authentic striatal neuronal lineage. Understanding the MSN differentiation pathway in the brain is therefore of paramount importance for the generation of accurate protocols to obtain striatal cells in vitro. The focus of this mini review will be on striatal development and current methods to generate MSNs from hPSCs.
Keywords: Activin, DARPP32, developmental biology, Huntington's disease, lateral ganglionic eminence, medium spiny neuron, neural differentiation, pluripotent stem cell, striatum, transplantation
Abbreviations
- ALKs
Activin-like kinase receptors
- BDNF
brain derived neurotrophic factor
- BMPs
bone morphogenic proteins
- CGE
caudal ganglionic eminence
- DARPP32
the dopamine- and cAMP-regulated phosphoprotein
- DRD1
D1 dopamine receptors Drd2
- DRD2
dopamine receptor 2
- E
embryonic day
- Enk
ENK enkephalin
- ESCs
embryonic stem cells
- GDFs
growth differentiation factors
- HD
Huntington's disease
- hPSCs
human pluripotent stem cells
- iN
induced neuron
- iPSCs
induced pluripotent stem cells
- LGE
lateral ganglionic eminence
- MGE
medial ganglionic eminence
- MSNs
medium spiny neurons
- pSmad2
phosphorylated Smad2
- RA
retinoic acid
- SHH
sonic hedgehog
- SVZ
subventricular zone
- TFs
transcription factors
- TGF β
Transforming Growth Factor β.
Introduction
Medium spiny neurons (MSNs) comprise 90% of the neuronal population in the striatum and are the main output projection cells of this brain region. Understanding how and when MSNs are born during normal human development is of particular interest to the Huntington's disease (HD) research community as this cell population is the main target of neurodegeneration in HD.1,2 Although tremendous effort has been made toward uncovering molecular mechanisms causing preferential MSN degeneration, modeling HD using patient-derived induced pluripotent stem cells (iPSCs) has been hampered by a low yield of these neurons in HD iPSC cultures.3 Fate committed neuronal progenitors have been demonstrated to survive and integrate better in the host brain than non-dividing neurons following transplantation.4 Thus, there is great demand for a source of MSN progenitor cells in order to develop cell transplantation therapies aimed at replenishing the lost neuronal population in patients' brains.
Human pluripotent stem cells (hPSCs) are emerging as an attractive donor cell source that can be standardized for clinical applications. MSNs derived from hPSCs present us with unlimited access to live human neurons. Their value extends beyond their use in the field of cell replacement therapy to the potential to study human development, as well as compare healthy and diseased neurons derived from patient iPSCs. This latter application provides a useful platform for high throughput screening of potential drugs before taking them into animal models. However, in order to exploit these possibilities, reliable and efficient differentiation paradigms are necessary to produce cell types of interest. This mini review will focus on current knowledge of striatal development and will provide an overview of existing protocols to generate MSNs from hPSCs.
Striatal Development
The striatum is developed from the lateral ganglionic eminence (LGE) in the ventral telencephalon, also known as the subpallium (Fig. 1). The LGE is the birthplace of MSNs and a small population of olfactory bulb interneurons.5 The two other neurogenic domains of the subpallium, the medial and caudal ganglionic eminences (MGE and CGE, respectively), give rise to cortical and striatal interneurons and globus pallidus projection neurons.6-8 The LGE is patterned under the influence of antagonistic morphogen gradients between sonic hedgehog (SHH) from the floorplate and the bone morphogenic proteins (BMPs) derived from the dorsal roofplate.9-11 Working downstream and in concert with the dorsal-ventral patterning morphogens are domain-specifically expressed transcription factors (TFs), such as PAX6 and GLI3 dorsally in the developing cortex and NKX2.1 in the MGE and CGE. A coordinated action between these extrinsic and intrinsic regulators is critical for the proper establishment of the LGE and striatum.
Figure 1.
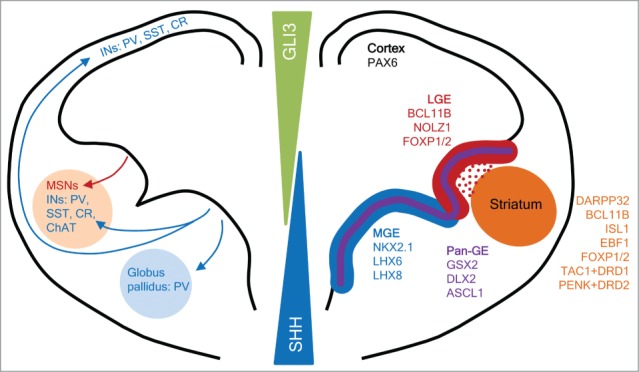
Schematic representation of a coronal hemisection of the developing telencephalon showing the cell migration (left) and gene expression (right) profile of morphologically defined progenitor domains of the cortex and medial (MGE) and lateral ganglionic eminences (LGE). Dorsal-ventral patterning of the telencephalon is achieved through gradients of morphogens SHH and GLI3. MSN progenitors develop in the LGE and populate the striatum, along with interneurons (INs) that migrate from the MGE. Cortical INs bypass the striatum and migrate from the MGE to the cortex. Parvalbumin+ (PV+) globus pallidus projection neurons also originate in the MGE. Abbreviations: GE: ganglionic eminence; SST: somatostatin; CR: calretinin; ChAT: choline acetyltransferase. Figure adapted from.56
GSX2 is a crucial homeodomain protein in early subpallial development involved in the maintenance of molecular identity of LGE progenitors.12 It is first detected in the forebrain from embryonic day 9 (E9) in mice and is expressed in the later developing ganglionic eminences, ventral thalamus and hypothalamus. In the absence of Gsx2, the mutant LGE is reduced in size and the subpallial markers DLX2 and ASCL1 (also named MASH1) are lost from all but the most ventral aspect of the LGE and replaced by the dorsal regulators PAX6 and NGN1/2 at E11.5.13 Furthermore, expression levels of Ebf1, important in the transition of progenitors from the subventricular zone (SVZ) to the striatal mantle, and Gad67, a biosynthetic enzyme that catalyzes the formation of the neurotransmitter GABA, are also greatly reduced in the Gsx2-/- LGE at E12.5.14 However, these molecular abnormalities are less prominent at later stages when aforementioned markers are expressed throughout the LGE up to the cortical-striatal boundary, suggesting that other genes are also at play.13,14 When expressed ectopically in the dorsal telencephalon, Gsx2 is able to up-regulate DLX1/2 and ASCL1 at the expense of cortical markers, further highlighting its participation in the specification of the LGE identity.5
The mis-specification of early LGE progenitors caused by the loss of Gsx2 affects the development of subsets of early born striatal neurons of the patch compartment. At E18.5, enkephalin (Enk) transcript levels are significantly decreased and dopamine receptor 2 (Drd2) expression is missing in the nucleus accumbens and perirhinal aspect of the ventral telencephalon, regions that are believed to be populated by early born striatal cells.14,15 This is further supported by a partial loss of cells positive for a mature MSN marker DARPP32 (the dopamine- and cAMP-regulated phosphoprotein) and fewer and smaller patches in the striatal SVZ at this age.14,16, 17 Although all MSNs express this protein in the adult striatum (i.e. in both the patch and matrix compartments), it is largely restricted to earlier born patch neurons at prenatal stages.18 The striatal defects in Gsx2−/− mutants are exacerbated by the loss of Gsx1, a gene closely related to Gsx2, resulting in no DARPP32+ neurons in the patch compartment of the double mutant striatum.16 This demonstrates a requirement for at least one of these genes for the differentiation of striatal patch MSNs. In contrast, later born striatal matrix neurons expressing calbindin and Ebf1 appear to be unaffected in Gsx2-/- and Gsx2/1-/- mice, indicating that Gsx-independent mechanisms might be important at later stages of striatal neurogenesis.14,16
Once the LGE progenitor domain is established, these cells give rise to the striatal complex through the activity of several regulatory genes, including DLX1/2, ASCL1 and EBF1. ASCL1 is a TF that is autonomously involved in neurogenesis and nonautonomously represses differentiation of adjacent progenitors through Notch signaling.19 Ascl1 mutants exhibit only moderate alterations in striatal development where Dlx1 and Gad67 are still expressed in the subpallium, while Gsx2/Ascl1 double mutants show severely affected LGE development similar to that seen in Gsx2/1-/- mice.20 After Ascl1 activity in the early stages of neurogenesis, Dlx1/2 repress its expression and Notch signaling, promoting later steps in LGE differentiation.21,22 Single knock outs of either Dlx1 or 2 do not show any evidence of forebrain defects, while Dlx1/2-/- mice have disrupted molecular identity of the SVZ in the LGE and reduced migration of later born striatal matrix neurons.23 EBF1 controls later striatal differentiation and loss of Ebf1 expression leads to defects in SVZ to mantle transition and increased progenitor death.24 A dramatic atrophy of the postnatal striatum is observed, characterized by the reduction of the matrix compartment, whereas the patch compartment remains relatively preserved.
GSX2 and DLX2 function upstream of other TFs such as HELIOS and NOLZ1, which promote cell cycle exit and terminal striatal differentiation.25,26 Although Helios is expressed downstream of Gsx2 and Dlx1/2, it is not directly regulated by these 2 genes. It is preferentially expressed in the developing matrix compartment co-localizing with BCL11B (also known as CTIP2) and FOXP1, well established markers of MSNs, however Helios's exact role in striatal development remains unknown.25 Nolz1 overexpression in neural progenitors promotes cell cycle exit and the acquisition of a neuronal phenotype.26 It relies on Gsx2 for normal expression since it is lost in Gsx2-/- mutants (thus, it may have a role in the development of MSNs of the patch compartment) and regulates retinoic acid (RA)-induced neurogenesis during LGE development. ASCL1, DLX1/2 and NOLZ1 in cooperation with RA signaling activate GAD67, initiating GABAergic fate specification in subpallial progenitors.26,27 While loss of RA signaling in Raldh3-/- embryos does not affect MAP2+ and DARPP32+ neurogenesis in the basal ganglia, it prevents striatal neurons from acquiring a GABAergic fate, suggesting that RA signaling is required to stimulate GABA synthesis in LGE-derived progenitors.27,28
After the active phase of neurogenesis (E14 in the rat and 50 d post fertilization in the human), juvenile post-mitotic neurons in the mantle zone of the LGE and striatal anlage cease to express GSX2 and DLX2 and are identified instead by the expression of BCL11B, FOXP1, FOXP2, ISL1 and EBF1 (Fig. 1).25,29 Mature MSNs can be identified by co-expression of BCL11B and DARPP32, while MSNs in patch and matrix compartments of the striatum can be distinguished by their expression of FOXP2 and FOXP1, respectively.30,31 Co-localization of these proteins is necessary to reliably identify cells as MSNs, as each of them is individually expressed in cortical neurons.29 Two circuits of the basal ganglia originate from distinct populations of MSNs expressing D1 dopamine receptors (DRD1) and substance P (direct) and DRD2 and ENK (indirect).32 Despite numerous studies of subpallial development described above our understanding of the induction of MSN fate in the developing LGE is still limited.
Derivation of MSNs from ESCs
Given the right cues embryonic stem cells (ESCs) have the capacity to differentiate into any cell types in the body. After ESCs exit the pluripotent state and become neuralized, different patterning factors are generally applied to direct newly derived naïve neuroectoderm cells toward a regionally-specific progenitor fate, which subsequently differentiate into a defined neuronal cell type. The first group to report a method for MSN differentiation in vitro used mouse stromal cell co-culture for neural induction of hESCs.33 Neural precursor cells were treated with BDNF, SHH and DKK1 to induce anterior-ventral identity, then neural rosettes were mechanically isolated and expanded in the same differentiation medium before maturation in the absence of SHH and DKK1. This method proved to be fairly inefficient at inducing a neuronal fate in hESCs (22% MAP2+ cells) but produced a >50% yield of DARPP32+ cells within the neuronal population after 60 d in culture. This suggested that SHH and DKK1 were somewhat effective in patterning hESCs-derivatives toward an MSN fate. Although differentiated progenitors gave rise to DARPP32+ neurons in vivo when transplanted into a quinolinic acid-lesioned rat striatum, there was overgrowth of neural progenitors 3–5 months after the graft. The ventralizing effect of SHH was further investigated by Ma et al.,34 who used an embryoid body method to generate neuroepithelial cells that were manually isolated and treated with a dose of SHH that effectively down-regulated PAX6 and up-regulated ASCL1, MEIS2 and GSX2, while keeping NKX2.1 expression to a minimum of 30%. This approach was far more efficient at producing neurons (93% βIII-tubulin1+ cells) and generation of MSNs with 80% of neurons co-labeling with DARPP32 and GABA. Four months after transplantation, no graft overgrowth was observed and DARPP32+ and GABA+ neurons represented more than half of grafted cells. Transplanted animals showed functional recovery on rotarod test and improvement in parameters of fine gait movements. Although this differentiation paradigm appears highly efficient in generating MSNs, the manual isolation of neural colonies makes this method unsuitable for large scale and automated MSN production.
Both Carri et al.35 and Nicoleau et al.36 took advantage of the highly efficient neural conversion of hESCs based on the dual inhibition of Smad signaling discovered by Chambers et al.,37 and confirmed the efficacy of the monolayer differentiation method (51% and 58% MAP2+ cells, respectively). Ventral telencephalic specification was also induced by SHH treatment, resulting in BCL11B+ and GABA+ neurons and an overall 20% DARPP32+ neurons in vitro.35 Transplanted MSN progenitors survived in vivo but showed a tendency to overgrow and no behavioral recovery. Nicoleau et al.36 optimized Wnt signaling inhibition by DKK1 or a chemical Wnt antagonist, XAV-939, and demonstrated an enhancement of FOXG1+ forebrain cell production in a dose-dependent manner. They also showed that a lower concentration of SHH was more efficient at inducing the LGE fate, while a higher concentration directed cells toward the MGE phenotype with 80% NKX2.1+ cells. Together, XAV-939 and SHH-treated cultures generated 28% DARPP32+ neurons with evidence of the 2 MSN subpopulations expressing DRD1 or DRD2 receptors in vitro. In addition, transplanted LGE progenitors matured into DARPP32+ neurons co-expressing BCL11B and FOXP1 5 months post-grafting, although behavior was not assessed on these animals.
In contrast to the above conceptually similar MSN differentiation methods, we developed a new protocol for generating MSNs from hPSC by using Transforming Growth Factor β (TGFβ) family protein Activin A (henceforth activin).38 The TGFβ superfamily members are secreted molecules that include TGFβs, Nodal, Activins, growth differentiation factors (GDFs) and BMPs.39 They regulate a variety of developmental processes through specific Activin-like kinase receptors (ALKs), which induce the phosphorylation and stimulation of regulatory Smads. TGFβs, Nodal and Activins act through ALKs 4/5 to recruit Smads 2/3, while GDFs and BMPs activate Smads 1/5/8 through ALKs 2/3/6. Activated Smad complexes translocate to the nucleus where they regulate gene expression through recruitment of co-Smad4 and other homeodomain proteins, including co-activators and co-repressors. Previous studies demonstrated the presence of Activin subunits, receptors and phosphorylated Smad2 (pSmad2) in the developing subpallium and co-localization of pSmad2 with DLX2 throughout.40,41 The two proteins were also confirmed to interact in vivo, and pSmad2 was found to bind to the DLX2 gene enhancer.40 In line with these findings, we treated hPSC-derived forebrain neural progenitors with activin to induce an LGE-derived MSN fate.38 We showed that activin induced markers specific to the LGE, including BCL11B, NOLZ1 and FOXP2 at progenitor stage. While it also upregulated pan-GE markers GSX2 and DLX2, it had no effect on the MGE markers NKX2.1 and LHX8. We demonstrated that induction of these LGE transcripts by activin can be completely blocked by a specific activin/Smad inhibitor, SB431542, suggesting that the specification of striatal characteristics in this system requires activation of the Smad2/3 pathway.
Furthermore, activin stimulated a large increase in BCL11B expression after just 12 hours of treatment, hinting that it may have a direct regulatory effect on its expression. Increase in the levels of LGE-specific TFs upon addition of activin remained higher for BCL11B than others, suggesting that it might be central to MSN fate specification. In vitro, activin-patterned neural precursors efficiently generated mature MSNs immunoreactive for BCL11B, GABA, GAD65/67 and PSD95 with 20–50% neurons co-expressing DARPP32 depending on the hPSC line. Upon transplantation in a rat model of HD, progenitors survived and differentiated into DARPP32+ neurons of both the direct and indirect subtypes of MSNs. Despite indications of glutamatergic and dopaminergic innervation from the host brain, the recipient animals did not show a behavioral improvement. It is worth noting that the behavioral recovery observed with SHH-treated cell-derived grafts might be attributed, at least in part, to the presence of interneurons generated by SHH.34,35 Also, LGE grafts derived with the activin protocol were generally lower in mass than those reported in SHH-based studies, which might affect the behavioral analysis. In fact, we demonstrated that activin acts as a potent pro-differentiation factor by promoting cell cycle exit via SHH signaling inhibition and by enhancing RA signaling.42 This 'pro-differentiation' effect of activin treatment might explain why we do not see overgrowth of neural progenitors after transplantation.
All of the studies using SHH-mediated generation of MSNs have faced the challenging trade-off between SHH and DKK1/XAV-939 dosage and the increase in levels of NKX2.1. While the treatment with patterning factors lead to up-regulated expression of pan-GE markers FOXG1, OTX2 and GSX2, LGE-specific TF BCL11B was unaffected and other LGE specific TFs not analyzed in MSN progenitors. It is possible that the observed results in MSN differentiation of hESCs are due to a generic ventral patterning of neural ectoderm by SHH rather than a specific promotion of striatal progenitor phenotype. This notion is supported by mouse genetics studies, which demonstrated that the primary role of SHH is to repress GLI3 function and vice versa to achieve normal dorsal-ventral patterning in the telencephalon.9 In the absence of either of them, the cortical-striatal border is shifted either ventrally (Shh mutants) or dorsally (Gli3 mutants) while expression of pan-ventral genes Gsx2 and Dlx2 remains persistent in homozygous mutants. We showed that cyclopamine-induced inhibition of SHH signaling in activin-treated cultures does not affect up-regulation of the LGE-enriched genes.38 This is in line with our previous findings that activin is initiating differentiation in forebrain progenitors via GLI3-mediated suppression of SHH pathway.42 Therefore, it seems that activin is able to induce the LGE characteristics in telencephalic neural precursors while simultaneously inhibiting the MGE fate.
Despite the robust effect of activin in LGE fate induction, very little is known about the TGFβ/Activin signaling pathway in subpallial development. Members of the TGFβ superfamily have been implicated in a variety of neurodevelopmental processes, including axon specification and synapse formation, as well as adult neurogenesis, and have been attributed broad neuroprotective functions in the adult brain.43-48 Moreover, TGFβ ligands, receptors and Smad signaling molecules show strong expression in the adult murine striatum, particularly in neurons of the striatal matrix compartment, and TGFβ signaling was found to be reduced in HD striatal tissues.49,50 Thus, this signaling pathway might play a role not only in LGE development but also in adult striatal homeostasis and its disruption might contribute to MSN vulnerability in HD.
Direct Conversion of Somatic Cells into MSNs
A non-hPSC route to generate neurons, direct reprogramming, or induced neuron (iN) technology, has been rapidly developing over recent years. The iN method converts post mitotic somatic cells (often fibroblasts) directly into functional neurons by combinatorial expression of lineage specific TFs.51 In a recent study, Victor et al.52 implemented this method to generate human MSNs via ectopic expression of brain-enriched microRNAs, miR-9/9* and miR-124, with striatal TFs. Interestingly, out of 16 TFs tested, only BCL11B induced DARPP32 expression in the neurons produced using the microRNAs. This further highlights the central role of BCL11B in MSN fate specification. DLX1 and DLX2 were added to promote GABAergic fate, while MYT1L increased the number of MAP2+ cells. Transplanted fibroblasts reprogrammed with the 2 microRNAs and all 4 of these factors survived in the murine striatum for 6 months and matured into MSNs expressing DARPP32, BCL11B, GABA and FOXP1.
The iN technology serves as a valuable model to study gene regulation in cell fate decisions but it has disadvantages over the current hPSC differentiation methods with regard to biomedical applications. Firstly, the iN method converts post mitotic somatic cells directly into neurons, hence it does not offer the opportunity for harvesting fate committed neuronal progenitors. These have been demonstrated to survive and integrate better in the host brain than non-dividing neurons following transplantation, and thus are better suited for applications in cell therapy.4 Secondly, iNs bypass developmental stages that precede final maturation and are therefore unsuitable for applications focusing on neuronal development. Finally, iN MSNs are derived from primary fibroblasts established from skin biopsies, which are generally limited in scale and proliferation capacity before undergoing senescence, unlike hPSCs that can be cultured indefinitely in vitro. There is an upper limit to how many iNs could be generated from a given tissue preparation. Thus, for applications in regenerative medicine and drug screening, which are currently the most sought-after applications of in vitro derived human neurons, the present iN approach cannot replace hPSC differentiation.
Ongoing Challenges
Stem cells hold great promise to become the source of striatal progenitors for clinical applications and a platform for in vitro disease modeling and high throughput drug screening. However, current MSN generation protocols are 40-50% efficient at best in generating striatal neurons in vitro, suggesting that there is still room to improve methods for MSN differentiation. Although mouse genetics studies have identified several TFs as important for MSN development, absence of these TFs does not prevent formation of the striatum. Thus, one of the main ongoing challenges remains to uncover the exact mechanism by which striatal neurons acquire their fate in vivo. It is possible that MSNs generated in SHH-based protocols might be a 'by-product' of general ventralization of forebrain progenitors induced by SHH. On the other hand, induction of the LGE fate by activin might be a more targeted event of striatal lineage specification. Further investigation is required to dissect the different components of this pathway that could be driving LGE characteristics in hPSC-derived neural progenitors. We found that BCL11B is the first striatal marker to be robustly upregulated within just 12 hours of activin treatment and it continues to increase with prolonged exposure to activin.38 This suggests that activin might have a direct regulatory effect on the expression of this TF. There is an increasing amount of evidence that supports BCL11B as a central factor for MSN fate specification and maturation. Loss of BCL11B in mice leads to dramatically reduced striatal DARPP32 expression and aberrant cellular organization into patches.53 Moreover, it has been implicated in BDNF (brain derived neurotrophic factor) signaling, which is also important for DARPP32 expression in mouse MSNs.54,55 Consensus binding sites for this factor include regions upstream of striatum-enriched genes such as DARPP32, FOXP1 and ARPP19. We consider BCL11B the likely mediator down-stream of Activin-Smad signaling in MSN fate specification and it would be interesting to demonstrate direct interaction and binding of this TF to striatal genes.
Conclusion
In conclusion, in vitro derivation of mature MSNs from hPSCs and iNs holds great promise for disease modeling and drug discovery. The development of cell therapies to slow down the progression of HD will benefit greatly from efficient generation of MSN progenitors, which survive better than their mature derivatives post-grafting. Moreover, the value of hPSCs also extends to the potential to study human MSN development in vitro. However, there is still room to improve MSN differentiation methods and our understanding of the induction of the LGE fate in vivo.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
The authors acknowledge the support of the UK Medical Research Council and European Union Framework 7 Neurostemcell Repair and Repair-HD programs in funding our own studies in this area.
References
- 1.Lange H, Thorner G, Hopf A, Schroder KF. Morphometric studies of neuropathological changes in choreatic diseases. J Neurol Sci 1976; 28:401-25; PMID:133209; http://dx.doi.org/ 10.1016/0022-510X(76)90114-3. [DOI] [PubMed] [Google Scholar]
- 2.Reiner A, Albin RL, Anderson KD, Damato CJ, Penney JB, Young AB. Differential loss of striatal projection neurons in Huntington disease. Proc Natl Acad Sci U S A 1988; 85:5733-7; PMID:2456581; http://dx.doi.org/ 10.1073/pnas.85.15.5733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.HD iPSC Consortium. Induced Pluripotent Stem Cells from Patients with Huntington's Disease Show CAG-Repeat-Expansion-Associated Phenotypes. Cell Stem Cell 2012; 11:264-78; PMID:22748968; http://dx.doi.org/ 10.1016/j.stem.2012.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thompson LH, Andersson E, Jensen JB, Barraud P, Guillemot F, Parmar M, Björklund A. Neurogenin2 identifies a transplantable dopamine neuron precursor in the developing ventral mesencephalon. Exp Neurol 2006; 198:183-98; PMID:16438966; http://dx.doi.org/ 10.1016/j.expneurol.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 5.Waclaw RR, Wang B, Pei Z, Ehrman LA, Campbell K. Distinct Temporal Requirements for the Homeobox Gene Gsx2 in Specifying Striatal and Olfactory Bulb Neuronal Fates. Neuron 2009; 63:451-65; PMID:19709628; http://dx.doi.org/ 10.1016/j.neuron.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marin O, Anderson SA, Rubenstein JLR. Origin and molecular specification of striatal interneurons. J Neurosci 2000; 20:6063-76; PMID:10934256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Butt SJB, Fuccillo M, Nery S, Noctor S, Kriegstein A, Corbin JG, Fishell G. The temporal and spatial origins of cortical interneurons predict their physiological subtype. Neuron 2005; 48:591-604; PMID:16301176; http://dx.doi.org/ 10.1016/j.neuron.2005.09.034. [DOI] [PubMed] [Google Scholar]
- 8.Xu Q, Cobos I, De la Cruz E, Rubenstein JL, Anderson SA. Origins of cortical interneuron subtypes. J Neurosci 2004; 24:2612-22; PMID:15028753; http://dx.doi.org/ 10.1523/JNEUROSCI.5667-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rallu M, Machold R, Gaiano N, Corbin JG, McMahon AP, Fishell G. Dorsoventral patterning is established in the telencephalon of mutants lacking both Gli3 and hedgehog signaling. Development 2002; 129:4963-74; PMID:12397105. [DOI] [PubMed] [Google Scholar]
- 10.Furuta Y, Piston DW, Hogan BLM. Bone morphogenetic proteins (BMPs) as regulators of dorsal forebrain development. Development 1997; 124:2203-12; PMID:9187146. [DOI] [PubMed] [Google Scholar]
- 11.Shimamura K, Hartigan DJ, Martinez S, Puelles L, Rubenstein JLR. Longitudinal organization of the anterior neural plate and neural tube. Development 1995; 121:3923-33; PMID:8575293. [DOI] [PubMed] [Google Scholar]
- 12.Szucsik JC, Witte DP, Li H, Pixley SK, Small KM, Potter SS. Altered forebrain and hindbrain development in mice mutant for the Gsh-2 homeobox gene. Dev Biol 1997; 191:230-42; PMID:9398437; http://dx.doi.org/ 10.1006/dbio.1997.8733. [DOI] [PubMed] [Google Scholar]
- 13.Toresson H, Potter SS, Campbell K. Genetic control of dorsal-ventral identity in the telencephalon: opposing roles for Pax6 and Gsh2. Development 2000; 127:4361-71; PMID:11003836. [DOI] [PubMed] [Google Scholar]
- 14.Corbin JG, Gaiano N, Machold RP, Langston A, Fishell G. The Gsh2 homeodomain gene controls multiple aspects of telencephalic development. Development 2000; 127:5007-20; PMID:11060228. [DOI] [PubMed] [Google Scholar]
- 15.DeCarlos JA, LopezMascaraque L, Valverde F. Dynamics of cell migration from the lateral ganglionic eminence in the rat. J Neurosci 1996; 16:6146-56; PMID:8815897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Toresson H, Campbell K. A role for Gsh1 in the developing striatum and olfactory bulb of Gsh2 mutant mice. Development 2001; 128:4769-80; PMID:11731457. [DOI] [PubMed] [Google Scholar]
- 17.Waclaw RR, Wang B, Campbell K. The homeobox gene Gsh2 is required for retinoid production in the embryonic mouse telencephalon. Development 2004; 131:4013-20; PMID:15269172; http://dx.doi.org/ 10.1242/dev.01272. [DOI] [PubMed] [Google Scholar]
- 18.Foster GA, Schultzberg M, Hokfelt T, Goldstein M, Hemmings HC, Ouimet CC, Walaas SI, Greengard P. Development of a dopamine-adrenosine and cyclic adenosine 3'-5'-monophosphate-regulated phosphoprotein (darpp-32) in the prenatal rat central-nervous-system, and its relationship to the arrival of presumptive dopaminergic innervation. J Neurosci 1987; 7:1994-2018; PMID:2886563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Casarosa S, Fode C, Guillemot F. Mash1 regulates neurogenesis in the ventral telencephalon. Development 1999; 126:525-34; PMID:9876181. [DOI] [PubMed] [Google Scholar]
- 20.Wang B, Waclaw RR, Allen ZJ II, Guillemot F, Campbell K. Ascl1 is a required downstream effector of Gsx gene function in the embryonic mouse telencephalon. Neural Dev 2009; 4:5; http://dx.doi.org/ 10.1186/1749-8104-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yun K, Fischman S, Johnson J, de Angelis MH, Weinmaster G, Rubenstein JLR. Modulation of the notch signaling by Mash1 and DIx1/2 regulates sequential specification and differentiation of progenitor cell types in the subcortical telencephalon. Development 2002; 129:5029-40; PMID:12397111. [DOI] [PubMed] [Google Scholar]
- 22.Long JE, Swan C, Liang WS, Cobos I, Potter GB, Rubenstein JLR. Dlx1&2 and Mash1 Transcription Factors Control Striatal Patterning and Differentiation Through Parallel and Overlapping Pathways. J Comp Neurol 2009; 512:556-72; PMID:19030180; http://dx.doi.org/ 10.1002/cne.21854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anderson SA, Qiu MS, Bulfone A, Eisenstat DD, Meneses J, Pedersen R, Rubenstein JL. Mutations of the homeobox genes Dlx-1 and Dlx-2 disrupt the striatal subventricular zone and differentiation of late born striatal neurons. Neuron 1997; 19:27-37; PMID:9247261; http://dx.doi.org/ 10.1016/S0896-6273(00)80345-1. [DOI] [PubMed] [Google Scholar]
- 24.Garel S, Marin F, Grosschedl R, Charnay P. Ebf1 controls early cell differentiation in the embryonic striatum. Development 1999; 126:5285-94; PMID:10556054. [DOI] [PubMed] [Google Scholar]
- 25.Martin-Ibanez R, Crespo E, Esgleas M, Urban N, Wang B, Waclaw R, Georgopoulos K, Martínez S, Campbell K, Vicario-Abejón C, et al. Helios Transcription Factor Expression Depends on Gsx2 and Dlx1&2 Function in Developing Striatal Matrix Neurons. Stem Cells Dev 2012; 21:2239-51; PMID:22142223; http://dx.doi.org/ 10.1089/scd.2011.0607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Urban N, Martin-Ibanez R, Herranz C, Esgleas M, Crespo E, Pardo M, et al. Nolz1 promotes striatal neurogenesis through the regulation of retinoic acid signaling. Neural Dev 2010; 5:22; PMID:20809932; http://dx.doi.org/ 10.1186/1749-8104-5-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chatzi C, Brade T, Duester G. Retinoic Acid Functions as a Key GABAergic Differentiation Signal in the Basal Ganglia. Plos Biol 2011; 9:e1000609; PMID:21532733; http://dx.doi.org/ 10.1371/journal.pbio.1000609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Molotkova N, Molotkov A, Duester G. Role of retinoic acid during forebrain development begins late when Raldh3 generates retinoic acid in the ventral subventricular zone. Dev Biol 2007; 303:601-10; PMID:17207476; http://dx.doi.org/ 10.1016/j.ydbio.2006.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Onorati M, Castiglioni V, Biasci D, Cesana E, Menon R, Vuono R, Talpo F, Goya RL, Lyons PA, Bulfamante GP, et al. Molecular and functional definition of the developing human striatum. Nat Neurosci 2014; 17:1804-15; PMID:25383901; http://dx.doi.org/ 10.1038/nn.3860. [DOI] [PubMed] [Google Scholar]
- 30.Takahashi K, Liu FC, Hirokawa K, Takahashi H. Expression of Foxp2, a gene involved in speech and language, in the developing and adult striatum. J Neurosci Res 2003; 73:61-72; PMID:12815709; http://dx.doi.org/ 10.1002/jnr.10638. [DOI] [PubMed] [Google Scholar]
- 31.Tamura S, Morikawa Y, Iwanishi H, Hisaoka T, Senba E. Foxp1 gene expression in projection neurons of the mouse striatum. Neuroscience 2004; 124:261-7; PMID:14980377; http://dx.doi.org/ 10.1016/j.neuroscience.2003.11.036. [DOI] [PubMed] [Google Scholar]
- 32.Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJ, Sibley DR. D1 and D2 dopamine receptor regulated gene-expression of striatonigral and striatopallidal neurons. Science 1990; 250:1429-32; PMID:2147780; http://dx.doi.org/ 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- 33.Aubry L, Bugi A, Lefort N, Rousseau F, Peschanski M, Perrier AL. Striatal progenitors derived from human ES cells mature into DARPP32 neurons in vitro and in quinolinic acid-lesioned rats. Proc Natl Acad Sci U S A 2008; 105:16707-12; PMID:18922775; http://dx.doi.org/ 10.1073/pnas.0808488105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ma L, Hu B, Liu Y, Vermilyea SC, Liu H, Gao L, Sun Y, Zhang X, Zhang SC, et al. Human Embryonic Stem Cell-Derived GABA Neurons Correct Locomotion Deficits in Quinolinic Acid-Lesioned Mice. Cell Stem Cell 2012; 10:455-64; PMID:22424902; http://dx.doi.org/ 10.1016/j.stem.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carri AD, Onorati M, Lelos MJ, Castiglioni V, Faedo A, Menon R, Camnasio S, Vuono R, Spaiardi P, Talpo F, et al. Developmentally coordinated extrinsic signals drive human pluripotent stem cell differentiation toward authentic DARPP-32(+) medium-sized spiny neurons. Development 2013; 140:301-12; http://dx.doi.org/ 10.1242/dev.084608. [DOI] [PubMed] [Google Scholar]
- 36.Nicoleau C, Varela C, Bonnefond C, Maury Y, Bugi A, Aubry L, Viegas P, Bourgois-Rocha F, Peschanski M, Perrier AL. Embryonic Stem Cells Neural Differentiation Qualifies the Role of Wnt/β-Catenin Signals in Human Telencephalic Specification and Regionalization. Stem Cells 2013; 31:1763-74; PMID:23818270; http://dx.doi.org/ 10.1002/stem.1462. [DOI] [PubMed] [Google Scholar]
- 37.Chambers SM, Fasano CA, Papapetrou EP, Tomishima M, Sadelain M, Studer L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol 2009; 27:275-80; PMID:19252484; http://dx.doi.org/ 10.1038/nbt.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arber C, Precious SV, Cambray S, Risner-Janiczek JR, Kelly C, Noakes Z, Fjodorova M, Heuer A, Ungless MA, Rodríguez TA, et al. Activin A directs striatal projection neuron differentiation of human pluripotent stem cells. Development 2015; 142:1375-86; PMID:25804741; http://dx.doi.org/ 10.1242/dev.117093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Massague J. TGF-β signal transduction. Annu Rev Biochem 1998; 67:753-91; PMID:9759503; http://dx.doi.org/ 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- 40.Maira M, Long JE, Lee AY, Rubenstein JLR, Stifani S. Role for TGF-β superfamily signaling in telencephalic GABAergic neuron development. J Neurodev Disord 2010; 2:48-60; PMID:20339443; http://dx.doi.org/ 10.1007/s11689-009-9035-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Feijen A, Goumans MJ, Vandeneijndenvanraaij AJM. Expression of activin subunits, activin receptors and follistatin in postimplantation mouse embryos suggests specific developmental functions for different activins. Development 1994; 120:3621-37; PMID:7821227. [DOI] [PubMed] [Google Scholar]
- 42.Cambray S, Arber C, Little G, Dougalis AG, de Paola V, Ungless MA, Li M, Rodríguez TA. Activin induces cortical interneuron identity and differentiation in embryonic stem cell-derived telencephalic neural precursors. Nature Communications 2012; 3:841; PMID:22588303; http://dx.doi.org/ 10.1038/ncomms1817. [DOI] [PubMed] [Google Scholar]
- 43.Yi JJ, Barnes AP, Hand R, Polleux F, Ehlers MD. TGF-β Signaling Specifies Axons during Brain Development. Cell 2010; 142:144-57; PMID:20603020; http://dx.doi.org/ 10.1016/j.cell.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Packard M, Mathew D, Budnik V. Wnts and TGF β in synaptogenesis: Old friends signalling at new places. Nat Rev Neurosci 2003; 4:113-20; PMID:12563282; http://dx.doi.org/ 10.1038/nrn1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.He Y, Zhang H, Yung A, Villeda SA, Jaeger PA, Olayiwola O, Fainberg N, Wyss-Coray T. ALK5-dependent TGF-β signaling is a major determinant of late-stage adult neurogenesis. Nat Neurosci 2014; 17:943-52; PMID:24859199; http://dx.doi.org/ 10.1038/nn.3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Unsicker K, Krieglstein K. TGF-β s and their roles in the regulation of neuron survival. Mol Cell Biol Neuroprot Cns 2002; 513:353-74; http://dx.doi.org/ 10.1007/978-1-4615-0123-7_13. [DOI] [PubMed] [Google Scholar]
- 47.Abdipranoto-Cowley A, Park JS, Croucher D, Daniel J, Henshall S, Galbraith S, Mervin K, Vissel B. Activin A Is Essential for Neurogenesis Following Neurodegeneration. Stem Cells 2009; 27:1330-46; PMID:19489097; http://dx.doi.org/ 10.1002/stem.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stayte S, Rentsch P, Li KM, Vissel B. Activin A Protects Midbrain Neurons in the 6-Hydroxydopamine Mouse Model of Parkinson Disease. Plos One 2015; 10:e0124325; PMID:25902062; http://dx.doi.org/ 10.1371/journal.pone.0124325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tapia-Gonzalez S, Giraldez-Perez RM, Isabel Cuartero M, Jose Casarejos M, Angeles Mena M, Wang X-F, Sánchez-Capelo A. Dopamine and α- synuclein dysfunction in Smad3 null mice. Mol Neurodegener 2011; 6:72; PMID:21995845; http://dx.doi.org/ 10.1186/1750-1326-6-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Battaglia G, Cannella M, Riozzi B, Orobello S, Maat-Schieman ML, Aronica E, Busceti CL, Ciarmiello A, Alberti S, Amico E, et al. Early defect of transforming growth factor β(1) formation in Huntington's disease. J Cell Mol Med 2011; 15:555-71; PMID:20082658; http://dx.doi.org/ 10.1111/j.1582-4934.2010.01011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Suedhof TC, Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature 2010; 463:1035-U50; PMID:20107439; http://dx.doi.org/ 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Victor MB, Richner M, Hermanstyne TO, Ransdell JL, Sobieski C, Deng PY, Klyachko VA, Nerbonne JM, Yoo AS. Generation of Human Striatal Neurons by MicroRNA-Dependent Direct Conversion of Fibroblasts. Neuron 2014; 84:311-23; PMID:25374357; http://dx.doi.org/ 10.1016/j.neuron.2014.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arlotta P, Molyneaux BJ, Jabaudon D, Yoshida Y, Macklis JD. Ctip2 controls the differentiation of medium spiny neurons and the establishment of the cellular architecture of the striatum. J Neurosci 2008; 28:622-32; PMID:18199763; http://dx.doi.org/ 10.1523/JNEUROSCI.2986-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tang B, Di Lena P, Schaffer L, Head SR, Baldi P, Thomas EA. Genome-Wide Identification of Bcl11b Gene Targets Reveals Role in Brain-Derived Neurotrophic Factor Signaling. Plos One 2011; 6:e23691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ivkovic S, Polonskaia O, Farinas I, Ehrlich ME. Brain-derived neurotrophic factor regulates maturation of the DARPP-32 phenotype in striatal medium spiny neurons: Studies in vivo and in vitro. Neuroscience 1997; 79:509-16; PMID:9200733; http://dx.doi.org/ 10.1016/S0306-4522(96)00684-7. [DOI] [PubMed] [Google Scholar]
- 56.Noakes Z, Fjodorova M, Li M. Deriving striatal projection neurons from human pluripotent stem cells with Activin A. Neural Regeneration Res (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]


